Abstract
Background
Patients with cancer are at high risk of severe or lethal COVID-19. The impact of SARS-COV-2 vaccination on the risk of developing COVID-19 was investigated in an exhaustive series of patients from a comprehensive cancer center.
Methods
This is a study of the exhaustive population of 2391 cancer patients who were prescribed SARS-COV-2 vaccination until 09/21. Patient characteristics, documented SARS-COV-2 infection with RT-PCR, and survival were collected. The primary endpoint was the rate of COVID-19 after vaccination. Secondary endpoints included risk factors to develop COVID-19 after vaccination, with a comparison with the cohort of vaccinated health care workers (HCW), and risk factors for death.
Results
From January to September 2021, among 2391 patients with cancer under active treatment in whom a SARS-COV-2 vaccine was prescribed, 659 (28%), 1498 (63%) and 139 (6%) received 1, 2, and 3 doses, respectively. Ninety five patients received a single dose of vaccine after a previous COVID-19. Two thousand two hundred eighty five health care workers (HCW) received one (N = 17, 0.7%), 2–3 (N = 2026, 88.7%) vaccine doses and one dose after COVID-19 (N = 242, 10.6%). With a median follow-up of 142 and 199 days for patients and HCW, respectively. Thirty nine (1.6%) patients and 35 (1.5%) HCW developed COVID-19 after vaccination. Six of 39 cancer patients and no HCW died because ofCOVID-19 within 50 days after diagnosis. Independent risk factors for COVID-19 in vaccinated patients were age, single dose of vaccine without previous COVID-19 and anti-CD20 treatment in the last three months. Independent risk factors for death included metastatic disease, gender, cancer type, but also documented COVID-19 before vaccination.
Conclusions
Patients receiving two or more doses of COVID-19 vaccine have reduced risk of COVID-19. The risk of death of vaccinated cancer patients presenting COVID-19 remains high. COVID-19 before vaccination is associated with an increased overall risk of death.
Keywords: COVID-19, Vaccination, Cancer patients, Survival, Health care workers
1. Introduction
Cancer patients are at high risk of death from COVID-19, with a 28-day mortality rate after documented SARS-COV-2 infection with RT-PCR ranging from 10 to 38% [[1], [2], [3], [4], [5], [6], [7], [8], [9]].
This increased risk of death is observed in all cancer types, of all stages [[1], [2], [3], [4], [5], [6], [7], [8], [9]], but has been reported to be higher in patients with associated comorbidities, with advanced lung cancer, in patients with hematological malignancies, in particular those treated with anti-CD20 Ab, in patients with lymphopenia, and a biological inflammation profile with CRP increase in particular [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]].
This increased risk of death is considered to result from immunodepression and inflammation associated with cancer as well as from cytotoxic and immunomodulating treatments, resulting in defective B and T cell response against SARS-COV-2 [[10], [11], [12], [13], [14]]. Lymphopenia is consistently associated with a worse outcome after COVID-19 infection in cancer patients [4,[6], [7], [8]].
Vaccination with mRNA based or recombinant virus vaccines have been shown to reduce the risk of documented SARS-COV-2 infection with long lasting protection in the general population [[15], [16], [17], [18]]. The protective effect of SARS-COV-2 vaccines have also been demonstrated in cancer patients in relatively small sized series [[19], [20], [21], [22]], although cancer patients develop less efficient antiviral immune response after vaccination [[23], [24], [25], [26]]. In a series of 1503 patients reported from our center, we reported on a higher incidence of COVID-19 in patients who received only one vaccine dose [21]. The impaired immune response observed in general in frail patients prompted researchers to investigate additional strategies for immunization. An additional vaccination boost has been proposed in transplanted patients with encouraging results [[26], [27], [28], [29], [30]]. Heterologous versus homologous boosts were also tested and reported to yield similar antibody response in patients without cancers [27]. A single dose of vaccine has been found to be efficient in patients who recovered from symptomatic COVID-19 infection [31].
Whether these strategies are efficient in cancer patients is still unclear. To address these questions, we analyzed the real-life efficacy of the vaccination program proposed for cancer patients in the comprehensive cancer Center Leon Berard (CLB). All patients were proposed for two doses of vaccines, homologous or heterologous depending on vaccine availability at that time, and a third dose after four months. Patients with a previous documented symptomatic COVID-19 infection were proposed for a single dose of vaccine in line with national recommendations.
We present here the clinical efficacy of SARS-COV-2 vaccination in this population of 2391 cancer patients receiving active cancer treatment in the Centre Leon Berard, and an analysis of the risk of documented SARS-COV-2 infections and death.
2. Material and methods
2.1. Study design and objectives
This is a retrospective study of the exhaustive series of patients with a prescribed and documented COVID-19 vaccination in the CLB until September 30, 2021. Data were collected until October 31, 2021.
