Abstract
Background
COVID-19 vaccines were authorised for emergency use to mitigate the impact of the pandemic. This study evaluated the effect of prior vaccination with either Oxford Astra Zeneca’s Covishield™ or Bharath Biotech’s Covaxin® on mortality among symptomatic COVID-19 patients during the second wave of the pandemic in India.
Methodology
In this cohort study comprising of RT-PCR confirmed symptomatic COVID-19 patients presenting during April and May 2021, the effect of prior vaccination on mortality (primary outcome), need for hospitalization, oxygen therapy, non-invasive ventilation (NIV) and intensive care unit (ICU) admission were assessed and expressed as risk ratio (RR) with 95% confidence intervals (CI).
Results
The mean (SD) age of the cohort (n = 4183) was 46.3 (15.5) years; 17.9% (748/4183) had received at least one dose of Covishield™ and 4.8% (201/4183) had received Covaxin®. Mortality was 0.2% (95% CI: 0.2% – 0.7%), 3.5% (1.9–5.2%), 6.2% (0.3–12%) and 12.9% (11.8–14.1%) among fully vaccinated (>2 weeks after two doses), partially vaccinated (>2 weeks after one dose or <2 weeks after two doses), indeterminate (<2 weeks after one dose) and unvaccinated patients respectively. The difference in mortality among unvaccinated vs. fully vaccinated was 12.7% (95% CI: 11.4–13.9%), unvaccinated vs. partially vaccinated was 9.4% (7.4–11.4%) and unvaccinated vs. indeterminate vaccinated was 6.8% (0.8–12.7%). On adjusted analysis, as compared to unvaccinated patients, at least one dose of vaccine reduced the need for hospitalization (RR: 0.40; 95% CI: 0.35–0.47), oxygen (0.33; 0.27–0.40), NIV (0.23; 0.17–0.32), ICU admission (0.18; 0.12–0.27) and mortality (0.18; 0.11–0.29).
Conclusion
Among symptomatic COVID-19 patients, prior vaccination with Covishield ™ or Covaxin® impacted the severity of illness and reduced mortality during a period of widespread delta variant circulation. Full vaccination conferred greater protection than partial vaccination.
Keywords: COVID-19, Vaccination, Corona virus, Covishield™, Covaxin®, Mortality
Abbreviations: COVID 19, Coronavirus disease19; SARS CoV 2, Severe Acute Respiratory Syndrome Coronavirus 2 virus
1. Introduction
With the emergence of the Coronavirus disease (COVID-19) pandemic, the world has witnessed the devastating consequences of the Severe Acute Respiratory Syndrome Coronavirus 2 virus (SARS-CoV-2), that has derailed life and made quarantine, isolation and social distancing the new norm. In the past 18 months, more than 200 million people have been infected and over 4 million people have died [1]. Despite improved understanding of the disease and better therapeutic options, the virus continues to pose challenges of resurgence, attributed to increased transmissibility and severity of illness due to the renegade Variants Of Concern (VOC). The B.1.617.2 (delta) variant, first detected in December 2020, was the predominant strain during the second wave of the pandemic which occurred between April and June 2021 in India [2], [3], [4]. The second wave overwhelmed the health system and resulted in a demand-supply mismatch of beds, medications and oxygen.
It was hoped that the emergency-use authorization of vaccines against COVID would mitigate subsequent waves of the pandemic. In India, the Oxford-AstraZeneca’s chimpanzee adenovirus vectored Chad0x1 vaccine, Covishield™, and the Bharat biotech’s whole virion inactivated BBV152, Covaxin® were approved [5]. Randomized phase 3 trials showed a 62–90% efficacy with Covishield™ and 78% efficacy with Covaxin® against infection [6], [7], [8]. The first phase of this nationwide vaccination campaign was launched on January 16, 2021, prioritizing healthcare workers (HCW) and other frontline workers. The second phase from March 2021 was extended to high-risk populations (>60 years, >45 years with co-morbidities) [5]. Despite best efforts and campaigns, by the time the second wave occurred in India, only 4% of the adult population of India had received at least one dose of vaccine by March 2021 [9]. This study was undertaken to evaluate if prior vaccination impacted mortality and the severity of illness at presentation in the real-world scenario outside vaccine trials during the second wave of the pandemic in India.
2. Patients and methods
2.1. Study design and setting
This cohort study of vaccinated and unvaccinated patients was conducted in a 2800-bed tertiary care referral hospital in South India during April and May 2021.
2.2. Participants
All symptomatic adult patients > 18 years of age, presenting to the Emergency Department (ED) and confirmed to have COVID-19 infection were included in the analysis. SARS CoV-2 RNA was detected in respiratory samples using reverse transcriptase polymerase chain reaction (RT-PCR) assays (Altona RealStar® SARS CoV-2) and/or Cartridge Based Nucleic Acid Amplification Test (CBNAAT) assay (Xpert® Xpress SARS CoV-2).
