Abstract
Platycodi radix is widely used in traditional herbal medicine for the treatment of bronchitis, asthma, pulmonary tuberculosis, hypertension, hyperlipidemia, and diabetes. This study aimed to investigate cell proliferation (Ki-67) and apoptosis (Caspase-3) potential in squamous cell hyperplasia of the stomach induced by a Platycodi radix water extract in a subchronic toxicity study. One hundred formalin-fixed, paraffin-embedded stomach tissues of rats treated with Platycodi radix at doses of 0, 500, 1,000, and 3,000 mg/kg body weight/day were used for the analysis. They were conventionally stained using hematoxylin and eosin (H&E) and immunohistochemically (IHC) stained using caspase-3 and Ki-67 antibodies. The incidence of squamous cell hyperplasia was significantly increased in the 3,000 mg/kg b.w./day treatment group in both sexes (p<0.01). However, the hyperplastic change was completely repaired after 4 weeks of recovery period. Ki-67 expression was similar in all groups, with no statistically significant differences among the groups. Caspase-3 expression was significantly increased in both sexes in the 3,000 mg/kg b.w./day treatment group (p<0.01), compared with the vehicle control groups, and then reduced to normal levels in the recovery groups in both sexes. In conclusion, this study showed that squamous cell hyperplasia induced by the Platycodi radix water extract in the limiting ridge of the stomach is not considered to be abnormal proliferative change; as a result, squamous cell hyperplasia is considered to be a non-adverse effect when induced by the oral administration of the Platycodi radix water extract once daily for 13 weeks in rats.
Keywords: Caspase-3, Ki-67 antigen, Sprague–Dawley rats, squamous cell hyperplasia, local irritation
Introduction
Lesions of the non-glandular stomach have increasingly attracted attention due to frequent findings in nonclinical toxicity studies. This is attributed to local irritation caused by gavage during the administration of a test article. The stomach of rodents are divided into non-glandular and glandular portions that are separated by the limiting ridge1. The function of the non-glandular stomach is the storage and predigestion of food. Histologically, the non-glandular stomach is covered with stratified squamous epithelium. In addition, the limiting ridge becomes gradually more thickened than the adjacent squamous mucosa, especially when induced by nongenotoxic irritating compounds that act specifically on this site. Squamous cell hyperplasia in the limiting ridge of the stomach is relatively common in oral gavage studies where the test article has an irritating effect1.
Platycodi radix is the root of Platycodon grandiflorum (Jacq.) A. DC., which is widely grown in northern Asia, China, Korea, Japan, and East Siberia2, 3 and it is typically used as a component for preparing salads, cold soups, and sauces in Asia4. Platycodi radix has been generally used in traditional herbal medicine for bronchitis, asthma, pulmonary tuberculosis, hypertension, hyperlipidemia, and diabetes5, 6, 7. The local irritating effect is presumably potentiated by the increased duration of exposure associated with intragastric storage and increased contact, when a test article precipitates in the gastric compartment. Squamous cell hyperplasia was consistent with proliferation or thickening of the stratum spinosum, often associated with a thickened keratin layer1. In certain rodent carcinogenicity studies8, 9, especially when the test compound was administered by gavage, tumor induction was found in the non-glandular stomach of rodents. In time-sequence studies, the pathogenesis appears frequently after initial necrosis and inflammation, with the occurrence of hyperplasia of squamous cells and/or acanthosis-hyperkeratosis, papilloma, and squamous cell carcinoma, with increasing concentration and/or time of exposure.
The proliferative activity of any tissue or neoplasm can be determined by its growth rate and by using antibodies directed against specific antigens, allowing the simultaneous analysis of cell proliferation and histology. Abnormal cell proliferation appears to be a possible predictor of tumorigenesis. Because Ki-67 protein level is closely related to cell proliferation, it can be used as a biomarker for indicating the growth of most proliferative lesions10. The expression of Ki-67 protein is strictly associated with cell proliferation, and this antigen can be detected in the nucleus. The fact that the Ki-67 protein is present during all active phases of the cell cycle (G1, S, G2, and mitosis) but absent from resting cells (G0) makes it an excellent marker for determining the growth fraction of a given cell population11, 12, 13.
Immunostaining using antibodies against the Ki-67 antigen has been well established as a quick and efficient method for evaluating growth fractions of various tissue types, including squamous epithelium, because of its distinctive reaction patterns that exclusively involve proliferating cells14.