The primary objective of the study was to describe the incidence of documented COVID-19 in cancer patients after vaccination against SARS-COV-2 in the exhaustive population of 2391 cancer patients in whom vaccination was prescribed on the site until September 2021. Patients who received one, two, or three doses of homologous or heterologous COVID-19 vaccines were compared, as well as patients who received a single dose of vaccine after a documented and symptomatic COVID-19 infection, as described [28]. The other objectives were the comparison of vaccine efficacy in patients versus health care workers (HCW) of the same center, the identification of predictive risk factors for the development of a documented COVID-19 after vaccination, the mortality of COVID-19 in cancer patients after SARS-COV-2 vaccination, and the overall risk of death in the different groups.
2.2. Patients
All cancer patients in whom vaccination was prescribed within the Comprehensive Cancer Center of Lyon (Centre Leon Berard, CLB) from January 1 to September 30, 2021 were included. Only patients who have agreed to have their personal data analyzed internally for academic research were analyzed, in agreement with the national and European laws. The study was approved by the institutional review board of the Centre Leon Berard on March 2021. The inclusion criteria were: a histological diagnostic of cancer, a documented vaccination using one of the four available vaccine options in the observation period prescribed by physicians in the center, i.e., the BNT162, the mRNA-1273, the Chadox1, the Ad26.Cov2s vaccines [[15], [16], [17], [18]], ongoing or active cancer treatment, a consultation in the center during the same observation period.
2.3. Vaccination
The national policy in France for doses and type of vaccination varied over time. From April 2021 it was recommended to reserve the AstraZeneca vaccine to patients aged above 55. However, the majority of patients received mRNA vaccines. Overall, the Pfizer, Moderna, Astra-Zeneca, and Johnson vaccines were used as first dose in 1914 (80%), 395 (16.5%), 67 (2.8%), and 15(0.6%) patients, respectively, with no significant difference of distribution between those aged under or above 60. Patients with a previous documented symptomatic COVID-19 infection were proposed for a single dose of vaccine in national recommendations.
2.4. Extracting electronic patient records (EPR) with the consore tool
The information on vaccination and patient characteristics were collected from the electronic patient records (EPR) with the CONSORE tool as previously described [32], see also https://www.sword-group.com/en/news/projet-consore). ConSoRe is a new data analytics solution aggregating diverse forms of structured and unstructured data extracted from EPR and structuring cancer management for all patients. It uses natural language processing to search aggregated data and perform advanced data mining. This tool enables the collection of clinical information integrated to the electronic patient record; these were subsequently validated manually if inconsistent data were collected. The EPR of all patients consulting the institution from January 2021 to October 2021 were screened. The EPR system of the CLBs includes a standard set of data (e.g., tumors characteristics, treatments, vaccines, deaths) as well as information present only in unstructured data sources. The extracted relevant structured data was integrated in Excel files.
2.5. Health care workers (HCW)
The cumulative incidence of COVID-19 in the 2285 vaccinated health care workers of the same institute was calculated from the date of the first vaccine dose, and analyzed according to the number of doses. Only dates of vaccination and of COVID-19 diagnosis (when relevant) available in the Occupational Medicine Department (OMD), were analyzed. The project was presented to the ethics Committee of the Centre Leon Berard and to the representative instances of workers of the hospital (Comite Social & Economique, CSE). HCW were all contacted and had the option to opt-out. Three opted out. Conversely, six HCW whom data were not declared at the OMD department (fellows, recent hiring) requested to participate.
2.6. Data
The following data were collected for cancer patients: demographic characteristics (age, weight, body mass index, gender, tobacco …), cancer characteristics (histotypes, primary site, stage), previous cancer treatments in the last months, dates and types of vaccines, patient outcome (date of documented SARS-COV-2 infection, survival, last news). Only dates of vaccinations, dates of documented RT-PCR + for SARS-COV-2 were analyzed for HCW. Only RT-PCR + documented SARS-COV-2 infections were retained as events.
2.7. Statistical analysis
The distribution of clinical characteristics was analyzed using Chi-squared test, Fisher exact test, Mann–Whitney U test. Survival was plotted from the date of the first dose (or for some analyses, from subsequent doses) of vaccine to the date of documented SARS-COV-2 using RT-PCR test, to the date of death or to the date of last news if alive at the time of the analysis (November 2021). Survival of cancer patients diagnosed with COVID-19 after the date of vaccination was plotted from the date of the positive SARS-COV-2 RT-PCR to the date of death or last news. Cumulative risk of death was plotted according to the inverse Kaplan–Meier method, and groups were compared using the logrank test. A landmark analysis 21 days after the first vaccine dose was performed to limit the biases related to the infections occurring in the first days after the first dose of vaccine. Independent risk factors for documented COVID-19 infection, or death were evaluated using Cox proportional hazard model in univariate and then multivariate analysis for parameters with significant statistical value in univariate analysis. Backwards selection procedure was used to determine the final model by removing non-significant variables (p > 0.05) one at a time. All statistical analyses will be performed using SPSS 23.0 software SPSS (IBM, Paris, France).
