Abstract
Endothelial-to-mesenchymal transition (EndMT) is involved in cardiac fibrosis induced by angiotensin II (Ang II). A disintegrin and metalloproteinase 8 (ADAM8), a member of ADAMs family, participates in cell adhesion, proteolysis and various signaling. However, its effects on the development of cardiac fibrosis remain completely unknown. This study aimed to reveal whether ADAM8 aggravates cardiac fibrosis induced by Ang II in vivo and in vitro. The C57BL/6J mice or cardiac endothelial cells were subjected to Ang II infusion to induce fibrosis. The results showed that systolic blood pressure and diastolic blood pressure were significantly increased under Ang II infusion, and ADAM8 was up-regulated. ADAM8 inhibition attenuated Ang II-induced cardiac dysfunction. ADAM8 knockdown suppressed Ang II-induced cardiac fibrosis as evidenced by the down-regulation of CTGF, collagen I, and collagen III. In addition, the endothelial marker (VE-cadherin) was decreased, whilst mesenchymal markers (α-SMA and FSP1) were increased following Ang II infusion. However, ADAM8 repression inhibited Ang II-induced EndMT. Moreover, ADAM8 silencing repressed the activation of TGF-β1/Smad2/Smad3 pathways. Consistent with the results in vivo, we also found the inhibitory effects of ADAM8 inhibition on EndMT in vitro. All data suggest that ADAM8 promotes Ang II-induced cardiac fibrosis and EndMT via activating TGF-β1/Smad2/Smad3 pathways.
Keywords: a disintegrin and metalloproteinase 8 (ADAM8), angiotensin II, cardiac fibrosis, endothelial-mesenchymal transition, TGF-β1/Smad2/Smad3 signaling
Introduction
Hypertension is one of the common cardiovascular diseases with complicated pathological mechanism and long treatment cycle, and it is difficult to cure [1]. Many patients need to take anti-hypertensive drugs throughout their lives. Hypertensive cardiovascular disease is an organic heart disease formed by the long-term increase of blood pressure, the augment of ventricular load, as well as the compensatory left ventricular (LV) hypertrophy and dilation [2]. Previous studies have shown that 50% of heart failure is caused by high blood pressure [3]. In addition, it may also be associated with coronary heart disease, atrial fibrillation and other complications.
Studies report that using angiotensin II (Ang II) can establish the hypertension model [4]. Growing evidence indicates that Ang II is a pivotal regulator in hypertensive cardiovascular disease [5]. Ang II up-regulates transforming growth factor-beta 1 (TGF-β1) through its type 1 receptor inducing cardiomyocyte hypertrophy and myofibroblast transformation [6]. Moreover, activation of TGF-β1/Smad signaling plays a central role in the pathogenesis of hypertensive cardiac disease [7]. Therefore, finding a target that can block Ang II [8] may open our eyes to the treatment of hypertensive cardiovascular disease.
Endothelial-to-mesenchymal transition (EndMT) is involved in the development of cardiac fibrosis [9]. During this process, endothelial cells gradually lose the adhesion between cells and endothelial characteristics, which is manifested by the down-regulation of VE-cadherin. Following, cells have acquired migration and invasion properties, as evidenced by the up-regulation of mesenchymal-specific markers, such as α-SMA, fibroblast-specific protein 1 (FSP1), and vimentin [10]. Improving endothelial function and inhibiting EnMT is beneficial for improving hypertensive cardiovascular disease induced by Ang II [11].
A disintegrin and metalloproteinase 8 (ADAM8) belongs to the ADAMs family and participates in cell adhesion, proteolysis and various signaling pathways [12]. Studies have shown that elevated ADAM8 expression and soluble ADAM8 (sADAM8) content in the blood are associated with the occurrence of some cardiovascular diseases [13, 14]. In addition, ADAM8 can induce epithelial-mesenchymal transition to facilitate the invasion of colon cancer cells via TGF-β/Smad2/3 signaling pathway [15]. Moreover, its family members play an important role in various fibrotic diseases. For instance, ADAM10 can accelerate pulmonary fibrosis [16]. ADAM17 inhibition suppresses renal fibrosis [17]. Therefore, we speculate that ADAM8, as a member of the same family, is likely to have similar functions. In this study, we investigated the functional role and mechanism of ADAM8 in Ang II-induced cardiac fibrosis in vivo and in vitro.
Materials and Methods
Animal experiments
All animal studies were approved by the Institutional Animal Use and Care Committee of Hebei General Hospital. Male 8–10-week-old C57BL/6J mice were housed at 22 ± 1°C and 45–55% humidity for 1 week. During this period, they were allowed access to food and water ad libitum for adapting to the environment. The mice in the Ang II group were treated with Ang II (Glbiochem, Shanghai, China) at a dose of 1.5 mg/kg/day for 14 days via subcutaneous infusion using osmotic minipumps (Alzet, Cupertino, CA, USA). The mice in the control (Con) group received saline infusion in the same way. The mice in the Ang II + LV-shADAM8 or Ang II + LV-shNC group were treated with lentivirus-shRNA targeting Adam8 or negative control via tail vein injection prior to Ang II administration. The mice were anesthetized to death by intraperitoneal injection of 200 mg/kg pentobarbital sodium. The heart tissues and serum were isolated for further experiments.