2.3. Exposure and outcomes
Vaccination history was obtained at presentation to the hospital. The available vaccines in India during the second wave were Oxford Astra Zeneca’s Covishield™ and Bharath Biotech’s Covaxin®; all vaccinated patients received one or two doses of either vaccine. Patients who received one dose of vaccination at least 2 weeks prior to onset of symptoms or the second dose of vaccine within 14 days of onset of symptoms were considered as ‘partially vaccinated’, while those who completed 2 doses of vaccine at least 2 weeks prior to onset of symptoms were considered to be ‘fully vaccinated’. Vaccination status was considered to be ‘indeterminate’ if a patient received one dose of vaccine, one to 13 days prior to onset of symptoms [10]. For the purposes of analysis, since the ‘intermediate group’ was a unique subset, they were not pooled with unvaccinated or partially vaccinated groups.
Patient data was collected from the hospital electronic database and the ED triage database and included demographic data, patient status (HCW or patient), vaccination status (no vaccination, indeterminate, partial or full vaccination), co-morbidities, requirement of oxygen, Non-invasive ventilation (NIV), inotropes and requirement of hospital admission or intensive care unit (ICU) care. HCW were defined as doctors, nurses, technical staff working in the hospital and medical, nursing and allied health students.
Patients were managed according to the treatment guidelines developed by the institution. The guidelines were reviewed periodically, revised based on new evidence and made available on the institutional intranet. Mildly symptomatic patients who fulfilled the criteria for management under a monitored home isolation (HI) program that was run by the institution, were advised HI. Inpatient treatment protocol included anti-viral therapy, anti-coagulation and corticosteroids. Remdesivir was part of the treatment protocol during the second wave of the pandemic and this was based on the available evidence at that time of a possible benefit in moderately ill patients who required oxygen therapy [11]. Prophylactic or therapeutic anti-coagulation was prescribed based on illness severity [12]. Corticosteroids were used as per published guidelines [13]. The choice of the corticosteroid was left to the treating physician.
Patients were initiated on NIV if they had evidence of respiratory failure with increasing tachypnea (respiratory rate > 24/min) and/or signs of increased work of breathing with accessory muscle use and were hemodynamically stable, conscious and co-operative [14]. Patients who failed trial of NIV were intubated unless there was a directive for non-escalation of care either from the patient or the next-of-kin. Patients who were intubated and ventilated received analgo-sedation and other organ support as required. Nosocomial infections and ventilator related adverse events were diagnosed and managed as per guidelines [15].
Every effort was made to provide ICU beds for those who were invasively ventilated. At the peak of the pandemic when there was critical shortage of beds, NIV was provided in Level 2 wards which were upgraded to semi-ICU facilities with the provision of additional manpower and monitoring equipment. Data on the number of patients who could not be admitted to hospital due to lack of beds or other reasons was also collected.
The primary outcome of the study was mortality. Secondary outcomes included disease severity at presentation, need for oxygen therapy, NIV and hospital admission. The severity of illness at presentation was assessed using the, SOFA score and the World Health Organization (WHO) severity scale [16], [17], [18].
2.4. Statistical analysis
Descriptive statistics were used to summarize the data; results are reported as mean and standard deviation (SD) for continuous variables such as age and vital signs. Categorical variables were summarized as number and proportions. Chi-square tests/Fisher’s exact test was used to test the association between the baseline characteristics among the groups of patients (indeterminate status vs. unvaccinated, partially vaccinated vs. unvaccinated and fully vaccinated vs. unvaccinated). The mean difference across the groups was compared using independent t-test. The difference between two proportions was done using z-test.
We used Poisson regression model to identify the protective effect of partial and full vaccination on various binary outcomes such as SOFA score, oxygen requirement in hospital, non-invasive ventilation, hospital admission, need for ICU admission and mortality. Forest plots were presented graphically using adjusted risk ratio (aRR) with 95% confidence intervals (CI).
Besides the above analysis, we also looked at the effect of any vaccination (subjects who were either partially or fully vaccinated) on illness severity (SOFA score, need for oxygen support) and outcomes (need for non-invasive ventilation, hospital or ICU admission, mortality). We modelled outcomes such as SOFA Score, oxygen requirement in hospital, non-invasive ventilation, hospital admission, requiring ICU stay and mortality using Poisson regression adjusted for any vaccination along with age and presence of more than two comorbidities. Associations were reported as RRs with 95% CIs. The difference between the deviance statistics of two models (with and without covariates) was used to study the significant improvement in the models. The Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) statistics were also used as fit statistics. [19]
A p-value less than 0.05 were considered to indicate statistical significance. Analyses were carried out using Stata software, version 16.0 (StataCorp) and R software, version 4.1.0 (R Foundation for Statistical Computing).