3. Results
3.1. Patients
From January 4 to September 30, 2021, 2391 cancer patients without previous documented COVID-19 were prescribed COVID-19 vaccination in the center, including 95 patients with a previously documented SARS-COV-2 infection and who received a single vaccine dose per national recommendations. For the remaining patients, 659 (28%), 1498 (63%), and 139 (6%) cancer patients received 1, 2, or 3 injections of COVID-19 vaccine at a median interval of 28 days (range 13–80) between dose 1 and 2, and 96 days (range 41–202) between dose 2 and 3, respectively. Nineteen hundred fourteen (80.1%), 395 (16.5%), 67 (2.8%), 15 (0.6%) received BNT162b2, mRNA-1273, Chadox1 and Ad26.cov2.S vaccines as first doses depending on availability [[15], [16], [17], [18]]. 31 of 2391 (1.6%) patients received a heterologous vaccine for dose 2. Table 1 describes the clinical characteristics of the patients according to the number of vaccines doses received. A significantly larger proportion of female patients, of smokers, of patients with hematological malignancies, receiving anti-CD20 received 2 or 3 doses of vaccines (Table 1). In the same hospital, 2285 health care workers (HCW) were vaccinated for COVID-19, with one (N = 259, 11.3%) or 2–3 doses (N = 2026, 88.7%). In France, vaccination is mandatory by law for all active HCW.
Table 1.
Patient characteristics and outcome according to vaccination status
| Alla | Vaccination dosesb |
p-value | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | One dose post COVID-19 | |||
| All | 2391 (100%) | 659 (28%) | 1498 (63%) | 139 (6%) | 95 (4%) | |
| Gender | ||||||
| Female | 1236 (52%) | 355 (29%) | 773 (63%) | 48 (4.5%) | 52 (4.2%) | 0.03 |
| Age | ||||||
| Median (SD) | 65 (13) | 62,6 (15) | 60,9 (14) | 64,7 (12) | 58,9 (15) | 0.001 |
| >60 | 1401 (59%) | 391 (28%) | 863 (62%) | 97 (7%) | 50 (4%) | 0.02 |
| BMI>30 | 314 (13%) | 88 (28%) | 185 (59%) | 22 (7%) | 19 (6%) | 0.13 |
| Smoking | 1078 (45%) | 252 (23%) | 719 (67%) | 83 (8%) | 24 (2%) | 0.000 |
| Haematological cancers | 517 (22%) | 136 (26%) | 306 (59%) | 51 (10%) | 24 (5%) | 0.000 |
| Breast Ca | 446 (19%) | 130 (29%) | 285 (64%) | 11 (3%) | 20 (5%) | 0.009 |
| Lung Ca | 284 (12%) | 72 (25%) | 179 (63%) | 26 (9%) | 7 (2%) | 0.04 |
| Prostate Ca | 126 (5%) | 53 (42%) | 67 (53%) | 5 (4%) | 1 (1%) | 0.001 |
| Anti-CD20 Ab | 69 (3%) | 12 (17%) | 41 (59%) | 14 (20%) | 2 (3%) | 0.000 |
| Metastases | 1214 (51%) | 319 (26%) | 782 (64%) | 68 (6%) | 45 (4%) | 0.34 |
| PCR + COVID-19c | 39 (2%) | 28 (4%) | 11 (0.7%) | 0 (0%) | 0 (0%) | 0.000 |
% of the whole population.
% of the subgroup.
% of the whole group of vaccination doses.
3.2. Cumulative incidence of documented COVID-19
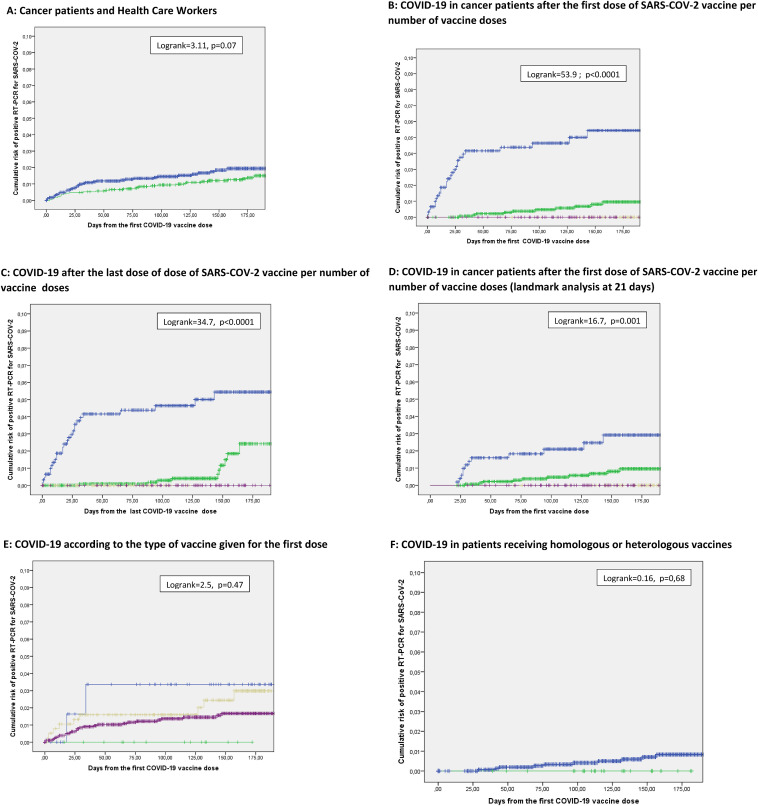
We first compared the incidence of COVID-19 after a first vaccine dose in cancer patient to that observed in the population of the 2285 health care workers of the hospital. Median follow-up for the 2 populations is 142 days (range: 1–253) and 199 days (range 3–288) respectively. Respectively 39 of 2391 (1.6%) patients versus 35 of 2285 (1.5%) HCW presented a RT-PCR documented COVID-19 infection: the cumulative incidence of post vaccination COVID-19 was numerically higher, not significantly in the patient population (Logrank = 3.11, p = 0.07; Fig. 1 A). The difference was not significant either when considering only populations who received 2 to 3 vaccine doses or vaccinated with a single dose after a documented COVID-19 (not shown).
