Abstract
The ongoing COVID-19 pandemic is raising great concern all over the world. The recent introduction of vaccines has offered reason for optimism, however, new issues have arisen, such as vaccine reluctance. The safety of vaccines for pregnant women is one of the most serious of these concerns. The purpose of this review article is to provide updated international vaccine recommendations, results of ongoing studies and clinical trials, and the role of gynecologists in counseling the women to understand the risks versus benefits as well as form an informed decision towards vaccine acceptance for COVID-19.
Although COVID-19 infection increases the risk of severe morbidity and mortality in pregnant women, pregnant women were not included in the initial vaccine trials. As a result, safety information is scarce. Nations have differing recommendations, though many have recently approved the COVID-19 immunization in pregnancy following a risk-benefit analysis. The Joint Committee on Vaccination and Immunization (JCVI) of the United Kingdom recently approved an mRNA vaccination for pregnant women. Vaccination is recommended by the CDC, ACOG, ARFM, and WHO. India recently took a stand, with the ICMR and the Ministry of Health and Family Welfare recommending vaccination during pregnancy and lactation.
Keywords: Pregnancy, COVID-19 vaccines, SARS-CoV-2, Informed consent, Counseling, Gynaecologist
Abbreviations: AEFI, Adverse Event Following Immunization; JCVI, Joint Committee on Vaccination and Immunization; CDC, Centers for Disease Control and Prevention; ACOG, American College of Obstetricians and Gynecologists; ASRM, American Society for Reproductive Medicine; WHO, World Health Organization; ICMR, Indian Council of Medical Research; MOHFW, Ministry of Health and Family Welfare; mRNA, messenger Ribonucleic Acid; SNFM, The Society for Maternal-Fetal Medicine; NACI, National Advisory Committee on Immunization
Introduction
COVID-19, widely referred to as coronavirus disease, is caused by the SARS-CoV-2 coronavirus strain, which was first detected in patients in Wuhan, China, in 2019 [1]. SARS-CoV-2 infections is expected to cause mild to moderate symptoms in both pregnant and non-pregnant women of the same age groups. There is now concrete evidence that pregnant women are at a higher risk of severe illness from COVID-19 than non-pregnant women, especially if infection occurs during the third trimester of pregnancy. In this cohort, the risk of ICU admission is around 1%, while the likelihood of invasive mechanical ventilation is around 0.3%. Maternal comorbidities such as age over 40, obesity, chronic hypertension, and diabetes mellitus constitute additional risk factors for the severity of the disease in this group. For all of the aforementioned reasons above, pregnant women should be considered a high-risk population for serious COVID-19 infection, and there are clear benefits to both mother and fetus from avoiding this disease during pregnancy [2]. Even though COVID-19 infection puts pregnant women at a higher risk of severe morbidity and mortality, they were excluded from the original vaccination trials [3].
Early COVID-19 vaccination studies excluded pregnant women, resulting in a paucity of safety data. However, in the United Kingdom, the Joint Committee on Vaccine and Immunisation (JCVI) presently recommends that if a pregnant woman meets the criteria for being particularly vulnerable to COVID-19, she should explore vaccination options with her obstetrician. Women who have had solid organ transplants, chronic kidney disease or a history of dialysis, homozygous sickle cell disease, severe respiratory distress such as cystic fibrosis/severe asthma, heart disease, or are on immunosuppressive drugs are at risk. Additionally, pregnant frontline healthcare or social service providers may discuss immunization opportunities. The dearth of safety data for approved vaccines for use during pregnancy should be considered against the fact that other non-live vaccines administered during pregnancy are not linked to risk [4].
Effect of COVID-19 in pregnancy
-
•
Maternal risk
While during pregnancy women's immunological, cardiovascular, and respiratory systems undergo significant physiological changes, perhaps a little amount of stress can exacerbate the disease severity. Pregnancy, on the other hand, produces hypercoagulability, endangering lives due to venous and arterial thromboembolism. According to early evidence and observational research, COVID-19 patients have a higher risk of thrombosis. Respiratory viral infections during pregnancy have been linked to factors such as low birth weight and preterm birth in several studies. Additionally, high fever in early pregnancy may increase the chances of certain birth defects [5]. According to some studies, pregnant women with COVID-19 have an increased risk of having premature and/or low-birthweight babies, postpartum hemorrhage, and problems that necessitate cesarean delivery [6].
In a systematic review [7], findings show a rate of preterm births among live births of 17% (95% CI 13%–21%), which is somewhat higher than the global rate of 11% in non-COVID-19 pregnancies. Surprisingly, when they investigated preterm births in pregnant women with COVID-19, they found that the incidences of premature rupture of membranes and spontaneous labour were only 5% and 6%, respectively. The rate of cesarean section in this review, on the other hand, appears alarming: 65% (95% CI 57%–73%). This is higher than the global report published in The Lancet, which shows cesarean section rates of 28.8% in East Asia and the Pacific, 32% in North America, and 26.9% in Western Europe, and contradicts the W·H.O. statement, which states that cesarean section rates greater than 15% are not associated with lower maternal and newborn mortality rates [8].
Additionally, several studies have shown the presence of viral RNA in the breast milk of infected mothers; however, no evidence that consuming breast milk from SARS CoV-2 infected mothers enhances the risk of transmission to their newborns [9].
-
•
Fetal risk
When assessing the risks and benefits, it's crucial to understand that there has not been a human trial to establish that the COVID-19 vaccines are safe for use in fetal and neonatal animals.
There are currently just a few unpublished animal developmental and reproductive toxicity studies available, however, over 1000 rats who got the Moderna COVID-19 immunization before or during pregnancy exhibited no adverse consequences [9].