Cleaved Caspase-3, the active form of Caspase-3, is a well-known marker of apoptosis in cells15. Activated Caspase-3 is the main executor of the apoptosis mechanism and results in the cleavage of multiple cytoplasmic and nuclear proteins16, 17. The determination of Caspase-3 level is a simple, reliable, and precise method for recognizing apoptosis in the early stages18, 19.
This study investigated the proliferation (Ki-67) and apoptosis (Caspase-3) potential of the squamous epithelium of the limiting ridge of the stomach in a subchronic toxicity study of the Platycodi radix water extract after repeated oral administration to Sprague–Dawley (SD) rats. Furthermore, we interpreted the relationship between Ki-67 and Caspase-3 expression and squamous cell hyperplasia in the limiting ridge of the stomach in the main treatment groups. Thus, we determined the proliferation tendency of squamous cell hyperplasia of the limiting ridge in the stomach induced by the Platycodi radix water extract in SD rats.
This article focuses on the reversibility assessment using immunohistochemistry endpoints (Caspase-3 and Ki-67) for the Platycodi radix water extract-induced gastric finding that was published in the Journal of Ethnopharmacology by Cha et al3.
Materials and Methods
Preparation of Platycodi radix water extract
Platycodi radix was collected in Korea, and discrimination of this plant was conducted by forming a herbal drug identification advisory committee. Platycodi radix water extract powder (Lot No. 2016-CUDP001) was supplied by the Marine Bio Industry Research Center of Daegu Catholic University (Daegu, Republic of Korea). Water was added twice to the Platycodi radix and refluxed for 2 h at 100 °C. The water extract was concentrated under vacuum and freeze-dried to obtain the final extract. Platycodin D content of the Platycodi radix water extract was measured using high-performance liquid chromatography (HPLC) systems with evaporative light scattering detection. A 100 mg/mL Platycodi radix water extract was used for experiments with the above conditions which was performed three times, and the Platycodi radix water extract showed an average content of 0.370% and the relative standard deviation (RSD) was superior to less than 5%3. The Platycodi radix water extract powder was prepared by suspension in sterile water (Daihan Pharm. Co., Seoul, Republic of Korea) for injection according to the doses of each group assigned in the repeated dose toxicity studies before administration.
Experimental animals and treatment
Specific pathogen-free 5-week-old Sprague–Dawley (Crl:CD(SD)) rats were used and included 50 animals of each sex. These animals were obtained from Orientbio Inc. (Seongnam-Si, Republic of Korea). The animals were housed in a room maintained at a temperature of 23 ± 3 °C and a relative humidity of 55 ± 15%, with artificial lighting from 08:00 to 20:00 h with a luminous intensity of 150–300 Lux and 10–20 air changes each hour. To maintain even conditions, the cages were rotated clockwise once a month. The animals were provided irradiation-sterilized pellet feed (Teklad-certified irradiated global 18% protein rodent diet, 2918C, ENVIGO, London, UK) purchased from Dooyeol Biotech, and groundwater, that was disinfected by an ultraviolet sterilizer and ultrafiltration, was provided ad libitum.
The animals were administered 500, 1,000, and 3,000 mg/kg b.w./day of Platycodi radix water extract for 13 weeks by oral gavage. The dose selection was based on the results of a preliminary 2-week repeated oral toxicity study (data not shown)3. This study also aimed to investigate the recovery potential during a 4-week recovery period. The main groups consisted of 10 animals of each sex in each group, and the recovery groups consisted of 5 animals of each sex in the vehicle control and 3,000 mg/kg b.w./day group. The vehicle control group was administered sterile distilled water only. According to the Organisation for Economic Co-operation and Development (OECD) Test Guideline 40820, the dose volume was 10 mL/kg/day3.
This experiment was conducted at the facility of ChemOn Inc. in the Republic of Korea. The study protocol was approved by our Institutional Animal Care and Use Committee based on the Animal Protection Act, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Samples
One hundred formalin-fixed, paraffin-embedded stomach specimens were used for both histological and immunohistochemical (IHC) evaluations. The sections were cut to a thickness of approximately 3 µm using a rotatory manual microtome. Three serial sections were cut from each block, one was stained with hematoxylin and eosin (H&E), and the others were used for IHC staining. For IHC staining, sections were placed on coated slides with poly-L-lysine and incubated for 1 h at 60 °C in an incubator before staining. All histology slides were reviewed by two pathologists (Li and Kang) to confirm the diagnoses. Squamous cell hyperplasia of the non-glandular stomach was diagnosed according to a study by Nolte et al1. Squamous cell hyperplasia of the limiting ridge was semi-quantitatively graded by examining the thickness of the squamous epithelium from the bottom of the rete pegs to the top of the keratinized layer as follows:
Normal - thickness less than approximately 100 µm.