Fig. 1.
Cumulative incidence of RT-PCR + documented COVID-19 after SARS-COV-2 vaccine. A: Incidence of documented COVID-19 from the first vaccination dose in health care workers (green) vs cancer patients (blue) (logrank = 3.11, p = 0.07). B: Since the first dose of SARS-COV-2 vaccine according to the number of vaccine doses. In blue: patients having received one dose of SARS-COV-2 vaccine (28 events/659); in green patients having received 2 doses of SARS-COV-2 vaccine (11 events/1498); in brown patients having received 3 doses of SARS-COV-2 vaccine (0 events/139); in purple: patients having received 1 dose of SARS-COV-2 vaccine after a previously documented COVID-19 (0 events/95) (Logrank = 53.9, p < 0.0001); C: Since the last dose of dose of SARS-COV-2 vaccine (blue: 1 dose; green: 2 doses; brown: 3 doses; purple 1 dose after a documented COVID-19; logrank = 34.7, p < 0.0001). D: COVID-19 in cancer patients after the first dose of SARS-COV-2 vaccine per number of vaccine doses (landmark analysis at 21 days) (blue: 1 dose; green: 2 doses; brown: 3 doses; purple 1 dose after a documented COVID-19; logrank = 16.7, p = 0.001); E: Since the first dose of the different vaccine types: Chadox1 (blue), BNT162b2 (green) mRNA-1273 (brown); Ad26.cov2.S (green); logrank = 2.5, p = 0.47). F: Incidence of documented COVID-19 from the first vaccination dose patients receiving homologous or heterologous vaccines (31/2391 (1.6%) patients) (logrank = 0.16, p = 0.68). Curves are plotted from the date of the first SARS-COV-2 vaccination dose (A,B,D,E,F) or from the date of the last SARS-COV-2 vaccination dose (C). Median follow-up 142 days for patients, 199 days for HCW.
Thirty nine of the 2391 cancer patients presented a documented SARS-COV-2 infection at a median of 27 days after the first vaccine dose. COVID-19 was documented in 28/659 (4%), 11/1498 (0.7%), and 0/139 (0%) who received one, two and three doses of vaccines respectively, and in 0/95 (0%) in those receiving the single vaccine dose after a documented COVID-19 (p < 0.0001, Table 1). Fig. 1B shows the cumulative risk of SARS-COV-2 infection in the same 4 subgroups from the first dose of vaccine (logrank = 55.0, p < 0.0001), from the last dose of vaccine (Fig. 1C, logrank = 41.6, p < 0.001). With a landmark analysis of 21 days after the first vaccine dose to limit the biases related to the infections occurring in the days after the first dose of vaccine, the incidence of COVID-19 was 13/515 (2.5%), 11/1446 (0.7%), 0/139 (0%) and 0/89 (0%) in the same 4 subgroups (logrank = 16.7; p = 0.001) (Fig. 1D). In the group of HCW, a single vaccine dose was also associated with an increased risk of post vaccination COVID-19 (not shown). In cancer patients, no significant difference was observed between the four vaccines products used for the first dose (Fig. 1E, logrank = 2.5, p = 0.47). No significant differences were observed for patients vaccinated twice or more with homologous or heterologous vaccines (Fig. 1F, logrank = 0.16, p = 0.68).
A multivariate analysis was conducted to determine independent risk factors for developing a documented COVID-19 after a first vaccination. Cancer histotype, primary site, stage, age, gender, anti-CD20 treatment, number of vaccine doses (1 vs 2 vs 3), comorbidities (obesity, smoking) were tested in univariate analysis. Significant risk factors in univariate analysis i.e. age, anti-CD20, number of vaccine doses > 1 and smoking were included as covariates in the multivariate model. Age younger than 60, treatment with anti-CD20 in the last 3 months, and a single vaccine dose were independently associated with increased risk of COVID-19 (Table 2 ). Of note, there was a significantly larger proportion of patients aged above 60 who had received 3 vaccine doses (97/1304 [6.9%] versus 42/948 [4.2%], p = 0.006). The same 3 parameters were retained by the model when the multivariate analysis was conducted with a landmark analysis of 21 days (not shown). In patients who received 2 or 3 vaccines or a single dose after a documented Covid-19, younger age (< 60) and treatment with anti-CD-20 in the last three months were retained as independent risk factors (not shown).
