Abstract
Background
The evolution of COVID-19 is a controversial topic in cancer patients. They have been designated by international organizations as a vulnerable population at greater risk for contracting SARS-CoV-2 and having a more severe clinical outcome.
Patients and methods
Active screening at our institution became routine early in the pandemic. We have examined the clinical data of 341 cancer patients, with a positive RT-PCR SARS-CoV-2 test between April 2020 and February 2021, in the prevaccination era.
Results
During the infection, 40.5% remained asymptomatic, 27.6% developed a mild form, 20.5% had a moderate form, and 11.4% a severe/critical form of COVID-19 that led to death in 7.6% of cases. Treatment was adapted to disease severity according to national guidelines. In our series, the incidence of COVID-19 infection was lower in cancer patients compared with the general population (P < 0.001), however, the mortality rate was higher in cancer patients in comparison with the general population (7.6% versus 2.9%, P < 0.001). The prognostic factors were assessed by three distinct univariate and multivariate analyses: (i) evolution to a moderate or severe/critical clinical manifestation, (ii) clinical worsening (severe/critical form or death), and (iii) overall survival. In the multivariate analysis, the prognostic factors associated with the evolution to a moderate or severe/critical clinical manifestation were: performance status (PS) (P < 0.0001) and no active treatment in the previous 3 months (P = 0.031). Factors associated with clinical worsening were: PS (P < 0.0001), peripheral arterial disease (P = 0.03), and chronic liver disease (P = 0.04). Factors associated with impaired overall survival were PS (P < 0.0001), ischemic cardiac disease (P = 0.0126), chronic liver disease (P = 0.001), and radiotherapy (P = 0.0027).
Conclusion
Our series confirms a more severe evolution for COVID-19 infection in cancer patients, with PS as the most prominent prognostic factor in all three multivariate analyses. By active screening, efforts should be in place to keep cancer units as coronavirus-free sanctuaries.
Key words: cancer, prognostic factors, COVID-19 pandemic
Highlights
-
•
This is the first comprehensive study addressing the impact of COVID-19 in a large cohort of cancer patients in Romania.
-
•
Oncological patients had a higher death rate after COVID-19 infection in comparison with the general population.
-
•
Decreased PS was the prominent prognostic factor correlated with worse outcomes and death in multiple multivariate analysis.
Introduction
The COVID-19 pandemic posed significant problems for the Romanian health system, with 730 056 positive cases and 18 402 deaths recorded until 1 February 2021 and an acceleration trend of the second wave in October to November 2020 with a peak of 10 269 new cases recorded on 18 November 2020.1
The Oncology Institute ‘Prof. Dr. Ion Chiricuta’ in Cluj-Napoca, with 550 hospital beds and 25 reusable places in the day hospital, is the oldest in the country and the second largest in Romania.
Here we present the effects of COVID-19 infection on a series of cancer patients who tested positive at our institute until 1 February 2021.
Patients and methods
Study population
Nasopharyngeal samples from patients examined in our institution between 1 April 2020 and 1 February 2021 (during the first two waves of the pandemic and before any vaccine was available for cancer patients in Romania) were collected.
The first 21 patients were diagnosed until 13 April 2020, at the initial active screening among asymptomatic hospitalized patients. From that point on, all patients were tested at admission, and those found positive were isolated and hospitalized in dedicated COVID-19 treatment units. Those patients who had a diagnosis of malignant tumor treated in our institution and had full clinical details of SARS-CoV-2 infection outcome were included in the present study.
SARS-CoV-2 PCR analysis
Samples were collected with cotton swabs in a 3 ml viral transport medium (ViroSan Transport Medium, SaniMed, Calugareni, Romania) and stored at 4°C before RNA extraction. The RNA extraction procedure was carried out with the PureLink Viral RNA/DNA Mini Kit (#12280050, Thermo Fisher Scientific, Waltham, MA) and Quick-RNA Viral Kit (#R1035, Zymo Research, Irvine, CA).
Real time quantitative PCR (RT-qPCR) assessment of SARS-CoV-2 was carried out with the EliGene COVID19 BASIC A RT Kit (#90077-RT-A, Elisabeth Pharmacon, Brno, Czech Republic) and the Coronavirus (COVID-19) Genesig Real-Time PCR assay (#Z-Path-COVID-19-CE, Primer Design, Chandler's Ford, UK). PCR data interpretation was done according to the manufacturer’s protocol. The RT-qPCR instruments used in this study were LightCycler480 and Cobas Z480 (Roche, Basel, Switzerland).
COVID-19 classification and treatment
The severity of the disease was defined as asymptomatic, mild (without pneumonia), medium (with non-severe pneumonia), and severe/critical (severe: tachypnea with >30 breaths/min or oxygen saturation <93% at rest or PaO2/FIO2 <300 mmHg; critical: respiratory failure requiring mechanical ventilation, shock, or other organ failure that requires intensive care), according to the first World Health Organization classification.2
Until August 2020, all patients diagnosed with SARS-CoV-2 infection were hospitalized in dedicated units, even if asymptomatic. Starting with September 2020 and the second wave of the pandemic, only symptomatic patients with moderate or severe/critical forms were hospitalized. The others were observed in isolation at home under the supervision of the family physician. The treatment, in accordance with the national protocol in use, stated that asymptomatic patients required no treatment or vitamin C, D, and zinc. Mild forms received antiviral treatment with lopinavir/ritonavir and antipyretics. Moderate forms received lopinavir/ritonavir + hydroxychloroquine +/− azithromycin and antipyretics. Severe/critical forms received antiviral treatment with remdesivir or favipiravir (if available) or lopinavir/ritonavir + hydroxychloroquine + azithromycin + corticosteroid therapy (dexamethasone), tocilizumab, +/− convalescent plasma (and anti-SARS-CoV-2 immunoglobulin G). The treatment of respiratory failure has been adapted to severity, with supplemental oxygen by nasal cannula or oxygen mask, continuous positive airway pressure, or mechanical ventilation. Appropriate anticoagulant therapy (prophylactic or curative) has been prescribed for obese patients at intermediate, high, or very high thromboembolic risk, with thromboembolic clinical manifestations or disseminated intravascular coagulation, as recommended. Analgesic, antipyretic or anti-inflammatory treatments [paracetamol, metamizole, or nonsteroidal anti-inflammatory drugs (NSAIDs)] and vitamin C, D, and zinc supplementation have also been added where appropriate at the physician’s choice.