In a previous study [10], authors investigated six miscarriages (2.3%), six intrauterine death (2.3%), and five (2.0%) neonatal deaths, with an overall rate of perinatal death of 4.2% (11/265), resulting in 17 cases experiencing the composite adverse fetal outcome and 248 cases not experiencing it. Congenital abnormalities were not detected during prenatal or postnatal screenings in either stillbirths or neonatal mortality. Furthermore, none of the IUD cases showed symptoms of impending death on arterial or venous Doppler scans (reverse end-diastolic flow in the umbilical artery, increased ductus venosus pulsality index, absent or reverse a wave in the ductus venosus). Prematurity-related adverse events included all neonatal deaths. One (0.4%) of the 250 live-born neonates was positive for RT-PCR pharyngeal swabs collected after delivery. During the third trimester of her pregnancy, the mother was found to be positive. After 14 days of life, the neonate was asymptomatic and had a negative RT-PCR test. There was no strong scientific evidence of vertical transmission of SARS-CoV-2 in research undertaken in China, Iran, and the United States. However, in a previous study [11], vertical transmission is possible, particularly in the third trimester, as evidenced by approximately 3.2% (22/936) of infant nasopharyngeal swab testing and SARS-CoV-2 RNA positivity ranging from 0% (0/51) in amniotic fluid and urine (0/17), 3.6% (1/28) in cord blood, 7.7% (2/26) by placental sample analysis, and 9.7% (3/31) by rectal or anal swab, and 3.7% (3/81) by serology [11].
Importance of vaccination in pregnancy
-
•
Role of Vaccine
Vaccines are biologics that provide active adaptive immunity against specific diseases. Vaccine development involves utilizing the microorganisms responsible for the disease either in the killed or attenuated form, or it involves the use of microorganisms' toxins or surface proteins. The vaccines are introduced in the body via the mouth, injection, or nasal route to incite the immune system against foreign bodies. In the process of immunity development, the body produces antibodies against specific microorganisms, which generates the defense mechanism. When a person encounters the same microorganisms later, the antibodies produced by the body in response to the microorganisms’ antigens either prevents the person from the disease induced by the microorganism or lessens the severity of the disease [12].
While vaccination during pregnancy boosts maternal immunity against vaccine-preventable illnesses and promotes vaccine-specific antibody transfer to the fetus [3].
Recommended COVID-19 vaccine in pregnancy
The U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the following vaccines [9] along with the Indian Central Drugs and Standards Committee (CDSCO) [13] in Table 1 (Type of COVID-19 vaccines authorized for emergency use for pregnant women).
Table 1.
Type of COVID-19 vaccines authorized for emergency use for pregnant women.
| S. No. | Name | Type | Age | Dose | Mechanism | Indirect safety data from vaccine | Theoretical safety concern |
|---|---|---|---|---|---|---|---|
| 1. | Pfizer-BioNTech | mRNA vaccine (BNT162b2) | 12 years and above | 2 doses are given 3 weeks apart. | mRNA – that encodes the critical fragment of the viral protein is injected into muscle cells, translate them to make the viral protein directly in the body. This gives a preview of the real virus without the disease [40]. | mRNA-based Zika virus vaccine used in pregnant mice showed no safety concerns | Transplacental passage of mRNA-containing lipids |
| 2. | Moderna | mRNA-1273 vaccine | 18 years and above | 2 doses given 1 month apart | mRNA-based Zika virus vaccine used in pregnant mice showed no safety concerns | Transplacental passage of mRNA-containing lipids | |
| 3. | Janssen Biotech, Inc. (Johnson & Johnson) | Ad26.COV2·S vaccine | 18 years and above | Single-dose regimen. | It uses double-stranded DNA. The genetic coding of spike proteins is encoded in an adenovirus. On contact with the cell surface, it is engulfed in a bubble that enters the nucleus, and the gene for spike protein is coded in mRNA which helps to bring an immune response [41]. | Adenovirus vector-based Zika virus vaccine used in pregnant mice showed no safety concerns. Other vaccines developed using chimpanzee adenovirus are Ebola, HIV | Safety of adenovirus vector |
| 4. | Serum Institute of India (Covishield) | ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant) | 18 years and above | 2 doses given 12–16 weeks apart | A chimpanzee adenovirus – ChAdOx1 is modified to carry the COVID-19 spike protein into human cells. This cold virus is incapable of infecting the receiver but teaches the immune system to prepare a mechanism against such viruses [20]. | The exact technology was used to prepare vaccines for viruses like Ebola. | Preliminary animal investigations show that there are no direct or indirect impacts on fertility. |
| 5. | Bharat Biotech (Covaxin) | Inactivated-virus vaccine | 18 years and above | 2 doses given 4–6 weeks apart | Whole-Virion Inactivated Vero Cell-derived technology was used to create it. They contain inactivated viruses that cannot infect a person but can teach the immune system how to prepare a defense against an active virus [23]. | Inactivated viruses are used in the production of vaccines for Seasonal influenza, Rabies, Polio, Pertussis, and Japanese encephalitis | Initial data did not include pregnant women in trials. |
mRNA COVID-19 vaccines (Pfizer-BioNtech & Moderna) [14]
These vaccines are made up of messenger RNA (mRNA) that has been encapsulated in a lipid nanoparticle (LNP) for delivery into the host cells. These vaccines use the body's cells to produce coronavirus spike protein (the relevant antigens), which induces immune cells to produce antibodies against COVID-19, much like all other vaccinations. These vaccines do not enter the nucleus and do not cause any changes to the human DNA of vaccine recipients. As a result, mRNA vaccinations are incapable of causing genetic alterations.
Efficacy of mRNA vaccines
The Pfizer-BioNtech COVID-19 vaccine was 91.3% vaccine efficacy observed against COVID-19, measured seven days through up to six months after the second dose [15]. The Moderna vaccine was 94.1% effective in preventing laboratory-confirmed COVID-19 infection in persons who received two doses and had no signs of being previously infected, as per clinical study results [16].
In a study [17], the BNT162b2 mRNA COVID-19 vaccine is projected to be as efficacious for pregnant women as previously reported for the general population at the same time period in this study: Effectiveness against confirmed infection is 96 percent, while effectiveness against symptomatic infection is 97 percent. 7–56 days after receiving the second dose of vaccination.
In a cohort study [18], serum antibodies were found in all of the women in the group after vaccination administration. Vaccination with mRNA vaccines produced spike antigen-specific IgA in a non-infected population with comparable kinetics of induction as IgG, however, spike antigen-specific IgA levels declined considerably with time. After the second dose, it was observed that there was a considerable rise in IgG response. The IgA response, on the other hand, diminished following the second dose.