Table 2.
Multivariate analysis of risk factors of post vaccine COVID-19 or death after the first vaccine dose
| Beta | SE | HR | p-value | |
|---|---|---|---|---|
| Risk factors for COVID-19 | ||||
| Only one vaccine dose | 2,235 | ,360 | 9,34 | ,000 |
| Anti-CD20 treatment | 1,422 | ,607 | 4,143 | ,019 |
| Age ≥ 60 | -,706 | ,325 | 0,494 | ,030 |
| Risk factors for death | ||||
| Metastatic disease | 1,547 | ,254 | 4,696 | ,000 |
| COVID-19 before 1st vaccine dose | ,782 | ,346 | 2,187 | ,024 |
| Hematological malignancy | ,648 | ,271 | 1,912 | ,017 |
| Male gender | ,451 | ,167 | 1,570 | ,007 |
3.3. Survival
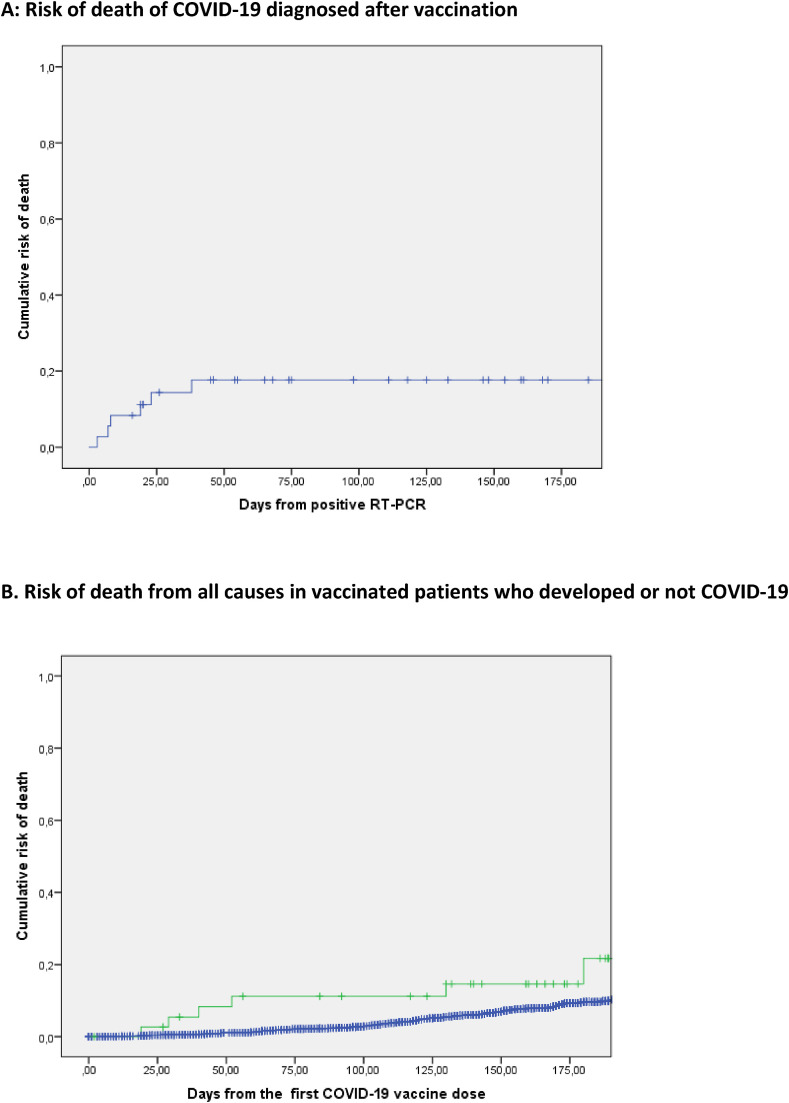
The survival of the 39 cancer patients with documented COVID-19 after vaccination was then explored. Fig. 2 A shows the survival of vaccinated cancer patients from the date of documented SARS-COV-2 infection. With six deaths due to COVID-19, the cumulative death-rate at day 50 post-infection (date of positive PCR) was 17.6% (Fig. 2A). All six patients are considered to have died from COVID-19 as the primary cause in the context of a solid tumor (N = 4) or lymphoid hemopathy (N = 2) in advanced stage. No significant difference was observed between patients who received one and > 1 dose of vaccine (not shown). No death is reported in the 35 HCW with documented COVID-19 post vaccination (0/35 vs 6/39, p = 0.015).
Fig. 2.
Mortality of COVID-19 diagnosed after vaccination. A: Time (days) from the positive SARS-COV-2 RT-PCR + post vaccine to the date of death or the date of last news. B: Cumulative risk of death from the first day of vaccination in patients who developed COVID-19 (green) or did not develop (blue) a documented COVID-19 after first vaccination dose.