Until August 2020, patients were discharged if apyretic, with the improvement of all other symptoms and two consecutive negative nasopharyngeal PCR SARS-CoV-2 tests, at >24 h interval, after at least 3 days of apyrexia and >7 days from the first positive test. Starting with September 2020, patients were discharged after 10-14 days if considered clinically healed and could leave isolation after 14 days if asymptomatic. Reinfection was defined as a second positive test result >180 days from the initial diagnosis.
Data analysis
The main purpose of this analysis was to characterize at diagnosis the prognostic factors for (i) evolution to moderate and severe/critical forms, (ii) clinical worsening (defined as severe/critical forms or death), and (iii) overall survival. Initially, a univariate analysis was carried out to identify prognostic factors using the chi-square test and log-rank test. In the multivariate analysis, the logistic model and the Cox model were used.3 The threshold for a significant P value was 0.05. All patient data were anonymized, our study being in accordance with the Declaration of Helsinki.
Results
In the period between 1 April 2020 and 1 February 2021, from a total of 21 893 nasopharyngeal swab samples carried out, 10 143 unique cancer patients were analyzed in our laboratory with a SARS-CoV-2 RT-PCR test, with 542 positive individual patients (test positivity rate 5.34%). This figure was significantly lower than the country-level positivity rate for the same period (730 056 positive cases out of 5 601 310 tests, 13.03%, P < 0.001).1 Complete data related to COVID-19 infection could be retrieved for 341 positive cancer patients, and their demographics are presented in Table 1. A subset of two patients had a documented reinfection with SARS-CoV-2 at 7 months after the first episode with a subsequent negative RT-PCR test.
Table 1.
Patients characteristics (n = 341)
| n (%) | |
|---|---|
| Gender | |
| Female | 189 (55.4) |
| Male | 152 (44.6) |
| Age (years), median (range) | 59 (9-89) |
| Age group | |
| 0-9 | 1 (0.3) |
| 10-19 | 4 (1.2) |
| 20-29 | 9 (2.6) |
| 30-39 | 24 (7) |
| 40-49 | 45 (13.2) |
| 50-59 | 95 (27.9) |
| 60-69 | 104 (30.5) |
| 70-79 | 51 (15) |
| 80-89 | 8 (2.3) |
| ECOG PS | |
| 0-1 | 247 (72.4) |
| 2-4 | 94 (27.6) |
| BMI, median (range) | 26 (13.6-46.4) |
| BMI group | |
| <20 | 27 (7.9) |
| 20-30 | 224 (65.7) |
| >30 | 90 (26.4) |
| Smoking status | |
| Active smoker | 60 (17.6) |
| Former smoker | 70 (20.5) |
| Nonsmoker | 202 (59.2) |
| Unknown | 9 (2.6) |
| Pack-years, median (range) | 25 (2-60) |
| Comorbidities | |
| Without comorbidities | 121 (35.5) |
| 1 Comorbidity | 42 (12.3) |
| 2 Comorbidities | 75 (22) |
| >2 Comorbidities | 103 (30.2) |
| Types of comorbidities | |
| Arterial hypertension | 125 (36.7) |
| Ischemic cardiac disease | 57 (16.7) |
| Diabetes mellitus | 52 (15.2) |
| Other cardiopathy | 32 (9.4) |
| Deep vein thrombosis and/or pulmonary embolism | 32 (9.4) |
| Chronic obstructive pulmonary disease | 28 (8.2) |
| Other comorbidities | 28 (8.2) |
| Endocrinopathies | 20 (5.9) |
| Chronic liver disease | 11 (3.2) |
| Bacterial co-infection | 10 (2.9) |
| Cerebrovascular disease | 8 (2.3) |
| Peripheral arterial disease | 7 (2.1) |
| Chronic kidney disease | 6 (1.8) |
| Primary tumor location | |
| Lung | 66 (19.4) |
| Breast | 60 (17.6) |
| Digestive | 54 (15.8) |
| Gynecological | 53 (15.5) |
| Hematological | 25 (7.3) |
| Genitourinary | 23 (6.7) |
| Skin, including melanoma | 20 (5.9) |
| Sarcoma | 11 (3.2) |
| Head and neck | 9 (2.6) |
| Endocrine | 9 (2.6) |
| Multiple primary tumors | 5 (1.5) |
| Neuroendocrine | 4 (1.2) |
| Central nervous system | 1 (0.3) |
| Unknown primary tumor | 1 (0.3) |
| Present status | |
| Remission | 37 (10.9) |
| Curative setting | 80 (23.5) |
| Advanced active disease or palliation | 224 (65.7) |
| Treatment in the previous 3 months | |
| No | 58 (17) |
| Yes | 283 (83) |
| Chemotherapy | 159 (46.6) |
| Targeted treatment | 70 (20.5) |
| Surgery | 53 (15.5) |
| Immunotherapy | 41 (12) |
| Radiotherapy | 34 (10) |
| Hormonal therapy | 34 (10) |
BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
At diagnosis, among the 341 patients, 164 (48.1%) were asymptomatic, and from the 177 (51.9%) symptomatic forms, 133 (39%) were mild, 37 (10.9%) moderate, and 7 (2.1%) severe/critical. The most common complaints were fatigue (n = 152 patients, 44.6%), dry cough (n = 98, 28.7%), fever (n = 71, 20.8%), dyspnea (n = 59, 17.3%), anosmia (n = 37, 10.9%), and diarrhea (n = 18, 5.3%).
During the course of the infection, 138 (40.5%) patients remained asymptomatic, while 94 (27.6%) developed a mild form, 70 (20.5%) developed a moderate form, and 39 (11.4%) developed a severe/critical form of COVID-19, Table 2.
Table 2.