Minimal (+1) thickness between more than 100 µm and less than 200 µm.
Mild (+2) thickness between approximately more than 200 µm and less than 300 µm.
Immunohistochemical (IHC) assay
Sections were deparaffinized using xylene, rehydrated using graded alcohol, and then transferred and rinsed twice with phosphate-buffered saline (PBS, Dako, Glostrup, Denmark A/S). The epitopes were retrieved by heating the sections in a microwave in ethylene diaminetetraacetic acid (EDTA; pH 8.0; Invitrogen: REF 00550) for up to 5 min after boiling starts. After cooling, the slides were washed three times with PBS and treated with 3% H2O2 (Thermo Fisher Scientific, Kalamazoo, MI, USA: REF TA-125-H2O2Q) for 10 min to block endogenous peroxidases. The protein was blocked with UltraVision Protein Block (Thermo: REF TA-125-PBQ) for 7 min, followed by washing with PBS. The sections were then incubated overnight at 4 °C with primary antibodies, rabbit monoclonal antibodies against Ki-67 (mouse Mki67; 1:200 dilution; PAB12127; Abnova, Taipei City, Taiwan) and rabbit polyclonal antibody against cleaved Caspase-3 (Asp175; 1:300 dilution; #9661S; Cell Signaling, Danvers, MA, USA). After that, the slides were rinsed gently three times with PBS and incubated for 10 min with the horseradish peroxidase (HRP)-labeled polymer conjugated with secondary antibody (Envision+System-HRP Labeled Anti-Rabbit, Dako K4003; Denmark A/S) at room temperature. The slides were washed thrice with PBS. Incubation with 3,3ʹ-diaminobenzidine tetrahydrochloride (DAB) was performed for 5 min at room temperature until a substrate chromogen solution produced a brown color. Finally, the slides were counterstained with Mayer’s hematoxylin, dehydrated, and mounted.
Lymph node specimens from SD rats were used as a positive control to confirm the sensitivity of the antibodies against cleaved Caspase-3 and Ki-67. Positive staining was observed in the germinal centers of apoptotic lymphocytes and reactivity to Ki-67 was observed in the lymphoid follicles. The specificity of staining was checked using negative control slides, in which the primary specific antibodies were substituted with a buffer solution. The stomach specimens, positive control, and negative control sections were processed in parallel.
Quantification of Immunohistochemistry
The appearance of brown precipitate in the cytoplasm and nucleus was taken as positivity. The cells with nuclear or cytoplasmic expression of Ki-67 and Caspase-3 were counted according to the staining results of the squamous epithelial cells of the limiting ridge. Areas that were poorly preserved, crushed, cauterized, folded, or retracted were specifically avoided at low-power magnification (×100). From the selected areas, three varied areas of non-overlapping fields at 400× magnification were captured using a microscope digital camera. Images were subsequently printed for quantitative analysis, which was performed by an observer blinded to the clinicopathological variables. Both positive and negative cells within the field were counted, and stromal or inflammatory cells were excluded. The percentage of Caspase-3- and Ki-67-expressing cells was defined as the ratio between the cells that positively stained for Caspase-3 and Ki-67, respectively, per the total number of cells counted in each case.
Statistical analysis
SPSS 10.1 K was used for all statistical analyses (Chicago, IL, USA). Data are presented as mean ± standard deviation (SD) and mean percentage of positive cells with 95% confidence intervals (CI). The level of significance was set at p<0.05, or p<0.01.
Caspase-3 expression was analyzed for significance using one-way analysis of variance (ANOVA) to compare the mean percentage of Caspase-3-expressing cells among the various study groups. The assumption of homogeneity was tested using Levene’s test21. If the assumption of homogeneity of variance was met, Duncan’s multiple range test was used as the post-hoc test22. If the assumption of homogeneity of variance was not met, Dunnett’s T3 test was used as the post-hoc test23. Fisher’s exact probability test was applied to the histopathological examination data24. Group comparisons were performed using the Student’s t-test.