There were respectively 43/616 (5.5%), 95/1403 (6.3%),7/132 (5.0%), 9/86 (9.5%) reported deaths in patients who had received 1, 2, 3, and 1 dose of vaccine post COVID-19, respectively. We investigated the overall survival from the first vaccination dose for patients who developed or not COVID-19 infection. Cumulative risk of death was numerically higher in the group of patients who were diagnosed with COVID-19 after vaccination (Fig. 2B). In Fig. 2B, the blue curve indicates the cumulative risk of death in patients in whom no diagnosis of COVID-19 was made: in this group, 148/2352 (6.3%) patients died at a median time of 109 days (range1–201) all from cancer or treatment related causes, compared to 6 the 39 (15.4%) patients diagnosed COVID-19 after vaccination.
Finally, we investigated the risk factors for any cause of death in this vaccinated cancer patient population in a multivariate Cox model. Cancer histotype, tumor site, metastatic stage, age, gender, anti-CD20 treatment in the last three months, number of vaccine doses, comorbidities (obesity, smoking) were tested in univariate analysis. Significant risk factors in univariate analysis i.e., metastatic stage, male gender, hematological malignancies, previous COVID-19 infection prior to vaccination were introduced as covariates in the model. Metastatic disease at vaccination, hematological malignancies, male gender, but also previous COVID-19 before vaccination was independent adverse risk factors for death (Table 2). Documented COVID-19 before vaccination was associated with an hazard ratio for death of all causes of 2.187 (Table 2).
4. Discussion
This series describes the efficacy of COVID-19 vaccine program in a real-life population of 2391 cancer patients treated in a comprehensive cancer center. It shows the efficacy of the full vaccination program in a large cohort of cancer patients, and also indicates that COVID-19 remains a significant risk factor for death of cancer patients even in the six months following vaccination. Pre-vaccination COVID-19 was also identified as an independent risk factor for death in this population.
With a median follow-up of 142 days, this analysis confirms the favorable impact of a complete vaccination program in cancer patients. It shows that the magnitude of protection conferred by a complete vaccine program is not significantly different in cancer patients and HCW working in the same hospital regarding the risk of COVID-19 post vaccination, even though a slightly higher cumulative incidence of COVID-19 is observed post vaccination in cancer patients. In the present study. There were no documented COVID-19 in patients who received three doses, nor in patients vaccinated once after a documented COVID-19 as per the national recommendations. For the group of patients vaccinated with 3 doses, the follow-up post last vaccine dose is obviously shorter than that of patients vaccinated twice, limiting the significance of the absence of any documented COVID-19 in this subgroup. Most COVID-19 were diagnosed at a short term after the vaccines, with a median of 27 days post first vaccine dose. A landmark analysis after 21 days confirms actually the increased risk of COVID-19 in patients receiving one dose versus others, in the patient population, as well as in the population of HCW.
The analysis of risk factors to develop COVID-19 after vaccination in this large series identified age < 60, anti-CD-20 treatment and single vaccine dose as parameters associated with an increased risk. While the negative impact of anti-CD20 [[22], [23], [24], [25]] and of a single dose of COVID-19 vaccine is documented [[19], [20], [21]], the observation of younger age being associated with an increased risk of post vaccination COVID-19 is surprising. It may reflect less rigorous protective measures post vaccination in young patients with good clinical condition or other not yet reported epidemiological specificities of this population. The significantly larger proportion of patients aged above 60 who had received 3 vaccine doses is also likely to contribute to these observations. Of note, there were no significant difference in the types of vaccine given to patients aged < 60 and≥60 (data not shown).
High mortality was however observed for cancer patients presenting with a COVID-19 after vaccination, with a 17.6% cumulative risk of death at day 50. Indeed, 6 of the 39 cancer patients died in the 50 days post documented RT-PCR. Conversely, none of the 35 HCW presenting with documented COVID-19 died, with one requiring a short hospitalization. In cancer patients, COVID-19 after vaccination remains a disease with a high risk of death in cancer patients. It is therefore essential to maintain precautions (masks, social distancing) in hospitalization and during all steps of cancer patient management. Third vaccination dose may also be recommended, since none of the 139 patients who received a third dose so far presented a COVID-19 after vaccination with the limit of the short follow-up discussed above. It is also important to note that no COVID-19 was observed either in patients who received a single vaccine dose after a documented episode of COVID-19 before vaccination.
Several parameters were found independently correlated with the risk of death from any cause in this population of vaccinated cancer patients. As expected, the presence of a metastatic disease was independently associated with a higher risk of death, but others such as male gender and hematological malignancy were not necessarily expected. For hematological cancer, this may reflect the aggressive natural history of these diseases as compared to the other neoplasia, but also most likely the frequent use of anti-CD20 for B cell malignancies. Though anti-CD20 treatment was not found correlated to overall survival, it is identified as an independent risk factor for post vaccination COVID-19, which are associated with a high mortality as discussed above.