Clinical evolution of COVID-19 infection
| Worst clinical state | |||||
|---|---|---|---|---|---|
| State at diagnosis |
Total n(%) |
Asymptomatic n (%) |
Symptomatic mild n (%) |
Symptomatic moderate n (%) |
Symptomatic severe/critical n (%) |
| Asymptomatic | 164 (48) | 138 (84.1) | 13 (7.9) | 11 (6.7) | 2 (1.2) |
| Symptomatic mild | 133 (39) | 81 (60.9) | 43 (32.3) | 9 (6.8) | |
| Symptomatic moderate | 37 (10.8) | 16 (43.2) | 21 (56.8) | ||
| Symptomatic severe/critical | 7 (2) | 7 (100) | |||
| Total | 341 (100) | 138 (40.5) | 94 (27.6) | 70 (20.5) | 39 (11.4) |
The 341 patients received the following main treatments: paracetamol (n = 183, 53.7%), vitamins (n = 178, 52.2%), anticoagulants (n = 102, 29.9%), antibiotics (other than azithromycin, n = 95, 27.9%), corticosteroids (n = 71, 20.8%), hydroxychloroquine (n = 70, 20.5%), azithromycin (n = 60, 17.6%), lopinavir/ritonavir (n = 49, 14.4%), NSAIDs (n = 30, 8.8%), metamizole (n = 16, 4.7%), remdesivir (n = 9, 2.6%), favipiravir (n = 6, 1.8%), tocilizumab (n = 6, 1.8%), and darunavir/ritonavir (n = 5, 1.5%). No treatment was given to 62 asymptomatic patients (18.2%).
At the time of data analysis, out of the total number of COVID-19 patients, 315 (92.4%) had returned home cured of COVID-19, and 26 patients (7.6%) who developed a severe/critical form died due to the infection. The median duration until the second negative test was 13 days (limits 7-54).
The median survival of the deceased patients was 17.5 days (range 2-60) after RT-PCR diagnosis. Four additional patients had a cancer-related death in the 30 days after being considered healed of COVID-19.
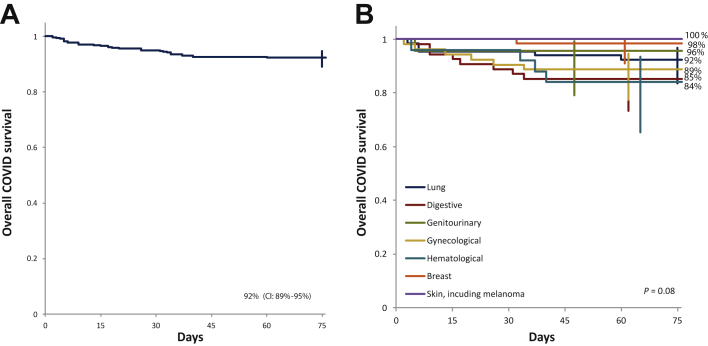
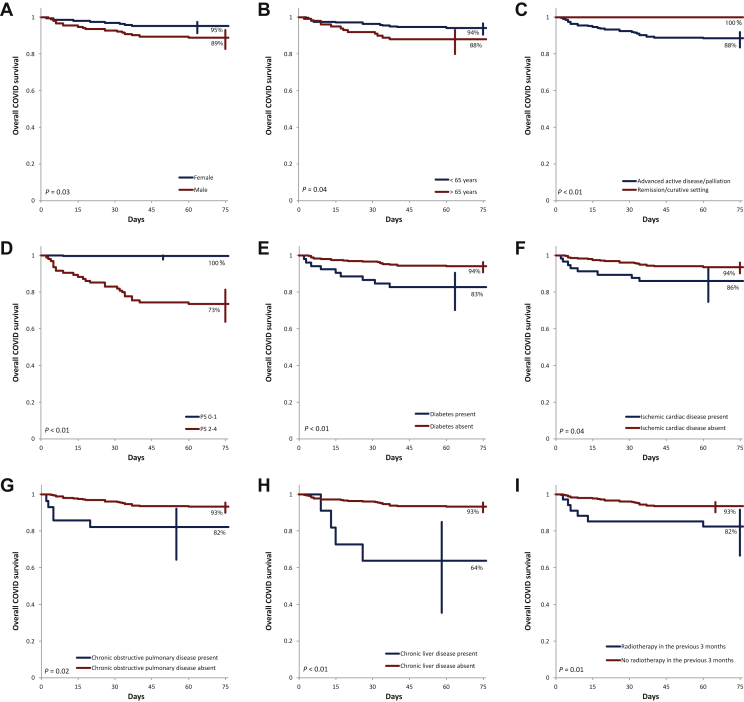
Until 1 February 2021, coinciding with database lock, 30 815 COVID-19-related deaths were recorded among a total of 1 073 713 closed cases in Romania (mortality rate 2.9%), which was significantly lower compared with the 7.6% mortality rate in our cancer patients series (P < 0.001).1 The overall survival curve of patients and specific cancer types after COVID-19 infection is presented in Figure 1. Subgroup analysis of overall survival is presented in Figure 2.
Figure 1.
Survival after COVID-19 infection.
(A) Overall survival. (B) Survival by primary tumor site.
CI, confidence interval.
Figure 2.
Overall survival according to (A) gender, (B) age, (C) cancer status and treatment intent, (D) performance status, (E) diabetes mellitus, (F) ischemic cardiac disease, (G) chronic obstructive pulmonary disease, (H) chronic liver disease, and (I) radiotherapy in the previous 3 months.
PS, performance status.