In another study [19], people 16 years and older, a two-dose regimen of BNT162b2 provided 95 percent protection against COVID-19. The safety of the vaccination was comparable to that of other viral vaccines after a median of two months.
a) Adenovirus-vector vaccine (Janssen Biotech Inc.) [14]
The COVID-19 vaccine (Ad26.COV2·S) from Janssen (Johnson & Johnson) is a monovalent vaccine based on the AdVac technology platform. It is made up of a recombinant, replication-incompetent human adenovirus type 26 (Ad26) vector that encodes a stabilized form of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike protein. Based on data from ongoing and completed clinical trials using Ad26-vectored vaccines for COVID-19, HIV, and Ebola in pregnant women, Ad26-based vaccinations have an acceptable safety and reactogenicity profile, with no notable safety concerns reported so far.
b) Adenovirus-vector vaccine (covishield) [20]
The vaccine developed by Oxford-AstraZeneca is based on the virus's genetic instructions for constructing the spike protein. The Oxford vaccine, unlike the Pfizer-BioNTech and Moderna vaccines, stores the instructions in double-stranded DNA rather than single-stranded RNA. The Oxford-AstraZeneca team utilized ChAdOx1, a modified variant of a chimp adenovirus. It can enter cells but not multiply within them. To prime the immune system to fight the coronavirus, the Oxford-AstraZeneca vaccine requires two doses, four weeks apart.
Efficacy of adenovirus-vector vaccines
Phase 3 data of Janssen Biotech Inc. demonstrated the vaccine was 85% effective in preventing severe disease across all regions of the study, and showed protection against COVID-19 related hospitalization and death, starting from 28 days after vaccination [21].
The data from the Serum Institute of India for the Covishield vaccine was reported to be of 78.29% efficacy after completing the second vaccination dosage at more than 12 weeks apart. Increased immunogenicity was linked to a longer dose interval in exploratory investigations [22].
Inactivated virus vaccines (COVAXIN) [23]
As inactivated vaccines do not multiply, therefore are unlikely to revert and cause disease. They include dead viruses that are unable to infect humans but activate the immune system to develop a defensive response against the infection. COVAXIN contains immune-potentiators, commonly known as vaccine adjuvants that are added to vaccines to augment their immunogenicity.
Efficacy of inactivated virus vaccines
The vaccine has an efficacy rate of 81%, from the preliminary data from its phase 3 trial [24].
Side effects of COVID-19 vaccines
The side effect profile of all the vaccines ranging from common to uncommon are shown in Table 2. These include milder symptoms ranging from pain at injection site, fever, fatigue, headache, muscle pain, nausea, vomiting, and allergic reactions to severe symptoms like lymphadenopathy, thrombotic events etc.
Table 2.
Side effects of COVID-19 vaccines.
| S. No. | Vaccine | Updated side effect profile |
|---|---|---|
| 1. | Pfizer-BioNTech COVID-19 Vaccine [31] | severe allergic reactions; non-severe allergic reactions such as rash, itching, hives, or swelling of the face; myocarditis (inflammation of the heart muscle); pericarditis (inflammation of the lining outside the heart); injection site pain; tiredness; headache; muscle pain; chills; joint pain; fever; injection site swelling; injection site redness; nausea; feeling unwell; swollen lymph nodes (lymphadenopathy); diarrhea; vomiting; arm pain. |
| 2. | Moderna COVID-19 Vaccine [32] | Injection site reactions- pain, tenderness and swelling of the lymph nodes in the same arm of the injection, swelling (hardness), and redness; General side effects- fatigue, headache, muscle pain, joint pain, chills, nausea and vomiting, and fever; Severe allergic reactions; myocarditis (inflammation of the heart muscle); pericarditis (inflammation of the lining outside the heart). |
| 3. | Janssen COVID-19 Vaccine [33] | Known history of a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine; injection site pain; headache; fatigue; myalgia; nausea; fever; injection site erythema and injection site swelling; thrombosis with thrombocytopenia; altered immunocompetence. |
| 4. | ChAdOx1 nCoV- 19 Corona Virus Vaccine (Covishield) [22] | Very common- Headache; nausea; myalgia; arthralgia; injection site pain, tenderness, erythema, pruritus, swelling, bruising; fatigue; malaise; pyrexia; chills. Common- Vomiting; injection site induration; influenza-like illness. Uncommon- Lymphadenopathy; decreased appetite; dizziness; abdominal pain; hyperhidrosis; pruritus; rash; thrombocytopenia and venous thrombotic events |
| 5. | Bharat Biotech COVID-19 Vaccine (Covaxin) [25] | Injection site pain, swelling, redness, itching; Headache; fever; malaise; nausea; vomiting; rashes; allergic reaction to the components of the vaccine. |
Available safety information related to the use of COVID-19 vaccines in pregnancy
The Developmental and Reproductive Toxicity (DART) experiments of the Pfizer-BioNtech COVID-19 vaccine have been reported in Europe. According to the study filed by the European Medicines Agency, there were no direct or indirect adverse effects on pregnancy, embryo/fetal development, parturition, or post-natal development in animals given the vaccination (EMA) [14].
FDA received a combined developmental, perinatal/postnatal reproductive toxicity (DART) study of Moderna's mRNA-1273 in rats on December 4, 2020. According to an FDA review of this study, mRNA1273 given at a dose of 100 μg before mating and during gestation periods had no adverse effects on female reproduction, fetal/embryonal development, or postnatal developmental outcomes, except for skeletal variations, which are common and typically resolve postnatal without intervention (FDA 2021) [14].
Female rabbits were given 1 mL of the Janssen COVID-19 vaccine in the reproductive developmental toxicity study and found no vaccine-related detrimental effects on female fertility, embryo-fetal development, or postnatal development were found (FDA 2021) [14].