Blood pressure and echocardiography
Blood pressure was detected by using a noninvasive tail cuff method (Non-invasive blood pressure system, AlcBio, Shanghai, China) after Ang II infusion at 14th day as previously described [18]. Cardiac function was measured by echocardiography using ultrasound imaging system (GE VOLUSON E8, Milwaukee, WI, USA) following Ang II infusion at 14th day. Left ventricular end-systolic diameter (LVESD) and LV end-diastolic diameter (LVEDD) were recorded. LV ejection fraction (LVEF)% and LV fraction shortening (LVFS)% were calculated using M-mode measurements.
Immunohistochemistry and Masson’s trichrome stain
The cardiac tissues were fixed in 4% paraformaldehyde, subsequently embedded in paraffin. Following the paraffin was sliced into 5 µm, it was covered with xylene for 15 min. After antigen retrieval, the sections were added with 3% H2O2. The sections were incubated with the anti-ADAM8 (Proteintech, Wuhan, China) after blocking with goat serum (Solarbio, Beijing, China) at 4°C overnight. The sections were washed with PBS followed by incubation with horseradish peroxidase goat anti-rabbit IgG (ThermoFisher, Waltham, MA, USA). The sections were stained with hematoxylin (Solarbio). The images were captured.
Masson’s trichrome stain was performed according to manufacturer’s description (Leagence, Huaibei, China). In brief, the 5 µm section was stained with hematoxylin for 6 min, subsequently, it was washed by water for 20 min. After staining with ponceaux, section was added with 1% phosphomolybdic acid for 5 min. The section was observed following aniline blue counterstaining. The fibrotic area was analyzed.
ELISA
The serum was obtained via centrifuging at 1,000 g for 20 min. The ELISA kit (Uscnk, Wuhan, China) was applied to measure the content of ADAM8. The OD value was examined at 450 nm via microplate reader (BioTek, Winooski, VT, USA).
Endothelial cell isolation and culture
The tissues were sliced into 1–3 mm and treated with 0.05% trypsin and 0.05% type II collagenase following incubation for 10 min at 37°C. After termination of digestion, the cell supernatant was removed through centrifuging at 1,000 g for 10 min. After repeating twice, the cells were resuspended in the DMEM (Gibco, Carlsbad, CA, USA) culture medium containing 10% FBS (Hyclone, Logan, UT, USA). After 24 h, the culture medium was changed and replaced every 3 days. Isolated cells were resuspended with buffer, then the CD45 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were added for incubation 15 min at 4°C. After washing, CD31 MicroBeads (Miltenyi Biotec) were used to bind endothelial cells. The endothelial cells were washed and cultured in complete medium.
Lentiviral infection and cell treatment
The cells were infected with the lentivirus-shRNA targeting Adam8 (LV-shADAM8) or negative control (LV-shNC) for 48 h. Following, cells were stimulated with 10 µM Ang II for 24 h.
Immunofluorescence
The cells were fixed in 4% paraformaldehyde for 15 min, and washing with PBS three times. The cells were covered with 0.1%tritonX-100 following incubation for 30 min. After washing with PBS, cells were blocked with goat serum. Subsequently, the primary antibodies (CD31, 1:200, Abcam, Cambridge, UK; FSP1, 1:200, Affinity, Jiangsu, China) were added with cells at 4°C overnight. After clearing the primary antibodies, cells were stained with secondary antibodies (Cy3-labeled goat-anti-rabbit IgG, 1:200, Beyotime, Haimen, China; FITC-labeled goat-anti-mouse IgG, 1:200, Beyotime) for 60 min at room temperature. Following staining with DAPI, cells were observed under a fluorescence microscope.
Real-time PCR
The total RNA of tissues and cells was extracted with TRIZOL reagent (BioTeke, Beijing, China) according to the manufacturer’s instructions. The messenger RNA (mRNA) was reverse transcribed to complementary DNA via M-MLV reverse transcriptase (TaKaRa, Tokyo, Japan). The Real-time PCR was performed using a SYBR Green staining (BioTeke). The primer sequences were listed as follows. ADAM8 forward 5’-GGGGTTGATTTCATTGGG-3’, reverse 5’- ACCCTCCCGTGGTTCAG-3’; ANP forward 5’-GGGCTTCTTCCTCGTCTT-3’, reverse 5’-CTTCTCCTCCAGGTGGTCTA-3’; BNP forward 5’-CAGCTCTTGAAGGACCAA-3’, reverse 5’-GTTACAGCCCAAACGACT-3’; collagen I forward 5’-AAGAACCCTGCCCGCACATG-3’, reverse 5’-GAATCCATCGGTCATGCTCT-3’; collagen III forward 5’-GCCCACAGCCTTCTACACCT-3’, reverse 5’-GATAGCCACCCATTCCTCCC-3’; CTGF forward 5’-TGTGAAGACATACAGGGCTAAG-3’, reverse 5’-ACAGTTGTAATGGCAGGCAC-3’; α-SMA forward 5’-GGGAGTAATGGTTGGAAT-3’, reverse 5’-TCAAACATAATCTGGGTCA-3’; FSP1 forward 5’-TACTCAGGCAAAGAGGGTG-3’, reverse 5’-CAATGCAGGACAGGAAGAC-3’; VE-cadherin forward 5’-CAGCCCTACGAACCTAAA-3’, reverse 5’-AAACTGCCCATACTTGACC-3’. β-actin was used as an internal reference.