Analysis for moderate and severe/critical forms
A first univariate analysis was conducted for the seriousness of the infection (asymptomatic or symptomatic mild versus symptomatic moderate or severe/critical form of COVID-19) presented in Table 3. Factors associated with a worse prognosis in univariate analysis were male gender, advanced active disease or palliation, older age (>65 years), active or former smoker, performance status (PS) 2-4, a high number of comorbidities (≥3), selected individual comorbidities (diabetes mellitus, ischemic cardiac disease), hematological malignancies, lung cancer, and no active cancer treatment in the previous 3 months. Specifically, asymptomatic moderate or severe/critical form of COVID-19 developed more frequently in men versus women (41.4% versus 24.3%, P < 0.01), patients with advanced active disease or palliation versus patients in remission or treated with curative intention (38.8% versus 18.8% P < 0.01), patients older than 65 years versus younger patients (40.4% versus 28.5%, P = 0.03), active or former smokers versus nonsmokers (40% versus 27%, P = 0.01), patients with a worse PS (2-4) versus good PS (0-1) (58.5% versus 21.9%, P < 0.01), patients with ≥3 versus 0-2 comorbidities (39.9% versus 23.3%, P < 0.01), patients with diabetes mellitus versus patients without (38.1% versus 29.1%, P < 0.01), patients with ischemic cardiac disease versus patients without (45.6% versus 29.2%, P = 0.02), patients with hematological malignancies versus patients with solid tumors (56% versus 30.1%, P < 0.01), and patients with lung cancer versus other cancers (42.4% versus 29.5%, P = 0.04). Patients without cancer-specific treatment in the 3 months previous to COVID-19 infection developed a moderate or severe/critical form of COVID-19 more frequently versus patients with treatment (44.8% versus 29.3%, P = 0.02). No other prognostic factors achieved statistical significance in univariate analysis with respect to the severity of COVID-19 infection. Other analyzed factors were not significant in univariate analysis and included body mass index, presence of some comorbidities (cerebrovascular disease, peripheral arterial disease, other cardiopathy, deep vein thrombosis and/or pulmonary embolism, chronic obstructive pulmonary disease, chronic kidney disease, chronic liver disease, infections, endocrinopathies or other comorbidities), solid cancers such as digestive, breast, gynecological, genitourinary, skin including melanoma, and individual cancer treatment methods in the previous 3 months (surgery, radiotherapy, chemotherapy, hormonal therapy, targeted molecular therapy, and immunotherapy).
Table 3.
Univariate and multivariate analysis of prognostic factors associated with COVID-19 severity and survival
| Asymptomatic or symptomatic mild (A) versus symptomatic moderate or severe/critical (B) COVID-19 |
Clinical worsening: asymptomatic or symptomatic mild/moderate (C) versus symptomatic severe/critical or death (D) |
Overall survival at 90 days (E) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prognosticfactor | A | B | Univariate analysis |
Multivariate analysis |
C | D | Univariate analysis |
Multivariate analysis |
E | Survival | Univariate analysis |
Multivariate analysis |
||||
| Category | n (%) | n (%) | P | OR (95% CI) | P | n (%) | n (%) | P | OR (95% CI) | P | n (%) | % | P | OR (95% CI) | P | |
| Gender | Female | 143 (75.7) | 46 (24.3) | <0.01 | 1.27 (0.71-2.28) | 0.43 | 176 (93.1) | 13 (6.9) | <0.01 | 1.87(0.76-4.61) | 0.17 | 189 (55.4) | 95 | 0.03 | ||
| Male | 89 (58.6) | 63 (41.4) | 126 (82.9) | 26 (17.1) | 152 (44.6) | 89 | ||||||||||
| Present status | Advanced active disease/palliation | 137 (61.2) | 87 (38.8) | <0.01 | 1.3 (0.68-2.49) | 0.42 | 188 (83.9) | 36 (16.1) | <0.01 | 0.48 (0.09-2.53) | 0.39 | 224 (65.7) | 88 | <0.01 | ||
| Remission/curative setting | 95 (81.2) | 22 (18.8) | 114 (97.4) | 3 (2.6) | 117 (34.3) | 100 | ||||||||||
| Age | ≤65 | 173 (71.5) | 69 (28.5) | 0.03 | 1.34 (0.75-2.4) | 0.33 | 219 (90.5) | 23 (9.5) | 0.08 | 242 (71) | 94 | 0.04 | ||||
| >65 | 59 (59.6) | 40 (40.4) | 83 (83.8) | 16 (16.2) | 99 (29) | 88 | ||||||||||
| Smoking status | Active/former smoker | 78 (60) | 52 (40) | 0.01 | 1.48 (0.81-2.72) | 0.21 | 112 (86.2) | 18 (13.8) | 0.21 | 130 (38.1) | 92 | 0.65 | ||||
| Nonsmoker | 154 (73) | 57 (27) | 183 (90.6) | 19 (9.4) | 202 (59.2) | 93 | ||||||||||
| ECOG PS | 0-1 | 193 (78.1) | 54 (21.9) | <0.01 | 3.82 (2.11-6.91) | <0.0001 | 242 (98) | 5 (2) | <0.01 | 34.1 (9.18-126.49) | <0.0001 | 247 (72.4) | 100 | <0.01 | 82.56 (11.26-605.24) | <0.0001 |
| 2-4 | 39 (41.5) | 55 (58.5) | 60 (63.8) | 34 (36.2) | 94 (27.6) | 73 | ||||||||||
| BMI | <30 | 166 (66.1) | 85 (33.9) | 0.21 | 219 (87.3) | 32 (12.7) | 0.2 | 251 (73.6) | 92 | 0.4 | ||||||
| ≥30 | 66 (73.3) | 24 (16.7) | 83 (92.2) | 7 (7.8) | 90 (26.4) | 94 | ||||||||||