2.5. Ethical considerations
This study was approved by the Institutional Review Board and Ethics committee of the institution (IRB No. 14085, 30th June 2021). We used unique identifiers and password-protected data entry software to maintain patient confidentiality
3. Results
During the months of April and May 2021, 4366 RT-PCR confirmed COVID-19 patient records of patients who presented to the ED were screened. After excluding missing/incomplete records (n = 154) and unknown vaccination status (n = 29), the cohort comprised of 4183 patients (2369 male) with a mean (SD) age of 46.3 (15.5) years. HCWs comprised 19.6% (821/4183) of the cohort. Diabetes mellitus (32.7%: 1368/4183) was the most common comorbidity while 20.1% (842/4183) had two or more co-morbidities (Table 1 ). Reported symptoms were fever (59.4%: 2486/4183), breathlessness (34.6%: 1451/4183) and cough (50.6%: 2118/4183); 72 patients were pregnant (1.7%: 72/4183). Based on the WHO severity scale, 55.6% (2328/4183) were categorized as mild disease (Grade 0) while 17.4% (727/4183) were categorized as critical disease; 5.5% (230/4183) had SOFA score > 4 (Table 1). Of the 4183 patients, 54.1% (2265/4183) required hospitalization. Among patients who fulfilled the criteria for HI and were willing to be enrolled in the program, 45.9% (1918/4183) were successfully managed at home. Of these, 92% (1825/1983) were successfully managed in our institution's HI program and the remaining 8% (93/1983) were managed in the Government program or other HI programs.
Table 1.
Comparison of baseline characteristics among fully vaccinated, partially vaccinated, indeterminate status group and unvaccinated patients.
| Variable |
Overall (N = 4183) |
Unvaccinated (N = 3234) |
Indeterminate status (N = 65) | P value& |
Partially vaccinated* (N = 481) |
P value$ |
Fully vaccinated# (N = 403) |
P value@ |
|---|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 46.3 (15.5) | 47.1 (15.5) | 41 (13.4) | 0.002 | 48.3 (15.9) | 0.106 | 38.7 (13.2) | <0.001 |
| Gender, Male sex | 2369 (56.6) | 1897 (58.7) | 36 (55.4) | 0.596 | 262 (54.5) | 0.082 | 174 (43.2) | <0.001 |
| Health care workers† | 821 (19.6) | 290 (9) | 25 (38.4) | <0.001 | 171 (35.6) | <0.001 | 335 (83.1) | <0.001 |
| Presence of ≥ 2 co-morbidities | 842 (20.1) | 691 (21.4) | 10 (15.4) | 0.246 | 101 (21) | 0.854 | 40 (9.9) | <0.001 |
| Diabetes Mellitus | 1368 (32.7) | 1143 (35.3) | 15 (23.1) | 0.043 | 149 (31) | 0.061 | 61 (15.1) | <0.001 |
| Hypertension | 1042 (24.9) | 827 (25.6) | 15 (23.1) | 0.648 | 135 (28.1) | 0.244 | 65 (16.1) | <0.001 |
| Ischemic heart disease | 226 (5.4) | 178 (5.5) | 1 (1.5) | 0.193 | 34 (7.1) | 0.171 | 13 (3.2) | 0.057 |
| Obstructive airway disease | 198 (4.7) | 141 (4.4) | 4 (6.2) | 0.487 | 26 (5.4) | 0.302 | 27 (6.7) | 0.043 |
| Type of vaccine received | ||||||||
| Covishield™ | 748 (17.9) | 0 (0) | 44 (67.7) | NA | 340 (70.7) | 364 (90.3) | NA | |
| Covaxin® | 201 (4.8) | 0 (0) | 21 (32.3) | 141 (29.3) | 39 (9.7) | |||
| Clinical parameters; n (%) | ||||||||
| Fever | 2486 (59.4) | 2034 (63) | 34 (52.3) | 0.078 | 243 (50.6) | <0.001 | 167 (41.4) | <0.001 |
| Breathlessness | 1451 (34.6) | 1305 (40.4) | 12 (18.5) | 0.001 | 97 (20.2) | <0.001 | 28 (7) | <0.001 |
| Cough | 2118 (50.6) | 1762 (54.5) | 26 (40) | 0.021 | 198 (41.3) | <0.001 | 124 (30.8) | <0.001 |
| Myalgia | 853 (20.3) | 635 (19.7) | 12 (18.5) | 0.806 | 100 (20.8) | 0.556 | 96 (23.9) | 0.056 |
| Pregnancy | 72 (1.7) | 69 (2.1) | 0 (0) | 0.987 | 3 (0.6) | 0.025 | 0 (0) | 0.001 |
| Preliminary vital signs, mean (SD) | ||||||||
| Pulse rate, /min | 95.8 (27.6) | 97.1 (28.5) | 94.2 (15.2) | 0.47 | 93.2 (31.4) | 0.006 | 89.1 (11.8) | <0.001 |
| Systolic Blood Pressure, mm Hg | 119.2 (14.7) | 118.8 (15.5) | 118.6 (9.4) | 0.93 | 120.9 (13.3) | 0.003 | 121.1 (9.2) | 0.003 |