Although there is no data available for breast milk antibodies and definitive animal studies have yet to be completed, preliminary animal tests for Covishield show no direct or indirect adverse effects on fertility, pregnancy, embryo-fetal development, parturition, or postnatal development [22]. Safety in pregnancy for Covaxin has been yet to be established [25]. Recently, the Ministry of Health & Family Welfare has approved the use of Covishield and Covaxin for vaccinating pregnant and lactating women and has been deemed safe [26,27].
In a study [28], antiplatelet antibodies may be produced after exposure to the “AstraZeneca COVID-19 vaccination,” leading in thrombocytopenia and venous thrombotic events (e.g., intracranial venous sinus thrombosis). The thromboembolic symptoms, coagulation dysfunction, and underlying immunological phenomena should all be addressed in these patients' treatment. Thrombosis linked with thrombocytopenia after immunisation is referred to as vaccine-induced prothrombotic immune thrombocytopenia (VIPT) or vaccine-induced immune thrombotic thrombocytopenia (VITT) in this study. Thromboembolic events, which can range from deep vein thrombosis to pulmonary embolism, splanchnic, portal or hepatic vein thrombosis, CVST, and ocular vein thrombosis, occurred 5–21 days after COVID-19 vaccine delivery in unexpected places such as the abdomen and brain [29].
Pregnancy also causes hypercoagulability, which increases the chance of thrombotic events, which are a documented side effect of SARS-CoV-2 infection [30]. The majority of the safety guidelines in the vaccine factsheet suggests the pregnant and lactating women consult with their obstetrician before undergoing vaccination for COVID-19 [[31], [32], [33]].
Recent studies supporting the use of the COVID-19 vaccine in pregnant women
In a cohort study [34] among pregnant participants, the mean gestational age at the first vaccine dose was 23.2 weeks, with 11 women (13%) receiving their first vaccine dose in the first trimester, 39 (46%) in the second trimester, and 34 (40%) in the third trimester. Side effect profiles between participant groups following vaccination were similar and Side effects of vaccine dose were injection site soreness, injection site reaction or rash, headache, muscle aches, fatigue, fever or chills, and allergic reactions. While delivery outcomes for the 13 pregnant participants who delivered during the study period were gestational age at delivery, median (IQR), week 39.3 (39–40.3 weeks), vaginal delivery were 10 (77) followed by cesarean 3 (23), Adverse pregnancy outcome most common are Preterm delivery 1 (8), among neonatal risk, composite infant morbidity, NICU admission 2 (15), supplemental oxygen/CPAP 1 (8), and transient tachypnea of the newborn 1 (8).
All 13 were vaccinated in the third trimester. Notably, 3 women delivered at hospitals other than the study sites, and cord blood samples were not available. Of the 10 umbilical cord blood samples available for analysis, 9 of 10 mothers had received both vaccine doses median, 36.5 days (IQR, 30–42) from the first vaccine and 14 days (IQR, 11–16) from the second vaccine). One participant delivered 17 days after vaccine 1, with spontaneous preterm labor at 35 weeks’ gestation [34].
In another study [35], the authors stated that in addition to protecting women against COVID-19 and its complications during pregnancy, new evidence suggests that maternal COVID-19 vaccination during the thirdtrimester results in the trans-placental transfer of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies, suggesting that maternal vaccination may provide some level of protection to the neonate.
It was reported that there were 827 completed pregnancies among the 3958 members in the v-safe pregnancy registry, with 115 (13.9%) resulting in a pregnancy loss and 712 (86.1%) resulting in a live birth where participants were vaccinated in the third trimester. Preterm birth in 9.4% of cases and small size for gestational age in 3.2% was among the negative neonatal outcomes; no newborn deaths were documented. Although not precisely comparable, computed percentage of adverse pregnancy and newborn outcomes in women who had a completed pregnancy after being vaccinated against COVID-19 were similar to incidences reported in
studies involving pregnant women conducted before the COVID-19 pandemic. The most common pregnancy-related adverse event reported to the VAERS was spontaneous abortion, which was reported 221 times.
Various ongoing clinical trials and pending safety data [36]
Pfizer has stated that a global phase 2/3 trial will be conducted to assess the safety, tolerability, and immunogenicity of the SARS-CoV-2 vaccination in pregnant women aged 18 and older. The study involved 4000 healthy women who were vaccinated between 24 and 34 weeks of pregnancy in a randomized, placebo-controlled, observer-blind study. Depending on whether she is randomized to receive the vaccine or the placebo, each woman will participate in the trial for 7–10 months, and their infants will be observed till they are 6 months old.
Moderna is preparing a prospective observational study to measure obstetric, neonatal, and infant outcomes. Janssen aims to conduct a phase 2 placebo-controlled trial in over 800 pregnant women.
The CDC has developed a voluntary smartphone-based registry for SARS-CoV-2 vaccine recipients called “v-safe,” which includes a post-vaccination “health checker” as well as a registry of pregnant women; as of March 15, 2021, over 50 000 pregnant people had registered in this monitoring program. So far, v-safe data has revealed no safety concerns. 73% of the reported adverse effects were unrelated to pregnancy. Miscarriage was the most commonly reported pregnancy-related adverse event among 29 participants; however, the reported miscarriage figures reflect background rates.
India's stand
Recently India took a stand to support COVID-19 vaccination in Pregnant and Lactating Women. MoHFW issued guidelines on 02nd July 2021 on the restricted use in an emergency and stated that vaccines like Covishield, Sputnik V, and Covaxin were safe in pregnancy [27].
Pre-existing co-morbidities in pregnant women such as Diabetes mellitus, Asthma, advanced maternal age, obesity, Organ transplant recipients, immunosuppression therapy, chronic kidney disease or on dialysis, sickle cell disease, presence of heart disease are all risk factors for developing severe COVID -19 infection during pregnancy. COVID-19 infection is more likely in health care workers or frontline workers, communities with a high or increasing rate of COVID-19 infections, and people who live in crowded conditions [27,37].
Special focus is given to the manpower development and training of the healthcare workers to provide seamless vaccine services to pregnant women. The role of household visits, antenatal check-ups have been encouraged to provide counseling and help the women understand the risks, benefits, and side effects of the vaccine. The role of obstetricians and pediatricians has been recognized for sensitization and management of an Adverse Event Following Immunization (AEFI) [24].