| Comorbidities | 0-2 | 125 (76.7) | 38 (23.3) | <0.01 | 1.28 (0.71-2.32) | 0.41 | 147 (90.2) | 16 (9.8) | 0.37 | 163 (47.8) | 94 | 0.31 | ||||
| ≥3 | 107 (60.1) | 71 (39.9) | 155 (87.1) | 23 (12.9) | 178 (52.2) | 91 | ||||||||||
| Arterial hypertension | Yes | 81 (64.8) | 44 (35.2) | 0.33 | 112 (89.6) | 13 (10.4) | 0.65 | 125 (36.7) | 92 | 0.83 | ||||||
| No | 151 (69.9) | 65 (30.1) | 190 (88) | 26 (12) | 216 (63.3) | 93 | ||||||||||
| Diabetes mellitus | Yes | 27 (51.9) | 25 (48.1) | <0.01 | 1.41 (0.7-2.86) | 0.34 | 39 (75) | 13 (25) | <0.01 | 2.17 (0.84-5.61) | 0.11 | 52 (15.2) | 83 | <0.01 | ||
| No | 205 (70.9) | 84 (29.1) | 263 (91) | 26 (9) | 289 (84.8) | 94 | ||||||||||
| Ischemic cardiac disease | Yes | 31 (54.4) | 26 (45.6) | 0.02 | 1.73 (0.83-3.58) | 0.14 | 48 (84.2) | 9 (15.8) | 0.26 | 57 (16.7) | 86 | 0.04 | 3.05 (1.28-7.3) | 0.0126 | ||
| No | 201 (70.8) | 83 (29.2) | 254 (89.4) | 30 (10.6) | 284 (83.3) | 94 | ||||||||||
| Cerebrovascular disease | Yes | 4 (50) | 4 (50) | 0.47 | 7 (87.5) | 1 (12.5) | 0.64 | 8 (2.3) | 88 | 0.6 | ||||||
| No | 228 (68.5) | 105 (31.5) | 295 (88.6) | 38 (11.4) | 333 (97.7) | 92 | ||||||||||
| Peripheral arterial disease | Yes | 3 (42.9) | 4 (57.1) | 0.3 | 4 (57.1) | 3 (42.9) | 0.04 | 9.7 (1.25-75.34) | 0.03 | 7 (2.1) | 86 | 0.53 | ||||
| No | 229 (68.6) | 105 (31.4) | 298 (89.2) | 36 (10.8) | 334 (97.9) | 93 | ||||||||||
| Other cardiopathy | Yes | 18 (56.2) | 14 (43.8) | 0.13 | 27 (84.4) | 5 (15.6) | 0.62 | 32 (9.4) | 88 | 0.26 | ||||||
| No | 214 (69.3) | 95 (30.7) | 275 (89) | 34 (11) | 309 (90.6) | 93 | ||||||||||
| Deep vein thrombosis and/or pulmonary embolism | Yes | 17 (53.1) | 15 (46.9) | 0.06 | 28 (87.5) | 4 (12.5) | 0.93 | 32 (9.4) | 88 | 0.25 | ||||||
| No | 215 (69.6) | 94 (30.4) | 274 (88.7) | 35 (11.3) | 309 (90.6) | 93 | ||||||||||
| Chronic obstructive pulmonary disease | Yes | 15 (53.6) | 13 (46.4) | 0.09 | 23 (82.1) | 5 (17.9) | 0.42 | 28 (8.2) | 82 | 0.02 | ||||||
| No | 216 (69.3) | 96 (30.7) | 279 (89.1) | 34 (10.9) | 313 (91.8) | 93 | ||||||||||
| Chronic kidney disease | Yes | 2 (33.3) | 4 (66.7) | 0.16 | 4 (66.7) | 2 (33.3) | 0.29 | 6 (1.8) | 83 | 0.41 | ||||||
| No | 230 (68.7) | 105 (31.3) | 298 (89) | 37 (11) | 335 (98.2) | 93 | ||||||||||
| Chronic liver disease | Yes | 5 (45.5) | 6 (54.5) | 0.19 | 7 (63.6) | 4 (36.4) | 0.03 | 6.48 (1.06-39.65) | 0.04 | 11 (3.2) | 64 | <0.01 | 6.85 (2.19-21.38) | 0.001 | ||
| No | 227 (68.8) | 103 (31.2) | 295 (89.4) | 35 (10.6) | 330 (96.8) | 93 | ||||||||||
| Infections | Yes | 5 (50) | 5 (50) | 0.37 | 7 (70) | 3 (30) | 0.17 | 10 (2.9) | 80 | 0.12 | ||||||
| No | 227 (68.6) | 104 (31.4) | 295 (89.1) | 36 (10.9) | 331 (97.1) | 93 | ||||||||||
| Endocrinopathies | Yes | 17 (85) | 3 (15) | 0.09 | 19 (95) | 1 (5) | 0.57 | 20 (5.9) | 95 | 0.65 | ||||||
| No | 215 (67) | 106 (33) | 283 (88.2) | 38 (11.8) | 321 (94.1) | 92 | ||||||||||
| Other comorbidities | Yes | 19 (67.9) | 9 (32.1) | 0.98 | 27 (96.4) | 1 (3.6) | 0.29 | 28 (8.2) | 100 | 0.12 | ||||||
| No | 213 (68.1) | 100 (31.9) | 275 (87.9) | 38 (12.1) | 313 (91.8) | 92 | ||||||||||
| Tumor type | Hematological | 11 (44) | 14 (56) | <0.01 | 2.64 (0.97-7.15) | 0.057 | 17 (68) | 8 (32) | <0.01 | 1.88 (0.61-5.88) | 0.27 | 25 (7.3) | 84 | 0.11 | ||
| Solid tumors | 221 (69.9) | 95 (30.1) | 285 (90.2) | 31 (9.8) | 316 (92.7) | 93 | ||||||||||
| Lung | Yes | 38 (57.6) | 28 (42.4) | 0.04 | 1.36 (0.68-2.74) | 0.39 | 58 (87.9) | 8 (12.1) | 0.85 | 66 (19.4) | 92 | 0.99 | ||||
| No | 194 (70.5 | 81 (29.5) | 244 (88.7) | 31 (11.3) | 275 (80.6) | 92 | ||||||||||
| Digestive | Yes | 33 (61.1) | 21 (38.9) | 0.23 | 44 (81.5) | 10 (18.5) | 0.07 | 54 (15.8) | 85 | 0.03 | ||||||
| No | 199 (69.3) | 88 (30.7) | 258 (89.9) | 29 (10.1) | 287 (84.2) | 94 | ||||||||||
| Breast | Yes | 47 (78.3) | 13 (21.7) | 0.06 | 59 (98.3) | 1 (1.7) | <0.01 | 0.41 (0.04-3.82) | 0.44 | 60 (17.6) | 98 | 0.06 | ||||
| No | 185 (65.8) | 96 (34.2) | 243 (86.5) | 38 (13.5) | 281 (82.4) | 91 | ||||||||||
| Gynecological | Yes | 40 (75.5) | 13 (24.5) | 0.21 | 46 (86.8) | 7 (13.2) | 0.66 | 53 (15.5) | 89 | 0.26 | ||||||
| No | 192 (66.7) | 96 (33.3) | 256 (88.9) | 32 (11.1) | 288 (84.5) | 93 | ||||||||||
| Genitourinary | Yes | 13 (56.5) | 10 (43.5) | 0.22 | 21 (91.3) | 2 (8.7) | 0.93 | 23 (6.7) | 96 | 0.55 | ||||||
| No | 219 (68.9) | 99 (31.1) | 281 (88.4) | 37 (11.6) | 318 (93.3) | 92 | ||||||||||
| Skin, including melanoma | Yes | 17 (85) | 3 (15) | 0.09 | 20 (100) | 0.33 | 20 (5.9) | 100 | 0.19 | |||||||
| No | 215 (67) | 106 (33) | 282 (87.9) | 39 (12.1) | 321 (94.1) | 92 | ||||||||||
| Treatment in the previous 3 months | Yes | 200 (70.7) | 83 (29.3) | 0.02 | 0.48 (0.25-0.94) | 0.031 | 253 (89.4) | 30 (10.6) | 0.28 | 58 (17) | 93 | 0.83 | ||||
| No | 32 (55.2) | 26 (44.8) | 49 (84.5) | 9 (15.5) | 283 (83) | 92 | ||||||||||
| Surgery | Yes | 40 (75.5) | 13 (24.5) | 0.21 | 52 (98.1) | 1 (1.9) | 0.02 | 0.13 (0.02-1.15) | 0.07 | 53 (15.5) | 98 | 0.09 | ||||
| No | 192 (66.7) | 96 (33.3) | 250 (86.8) | 38 (13.2) | 288 (84.5) | 91 | ||||||||||