| Respiratory rate, /min | 25.5 (7.6) | 26.5 (8.0) | 22.9 (5.4) | <0.001 | 23.0 (5.9) | <0.001 | 21.0 (3) | <0.001 |
| SpO2, % | 93.4 (9.6) | 92.5 (10.4) | 96.4 (4.9) | 0.003 | 95.9 (6.6) | <0.001 | 97.9 (2.8) | <0.001 |
|
WHO severity scale 0: Mild disease 1: Moderate disease 2: Severe disease 3/ 4 / 5: Critical disease |
2328 (55.6) 526 (12.6) 602 (14.4) 727 (17.4) |
1551 (47.9) 459 (14.2) 540 (16.7) 684 (21.2) |
48 (73.8) 8 (12.3) 3 (4.6) 6 (9.2) |
<0.001 |
357 (74.2) 43 (8.9) 50 (10.4) 31 (6.4) |
<0.001 |
371 (92.1) 17 (4.2) 10 (2.5) 5 (1.2) |
<0.001 |
| Admission SOFA score > 4 | 230 (5.5) | 217 (6.7) | 0 (0) | 0.995 | 11 (2.3) | <0.001 | 2 (0.5) | 0.001 |
All values are expressed as number (n) and percentage unless indicated; † Healthcare workers and medical, nursing and allied health students were taken together; * partially vaccinated indicates that patient received one dose of vaccination at least 2 weeks prior to onset of symptoms or the second dose of vaccine within 14 days of onset of symptoms; # fully vaccinated indicates completion of 2 doses of vaccine at least 2 weeks prior to onset of symptoms;& p value: compares indeterminate status and unvaccinated patients $ p value: compares partially vaccinated and unvaccinated patients; @ p value: compares fully vaccinated and unvaccinated patients; WHO: World Health Organization; SOFA: Sequential Organ Failure Assessment; NA – not applicable
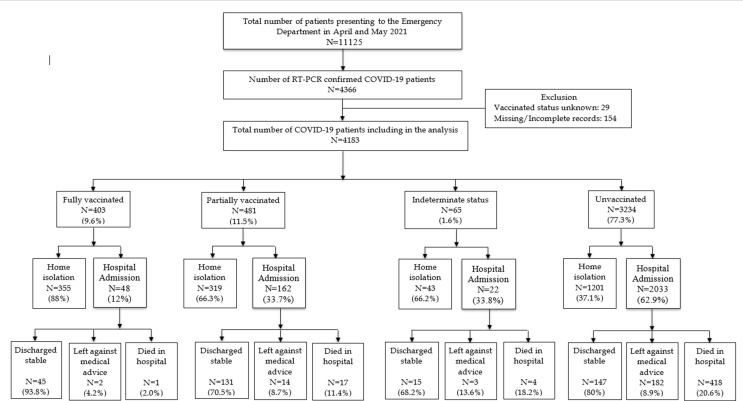
Based on the vaccination status, 77.3% (3234/4183) were unvaccinated, 11.5% (481/4183) were partially vaccinated, 1.6% (65/4183) had indeterminate vaccination status and 9.6% (403/4183) were fully vaccinated (Fig. 1 ). Among the vaccinated patients, 78.8% (748/949) received Covishield™ while 21.2% (201/949) received Covaxin®. (Table 1) The majority of patients who required hospitalization (89.8%: 2033/4183) were unvaccinated.
Fig. 1.
STROBE diagram showing the outcome among fully vaccinated, partially vaccinated and unvaccinated patients Of the 4183 patients who presented during the study period, among the 403 fully vaccinated patients, one patient died (2%), while among the 451 partially vaccinated patients 17 died (11.4%) and 418 (20.6%) died among the unvaccinated group (n = 3234). The mortality was 18.2% (4/65) in the indeterminate vaccination status group. More patients were managed on home isolation among fully vaccinated (88%) that among unvaccinated (37.1%).
When compared with unvaccinated patients, fully vaccinated patients were significantly younger and had fewer co-morbidities. A significantly higher proportion of patients who were unvaccinated presented with fever, cough and breathlessness when compared with those who were partially or fully vaccinated (Table 1). The majority of patients in the fully vaccinated subgroup (83.1%: 335/403) were HCW. Among the fully vaccinated, 92.1% (371/403) presented with mild disease and only 1.2% (5/403) had critical disease, while among the partially vaccinated subgroup, 74.2% (357/481) presented with mild disease and 6.4% (31/481) with critical disease. Among unvaccinated, only 47.9% (1551/3234) presented with mild disease while 21.2% (684/3234) presented with critical disease (Table 1). The mortality rate was 0.2% (95% CI: −0.2% – 0.7%), 3.5% (1.9–5.2%), 6.2% (0.3–12%) and 12.9% (11.8–14.1%) among fully vaccinated, partially vaccinated, indeterminate and unvaccinated patients respectively. When the impact of vaccination was analysed (Fig. 2 ), compared with unvaccinated patients, partially vaccinated patients had milder disease (SOFA > 4), reduced requirement of oxygen, NIV, hospital admission, ICU admission and mortality (p < 0.001). Again, when fully vaccinated patients were compared with unvaccinated individuals, full vaccination was associated with significantly less disease severity, requirement of respiratory supports, hospital admission, ICU admission and mortality (p < 0.001).