Results
Histopathological examination
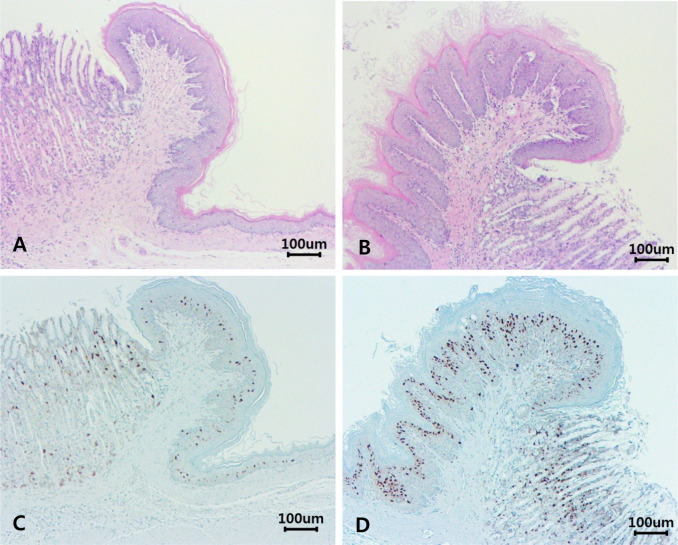
The stomach was thin-walled and separated into glandular and non-glandular regions. Microscopically, the normal epithelium of the stomach had a solitary papillary fold (limiting ridge) at the junction between the non-glandular stomach and the glandular stomach (Fig. 1A). Squamous cell hyperplasia was evident in the limiting ridge of the stomach in the treatment groups. This squamous cell hyperplasia was characterized by thickening of the stratified squamous epithelium, hyperkeratosis, and elongated rete pegs. A few apoptotic cells were observed on the surface of stratified squamous epithelium, and the incidence of apoptosis did not increase in the treatment groups compared to the control group. However, papilloma-like projections and mitotic figures were not observed. Hyperplasia was not accompanied by dysplasia or cellular atypia. Furthermore, reactive changes, such as local erosion, ulceration, or inflammation, were not observed in the mucosa of the entire stomach (Fig. 1B).
Fig. 1.
The normal epithelium of stomach was observed in a male of control group (A and C). The stomach was divided into the non-glandular (right) and glandular stomach (left). The limiting ridge had solitary papillary fold at the junction between non-glandular and glandular stomach. Squamous cell hyperplasia of limiting ridge (left) was observed in the stomach in a male of 3,000 mg/kg b.w./day treatment group (B and D). The squamous cell hyperplasia was evident in the limiting ridge of stomach and was characterized by thickening of the stratified squamous epithelium and hyperkeratosis. Caspase-3 expression were mainly located in the spinous layers of squamous epithelium in the limiting ridge of stomach (C and D). Caspase-3 was highly expressed in the epithelium with squamous cell hyperplasia (D) than the normal epithelium (C). A and B: H&E staining, C and D: Caspase-3 staining.
Squamous cell hyperplasia of the limiting ridge in the stomach was observed in 3 and 2 cases in males and females, respectively, in the 1,000 mg/kg b.w./day treatment group and 7 cases each in males and females in the 3,000 mg/kg b.w./day treatment group. The incidence of squamous cell hyperplasia in the stomach was significantly increased in the 3,000 mg/kg b.w./day group in both sexes. However, squamous cell hyperplasia was not observed in the recovery groups, indicating complete repair during the 4-week recovery period (Table 1).
Table 1. Incidence of Squamous Cell Hyperplasia in Limiting Ridge of the Stomach Affected by the Platycodi Radix Water Extract in SD Rats in Both Sexes.
The test article induced adaptive non-adverse changes, centrilobular hepatocellular hypertrophy in the liver, and diffuse follicular cell hypertrophy in the thyroid gland3; however, they were not analyzed in this study.
IHC examination
Nuclear Ki-67 positivity was detected in all cases of the squamous epithelium of the stomach and was restricted to the basal layers of the squamous epithelium. Ki-67 expression was similar in all groups, with no statistically significant difference between groups, including between normal epithelium and hyperplasia (<5%, data not shown).