Worldwide recommendations for COVID-19 vaccination in pregnancy and lactation
Various governmental and professional organisations came forward from different geopolitical regions and presented their stand on the safety and efficacy of COVID-19 vaccinations in pregnant and lactating women. The updated guidelines and recommendations are compiled in Table 3.
Table 3.
Worldwide updated recommendations from government and professional organizations.
| International |
|---|
| World Health Organisation |
| When the benefits of vaccination to the pregnant woman outweigh the potential dangers, it is recommended that the COVID-19 vaccine be used. Pregnant women should be informed about the dangers of COVID-19 during pregnancy, the likely advantages of vaccination in the local epidemiological environment, and the present limits of safety data in pregnant women to assist them in making this decision. Pregnancy testing is not recommended before vaccination, according to the World Health Organization. The World Health Organization does not advocate delaying or terminating a pregnancy due to immunization [42]. |
| Asia |
| India |
| ICMR All pregnant women who come in for antenatal care may be advised about the risks and benefits of the COVID-19 vaccines (Covishield and Covaxin) that are available in the country. A pregnant woman may be offered the COVID-19 vaccination at the nearest centre based on the information provided. During pregnancy, the COVID-19 vaccine can be given at any time. COVID-19 vaccinations are available to all lactating women at any time following delivery [43]. |
| Japan |
| The Japanese Society of Obstetrics and Gynaecology and the Japanese Society of Infectious Diseases in Obstetrics and Gynaecology have issued recommendations for the inclusion of pregnant women to be vaccinated against COVID-19. Informed consent should be obtained. Healthcare workers and pregnant women with complications such as diabetes, hypertension, or obesity have to be prioritized. Vaccination should be avoided during organogenesis until 12 weeks of pregnancy [44]. |
| Europe |
| United Kingdom |
| JCVI advises that vaccination in pregnancy should be considered for women who are offered the Pfizer-BioNTech or AstraZeneca COVID-19 vaccines in cases of high risk of exposure to SARS-CoV2 infection, or if the woman has underlying conditions that put her at greater risk of serious COVID-19 complications. Clinicians should discuss the risks and benefits of vaccination with the woman in these circumstances, as well as the vaccine's lack of adequate data in pregnant women [45]. The Joint Committee on Vaccination and Immunization (JCVI) has recently recommended that COVID-19 vaccines be given to pregnant women at the same time as the rest of the population, following the age group rollout. Women may want to explore the vaccine's benefits and hazards with their healthcare providers and come to a mutual choice based on their unique circumstances. Pregnant women, on the other hand, can receive the COVID-19 vaccine even if they have not discussed it with a healthcare expert as well as breastfeeding women [46]. |
| Netherland |
| The Dutch public health organization RIVM recommends that pregnant women be provided COVID-19 vaccinations, specifically mRNA vaccines such as Pfizer and Moderna. All four vaccines approved for use in Europe are currently used in the country, including AstraZeneca, Johnson & Johnson, Moderna, and Pfizer-BioNTech products [47]. |
| Austria |
| For pregnant women, Austrian authorities have stated that mRNA vaccines such as Moderna and Pfizer-BioNtech are preferred. The vaccination committee underlines the benefits of mRNA vaccines in particular [48]. |
| France |
| The National Academy of Medicine advises to consider pregnancy as a serious risk factor in the event of SARS-CoV-2 infection, and to protect each pregnant woman from any potential source of contamination; to vaccinate any professionally or family-exposed pregnant woman, or pregnant woman with comorbidity (age >35 years, BMI >25, hypertension, diabetes); not to postpone or terminate a pregnancy due to vaccination; to encourage women who have been infected with SARS-CoV2 or vaccinated during their pregnancy to continue breastfeeding, as antibodies delivered through breast milk protect the newborn [49]. |
| Germany |
| Because pregnant women are often excluded from clinical trials, according to Germany's standing committee on vaccination (STIKO), there is a dearth of data on pregnant women and COVID vaccinations. It's a routine precaution meant to protect both the mother and the child. Saxony has initiated the rollout of vaccinations in pregnant women ahead of STIKO's recommendations [50]. |
| Ireland |
| Pregnant women are provided mRNA COVID-19 vaccine between 14 and 36 weeks of pregnancy, according to the National Immunisation Advisory Committee (NIAC), after an individual benefit/risk conversation with their obstetric caregiver [51]. |
| North America |
| United States of India |