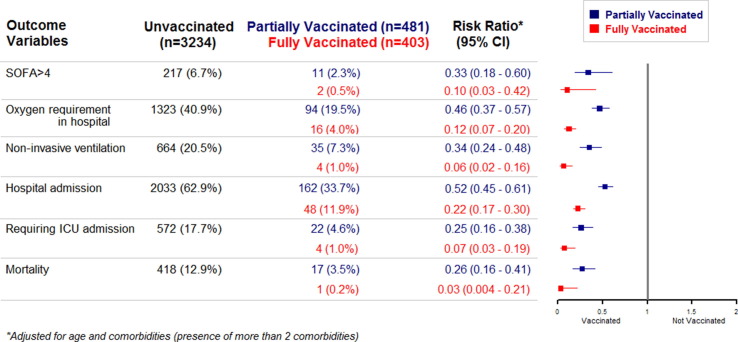
Fig. 2.
Forest plot illustrating the adjusted effect of partial and full vaccination, compared to unvaccinated patients, on illness severity and mortality 2a: Forest plots demonstrating the adjusted effect of partial vaccination versus unvaccinated individuals on proportion presenting with Sequential Organ Failure Assessment (SOFA) score > 4 and need for oxygen in hospital, non-invasive ventilation, hospitalization, intensive care unit (ICU) admission and mortality, expressed as risk ratio (RR) with 95% confidence intervals (CI). Partial vaccination was associated with significantly reduced risk (p < 0.001) in mortality and morbidity parameters when compared with unvaccinated individuals. 2b: Forest plots demonstrating the effect of full vaccination versus unvaccinated individuals on proportion presenting with SOFA score > 4 and need for oxygen in hospital, non-invasive ventilation, hospitalization, ICU admission and mortality, expressed as risk ratio (RR) with 95% confidence intervals (CI). Full vaccination was associated with significantly reduced risk (p < 0.001) in mortality and morbidity parameters when compared with unvaccinated individuals.
On adjusted analysis (Table 2 ), at least one dose of vaccine reduced the need for oxygen therapy (RR: 0.33; 95 %CI: 0.27–0.40), NIV (RR: 0.23; 95 %CI: 0.17–0.32), hospitalization (RR: 0.40; 95 %CI: 0.35–0.47), ICU admission (RR: 0.18; 95 %CI: 0.12–0.27) and mortality (RR: 0.18; 95 %CI: 0.11–0.29). In our cohort, prior vaccination also significantly reduced (RR: 0.25, 95 %CI: 0.14–0.43) the number of patients who presented with severe illness (SOFA > 4).
Table 2.
Unadjusted and adjusted analysis of the effect of vaccination on illness severity and outcome using Poisson regression.
| Variables |
Any Vaccination‡ |
Unadjusted Analysis | Adjusted Analysis† | |
|---|---|---|---|---|
|
Yes (n = 884) |
No (n = 3234) |
RR (95% CI) | RR (95% CI) | |
| n (%) | n (%) | |||
| SOFA > 4 | 13 (1.5) | 217 (6.7) | 0.22 (0.13–0.38) | 0.25 (0.14–0.43) |
| Oxygen requirement in hospital | 110 (12.4) | 1323 (40.9) | 0.30 (0.25–0.37) | 0.33 (0.27–0.40) |
| Non-invasive Ventilation | 39 (4.4) | 664 (20.5) | 0.21 (0.16–0.30) | 0.23 (0.17–0.32) |
| Hospital Admission | 210 (23.8) | 2033 (62.9) | 0.38 (0.33–0.44) | 0.40 (0.35–0.47) |
| Requiring ICU stay | 26 (2.9) | 572 (17.7) | 0.17 (0.11–0.25) | 0.18 (0.12–0.27) |
| Mortality | 18 (2.0) | 418 (12.9) | 0.16 (0.10–0.25) | 0.18 (0.11–0.29) |
‡Only patients who had taken at least one dose of vaccine at least 2 weeks prior to presentation to hospital were included; the indeterminate group of patients were excluded from this analysis; RR: Risk Ratio; n: number of patients; CI: Confidence Interval; SOFA: Sequential Organ Failure Assessment; †Adjusted for age and comorbidities (presence of more than 2 comorbidities)
4. Discussion
This study demonstrated the impact of COVID-19 vaccination with Covishield™ and Covaxin® in the real-world scenario, outside clinical trials, during the second wave of the COVID-19 pandemic in India in the months of April and May 2021. The key finding was a graded reduction in mortality with vaccination from unvaccinated to partially vaccinated to fully vaccinated patients. When compared with the mortality rate of 12.9% among unvaccinated patients, mortality was 6.2%. 3.5% and 0.2% in the indeterminate, partially vaccinated and fully vaccinated groups respectively. Full vaccination with two doses also significantly decreased the requirement of oxygen, need for hospitalization, NIV and ICU admission. On adjusted analysis, at least one dose of vaccine decreased oxygen requirement, hospitalization and mortality.