Western blot
The protein of tissues and cells were extracted via RIPA lysis (Beyotime). The concentration of protein was measured using BCA kits (Beyotime). Following, the protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and were transferred onto polyvinylidene fluoride (PVDF) membranes. After blocking, the membranes were incubated with the primary antibodies (ADAM8, 1:1,000, Proteintech; α-SMA, 1:1,000, Affinity; FSP1, 1:1,000, Affinity; VE-cadherin, 1:1,000, ABclonal, Wuhan, China; TGF-β1, 1:1,000, ABclonal; Smad2, 1:1,000, Affinity; p-Smad2, 1:1,000, Affinity; Smad3, 1:1,000, ABclonal; p-Smad3, 1:1,000, ABclonal) at 4°C overnight. The membrane was washed using PBS following incubation with corresponding secondary antibodies. The image was analyzed by Gel-Pro Analyzer (Media Cybernetics, Bethesda, MD, USA).
Statistical analysis
Data are described as the mean ± SD. Student’s t-test was used for two-group comparisons. Multiple comparisons were performed using one-way ANOVA followed by Bonferroni’s post hoc test from GraphPad Prism 8.0.
Results
Ang II influences cardiac function in vivo
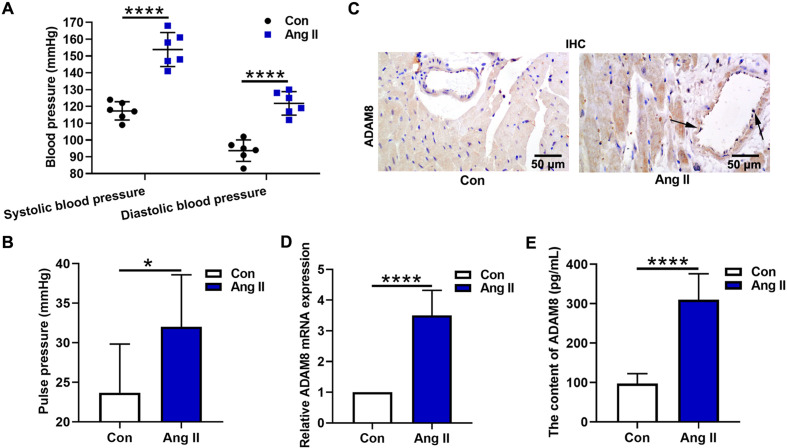
In this study, Ang II was used to establish the hypertension model. Systolic blood pressure and diastolic blood pressure were recorded via a non-invasive blood pressure monitor. The results showed that systolic blood pressure and diastolic blood pressure were significantly increased after Ang II treatment (Fig. 1A, P<0.0001). The mean pulse pressure of mice in the Ang II group (more than 30 mmHg) was higher than that in the Con group (less than 25 mmHg) (Fig. 1B, P<0.05). In addition, we detected the ADAM8 expression in the myocardial tissues. The results of immunochemistry analysis indicated that ADAM8 was up-regulated in the presence of Ang II (Fig. 1C), and ADAM8 expression was evident in vascular endothelial cells (arrow pointed). Further, the mRNA expression of Adam8 was measured by real-time PCR. It was also found that Adam8 expression in the Ang II group was obviously enhanced compared with that in the Con group (Fig. 1D). Consistent with these results, ELISA assays showed the increased ADAM8 level in the Ang II group.
Fig. 1.
Effect of Ang II on cardiac function in vivo. (A) Systolic blood pressure and diastolic blood pressure were recorded via a non-invasive blood pressure monitor. (B) The pulse pressure was analyzed. (C) The immunochemistry assay was applied to detect the ADAM8 expression level in myocardial tissues. (D) The mRNA expression of Adam8 was measured by real-time PCR. (E) ELISA kits were used to examine the content of ADAM8. *P<0.05 and ****P<0.0001. Student’s t-test was used for two-group comparisons.