| People who are pregnant and part of a group recommended to receive the COVID-19 vaccine may choose to be vaccinated, according to the US Centres for Disease Control (CDC). A talk with a healthcare expert may help them make an informed decision if they have reservations about getting vaccinated [51]. According to the American College of Obstetricians and Gynaecologists states (ACOG), pregnant women who meet the requirements for vaccination based on ACIP-recommended priority groups should not be denied COVID-19 vaccines. They also declare that “COVID-19 vaccinations should be administered to lactating persons in the same way that non-lactating individuals are offered the vaccine when they fit the criteria for receiving the vaccine based on the ACIP's prioritizing groups. The Society for Maternal-Fetal Medicine (SMFM) states that pregnant, and healthcare personnel would be considered a priority for vaccination. They also suggest that pregnant and breastfeeding women should be administered the vaccine if they are otherwise eligible. American Society for Reproductive Medicine. Because the vaccine does not contain a live virus, there is no need to postpone pregnancy attempts or delay therapy until the second dose has been administered. Patients and clinicians should employ a shared decision-making model that considers the ethical principles of autonomy, beneficence, and nonmaleficence [38]. |
| Canada |
| Individuals in the permitted age category who are pregnant should be administered a complete immunization series with an mRNA COVID-19 vaccine, according to NACI. If an mRNA vaccination is not recommended, another COVID-19 vaccine that has been approved should be provided. If the conditions outlined in recommendations are met and a risk assessment dictates that the benefits outweigh the potential risks for the individual and the fetus, the pregnant woman can undergo vaccination [52]. |
| Middle East |
| Israel |
| Vaccination is advised for all pregnant women in their second or third trimester. It is also suggested that breastfeeding mothers, as well as those contemplating pregnancy or undergoing reproductive treatments, receive the two vaccine doses before the start of the pregnancy [53]. |
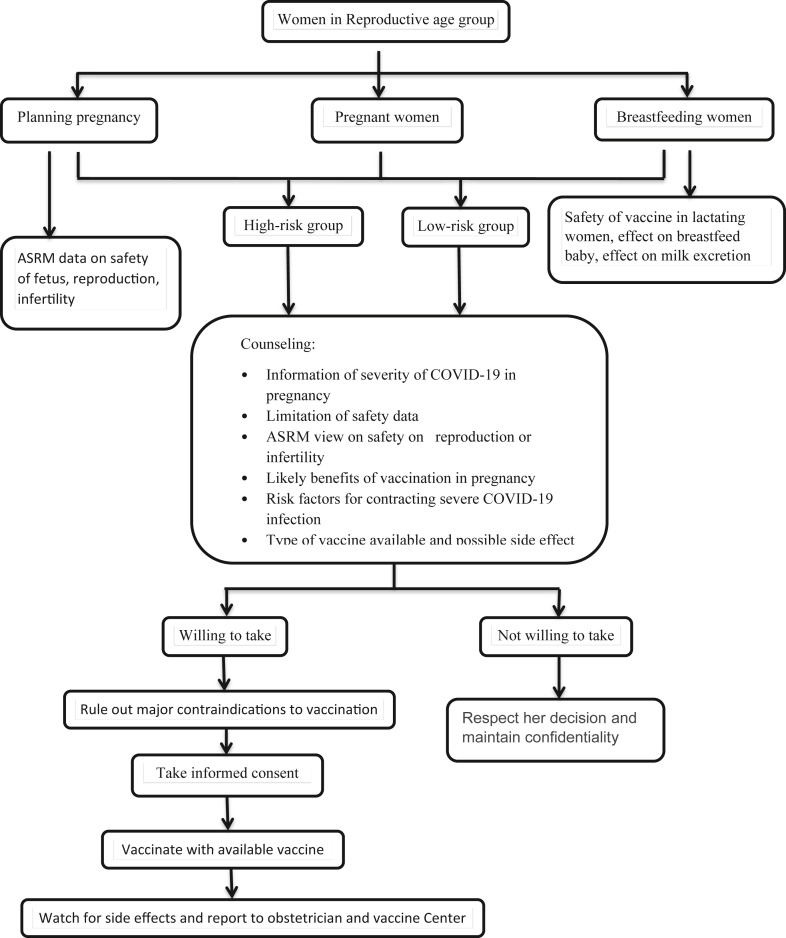
Role of obstetrician and gynecologists in making a decision
As Obstetrician and gynecologists, we are facing three groups of patients in our clinical practice:
1) Pregnant women 2) plan to become pregnant 3) Breastfeeding or plan to do so.
Professional ethics in obstetrics and gynecology provide practical tools for counseling eligible patients.
Counseling of pregnant patient
The informed consent procedure embodies the ethical premise of respect for autonomy, which requires the obstetrician-gynecologist to equip patients with the knowledge they need to make informed decisions. When the informed consent procedure is based on the patient's values and beliefs, the patient is empowered to make informed decisions [38]. Patients should be informed about available information and advised not to make decisions purely based on speculative danger. As a result, the risk of complications should be weighed against the enormous benefit of preventing infection, asymptomatic infections, and potential transmission of infection to others, as well as serious sickness, long-term repercussions, and death [38].
Counseling of patients planning to become pregnant
Some individuals who are anticipating a pregnancy may be apprehensive to get vaccinated. Vaccine apprehension results from several factors that differ from person to person and community to community. An obstetrician should inform a patient who is planning to become pregnant that there is no evidence that the vaccine impacts current or future fertility, and the ASRM recommends that all eligible patients who are planning to become pregnant be vaccinated [38].
Counseling to lactating women
The safety of COVID-19 vaccines in lactating people, the effects of vaccination on the breastfed baby, and the effects on milk supply or excretion should all be discussed with an obstetrician [39].
Fig. 1: depicting our views on the process of counseling and guidance of expecting women.
Fig. 1.
Obstetrician & Gynecologist counseling and guidance flowchart.
Conclusion
The most efficient strategy to combat the present COVID-19 pandemic is to vaccinate everyone. Although we don't have adequate clinical data on long-term adverse effects, we can expect vaccines to end the pandemic because the number of new cases, critically ill patients, and mortality in many countries has reduced.
In the instance of COVID-19, pregnant women having high-risk factors such as diabetes, cardiovascular disease, hypertension, advanced maternal age, and obesity were associated with serious consequences of COVID-19 infection.
In our expert opinion, a comprehensive risk-benefit discussion regarding the lack of safety data before COVID-19 vaccine administration in pregnant women is recommended, with a preference for pregnant women at the highest risk of more severe infection-related diseases until the safety and efficacy of these novel COVID-19 vaccines are established.
Consent
It is not applicable.
Ethical approval
It is not applicable.
Funding
No funding was received.
Declaration of competing interest
Authors have declared that no conflict of interests exists.
Acknowledgments
We are highly thankful to our colleagues who provided valuable comments about the manuscript.