| Chemotherapy | Yes | 108 (67.9) | 51 (32.1) | 0.97 | 138 (86.8) | 21 (13.2) | 0.34 | 159 (46.6) | 91 | 0.25 | ||||||
| No | 124 (68.1) | 58 (31.9) | 164 (90.1) | 18 (9.9) | 182 (53.4) | 94 | ||||||||||
| Radiotherapy | Yes | 25 (73.5) | 9 (26.5) | 0.47 | 28 (82.4) | 6 (17.6) | 0.36 | 34 (10) | 82 | 0.01 | 4.34 (1.67-11.28) | 0.0027 | ||||
| No | 207 (67.4) | 100 (32.6) | 274 (89.3) | 33 (10.7) | 307 (90) | 93 | ||||||||||
| Hormonal therapy | Yes | 26 (76.5) | 8 (23.5) | 0.27 | 33 (97.1) | 1 (2.9) | 0.17 | 34 (10) | 97 | 0.28 | ||||||
| No | 206 (67.1) | 101 (32.9) | 269 (87.6) | 38 (12.4) | 307 (90) | 92 | ||||||||||
| Targeted therapy | Yes | 49 (70) | 21 (30) | 0.69 | 63 (90) | 7 (10) | 0.67 | 70 (20.5) | 94 | 0.49 | ||||||
| No | 183 (67.5) | 88 (32.5) | 239 (88.2) | 32 (11.8) | 271 (79.5) | 92 | ||||||||||
| Immunotherapy | Yes | 31 (75.6) | 10 (24.4) | 0.27 | 38 (92.7) | 3 (7.3) | 0.53 | 41 (12) | 95 | 0.49 | ||||||
| No | 201 (67) | 99 (33) | 264 (88) | 36 (12) | 300 (88) | 92 | ||||||||||
BMI, body mass index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; OR, odds ratio.
A first multivariate analysis was carried out for the 11 factors found significant from the previous univariate analysis. Factors with an independent prognostic value in the multivariate analysis were Eastern Cooperative Oncology Group (ECOG) PS [hazard ratio (HR) 3.82, 95% confidence interval (CI) 2.11-6.91 for ECOG PS 2-4, P < 0.0001] and if a previous cancer treatment was given in the 3 previous months (HR 0.48, 95% CI 0.25-0.94 for treatment given, P = 0.031).
Analysis for clinical worsening
A second univariate analysis was carried out for clinical worsening during COVID-19 infection. Clinical worsening was defined as patients who developed a severe/critical form or died due to COVID-19 infection. Factors with a significant negative prognosis in univariate analysis in relation to clinical worsening were male gender, advanced active disease or palliation, PS 2-4, selected individual comorbidities (diabetes mellitus, peripheral arterial disease, chronic liver disease), hematological malignancies, and no surgical treatment in the last 3 months, whereas breast cancer diagnosis had less clinical worsening. Specifically, a symptomatic severe/critical form of COVID-19 or death occurred more frequently in men versus women (17.1% versus 6.9%, P < 0.01), patients with advanced active disease or palliation versus patients in remission or treated with curative intention (16.1% versus 2.6% P < 0.01), patients with a worse PS (2-4) versus good PS (0-1) (36.2% versus 2%, P < 0.01), patients with diabetes mellitus versus patients without (25% versus 9%, P < 0.01), patients with peripheral arterial disease versus patients without (42.9% versus 10.8%, P = 0.04), patients with chronic liver disease versus patients without (36.4% versus 10.6%, P = 0.04), and patients with hematological malignancies versus patients with solid tumors (32% versus 9.8%, P < 0.01). Patients without surgical treatment in the 3 months previous to COVID-19 infection developed a severe/critical form of COVID-19 more frequently or died versus patients with surgical treatment (13.2% versus 1.9%, P = 0.02). Breast cancer diagnosis versus other cancer diagnosis was associated with less clinical worsening (1.7% versus 13.5%, P < 0.01).
A second multivariate analysis related to clinical worsening during COVID-19 infection was carried out and included the nine factors identified in the univariate analysis. The multivariate analysis retained three independent prognostic factors: ECOG PS (HR 34.1, 95% CI 9.18-126.49 for PS 2-4, P < 0.0001), peripheral arterial disease (HR 9.7, 95% CI 1.25-75.34 for the presence of disease, P = 0.03), and chronic liver disease (HR 6.48, 95% CI 1.06-39.65 for the presence of disease, P = 0.04), Table 3.
Analysis for overall survival
A third univariate analysis was carried out for overall survival after COVID-19 infection. Factors significantly related to a worse overall survival at 90 days following COVID-19 infection were male versus female gender (89% versus 95%, P = 0.03), advanced active disease or palliation versus remission or curative setting (88% versus 100%, P < 0.01), age >65 versus ≤65 years (88% versus 94%, P = 0.04), patients with a worse PS (2-4) versus good PS (0-1) (73% versus 100%, P < 0.01), patients with diabetes mellitus versus patients without (83% versus 94%, P < 0.01), patients with ischemic cardiac disease versus patients without (86% versus 94%, P = 0.04), patients with chronic obstructive pulmonary disease versus patients without (82% versus 93%, P = 0.02), patients with chronic liver disease versus patients without (64% versus 93%, P < 0.01), patients with digestive tumors versus patients with other tumors (85% versus 94%, P = 0.03), and patients with radiotherapy treatment given in the last 3 months versus patients without (83% versus 92%, P < 0.01).