The effect of ADAM8 on Ang II-induced cardiac dysfunction in vivo
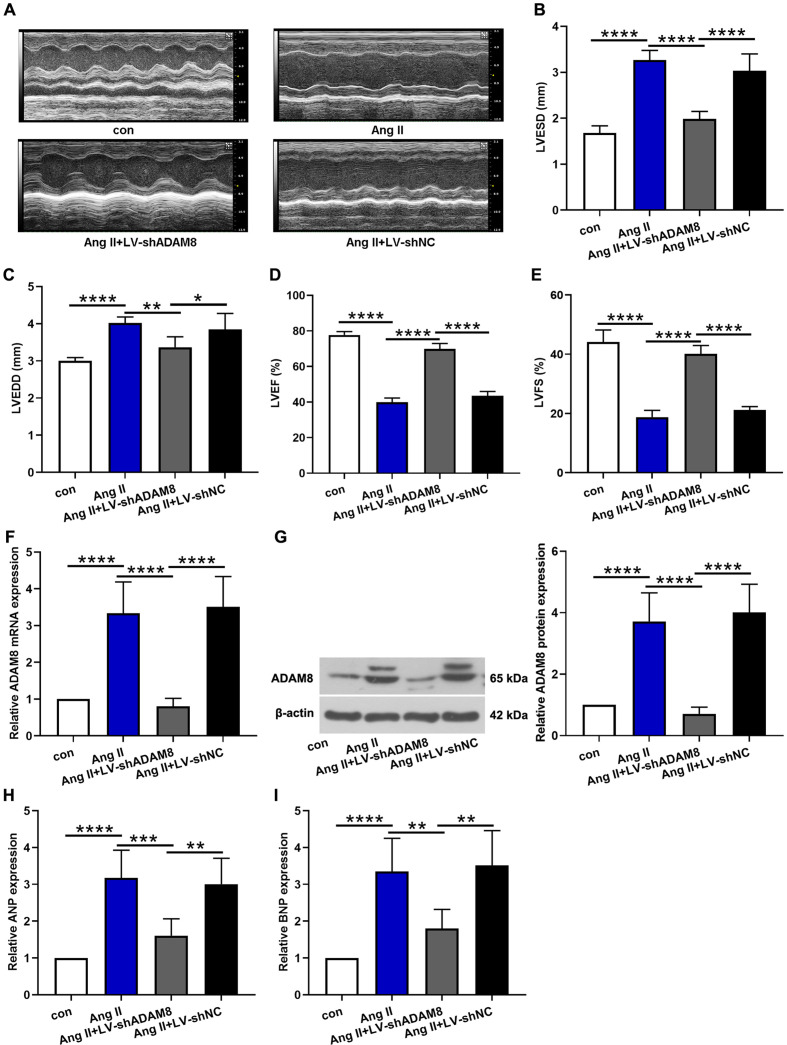
After two weeks of Ang II administration, echocardiography was used to examine LVESD, LVEDD, LVEF%, and LVFS%. Representative echocardiographic images from M-mode were shown as Fig. 2A. We found that the LVESD and LVEDD were elevated following Ang II infusion, whilst ADAM8 silencing reversed the undesirable elevation (Figs. 2B and C). Meanwhile, Ang II infusion decreased dramatically LVEF% and LVFS%, and ADAM8 knockdown increased the reduction of LVEF% and LVFS% caused by Ang II (Figs. 2D and E). We further confirmed that Ang II infusion upregulated the mRNA and protein expression levels of ADAM8. Both the mRNA and protein expression levels of ADAM8 were down-regulated after LV-shADAM8 treatment (Figs. 2F and G). Subsequent analysis on mRNA expression levels of ANP and BNP, which were responsible for hypertension heart disease, showed similar trend (Figs. 2H and I). These findings reveal that ADAM8 contributes to the Ang II induced cardiac dysfunction.
Fig. 2.
The effect of ADAM8 on Ang II induced cardiac dysfunction in vivo. (A) Representative echocardiographic images from M-mode. (B-E) Quantitative changes of LVESD, LVEDD, LVEF%, and LVFS% in each group. (F, G) The mRNA and protein expression levels of ADAM8 were determined via real-time PCR and western blot, respectively. (H, I) ANP and BNP expression levels were also tested. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. Multiple comparisons were performed using one-way ANOVA followed by Bonferroni’s post hoc test.
ADAM8 knockdown attenuates Ang II-induced cardiac fibrosis in vivo
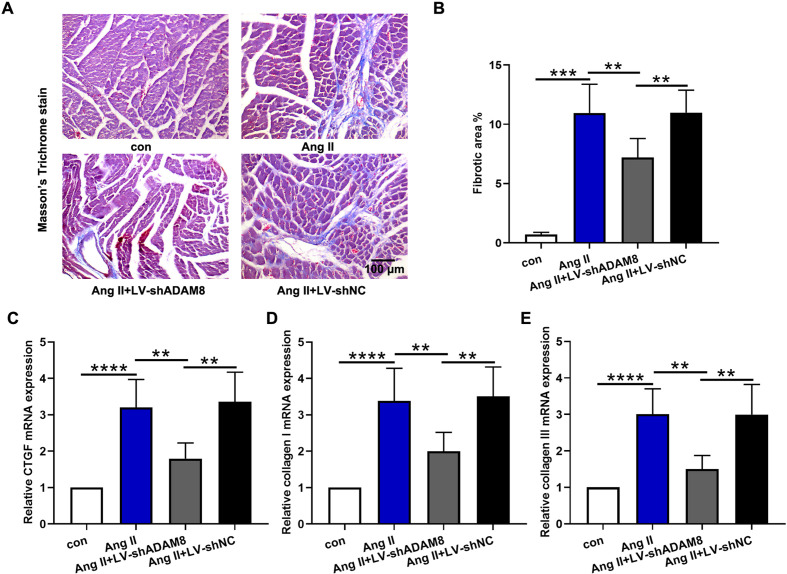
Myocardial fibrosis is an important pathological manifestation of hypertensive heart disease. The results of Masson’s trichrome staining demonstrated that Ang II infusion aggravated cardiac fibrosis with an increase in fibrotic area, nevertheless, this effect was attenuated by ADAM8 inhibition (Figs. 3A and B). Additionally, collagen deposition was evaluated. Compared with those in the Ang II group, the mRNA expression levels of CTGF, collagen I, and collagen III in the Ang II + LV-shADAM8 group were markedly diminished (Figs. 3C–E). All data suggest that ADAM8 knockdown attenuates Ang II induced cardiac fibrosis.
Fig. 3.
ADAM8 knockdown attenuates Ang II induced cardiac fibrosis in vivo. (A) Collagen deposition was assessed through Masson’s trichrome staining. (B) The fibrotic area was analyzed. (C-E) Quantitative PCR analysis of mRNA levels of CTGF, collagen I, and collagen III in the hearts. **P<0.01, ***P<0.001 and ****P<0.0001. Multiple comparisons were performed using one-way ANOVA followed by Bonferroni’s post hoc test.