Caspase-3 expression in the squamous epithelium was detected in all cases and was restricted to the spinous layers of the squamous epithelium of the stomach in all cases. Apoptosis was also observed on the surface of stratified squamous epithelium (Fig. 1C, 1D). The percentage of Caspase-3-expressing cells in each group is presented in Tables 2and 3 and Fig. 2. Caspase-3 expression was significantly increased in the 3000 mg/kg b.w./day treatment group in both sexes (p<0.01; Table 2) and then decreased in the recovery groups in both sexes (Table 3, Fig. 2).
Table 2. Comparison of Percentage of Cells Expressing Caspase-3 among the Main Groups Treated with Platycodi Radix Water Extract in SD Rats in Both Sexes.
Table 3. Comparison of Percentage of Cells Expressing Caspase-3 in the Recovery Groups in SD Rats in Both Sexes.
Fig. 2.
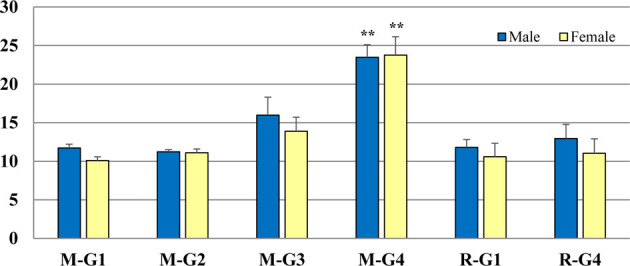
In the main group of both sexes, C aspase-3 expression was significantly increased in 3,000 mg/kg b.w./day (M-G4) group than in control or any other treated group (M-G1, M-G2, M-G3, **: p<0.01). No significant change in the recovery group was observed in 3,000 mg/kg b.w./day (R-G4) compared to recovery control group (R-G1) of both sexes. M: main; G: group; R: recovery.
A total of 60 individuals in the main groups treated with Platycodi radix water extract (20 males with normal epithelium, 10 males with squamous cell hyperplasia, 21 females with normal epithelium, and 9 females with squamous cell hyperplasia) were examined in the present study (Tables 1 and 4).
Table 4. Comparison of Percentage of Cells Expressing Caspase-3 between the Normal Epithelium and Epithelium with Squamous Cell Hyperplasia in the Main Groups Treated with Platycodi Radix Water Extract in SD Rats in Both Sexes.
The percentage of Caspase-3-expressing cells with squamous cell hyperplasia of the limiting ridge of the stomach accounted for 26.46% (n=10) in males and 27.48% (n=9) in females of the treated main groups, while Caspase-3 expression with normal squamous epithelium of the limiting ridge accounted for 11.98% (n=20) in males and 11.00% (n=21) in females of the administered main groups (Table 4; Fig. 2). Therefore, the percentage of Caspase-3-expressing cells showed a statistically significant increase (p<0.01) in the epithelium with squamous cell hyperplasia, compared with that in the normal squamous epithelium (Fig. 3).
Fig. 3.
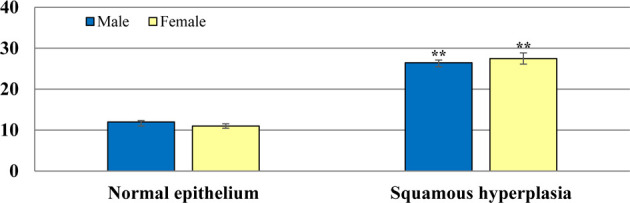
Caspase-3 expression was significantly increased in the squamous cell hyperplasia (**: p<0.01) than in the normal epithelium in the stomach in both sexes.
The expression of caspase 3 and Ki-67 was analyzed in the glandular stomach, but significant differences were not observed among the groups (data not shown).
Discussion
It is required that the findings in nonclinical toxicity studies as either “adverse” or “non-adverse” be consistent and based on a clear scientific rationale. In current practice, however, it is often challenging to reliably determine how and why a particular biological response qualifies. In recent years, test article-induced effects in the non-glandular stomach have increasingly attracted attention because they were frequently observed to be attributable to local irritation in oral administration toxicity studies.
The stomach of rodents is divided into the non-glandular stomach and glandular stomach, which is different from other laboratory animal species and humans. The non-glandular stomach is lined by stratified squamous epithelium and functionally serves as a temporary storage organ. In addition, the non-glandular stomach is separated from the glandular stomach by the limiting ridge1, 10. Exposure to the rodent non-glandular stomach by gavage resulted in the direct contact of a test article with keratinized squamous epithelium1, especially in the protruded structure of the limiting ridge. Therefore, squamous cell hyperplasia can be a frequent observation which is attributed to local irritation caused by orally administered test articles in toxicity studies.