References
- 1.Mahato C., Suryavanshi S. Knowledge, attitude, and practices towards COVID-19 among nurses, ward attendants, and housekeeping staff at a tertiary psychiatric institute in India. Int J Community Med Public Heal. 2020;7(12):5035. doi: 10.18203/2394-6040.ijcmph20205181. [DOI] [Google Scholar]
- 2.Martins I., Louwen F., Ayres-de- Campos D., Mahmood T. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;(xxxx):19–21. doi: 10.1016/j.ejogrb.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu-Raya B. Am Acad Pediatr; 2021. Vaccination of pregnant women against coronavirus disease 2019 during the pandemic. Published online. [DOI] [Google Scholar]
- 4.Sebghati M., Khalil A. Uptake of vaccination in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2021;(xxxx) doi: 10.1016/j.bpobgyn.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samji P., K R M Effect of COVID-19 on pregnancy and childbirth. Indian J Obstet Gynecol Res. 2020;7(2):296–299. doi: 10.18231/j.ijogr.2020.065. [DOI] [Google Scholar]
- 6.Elsaddig M., Khalil A. Effects of the COVID pandemic on pregnancy outcomes Maab. Best Pract Res Clin Obstet Gynaecol. 2020;73(January):125–136. doi: 10.1016/j.bpobgyn.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:6–8. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vergara-Merino L., Meza N., Couve-Pérez C., Carrasco C., Ortiz-Muñoz L., Madridet E., et al. Maternal and perinatal outcomes related to COVID-19 and pregnancy: an overview of systematic reviews. Acta Obstet Gynecol Scand. 2021;(February):1–19. doi: 10.1111/aogs.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stafford I.A., Parchem J.G., Sibai B.M. The coronavirus disease 2019 vaccine in pregnancy: risks, benefits, and recommendations. Am J Obstet Gynecol. 2021;224(5):484–495. doi: 10.1016/j.ajog.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Mascio D., Sen C., Saccone G., Galindo A., Grünebaum A., Yoshimatsu J., et al. Risk factors associated with adverse fetal outcomes in pregnancies affected by Coronavirus disease 2019 (COVID-19): a secondary analysis of the WAPM study on COVID-19. J Perinat Med. 2020;48(9):950–958. doi: 10.1515/jpm-2020-0355. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R., Yeni C.M., Utami N.A., Masand R., Asrani RK, Patel SK, et al. SARS-CoV-2 infection during pregnancy and pregnancy-related conditions: concerns, challenges, management and mitigation strategies–a narrative review. J Infect Public Health. 2021;14(7):863–875. doi: 10.1016/j.jiph.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashte S., Gulbake A., El-Amin S.F., Gupta A. COVID-19 vaccines: rapid development, implications, challenges and future prospects. Hum Cell. 2021;34(3):711–733. doi: 10.1007/s13577-021-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coronavirus . The Hindu; 2021. India approves COVID-19 vaccines Covishield and Covaxin for emergency use.https://www.thehindu.com/news/national/drug-controller-general-approves-covishield-and-covaxin-in-india-for-emergency-use/article33485539.ece Published. [Google Scholar]
- 14.Riley L.E., Beigi R., Jamieson D.J., Hughes BL., Swamy G., O'Neal Eckert L., et al. The American College of Obstetricians and Gynecologists; 2021. Vaccinating pregnant and lactating patients against COVID-19 summary of key information and recommendations COVID-19 infection risk in pregnancy COVID-19 vaccines in development.https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care Published. [Google Scholar]
- 15.Pfizer and biontech confirm high efficacy and NO serious safety concerns through UP to six months following second dose IN updated topline analysis OF landmark COVID-19 vaccine study. Pfizer Inc. 2021 https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-confirm-high-efficacy-and-no-serious Published. [Google Scholar]
- 16.Moderna announces primary efficacy analysis in phase 3 COVE study for its COVID-19 vaccine candidate and filing today with U.S. FDA for emergency use authorization. Moderna, Inc. 2020 https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-primary-efficacy-analysis-phase-3-cove-study [Google Scholar]
- 17.Dagan N., Barda N., Biron-Shental T., Makov-Assif M., Key C., Kohane IS., et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 18.Charepe N., Gonçalves J., Juliano A.M., Lopes DG., Canhão H., Soares H., et al. COVID-19 mRNA vaccine and antibody response in lactating women: a prospective cohort study. BMC Pregnancy Childbirth. 2021;21(1):1–9. doi: 10.1186/s12884-021-04051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corum J., Zimmer C. How the oxford-AstraZeneca vaccine works. The New York Times; 2021. https://www.nytimes.com/interactive/2020/health/oxford-astrazeneca-covid-19-vaccine.html Published. [Google Scholar]
- 21.Johnson & Johnson COVID-19 vaccine authorized by U.S. FDA for emergency use - first single-shot vaccine in fight against global pandemic. JOHNSON & JOHNSON; 2021. https://www.jnj.com/johnson-johnson-covid-19-vaccine-authorized-by-u-s-fda-for-emergency-usefirst-single-shot-vaccine-in-fight-against-global-pandemic Published. [Google Scholar]
- 22.Serum Institute of India Pvt. Ltd . 2021. ChAdOx1 NCoV- 19 corona virus vaccine (recombinant)https://www.seruminstitute.com/pdf/covishield_fact_sheet.pdf [Google Scholar]
- 23.COVAXIN® - India's first indigenous COVID-19 vaccine. 2021. https://www.bharatbiotech.com/covaxin.html Published. [DOI] [PMC free article] [PubMed]
- 24.Moderna Covovax. BBC NEWS; 2021. Biological E: what we know about India's new Covid vaccines.https://www.bbc.com/news/world-asia-india-55748124 Published. [Google Scholar]
- 25.Fact sheet for vaccine recipients & caregivers restricted use in emergency situation for covid-19 covaxin SARS-CoV-2 vaccine by bharat biotech. 2021. https://www.bharatbiotech.com/images/covaxin/covaxin-fact-sheet.pdf [Google Scholar]
- 26.Mordani S. Dr VK Paul. India Today; 2021. Covishield, Covaxin, Sputnik V, Moderna vaccines safe for pregnant women, lactating moms.https://www.indiatoday.in/coronavirus-outbreak/story/covishield-covaxin-sputnik-v-moderna-vaccines-safe-for-pregnant-women-lactating-moms-dr-vk-paul-1820888-2021-06-29 Published. [Google Scholar]
- 27.Operational guidance for COVID-19 vaccination of pregnant women. Ministry of Health and Family Welfare; 2021. https://www.mohfw.gov.in/pdf/OperationalGuidanceforCOVID19vaccinationofPregnantWoman.pdf Published. [Google Scholar]