Surprisingly, a documented previous COVID-19 infection before the single vaccine dose was also associated with an increased risk of death in this series. The medical explanation of this observation remains unclear, but similar findings were recently reported for cancer patient series with a previously documented COVID-19 [33]. Cancer patient remain fragile for a long period of time after COVID-19, and therefore remain at higher risk of complications or death, in particular patients with long lasting symptoms [33]. In the present study, documented COVID-19 before the first vaccine dose was associated with a hazard ratio for death of all causes above 2, while none of these patients presented a COVID-19 after vaccination. While the description of the possible causes of increased mortality remain unclear, one can hypothesize the delays to cancer treatment [34], adapted treatment, poor PS or increased risk of DVT/PE may all have contributed to this observation. Death rate after documented COVID-19 was high confirming the importance to maintain protective measures in cancer patients after vaccination.
The results presented in this study are consistent with previous reports showing the importance of a full vaccination program [[19], [20], [21], [22]]. It is consistent with the reports of the value of a third vaccines both in terms of clinical efficacy and immunological response [[26], [27], [28], [29], [30],35,36]. It further indicates the severity of the rare post-vaccination COVID-19 in cancer patients, with a mortality rate of post-vaccination COVID-19, which is not different from that of non-vaccinated patients [[1], [2], [3], [4], [5], [6], [7], [8], [9]]. The immunosuppression associated with cancer, and cancer treatments, are correlated to a less efficient immune response in other studies, with baseline lymphopenia, and inadequate Ab response or T-cell response now well documented for these patients [[1], [2], [3], [4], [5], [6], [7], [8], [9],[23], [24], [25], [26]]. The present report indicates that cancer patients remain at high risk of death when presenting a documented COVID-19 and should persist on protective measures, within and outside the hospital.
This study has several limitations. It focuses on patients in whom vaccination was prescribed in the center and does not take in account patients vaccinated outside the center. This is not a randomized study testing the value of the number of vaccine doses, which would be needed to demonstrate the impact of the number of vaccine doses, in particular the third dose, in the population of cancer patients [26]. For the survival analyses, not all information of comorbidities were collected, for example, cardiovascular disease or diabetes. The CONSORE tool has limitations for the collection of information on follow-up and require manual verification when data are missing or inconsistent [32]. As a descriptive real-life study, vaccinations were proposed and applied to patients at different time of the disease course, explaining heterogeneous outcomes. The vaccine products applied were variable according to supplies with sometimes heterologous second vaccines, though is a limited proportion of patients. Finally, the follow-up remains relatively limited and will need to be updated in the years to come.
Nevertheless, this real-life series of 2391 patients provides important and reassuring information of the protection conferred by vaccines in cancer patients. This analysis indicates however the remaining severity of COVID-19 in cancer patients after vaccination, in marked contrast with HCW of the same hospital. It also indicates that protective measures should be maintained in cancer patients, including consideration of additional third vaccine doses as suggested for immunosuppressed patients [[26], [27], [28], [29], [30]], in particular young patients, and those recently treated with anti-CD20. The observation that a documented COVID-19, prior to vaccination is associated with an increased risk of death in the period after vaccination is of concern, and suggest a long term impact of COVID-19 of the natural history of neoplastic diseases.
Funding
LYRICAN (INCA-DGOS-INSERM 12563), NetSARC (INCA & DGOS), InterSARC (INCA), LabEx DEvweCAN (ANR-10-LABX 0061), PIA Institut Convergence Francois Rabelais PLAsCAN (PLASCAN, 17-CONV-0002), Fondation ARC contre le Cancer, La Ligue contre le Cancer (Canopée), EURACAN (EC 739521) contributed to fund this study.
Author contribution
Conceptualization: JYB, PH, MLS. Methodology: PH, BF, M-LS, 5, SC, DP, J-YB. Software: PH, SC, DP, JYB. Data curation: PH, DF, MLS, SA, NC, OT, TB, IRC,BR, MLF, VA, ASM, PZ, JYB. Writing- Original draft preparation: JYB. Investigation: PH, DF, MLS, SA, NC, OT, TB, IRC,BR, MLF, VA, ASM, PZ, JYB. Supervision: JYB, BF, MLS. Software, Validation: PH, DP, SC. Writing- Reviewing and Editing: all authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: All authors: research support from Astra-Zeneca and Innate Pharma for a research program not directly related to the present work.