ADAM8 silencing suppresses Ang II-induced EndMT in vivo
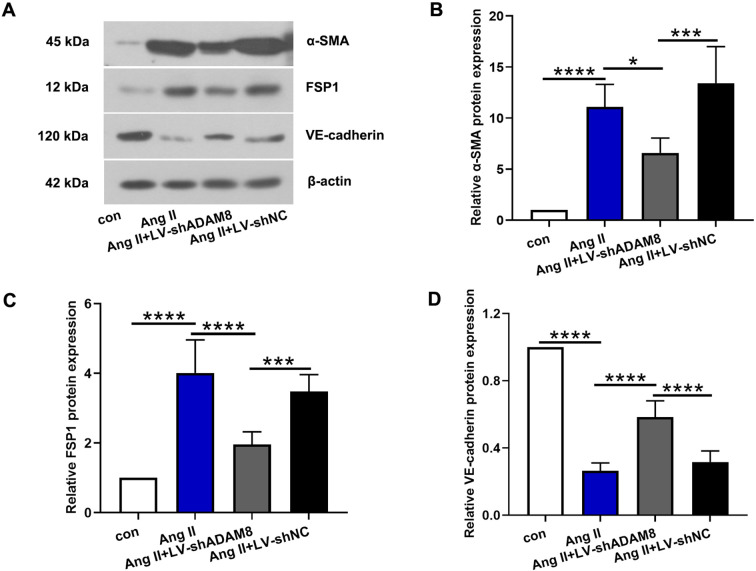
As evidenced by the previous studies, EndMT plays an important role in the cardiac fibrosis. Therefore, we measured that EndMT-related makers, α-SMA, FSP1 and VE-cadherin. The results of western blot revealed that the endothelial marker (VE-cadherin) was decreased, whilst mesenchymal markers (α-SMA and FSP1) were increased following Ang II infusion. However, ADAM8 repression suppressed Ang II-induced EndMT (Figs. 4A–D).
Fig. 4.
ADAM8 silencing suppresses Ang II-induced EndMT in vivo. (A) Representative western blot bands of α-SMA, FSP1 and VE-cadherin were displayed. (B-D) The quantitative analysis of α-SMA, FSP1 and VE-cadherin was showed. *P<0.05, ***P<0.001 and ****P<0.0001. Multiple comparisons were performed using one-way ANOVA followed by Bonferroni’s post hoc test.
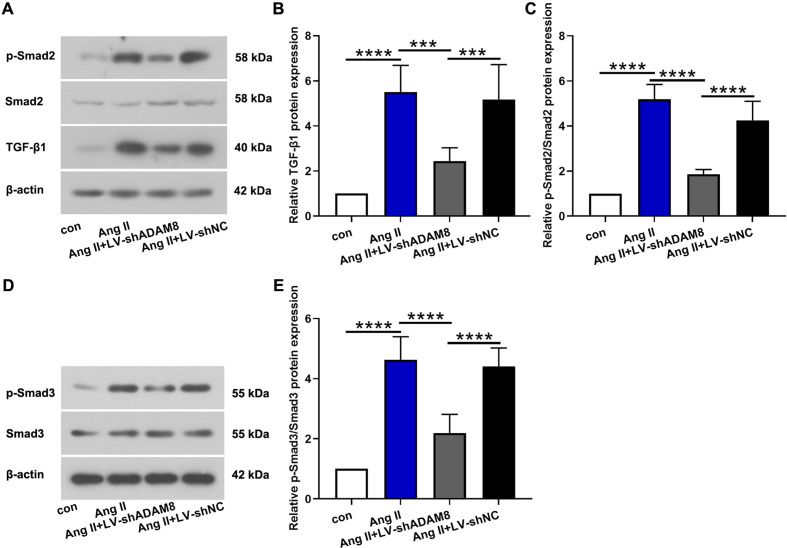
ADAM8 modulates cardiac TGF-β1/Smad2/Smad3 pathways in vivo
Following, we investigated the effect of ADAM8 on TGF-β1/Smad2/Smad3 pathways in Ang II-induced cardiac dysfunction. The results demonstrated that Ang II infusion obviously activated TGF-β1/Smad2/Smad3 pathways, according to the upregulation of TGF-β1, phosphorylated Smad2 and Smad3 in the heart tissues. On the contrary, ADAM8 knockdown repressed TGF-β1/Smad2/Smad3 signaling as evidenced by downregulating the cardiac protein expression of TGF-β1, phosphorylated Smad2 and Smad3 (Figs. 5A–E).
Fig. 5.
ADAM8 modulates cardiac TGF-β1/Smad2/Smad3 pathways in vivo. (A-C) Western blot analysis for TGF-β1, Smad2, and phosphorylated Smad2 in the heart. (D, E) Representative images and quantitative analysis of Smad3 and phosphorylated Smad3. ***P<0.001 and ****P<0.0001. Multiple comparisons were performed using one-way ANOVA followed by Bonferroni’s post hoc test.