During the first half of 2021, only two vaccines were available in India, namely Covishield™ and Covaxin®. The supply of these vaccines was controlled centrally and distributed to all the states by the Government of India. In our district, Covishield™ was initially rolled out and Covaxin® was available only subsequently and hence a majority received Covishield™. Covishield™ is a recombinant ChAdOx1 nCoV-19 corona virus vaccine manufactured by Serum Institute of India Pvt Ltd under licence from AstraZeneca [20]. Covaxin® is India’s indigenous COVID-19 vaccine developed in collaboration with the Indian Council of Medical Research (ICMR) - National Institute of Virology (NIV) and is manufactured by Bharat Biotech [21]. Both the vaccines are available as liquid preparations in multidose vials and can be stored at regular fridge temperature [22].
Recent publications have evaluated the effectiveness of Covishield™ and Covaxin® in the real world setting outside clinical trials. In a large retrospective cohort of 15,244 HCWs conducted during the delta variant (B.1.617.2) predominant months of March to June 2021 in North India, recipients of 2 doses of Covaxin were found to have higher protection against symptomatic and asymptomatic reinfection [23]. In a cohort of patients on haemodialysis during the months of March to June 2021, patients who received at least one dose of either Covishield or Covaxin had a 46% (95% CI: 17%-62%) lower risk of mortality due to COVID 19 infection on adjusted models [24].
In a study from the UK, a test-negative case-control design was used to estimate the effectiveness of vaccination (Chad0x1 nCOV19 Astra Zeneca and BNT162b2 Pfizer vaccines) against symptomatic disease caused by the delta variant or the alpha variant over the period that the delta variant began circulating [25]. In this study 19,109 patients tested positive. The effectiveness of full vaccination (any vaccine) against infection was 87.5% (95 %CI 85.1–89.5) for the alpha variant and 79.6% (95 %CI 76.7–82.1) for the delta variant. Another study from the United States using a test negative design showed a 90% decreased requirement of ICU admission among fully vaccinated individuals [10].
The significant benefits offered by full vaccination over partial vaccination was also demonstrated in the current study. However, there are several differences between these studies (Supplementary table). In particular, the study designs were different. The current study evaluated the effect of prior vaccination (full or partial) on mortality in a cohort of symptomatic patients who presented to the ED while the US and UK studies were community-based test-negative design case-control studies to estimate vaccine effectiveness by comparing the odds of a positive test for SARS-CoV2 infection among vaccinated patients with those among unvaccinated patients. In the light of different study designs, although it may not be appropriate to compare the relative protective effect of vaccination in the various studies, it is important to note that among symptomatic presentations to the ED, prior vaccination significantly reduced mortality.
In another study in a large community-based survey of randomly selected households across the United Kingdom (available online as pre-print), a decreased effectiveness against new PCR positive cases of BNT162b2 (Pfizer) and ChAd0x1 (AstraZeneca) was reported against the delta variant [26]. Vaccine effectiveness against new PCR positives for both the vaccines was greater with two doses than with one dose, but found no variation in effectiveness with dosing interval. However, prior infection with SARS CoV2 offered higher protection against re-infection and hospitalization. This study focused primarily on the risk of infection at different time points following vaccination as well as different time periods in the course of the pandemic and did not report ED presentations or ICU admissions [26].
The proportion of patients presenting with severe (WHO category critical, Table 1) disease among the unvaccinated group was 21.2% in our study. In a systematic review of 12 studies that included 2794 COVID-19 patients, 21.33% presented with severe disease [27]. In another meta-analysis that also included 12 studies involving 2445 patients who were admitted to hospitals in China between 1 January 2020 and 14 April 2020, 479 (19.9%) had severe illness or were admitted to the ICU [28]. These studies pertained to cohorts prior to vaccination. Although the definition of “severe disease” was different in these studies and defined as Acute Respiratory Distress Syndrome (ARDS), ICU admission or death and more likely to be consistent with the WHO severity of “critical”, the observation that 21.2% of the patients in the unvaccinated group presented with critical disease is consonant with observations from other systematic reviews of 19.9% and 21.33% [27]. In contrast to the higher mortality in the unvaccinated groups in our study and the systematic reviews, among the partial and fully vaccinated groups in the current study, the proportion of patients presenting with critical illness was only 6.4% and 1.2% respectively, strengthening the observation of a protective effect of vaccination on illness severity.