A third multivariate analysis was carried out and included the 10 factors from the univariate analysis. The multivariate analysis retained four independent prognostic factors: ECOG PS (HR 82.56, 95% CI 11.26-605.24 for PS 2-4, P < 0.0001), ischemic cardiac disease (HR 3.05, 95% CI 1.28-7.3 for the presence of disease, P < 0.0126), chronic liver disease (HR 6.85, 95% CI 2.19-21.38 for the presence of disease, P < 0.001), and comorbidities associated with a negative prognosis together with radiotherapy treatment in the last 3 months (HR 4.34, 95% CI 1.67-11.28 for having radiotherapy, P = 0.0027), Table 3.
Discussion
The Oncology Institute ‘Prof. Dr. Ion Chiricuta’ from Cluj-Napoca has been significantly affected by the COVID-19 pandemic, however, sustained measures have been taken to counteract the disruption of activity through bimonthly PCR screening of all staff, PCR screening of patients with symptoms suggestive for COVID-19 infection, PCR screening of asymptomatic patients before inpatient care, surgical or interventional radiology procedures, radiotherapy sessions or systemic therapy (chemotherapy, immunotherapy, targeted therapy), creation of COVID-19-free pathways and treatment spaces, and regular staff and patient information on the correct use of protective equipment.
Analysis of our series of patients infected with COVID-19 showed that the incidence among cancer patients was lower than the national incidence. This finding could be explained by the more active prophylactic measures taken by cancer patients (self-isolation, social distancing, mask wearing, washing hands) who by all means try to avoid COVID-19 infection due to the risks of a more severe evolution and interference with anticancer treatments. The higher positivity rate of the national incidence could also be explained by the strict selection criteria for cases tested at the national level, according to the National Institute for Public Health methodology. At a national level, most tests were carried out on suspect symptomatic cases, according to the case definition, while in our institute most cases tested were asymptomatic and were scheduled for inpatient care or oncological treatments. Active screening with nasopharyngeal PCR was used to screen for SARS-CoV-2 in the asymptomatic phase in almost half of the cases (48%). At the time of diagnosis, 51.9% had one or more symptoms: fatigue, dry cough, fever, dyspnea, anosmia/ageusia, or diarrhea.
Confirming the results reported by Chinese and Italian authors4, 5, 6, 7, 8 and not in line with the initial results from the Gustave Roussy Institute,9 in our series the mortality rate for the closed cases of COVID-19 in patients with cancer was significantly higher compared with the general population in Romania (7.6% versus 2.9%, P < 0.01, risk ratio = 2.7). These local results can constitute a quantitative argument for Romanian doctors to recommend vaccination as an efficient weapon to transform COVID-19 into a preventable disease for their cancer patient population. None of the patients included in the present analysis were vaccinated, given that the vaccination program for oncological patients started on 1 February 2021 in Romania.
In univariate analysis, in our series, the factors with a pejorative prognosis related to a moderate or severe/critical evolution of COVID-19 infection, clinical worsening (severe/critical form and/or death), and an impaired survival with COVID-19 infection were male gender, advanced active cancer, a declined PS (2-4), and presence of diabetes as an individual comorbidity.
We identified additional factors that were predictive for a moderate or severe/critical evolution of COVID-19 infection such as age >65 years, active or former smoker, more than three comorbidities, ischemic cardiac disease, hematological malignancies, lung cancer, and no specific cancer treatment in the previous 3 months.
Factors that were predictive for clinical worsening of COVID-19 infection included peripheral arterial disease, chronic liver disease, hematological malignancies, and no specific surgical cancer treatment in the previous 3 months, with breast cancer having a better outcome.
Factors that were negatively correlated with survival due to COVID-19 included age >65 years, ischemic cardiac disease, chronic obstructive pulmonary disease, chronic liver disease, digestive tumors, and radiotherapy in the last 3 months.
Some analyzed factors were found to have positive prognostic value in other studies (presence of obesity and certain comorbidities, surgical treatment, or chemotherapy in the previous 3 months), however, we found no significant positive relationship in our study.
On multivariate analysis, PS 2-4 was the only independent predictor for a moderate or severe/critical evolution, clinical worsening, and overall survival (P < 0.0001) due to SARS-CoV-2 in our series. Additionally, the multivariate analysis highlighted other independent prognostic factors for a moderate or severe/critical evolution of COVID-19 (absence of cancer treatment in the previous 3 months, statistical trend for hematological malignancies), for clinical worsening (peripheral arterial disease, chronic liver disease), and for overall survival (ischemic cardiac disease, chronic liver disease, radiotherapy in the previous 3 months).
The Gustave Roussy Institute reported, in a series of 137 cancer patients, a positivity rate of the RT-PCR test and mortality that was similar to the ones in the general population. Prognostic factors for clinical worsening in a univariate analysis were PS >1, hematological malignancies, cancer treatment in the last 3 months, and chemotherapy in the last 3 months. Only PS remained significant for clinical worsening in a multivariate analysis. In the same series, prognostic factors for COVID-19 survival in a univariate analysis were PS, disease status (active/metastatic), and chemotherapy treatment in the last 3 months. Again, only PS remained significant for survival in the multivariate analysis.9
In a multicenter cohort study in the province of Hubei, China, 105 COVID-19 patients with cancer were compared with 653 COVID-19 patients without cancer. Patients with cancer appeared significantly more vulnerable to SARS-CoV-2 (at 3.5 times the risk compared with the general population) in terms of necessity of invasive ventilation, admission to the intensive care unit, severe/critical forms, and death.5 The fatality rate for infected cancer patients in China is 28.6%,10 compared with a 2.3% fatality rate for all COVID-19 patients.8 The major risk factor for cancer patients during the COVID-19 pandemic is their inability to receive enough medical care.11
The largest international registry of patients with thoracic tumors and COVID-19 infection, TERAVOLT, included 1012 patients from 20 countries (Europe 74% and North America 23%) and found a very high mortality for this category of patients (32%). Patients presenting with pneumonia [odds ratio (OR) 2.7], consolidation (OR 2), bilateral involvement of the lungs (OR 2.8), and pleural effusion (OR 2.7) had a higher risk of death. In multivariate analysis, the factors significantly related to a fatal evolution of COVID-19 infection were PS ≥2 (OR 3.7), stage IV (OR 1.9), active smoker or ex-smoker status (OR 2), corticosteroid use before the diagnosis of COVID-19 (OR 1.8), and age >65 years (OR 1.5). Chemotherapy and targeted molecular therapy were not correlated to a higher risk of death and immunotherapy had a lower risk of mortality (OR 0.6).12
Based on these results, the authors developed a nomogram that predicts mortality in patients with chest tumors and COVID-19. Patients receive a score based on ECOG PS, stage, smoking/nonsmoking status, age, steroid use, and the type of systemic treatment. For example, a 70-year-old smoker with an ECOG 2 PS who received third-line chemotherapy with docetaxel for a stage IV squamous carcinoma has a score of 260 that translates into a risk of death of >60%. A 50-year-old nonsmoker with an ECOG PS 0 who receives first-line therapy with osimertinib for stage IV NSCLC has a score of 55 points, which translates into a lower risk of death of 20%.