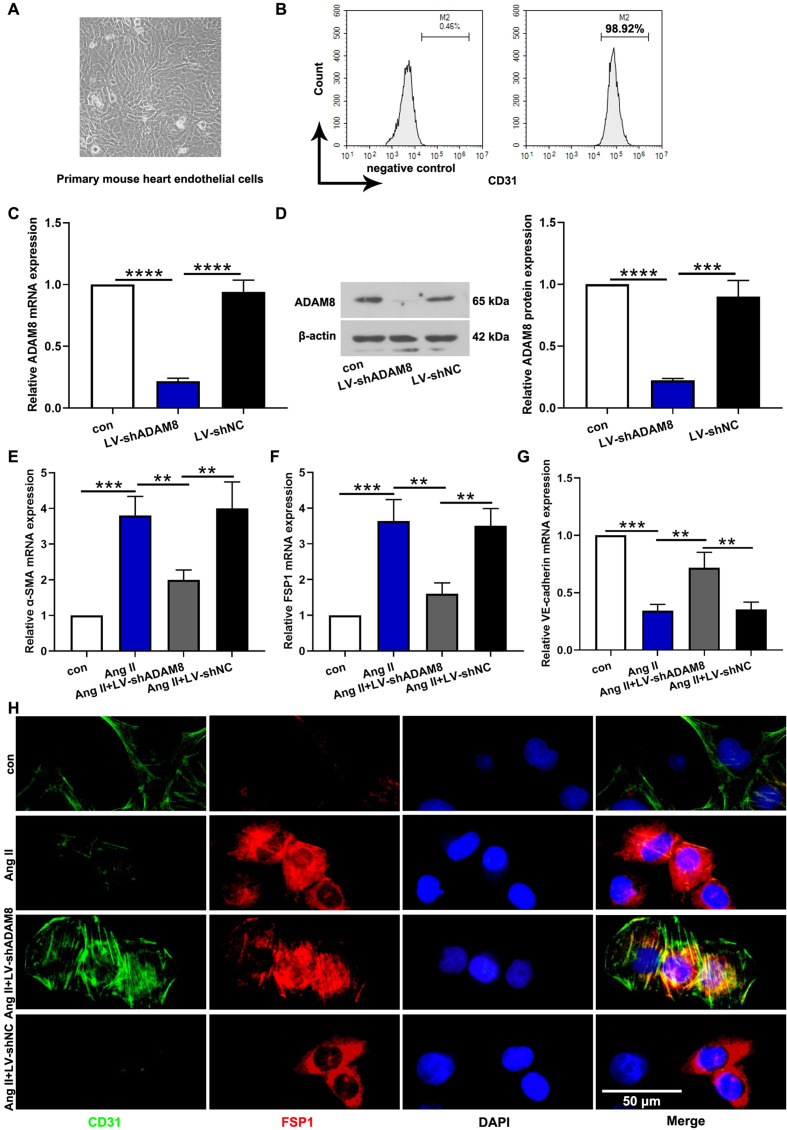
ADAM8 inhibition ameliorated Ang II-induced EndMT in vitro
The primary mouse heart endothelial cells were isolated and purified. The representative image was shown in Fig. 6A. Meantime, the results of flow cytometry analysis confirmed that the purify of endothelial cells was up to standard (Fig. 6B). To further verify the effect of ADAM8 in EndMT, we knocked down the ADAM8 in cardiac endothelial cells. Lentivirus infection efficiency was confirmed by the diminishment of ADAM8 both in mRNA and protein expression (Figs. 6C and D). Similar with the results in vivo, we also found that Ang II infusion induced EndMT with the reduction of VE-cadherin and the elevation of α-SMA and FSP1 (Figs. 6E–G). In the meantime, the results from double immunofluorescence staining further confirmed inhibitory effects of ADAM8 inhibition on EndMT (Fig. 6H).
Fig. 6.
ADAM8 inhibition ameliorated Ang II-induced EndMT in vitro. (A) Primary mouse heart endothelial cells. (B) The purity of endothelial cells was measured via flow cytometry. (C, D) The endothelial cells were infected with lentivirus-shRNA targeting ADAM8. Lentivirus infection efficiency was detected via real-time PCR and western blot. (E-G) The EndMT-related makers, α-SMA, FSP1 and VE-cadherin, were determined by real-time PCR. (H) Endothelial cells were co-immunostained to detect CD31 (green) and FSP-1 (red). **P<0.01, ***P<0.001 and ****P<0.0001. Multiple comparisons were performed using one-way ANOVA followed by Bonferroni’s post hoc test.
Discussion
In this study, it was found that Ang II induced cardiac fibrosis and impaired cardiac function by upregulating cardiac ADAM8. On the contrary, knocking down the ADAM8 can ameliorate Ang II-induced cardiac fibrosis and EndMT via blocking TGF-β1/Smad2/Smad3 signaling, which implied a negative role for ADAM8 in hypertensive cardiac fibrosis.
Hypertensive cardiovascular disease has become the main cause of heart failure. Compared with other diseases that cause heart failure, the heart tissue of hypertensive heart disease is more prone to diffuse fibrosis, which eventually leads to diastolic and systolic failure. We found that systolic blood pressure and diastolic blood pressure were significantly enhanced after Ang II infusion for 14 days. In the meantime, Ang II infusion decreased dramatically LVEF% and LVFS%, which demonstrated the damage of myocardial function. The above results showed that the hypertension model was successfully replicated. Consistent with our results, Xu et al. [11] and Zhang et al. [18] also established a model of hypertensive cardiac fibrosis via using Ang II with the same time and similar dose.