Vaccination also appears to reduce the need for oxygen therapy and NIV. When compared with the meta-analysis that reported that 14% of patients with COVID-19 required oxygen therapy, only 4% of the fully vaccinated patients in our cohort required oxygen (Table 2) [27]. However, the proportion of unvaccinated and partially vaccinated patients who required oxygen was much higher at 40.9% and 19.5% respectively, reflecting the severity of presentations and overwhelmed health system during the second wave of the pandemic in India. These findings of benefits of vaccines being maintained outside trial settings underscores the critical importance of receiving the second dose to complete the currently recommended vaccination schedule.
A few limitations merit mention. Certain demographic variables like immunosuppression, that could impact vaccine response, were not collected and hence could not be analysed. Our study was a hospital-based cohort study and not a test negative study design that is ideal to assess post-licensure evaluation of vaccine effectiveness, although cohort or case-control study designs are also used for this purpose [29], [30]. Some of the methodological challenges in measuring vaccine effectiveness using population cohorts in low resource settings have been described [31]. The authors argued that while no single set of definitions or analytic approach can address all possible biases and confounding with cohort studies, the careful prior consideration of the denominator (in our situation all patients presenting with symptomatic illness), exposure (prior vaccination) and outcome definitions (mortality) with a balanced per-protocol primary analysis provides reasonably conservative estimates of vaccine effectiveness [31]. The cohort design in our study also leads to a selection bias since it is dependent on patients who accessed healthcare in our centre during the time of the second wave of the pandemic and not truly a reflection of community level protection of vaccination. The small numbers receiving Covaxin® (4.8% of the cohort) precluded meaningful subgroup analysis to assess superiority of either vaccine. However, this large cohort of symptomatic presentations during the peak of the pandemic offered the opportunity to assess if fully vaccinated symptomatic individuals presenting to hospital fared had lower mortality and lower illness severity when compared with unvaccinated symptomatic individuals presenting at the same time. Thus, this study provides confidence that prior vaccination reduces the probability of death and alters the course of illness in symptomatic COVID-19 infection presenting to hospital. Another limitation of this study is not adjusting for the effect of prior infection on the severity and outcome of the current episode, a significant finding in a prior study [32]. Further, this study is not generalizable to the period during which Omicron has expanded and should be interpreted as estimates of effectiveness of Covishield™ and Covaxin® during a period of widespread delta variant transmission.
5. Conclusion
Among symptomatic COVID-19 patients, prior vaccination with either Covishield™ or Covaxin® impacted the severity of illness and reduced mortality when compared with unvaccinated patients. Full vaccination conferred a substantially higher protective effect over partial vaccination
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. There was no funding or grant for this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.02.023.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.COVID Live Update: 218,695,446 Cases and 4,536,792 Deaths from the Coronavirus - Worldometer [Internet] [cited 2021 Sep 1]. Available from: https://www.worldometers.info/coronavirus/.
- 2.CDC. Coronavirus Disease 2019 (COVID-19) [Internet]. Centers for Disease Control and Prevention; 2020 [cited 2021 Sep 1]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html.
- 3.Kunal S., Aditi, Gupta K., Ish P. COVID-19 variants in India: Potential role in second wave and impact on vaccination. Heart Lung. 2021;50(6):784–787. doi: 10.1016/j.hrtlng.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revealed: B.1.617.2 variant found in healthcare workers of Delhi’s Apollo Hospital | Cities News, The Indian Express [Internet] [cited 2021 Sep 1]. Available from: https://indianexpress.com/article/cities/delhi/revealed-b-1-617-2-variant-found-in-healthcare-workers-of-apollo-hospital-7330960/.
- 5.Press Statement by the Drugs Controller General of India (DCGI) on Restricted Emergency approval of COVID-19 virus vaccine [Internet] [cited 2021 Sep 1]. Available from: https://pib.gov.in/Pressreleaseshare.aspx?PRID=1685761.
- 6.Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK - The Lancet [Internet] [cited 2021 Sep 1]. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32661-1/fulltext. [DOI] [PMC free article] [PubMed]
- 7.Bharat Biotech’s Phase III interim results of COVAXIN demonstrate efficacy of 81% [Internet] [cited 2021 Sep 1]. Available from: https://www.pharmaceutical-business-review.com/news/bharat-biotechs-phase-iii-interim-results-of-covaxin-demonstrate-efficacy-of-81/.
- 8.Ella R., Reddy S., Jogdand H., Sarangi V., Ganneru B., Prasad S., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21(7):950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Update on COVID Vaccine Allocation [Internet] [cited 2021 Sep 1]. Available from: https://pib.gov.in/pib.gov.in/Pressreleaseshare.aspx?PRID=1718543.