Zhang et al.10 studied the outcomes of cancer patients with COVID-19 and found more than fourfold higher likelihood of experiencing severe events in those who received therapy in the preceding 14 days of COVID-19 diagnosis.
To assist health care facilities, leading oncology societies such as the European Society of Medical Oncology, the American Society of Clinical Oncology, and the National Comprehensive Cancer Network have developed guidelines to mitigate the negative effects of the COVID-19 pandemic on the diagnosis and treatment of cancer patients.13, 14, 15
The drugs used in >50% of patients were paracetamol and vitamins and in 20%-30% were anticoagulants, antibiotics, and hydroxychloroquine. Among the approved drugs for SARS-CoV-2, corticoids, authorized in severe forms following the RECOVERY study,16,17 were used in 20.8% of patients in our series. Other authorized medications, the antiviral agent remdesivir and also the monoclonal anti-interleukin 6 antibody tocilizumab,18, 19, 20, 21 considered active in severe/critical forms, and convalescent plasma transfusion were administered in <5% of patients. No anti-SARS-CoV-2 therapeutic antibodies and no vaccines were available in the studied period. The number of patients treated with each drug and the retrospective nature of the study does not support any conclusions about their effectiveness.
Conclusion
Although the COVID-19 pandemic has posed obstacles to the conduct of the activity at the Oncology Institute ‘Prof. Dr. Ion Chiricuta’, the introduction of protective measures and systematic screening of the virus for staff and patients (before inpatient treatment and major diagnostic and therapeutic procedures) were implemented in an effort to keep the institute virus-free with a dedicated buffer department.
In our series the mortality of COVID-19 infection appeared to be greater among cancer patients compared with the general population.
PS was the only independent prognostic factor found in all our multiple multivariate analyses, related both to an evolution towards a moderate or severe/critical form, to clinical worsening, and to an impaired survival with COVID-19.
Acknowledgments
Funding
This work was supported by the Iuliu Hatieganu University of Medicine and Pharmacy Cluj-Napoca [grant number 4556/01.10.2020].
Disclosure
ACP reports personal fees from Roche, Merck Serono, Sandoz, support for attending meetings and/or travel from Accord, Pfizer, grants from University of Medicine and Pharmacy Iuliu Hatieganu Cluj-Napoca, outside the submitted work. TC reports personal fees from Astellas Pharma, Janssen, Merck Sharp & Dohme, Merck Serono, Amgen, Roche, Pfizer, Sanofi Genzyme, Servier, Ipsen, AstraZeneca, Lilly, Novartis, Boehringer Ingelheim, Bristol Myers Squibb, grants from University of Medicine and Pharmacy Iuliu Hatieganu Cluj-Napoca, outside the submitted work. PK reports personal fees from Roche, Sandoz, Bristol Myers Squibb, AstraZeneca, Merck Serono, Pproevents, Astellas Pharma, support for attending meetings and/or travel from Sandoz, Elli Lilly, Roche, Boehringer Ingelheim, grants from University of Medicine and Pharmacy Iuliu Hatieganu Cluj-Napoca, Romanian National Authority for Scientific Research, COST Association, outside the submitted work. CM reports support for attending meetings and/or travel from Roche, Pfizer, Accord, outside the submitted work. MBV reports support for attending meetings and/or travel from Roche, Pfizer, Accord, Servier, outside the submitted work. CV reports grants from the Romanian National Authority for Scientific Research, COST Association, outside the submitted work. All other authors have declared no conflicts of interest.
References
- 1.Coronavirus Pandemic (COVID-19 Our World in Data. Available at. https://ourworldindata.org/coronavirus/country/romania
- 2.Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) [Internet]. Available at. https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19)
- 3.Rosner B. Cengage Learning; 2010. Fundamentals of Biostatistics. [Google Scholar]
- 4.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.-j., Ni Z.-y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.J., Liang W.H., Zhao Y., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):200547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 9.Barlesi F., Foulon S., Bayle A., et al. Abstract CT403: outcome of cancer patients infected with COVID-19, including toxicity of cancer treatments. Cancer Res. 2020;80(suppl 16):CT403. [Google Scholar]
- 10.Zhang L., Zhu F., Xie L., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H., Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21(4):e181. doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garassino M.C., Whisenant J.G., Huang L.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCCN. Coronavirus disease 2019 (COVID-19) resources for the cancer care community. Available at. https://www.nccn.org/covid-19/
- 14.ASCO. ASCO coronavirus resources. Available at. https://www.asco.org/asco-coronavirus-information
- 15.ESMO. ESMO COVID-19 and cancer. Available at. https://www.esmo.org/covid-19-and-cancer
- 16.Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahase E. Covid-19: low dose steroid cuts death in ventilated patients by one third, trial finds. BMJ. 2020;369:m2422. doi: 10.1136/bmj.m2422. [DOI] [PubMed] [Google Scholar]
- 18.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman J.D., Lye D.C.B., Hui D.S., et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guaraldi G., Meschiari M., Cozzi-Lepri A., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]