A variety of cells are involved in the occurrence and development of cardiac fibrosis, including cardiac fibroblasts, endothelial cells, hematopoietic stem cells, and so on, among which the conversion of endothelial cells to mesenchymal cells plays an important role in cardiac fibrosis [19, 20]. A number of studies have found that inhibiting EndMT can suppress the development of cardiac fibrosis [21, 22]. For instance, Liu et al. reported that SIRT1 activated by resveratrol attenuated isoproterenol-induced cardiac fibrosis by downregulating EndMT via the TGF-β/Smad2/3 pathway [23]. You et al. found that EndMT contributed to vascular injury in Ang II-treated mice, and it can be prevented via suppressing NF-κB activation by Schizandrin B treatment [24]. In addition, previous studies have reported that ADAM8 participates in the epithelial-mesenchymal transition (EMT) process during colon cancer formation [15]. Our data indicated that ADAM8 can promote the formation of cardiac fibrosis, thereby impairing cardiac function. Furthermore, inhibition of ADAM8 blocked the development of cardiac fibrosis by reducing EndMT, as characterized by the reduction of endothelial maker, VE-cadherin, as well as the acquisition of mesenchymal markers, including α-SMA and FSP1 in cardiac endothelial cells. Taken together, ADAM8 plays an important role in the regulation of EndMT, especially by promoting cardiac fibrosis in response to Ang II infusion.
TGF-β1, a pro-fibrotic cytokine, which plays a vital role in the pathogenesis of fibrosis and takes part in the modulation of EndMT [25]. Increasing evidence displays that TGF-β1 transcription and secretion were obviously elevated in the process of hypertension-induced cardiac fibrosis [26]. Additionally, TGF-β1 binds to the receptor of Smad2/3 inducing the activation of Smad2/3, displaying the down-stream effect of TGF-β1-induced cardiac fibrosis [27]. Previous studies indicated that ADAM8 acted as an agonist of the profibrotic effects of TGF-β1 and activated the TGF-β/Smad2/3 pathways [15]. Our data showed that Ang II infusion significantly activated TGF-β1/Smad2/Smad3 pathways. In contrast, ADAM8 suppression inhibited TGF-β1/Smad2/Smad3 signaling as evidenced by decreasing the cardiac protein expression of TGF-β1 and phosphorylated Smad2 and Smad3. Similar with our finding, Meng et al. [28] studied that Asiatic acid inhibited cardiac fibrosis through suppressing TGF-β1/Smads signaling. Zhu et al. [29] demonstrated that BRD4 inhibition exerted an anti-fibrotic role in Ang II-incubated cardiomyocytes by repressing TGF-β1/SMADs signaling.
Conclusion
In conclusion, the present study has revealed that ADAM8 exacerbates Ang II-induced cardiac fibrosis and EndMT via activating TGF-β1/Smad2/Smad3 pathways. Our study may inform development of novel strategies targeting the EndMT to treat hypertensive cardiac injury.
Conflicts of Interests
The authors declare that they have no competing interests.
Authors’ Contributions
Lixia Yao conceived the study. Lixia Yao, Weihua Shao, Yan Chen, Suxing Wang, and Dai Huang carried out the experiments and analyzed the data. Lixia Yao drafted the manuscript. All authors approved the final version of the manuscript.
Acknowledgments
This research was supported by the Key Science and Technology Research Plan of Hebei Provincial Health Commission (No. 20211209).
References
- 1.Perumareddi P. Prevention of Hypertension Related to Cardiovascular Disease. Prim Care. 2019; 46: 27–39. doi: 10.1016/j.pop.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 2.Fuchs FD, Whelton PK. High Blood Pressure and Cardiovascular Disease. Hypertension. 2020; 75: 285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Zhang J, Butler J, Yang X, Xie P, Guo D, et al. China-HF Investigators. Contemporary Epidemiology, Management, and Outcomes of Patients Hospitalized for Heart Failure in China: Results From the China Heart Failure (China-HF) Registry. J Card Fail. 2017; 23: 868–875. doi: 10.1016/j.cardfail.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 4.Lerman LO, Kurtz TW, Touyz RM, Ellison DH, Chade AR, Crowley SD, et al. Animal Models of Hypertension: A Scientific Statement From the American Heart Association. Hypertension. 2019; 73: e87–e120. doi: 10.1161/HYP.0000000000000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urata H, Ganten D. Cardiac angiotensin II formation: the angiotensin-I converting enzyme and human chymase. Eur Heart J. 1993; 14:(Suppl I): 177–182. [PubMed] [Google Scholar]
- 6.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004; 63: 423–432. doi: 10.1016/j.cardiores.2004.04.030 [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Jin H, Sun L, Chen S, Huang Y, Liu J, et al. Hydrogen sulfide alleviates myocardial collagen remodeling in association with inhibition of TGF-β/Smad signaling pathway in spontaneously hypertensive rats. Mol Med. 2015; 20: 503–515. doi: 10.2119/molmed.2013.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Y, Zhang JQ, Zhang J, Ramires FJ. Angiotensin II, transforming growth factor-beta1 and repair in the infarcted heart. J Mol Cell Cardiol. 1998; 30: 1559–1569. doi: 10.1006/jmcc.1998.0721 [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Lui KO, Zhou B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat Rev Cardiol. 2018; 15: 445–456. doi: 10.1038/s41569-018-0023-y [DOI] [PubMed] [Google Scholar]