Traditional herbal medicines are believed to be safe because of the established history of natural product compounds25, 26. However, concerns about their potential adverse effects, such as hepatotoxicity and genotoxicity, have been increasingly described27, 28. Therefore, many efforts have been actively undertaken to prevent potential adverse effects arising from the misuse of herbal medicines that do not have accurate quality assessments or toxicity evaluations. Furthermore, the potential subchronic toxicity of the Platycodi radix water extracts was previously investigated in SD rats by Cha et al3.
In the present study, repeated oral administration of the Platycodi radix water extract to SD rats resulted in an increased incidence of squamous cell hyperplasia in the limiting ridge of the stomach at dose levels of ≥1,000 mg/kg bw/day in both sexes. Furthermore, the incidence of squamous cell hyperplasia was significantly increased in the 3,000 mg/kg b.w./day group in both sexes. However, hyperplastic changes were not observed in the recovery groups, indicating complete repair after withdrawal of the Platycodi radix water extract for 4 weeks.
In this study, squamous cell hyperplasia was observed, showing consistent thickening of the stratum spinosum and hyperkeratosis. However, the polarity of differentiation to keratinocytes is orderly and complete, and evidence of cellular atypia, dysplasia, and papillary projection were not observed. In addition, reactive changes such as local erosion, ulceration, or inflammation were not observed.
As mentioned above, squamous cell hyperplasia in the limiting ridge of the stomach is considered an adaptive change to an irritating stimulus by gavage when the Platycodi radix water extract was used in the present study.
Tissue homeostasis is regulated by cell proliferation and apoptosis. The present study aimed to evaluate the expression of the proliferative marker Ki-67 and to identify apoptosis by cleaved Caspase-3 expression between the squamous epithelium with squamous cell hyperplasia and normal squamous epithelium of the limiting ridge in the stomach. Cell proliferation markers have been used in several studies as diagnostic aids to understand biological behavior in many stages of the disease. Ki-67 is considered a classic marker of cell proliferation and is routinely used by pathologists. Furthermore, Ki-67 has been used in several studies to evaluate cell proliferation in different tumors of various origins13, 29, 30. The antibody against Ki-67 is now considered as the standard for the assessment of cell proliferation and is extensively used to examine squamous epithelium in humans14, 31. In this study, expression of the Ki-67 protein was observed at the basal layers of the squamous epithelium of the stomach, where proliferation is a property of stem cells of the basal layers. Furthermore, Ki-67 expression was not significantly different between the treatment groups and control group, and in squamous cell hyperplasia and normal squamous epithelium in the stomach. Therefore, this study suggests that appropriate growth control of proliferation exists in the squamous epithelium of the stomach in subchronic toxicity studies of the Platycodi radix water extract in SD rats.
Apoptosis is a well-known process of programmed cell death, which plays an important role in the development of multicellular organisms and in the maintenance of cellular homeostasis32. Many of the biochemical and morphological alterations that occur during apoptosis are a consequence of the breakdown of cells by a family of cysteine proteases called caspases, which cleave various substrate proteins that regulate programmed cell death (apoptosis). Evaluation of cleaved caspase-3 expression is a precise method for identifying apoptosis17, 19, 33. In the present study, the expression of Caspase-3 protein was significantly increased in the 3,000 mg/kg b.w./day treatment group compared to the vehicle control group in both sexes (p<0.01). The increase in the expression of Caspase-3 protein was in parallel with the histopathological result of squamous cell hyperplasia (p<0.05, p<0.01). Furthermore, the expression of Caspase-3 protein was significantly higher in the epithelium with squamous cell hyperplasia in the limiting ridge of the stomach in all treatment groups (male: 26.46%; female: 27.48%) than in normal epithelium (male: 11.98%; female: 11.00%) in both sexes (p<0.01). Therefore, it is suggested that increased Caspase-3 expression indicates increased apoptosis that eliminates hyperplastic squamous epithelium. Based on the above information, it can be concluded that squamous cell hyperplasia is not aggressive in behavior and does not have active proliferative activity.
Ki-67 expression was detected in regions similar to that of Caspase-3, and no statistically significant difference was observed in all groups. We have not investigated tissue renewal cycle factors other than Ki-67 and Caspase-3, and have not examined other biological changes. However, the squamous cell hyperplasia in the limiting ridge may be related to the accelerated tissue renewal cycle and is considered to be an adaptive reaction to the physical stimulus of Platycodi radix water extract, rather than genetic events. This is because the lesion disappeared with the discontinuation of the extract administration.