- 28.Wolf M.E., Luz B., Niehaus L., Bhogal P., Bäzner H., Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “covid-19 vaccine astrazeneca” exposure. J Clin Med. 2021;10(8) doi: 10.3390/jcm10081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aghabaklou S., Razavi S., Mohammadi P. Cerebral coagulation complications following COVID-19 adenoviral vector vaccines : a systematic review. J Endo Metabol. 2021 doi: 10.14740/jnr700. 000(000) [DOI] [Google Scholar]
- 30.Chavan M., Qureshi H., Karnati S., Kollikonda S. COVID-19 vaccination in pregnancy: the benefits outweigh the risks. J Obstet Gynaecol Can. 2021;3:814–816. doi: 10.1016/j.jogc.2021.03.010. http://repositorio.unan.edu.ni/2986/1/5624.pdf July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fact sheet for recipients and caregivers emergency use authorization (EUA) OF the pfizer-biontech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) IN individuals 12 years OF age and older. 2021. https://www.fda.gov/media/144414/download [Google Scholar]
- 32.(FDA) USF and DA . vol. 2019. 2020. Fact sheet for recipients and caregivers emergency-moderna; p. 6. [Google Scholar]
- 33.Fact sheet for healthcare providers administering vaccine (vaccination providers) emergency use authorization (EUA) OF the janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19. 2021. https://www.fda.gov/media/146304/download [Google Scholar]
- 34.Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N., et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021:1–17. doi: 10.1016/j.ajog.2021.03.023. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro PL., Oduyebo T., Panagiotakopoulos L., et al. Preliminary findings of mRNA covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–2282. doi: 10.1056/nejmoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang E.W., Parchem J.G., Atmar R.L., Clark E.H. SARS-CoV-2 vaccination during pregnancy: a complex decision. Open Forum Infect Dis. 2021;8(5):1–6. doi: 10.1093/ofid/ofab180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization Guidance for the development of evidence-based vaccination-related recommendations. WHOI. 2017;8(January) http://www.who.int/immunization/sage/Guidelines_development_recommendations.pdf [Google Scholar]
- 38.Chervenak F.A., McCullough L.B., Bornstein E., Johnson L., Katz A., McLeod-Sordjan R., et al. Professionally responsible coronavirus disease 2019 vaccination counseling of obstetrical and gynecologic patients. Am J Obstet Gynecol. 2021;224(5):470–478. doi: 10.1016/j.ajog.2021.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.COVID-19 vaccines while pregnant or breastfeeding. National center for immunization and respiratory diseases (NCIRD) Division of Viral Diseases. 2021 https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html Published. [Google Scholar]
- 40.Mishra Sanjay. How mRNA vaccines from Pfizer and Moderna work, why they’re a breakthrough and why they need to be kept so cold. The Conversation. 2020 https://theconversation.com/how-mrna-vaccines-from-pfizer-and-moderna-work-why-theyre-a-breakthrough-and-why-they-need-to-be-kept-so-cold-150238 [Google Scholar]
- 41.Corum J., Zimmer C. How the Johnson & Johnson vaccine works. The New York Times; 2021. https://www.nytimes.com/interactive/2020/health/johnson-johnson-covid-19-vaccine.html Published. [Google Scholar]
- 42.The Moderna COVID-19 (mRNA-1273) vaccine: what you need to know. World Health Organization; 2021. https://www.who.int/news-room/feature-stories/detail/the-moderna-covid-19-mrna-1273-vaccine-what-you-need-to-know Published. [Google Scholar]
- 43.Minutes of the meeting of national advisory group on immunization (NTAGI), held on 28th May, 2021 under the chairpersonship of secretary (health & family Welfare) at nirman bhawan. 2021. https://main.mohfw.gov.in/sites/default/files/MoM NTAGI - May 28%2C 2021.pdf New Delhi. [Google Scholar]
- 44.Hayakawa S., Komine-aizawa S., Takada K., Kimura T., Yamada H. Anti-SARS-CoV-2 vaccination strategy for pregnant women in Japan. J Obstet Gynaecol Res. 2021;47(6):1958–1964. doi: 10.1111/jog.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joint committee on vaccination and immunisation: advice on priority groups for COVID-19 vaccination, 30 december 2020. Department of Health & Social Care; 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020 Published. [Google Scholar]
- 46.COVID-19 vaccines, pregnancy and breastfeeding. Royal College of Obstetricians and Gynaecologists; 2021. https://www.rcog.org.uk/en/guidelines-research-services/coronavirus-covid-19-pregnancy-and-womens-health/covid-19-vaccines-and-pregnancy/covid-19-vaccines-pregnancy-and-breastfeeding/ Published. [Google Scholar]
- 47.Pregnant women can use Pfizer or Moderna Covid vaccine, RIVM advises. NL Times; 2021. https://nltimes.nl/2021/04/29/pregnant-women-can-use-pfizer-moderna-covid-vaccine-rivm-advises Published. [Google Scholar]
- 48.Reader question: can pregnant women get vaccinated for Covid in Austria? The Local. 2021 https://www.thelocal.at/20210512/reader-question-can-pregnant-women-get-vaccinated-for-covid-in-austria/ [Google Scholar]
- 49.Should pregnant women be vaccinated against covid-19? Press release of the French national academy of medicine. ACADÉMIE NATIONALE DE MÉDECINE; March 2, 2021. https://www.academie-medecine.fr/wp-content/uploads/2021/03/21.3.2-Should-pregnant-women-be-vaccinated-against-Covid.pdf Published 2021. [Google Scholar]
- 50.Facts: COVID-19 vaccination in pregnancy. DW. https://www.dw.com/en/facts-covid-19-vaccination-in-pregnancy/a-57486122
- 51.Questions and answers for pregnant or breastfeeding women about COVID-19 vaccination. 2021. https://rcpi-live-cdn.s3.amazonaws.com/wp-content/uploads/2021/05/QA-for-pregnant-or-breastfeeding-women-about-COVID-19-vaccination_May2021.pdf [Google Scholar]
- 52.Recommendations on the use of COVID-19 vaccines. Government of Canada; 2021. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html Published. [Google Scholar]
- 53.Vaccinating women who are planning a pregnancy, pregnant or breastfeeding with the COVID-19 vaccine – clarification. Ministry of Health, Government of Israel; 2021. https://www.gov.il/en/departments/news/28012021-03 Published. [Google Scholar]