- 10.Thompson M.G., Stenehjem E., Grannis S., Ball S.W., Naleway A.L., Ong T.C., et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. N Engl J Med. 2021 doi: 10.1056/NEJMoa2110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remdesivir – Covid Guidelines India [Internet] [cited 2021 Sep 14]. Available from: https://indiacovidguidelines.org/remdesivir/.
- 12.Prophylactic vs Therapeutic dose anticoagulation – Covid Guidelines India [Internet] [cited 2021 Sep 14]. Available from: https://indiacovidguidelines.org/anti-coagulation/.
- 13.Systemic Corticosteroids – Covid Guidelines India [Internet] [cited 2021 Sep 14]. Available from: https://indiacovidguidelines.org/systemic-corticosteroids/.
- 14.Nava S., Hill N. Non-invasive ventilation in acute respiratory failure. Lancet Lond Engl. 2009;374(9685):250–259. doi: 10.1016/S0140-6736(09)60496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Types of Healthcare-associated Infections | HAI | CDC [Internet]. 2019 [cited 2021 Apr 8]. Available from: https://www.cdc.gov/hai/infectiontypes.html.
- 16.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent J.-L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H., et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance Pediatr Med Rodz. 2020;16(1):9–26. [Google Scholar]
- 19.Hilbe J.M. Cambridge University Press; New York, NY: 2014. Modeling count data; pp. 116–122. [Google Scholar]
- 20.Serum Institute Of India - ChAdOx1 nCoV- 19 Corona Virus Vaccine (Recombinant) - COVISHIELD [Internet] [cited 2022 Jan 26]. Available from: https://www.seruminstitute.com/product_covishield.php. [DOI] [PubMed]
- 21.COVAXIN - India’s First Indigenous Covid-19 Vaccine | Bharat Biotech [Internet] [cited 2022 Jan 26]. Available from: https://www.bharatbiotech.com/covaxin.html.
- 22.Abhilash K.P. COVID-19 vaccines: Hope on the horizon with doubts. Curr Med Issues. 2021;19(2):67. doi: 10.4103/cmi.cmi_14_21. [DOI] [Google Scholar]
- 23.Malhotra S., Mani K., Lodha R., Bakhshi S., Mathur V.P., Gupta P., et al. SARS-CoV-2 Reinfection Rate and Estimated Effectiveness of the Inactivated Whole Virion Vaccine BBV152 Against Reinfection Among Health Care Workers in New Delhi, India. JAMA Netw Open. 2022;5(1):e2142210. doi: 10.1001/jamanetworkopen.2021.42210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav A.K., Sankarasubbaiyan S., Gowda BG M., Shah K., Jha V. The High Mortality and Impact of Vaccination on COVID-19 in Hemodialysis Population in India During the Second Wave. Kidney Int Rep. 2021;6(10):2731. doi: 10.1016/j.ekir.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta K-D, et al. Impact of Delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK [Internet]. 2021 Aug [cited 2021 Sep 14] p. 2021.08.18.21262237. Available from: https://www.medrxiv.org/content/10.1101/2021.08.18.21262237v1. [DOI] [PMC free article] [PubMed]
- 27.Features of severe COVID‐19: A systematic review and meta‐analysis - Del Sole - 2020 - European Journal of Clinical Investigation - Wiley Online Library [Internet]. [cited 2021 Sep 1]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/eci.13378. [DOI] [PMC free article] [PubMed]
- 28.Li J., He X., Yuan Yuan, Zhang W., Li X., Zhang Y., et al. Meta-analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. Am J Infect Control. 2021;49(1):82–89. doi: 10.1016/j.ajic.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.COVID-19 Vaccine Effectiveness Research | CDC [Internet]; 2021 [cited 2021 Oct 5]. Available from: https://www.cdc.gov/vaccines/covid-19/effectiveness-research/protocols.html.
- 30.Patel M.M., Jackson M.L., Ferdinands J. Postlicensure Evaluation of COVID-19 Vaccines. JAMA. 2020 Nov 17;324(19):1939–1940. doi: 10.1001/jama.2020.19328. [DOI] [PubMed] [Google Scholar]
- 31.King C., Beard J., Crampin A.C., Costello A., Mwansambo C., Cunliffe N.A., et al. Methodological challenges in measuring vaccine effectiveness using population cohorts in low resource settings. Vaccine. 2015;33(38):4748–4755. doi: 10.1016/j.vaccine.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.León T.M., Dorabawila V., Nelson L., Lutterloh E., Bauer U.E., Backenson B., et al. COVID-19 Cases and Hospitalizations by COVID-19 Vaccination Status and Previous COVID-19 Diagnosis — California and New York, May–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71(4):125–131. doi: 10.15585/mmwr.mm7104e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.