In conclusion, the squamous cell hyperplasia of the limiting ridge in the stomach induced by the Platycodi radix water extract was completely reversible following withdrawal of the stimulation and did not show active proliferative potency and reactive changes, such as local erosion, ulceration, or inflammation. Therefore, squamous cell hyperplasia is considered a non-adverse effect of Platycodi radix water extract administration in SD rats after a 13-week repeated-dose oral administration, and the non-adverse effect in the stomach was confirmed using IHC endpoints.
Funding
This study was supported by a Chemon Inc.
Disclosure of Potential Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
The authors gratefully acknowledge the technicians at the Toxicologic Pathology Center, ChemOn Inc., for their technical assistance.
References
- 1.Nolte T, Brander-Weber P, Dangler C, Deschl U, Elwell MR, Greaves P, Hailey R, Leach MW, Pandiri AR, Rogers A, Shackelford CC, Spencer A, Tanaka T, and Ward JM. Nonproliferative and proliferative lesions of the gastrointestinal tract, pancreas and salivary glands of the rat and mouse. J Toxicol Pathol. 29(Suppl): 1S–125S. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urbańska N, Giebułtowicz J, Olszowska O, and Szypuła W. The growth and saponin production of Platycodon grandiflorum (Jacq.) A. DC. (Chinese bellflower) hairy roots cultures maintained in shake flasks and mist bioreactor. Acta Soc Bot Pol. 83. 2014. [Google Scholar]
- 3.Cha S-B, Li Y, Bae J-S, Song S-W, Lee I-C, and Kim J-C. Evaluation of 13-week subchronic toxicity of Platycodon grandiflorus (Jacq.) A.DC. root extract in rats. J Ethnopharmacol. 267: 113621. 2021. [DOI] [PubMed] [Google Scholar]
- 4.Kim JI, Jeon SG, Kim KA, Kim JJ, Song EJ, Jeon Y, Kim E, Lee KB, Kwak JH, and Moon M. Platycodon grandiflorus root extract improves learning and memory by enhancing synaptogenesis in mice hippocampus. Nutrients. 9: 9. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong C-H, Choi GN, Kim JH, Kwak JH, Kim DO, Kim YJ, and Heo HJ. Antioxidant activities from the aerial parts of Platycodon grandiflorum. Food Chem. 118: 278–282. 2010. [Google Scholar]
- 6.Lee KJ, Choi CY, Chung YC, Kim YS, Ryu SY, Roh SH, and Jeong HG. Protective effect of saponins derived from roots of Platycodon grandiflorum on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Toxicol Lett. 147: 271–282. 2004. [DOI] [PubMed] [Google Scholar]
- 7.Moon M-K, Ahn J-Y, Kim S, Ryu S-Y, Kim Y-S, and Ha TY. Ethanol extract and saponin of Platycodon grandiflorum ameliorate scopolamine-induced amnesia in mice. J Med Food. 13: 584–588. 2010. [DOI] [PubMed] [Google Scholar]
- 8.Cantoreggi S, Dietrich DR, and Lutz WK. Induction of cell proliferation in the forestomach of F344 rats following subchronic administration of styrene 7,8-oxide and butylated hydroxyanisole. Cancer Res. 53: 3505–3508. 1993. [PubMed] [Google Scholar]
- 9.Wester PW, and Kroes R. Forestomach carcinogens: pathology and relevance to man. Toxicol Pathol. 16: 165–171. 1988. [DOI] [PubMed] [Google Scholar]
- 10.Greaves P. Chapter 8—Digestive system. In: Histopathology of Preclinical Toxicity Studies, 4th ed. P Greaves (ed). Academic Press, Boston. 325–431. 2012. [Google Scholar]
- 11.Scholzen T, and Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 182: 311–322. 2000. [DOI] [PubMed] [Google Scholar]
- 12.Takkem A, Barakat C, Zakaraia S, Zaid K, Najmeh J, Ayoub M, and Seirawan MY. Ki-67 prognostic value in different histological grades of oral epithelial dysplasia and oral squamous cell carcinoma. Asian Pac J Cancer Prev. 19: 3279–3286. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bologna-Molina R, Mosqueda-Taylor A, Molina-Frechero N, Mori-Estevez A-D, and Sánchez-Acuña G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med Oral Patol Oral Cir Bucal. 18: e174–e179. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birajdar SS, Radhika M, Paremala K, Sudhakara M, Soumya M, and Gadivan M. Expression of Ki-67 in normal oral epithelium, leukoplakic oral epithelium and oral squamous cell carcinoma. J Oral Maxillofac Pathol. 18: 169–176. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budihardjo I, Oliver H, Lutter M, Luo X, and Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 15: 269–290. 1999. [DOI] [PubMed] [Google Scholar]