References
- 1.Yang K., Sheng Y., Huang C., et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee L.Y., Cazier J.B., Angelis V., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assaad S., Avrillon V., Fournier M.L., et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer. 2020;135:251–259. doi: 10.1016/j.ejca.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee L.Y.W., Cazier J.B., Starkey T., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lièvre A., Turpin A., Ray-Coquard I., et al. GCO-002 CACOVID-19 collaborators/investigators. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19) Eur J Cancer. 2020;141:62–81. doi: 10.1016/j.ejca.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assaad S., Zrounba P., Cropet C., et al. Mortality of patients with solid and haematological cancers presenting with symptoms of COVID-19 with vs without detectable SARS-COV-2: a French nationwide prospective cohort study. Br J Cancer. 2021;125:658–671. doi: 10.1038/s41416-021-01452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grivas P., Khaki A.R., Wise-Draper T.M., et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32:787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukkada S., Bhakta N., Chantada G.L., et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol. 2021;22:1416–1426. doi: 10.1016/S1470-2045(21)00454-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solodky M.L., Galvez C., Russias B., et al. Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol. 2020;31:1087–1088. doi: 10.1016/j.annonc.2020.04.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goubet A.G., Dubuisson A., Geraud A., et al. Prolonged SARS-CoV-2 RNA virus shedding and lymphopenia are hallmarks of COVID-19 in cancer patients with poor prognosis. Cell Death Differ. 2021;6:1–19. doi: 10.1038/s41418-021-00817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melenotte C., Silvin A., Goubet A.G., et al. Immune responses during COVID-19 infection. OncoImmunology. 2020;9:1807836. doi: 10.1080/2162402X.2020.1807836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dettorre G.M., Dolly S., Loizidou A., et al. Systemic pro-inflammatory response identifies patients with cancer with adverse outcomes from SARS-CoV-2 infection: the OnCovid Inflammatory Score. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansi L., Spehner L., Daguindau E., et al. Study of the SARS-CoV-2-specific immune T-cell responses in COVID-19-positive cancer patients. Eur J Cancer. 2021;150:1–9. doi: 10.1016/j.ejca.2021.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadoff J., Gray G., Vandebosch A., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;S1470–2045(21):213–218. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrière J., Chamorey E., Adjtoutah Z., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;28(21):1183–1192. doi: 10.1016/j.annonc.2021.04.019. S0923-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heudel P., Favier B., Assaad S., Zrounba P., Blay J.Y. Reduced SARS-CoV-2 infection and death after two doses of COVID-19 vaccines in a series of 1503 cancer patients. Ann Oncol. 2021 Jul 30;S0923–7534(21):2210–2219. doi: 10.1016/j.annonc.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roeker L.E., Knorr D.A., Thompson M.C., et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35:2703–2705. doi: 10.1038/s41375-021-01270-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim S.H., Campbell N., Johnson M., et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. 2021;8:e542–e544. doi: 10.1016/S2352-3026(21)00199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grinshpun A., Rottenberg Y., Ben-Dov I.Z., Djian E., Wolf D.G., Kadouri L. Serologic response to COVID-19 infection and/or vaccine in cancer patients on active treatment. ESMO Open. 2021;6:100283. doi: 10.1016/j.esmoop.2021.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavanna L., Citterio C., Toscani I. COVID-19 vaccines in cancer patients. Seropositivity and safety. Systematic review and meta-analysis. Vaccines (Basel) 2021;9:1048. doi: 10.3390/vaccines9091048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall V.G., Ferreira V.H., Ku T., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X., Shaw R.H., Stuart A.S.V., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gounant V., Ferré V.M., Soussi G., et al. Efficacy of severe acute respiratory syndrome Coronavirus-2 vaccine in patients with thoracic cancer: a prospective study supporting a third dose in patients with minimal serologic response after two vaccine doses. J Thorac Oncol. 2022;17:239–251. doi: 10.1016/j.jtho.2021.10.015. 16:S1556-0864(21)03286-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Re D., Seitz-Polski B., Carles M., et al. Humoral and cellular responses after a third dose of BNT162b2 vaccine in patients treated for lymphoid malignancies. medRxiv. 2021 doi: 10.1101/2021.07.18.21260669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrière J., Carles M., Audigier-Valette C., et al. Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: should humoral responses be monitored? A position article. Eur J Cancer. 2021;162:182–193. doi: 10.1016/j.ejca.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levi R., Azzolini E., Pozzi C., et al. One dose of SARS-CoV-2 vaccine exponentially increases antibodies in individuals who have recovered from symptomatic COVID-19. J Clin Invest. 2021;131 doi: 10.1172/JCI149154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heudel P., Chabaud S., Perol D., et al. Immune checkpoint inhibitor treatment of a first cancer is associated with a decreased incidence of second primary cancer. ESMO Open. 2021;6:100044. doi: 10.1016/j.esmoop.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinato D.J., Tabernero J., Bower M., et al. OnCovid study group. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021;S1470–2045(21):573–578. doi: 10.1016/S1470-2045(21)00573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blay J.Y., Boucher S., Le Vu B., et al. Delayed care for patients with newly diagnosed cancer due to COVID-19 and estimated impact on cancer mortality in France. ESMO Open. 2021;6:100134. doi: 10.1016/j.esmoop.2021.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tran S., Truong T.H., Narendran A. Evaluation of COVID-19 vaccine response in patients with cancer: an interim analysis. Eur J Cancer. 2021;159:259–274. doi: 10.1016/j.ejca.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corti C., Antonarelli G., Scotté F., et al. Seroconversion rate after vaccination against COVID-19 in cancer patients-a systematic review. Ann Oncol. 2021;S0923–7534(21):4550–4556. doi: 10.1016/j.annonc.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]