- 10.Xiao L, Dudley AC. Fine-tuning vascular fate during endothelial-mesenchymal transition. J Pathol. 2017; 241: 25–35. doi: 10.1002/path.4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Fu M, Chen D, Han W, Ostrowski MC, Grossfeld P, et al. Endothelial-specific deletion of Ets-1 attenuates Angiotensin II-induced cardiac fibrosis via suppression of endothelial-to-mesenchymal transition. BMB Rep. 2019; 52: 595–600. doi: 10.5483/BMBRep.2019.52.10.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008; 29: 258–289. doi: 10.1016/j.mam.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vuohelainen V, Raitoharju E, Levula M, Lehtimaki T, Pelto-Huikko M, Honkanen T, et al. Myocardial infarction induces early increased remote ADAM8 expression of rat hearts after cardiac arrest. Scand J Clin Lab Invest. 2011; 71: 553–562. doi: 10.3109/00365513.2011.591424 [DOI] [PubMed] [Google Scholar]
- 14.Schick D, Babendreyer A, Wozniak J, Awan T, Noels H, Liehn E, et al. Elevated expression of the metalloproteinase ADAM8 associates with vascular diseases in mice and humans. Atherosclerosis. 2019; 286: 163–171. doi: 10.1016/j.atherosclerosis.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 15.Jin Q, Jin X, Liu T, Lu X, Wang G, He N. A disintegrin and metalloproteinase 8 induced epithelial-mesenchymal transition to promote the invasion of colon cancer cells via TGF-β/Smad2/3 signalling pathway. J Cell Mol Med. 2020; 24: 13058–13069. doi: 10.1111/jcmm.15907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagares D, Ghassemi-Kakroodi P, Tremblay C, Santos A, Probst CK, Franklin A, et al. ADAM10-mediated ephrin-B2 shedding promotes myofibroblast activation and organ fibrosis. Nat Med. 2017; 23: 1405–1415. doi: 10.1038/nm.4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kefaloyianni E, Muthu ML, Kaeppler J, Sun X, Sabbisetti V, Chalaris A, et al. ADAM17 substrate release in proximal tubule drives kidney fibrosis. JCI Insight. 2016; 1: e87023. doi: 10.1172/jci.insight.87023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM, Lan HY. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol Ther. 2014; 22: 974–985. doi: 10.1038/mt.2014.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Nkennor B, Mastikhina O, Soon K, Nunes SS. Endothelium-mediated contributions to fibrosis. Semin Cell Dev Biol. 2020; 101: 78–86. doi: 10.1016/j.semcdb.2019.10.015 [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Weng H, Zheng J. NAD+ repletion inhibits the endothelial-to-mesenchymal transition induced by TGF-β in endothelial cells through improving mitochondrial unfolded protein response. Int J Biochem Cell Biol. 2019; 117: 105635. doi: 10.1016/j.biocel.2019.105635 [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Wang Z, Gao L, Xiao L, Yao R, Du B, et al. miR-222 inhibits cardiac fibrosis in diabetic mice heart via regulating Wnt/β-catenin-mediated endothelium to mesenchymal transition. J Cell Physiol. 2020; 235: 2149–2160. doi: 10.1002/jcp.29119 [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Gao L, Zhao X, Guo S, Liu Y, Li R, et al. Saikosaponin A Protects From Pressure Overload-Induced Cardiac Fibrosis via Inhibiting Fibroblast Activation or Endothelial Cell EndMT. Int J Biol Sci. 2018; 14: 1923–1934. doi: 10.7150/ijbs.27022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu ZH, Zhang Y, Wang X, Fan XF, Zhang Y, Li X, et al. SIRT1 activation attenuates cardiac fibrosis by endothelial-to-mesenchymal transition. Biomed Pharmacother. 2019; 118: 109227. doi: 10.1016/j.biopha.2019.109227 [DOI] [PubMed] [Google Scholar]
- 24.You S, Qian J, Wu G, Qian Y, Wang Z, Chen T, et al. Schizandrin B attenuates angiotensin II induced endothelial to mesenchymal transition in vascular endothelium by suppressing NF-κB activation. Phytomedicine. 2019; 62: 152955. doi: 10.1016/j.phymed.2019.152955 [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Hu ZF, Liao HH, Liu W, Liu J, Ma ZG, et al. Toll-like receptor 5 deficiency attenuates interstitial cardiac fibrosis and dysfunction induced by pressure overload by inhibiting inflammation and the endothelial-mesenchymal transition. Biochim Biophys Acta. 2015; 1852: 2456–2466. doi: 10.1016/j.bbadis.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Gupte M, Umbarkar P, Singh AP, Sui JY, Force T, et al. Entanglement of GSK-3β, β-catenin and TGF-β1 signaling network to regulate myocardial fibrosis. J Mol Cell Cardiol. 2017; 110: 109–120. doi: 10.1016/j.yjmcc.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santibañez JF, Quintanilla M, Bernabeu C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2011; 121: 233–251. doi: 10.1042/CS20110086 [DOI] [PubMed] [Google Scholar]
- 28.Meng Z, Li HY, Si CY, Liu YZ, Teng S. Asiatic acid inhibits cardiac fibrosis throughNrf2/HO-1 and TGF-β1/Smads signaling pathways in spontaneous hypertension rats. Int Immunopharmacol. 2019; 74: 105712. doi: 10.1016/j.intimp.2019.105712 [DOI] [PubMed] [Google Scholar]
- 29.Zhu W, Wu RD, Lv YG, Liu YM, Huang H, Xu JQ. BRD4 blockage alleviates pathological cardiac hypertrophy through the suppression of fibrosis and inflammation via reducing ROS generation. Biomed Pharmacother. 2020; 121: 109368. doi: 10.1016/j.biopha.2019.109368 [DOI] [PubMed] [Google Scholar]