- 16.Lu X, Arbiser JL, West J, Hoedt-Miller M, Sheridan A, Govindarajan B, Harral JW, Rodman DM, and Fouty B. Tumor necrosis factor-related apoptosis-inducing ligand can induce apoptosis in subsets of premalignant cells. Am J Pathol. 165: 1613–1620. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heshiki W, Tomihara K, Yamazaki M, Arai N, Nakamori K, and Noguchi M. Constitutive activation of caspase-3 in non-apoptotic oral squamous cell carcinoma cells. J Cancer Sci Ther. 7: 1–6. 2015. [Google Scholar]
- 18.Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, and Blomme EA. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol. 199: 221–228. 2003. [DOI] [PubMed] [Google Scholar]
- 19.Veeravarmal V, Austin RD, Siddavaram N, Thiruneelakandan S, and Nassar MHM. Caspase-3 expression in normal oral epithelium, oral submucous fibrosis and oral squamous cell carcinoma. J Oral Maxillofac Pathol. 20: 445–452. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.OECD. Guidelines for Testing of Chemicals. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents. 1998.
- 21.Levene H. Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Stanford University Press. 1960. [Google Scholar]
- 22.Duncan DB. Multiple range and multiple F tests. Biometrics. 11: 1–42. 1955. [Google Scholar]
- 23.Dunnett CW. New tables for multiple comparisons with a control. Biometrics. 20: 482–491. 1964. [Google Scholar]
- 24.Fisher RA. Statistical Methods for Research Workers. Oliver and Boyd, Edinburgh. 1970. [Google Scholar]
- 25.Lee M-J, Jung H-K, Lee K-H, Jang J-H, Sim M-O, Seong T-G, Ahn B-K, Shon J-H, Ham S-H, Cho H-W, Kim Y-M, Park S-J, Yoon J-Y, Ko J-W, and Kim J-CA. A 90-day repeated oral dose toxicity study of Alismatis Rhizoma aqueous extract in rats. Toxicol Res. 35: 191–200. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin NR, Jung TY, Seo CS, Park SW, Ko JW, Kim JC, and Shin IS. Protective effect of water extract of guibi-tang against pulmonary inflammation induced by cigarette smoke and lipopolysaccharide. Lab Anim Res. 34: 92–100. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bent S. Herbal medicine in the United States: review of efficacy, safety, and regulation: grand rounds at University of California, San Francisco Medical Center. J Gen Intern Med. 23: 854–859. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Ouedraogo M, Qu F, and Duez P. Potential genotoxicity of traditional chinese medicinal plants and phytochemicals: an overview. Phytother Res. 27: 1745–1755. 2013. [DOI] [PubMed] [Google Scholar]
- 29.Mateoiu C, Pirici A, and Bogdan F. Immunohistochemical nuclear staining for p53, PCNA, Ki-67 and bcl-2 in different histologic variants of basal cell carcinoma. Rom J Morphol Embryol. 52(Suppl): 315–319. 2011. [PubMed] [Google Scholar]
- 30.Oka S, Uramoto H, Shimokawa H, Iwanami T, and Tanaka F. The expression of Ki-67, but not proliferating cell nuclear antigen, predicts poor disease free survival in patients with adenocarcinoma of the lung. Anticancer Res. 31: 4277–4282. 2011. [PubMed] [Google Scholar]
- 31.Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, and Sloan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 42: 987–993. 2006. [DOI] [PubMed] [Google Scholar]
- 32.Langlois NE, Eremin O, and Heys SD. Apoptosis and prognosis in cancer: rationale and relevance. J R Coll Surg Edinb. 45: 211–219. 2000. [PubMed] [Google Scholar]
- 33.Krajewska M, Wang HG, Krajewski S, Zapata JM, Shabaik A, Gascoyne R, and Reed JC. Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res. 57: 1605–1613. 1997. [PubMed] [Google Scholar]