Abstract
Lymphoma is the third most common cancer diagnosed in children, and T-cell lymphoma has the worst prognosis based on clinical observations. To date, a lymphoma model with uniform penetrance has not yet been developed. In this study, we generated a p53 deficient mouse model by targeting embryonic stem cells derived from a C57BL/6J mouse strain. Homozygous p53 deficient mice exhibited a higher rate of spontaneous tumorigenesis, with a high spontaneous occurrence rate (93.3%) of malignant lymphoma. Because tumor models with high phenotypic consistency are currently needed, we generated a lymphoma model by a single intraperitoneal injection of 37.5 or 75 mg/kg N-methyl-N-nitrosourea to p53 deficient mice. Lymphoma and retinal degeneration occurred in 100% of p53+/− mice administered with higher concentrations of N-methyl-N-nitrosourea, a much greater response than those of previously reported models. The main anatomic sites of lymphoma were the thymus, spleen, bone marrow, and lymph nodes. Both induced and spontaneous lymphomas in the thymus and spleen stained positive for CD3 antigen, and flow cytometry detected positive CD4 and/or CD8 cells. Based on our observations and previous data, we hypothesize that mice with a B6 background are prone to lymphomagenesis.
Keywords: p53 deficient mouse, lymphoma model, MNU induction, C57BL/6J genetic background, T cell lineage
Introduction
Hodgkin lymphoma and non-Hodgkin lymphoma are the third most common cancers diagnosed in children every year worldwide1. Although the prognosis of patients has significantly improved because of advancements in the study of pathogenesis and development of new therapies, the overall outcomes of treatment remain poor. An appropriate animal model mimicking human disease conditions is crucial for facilitating our understanding of the basic theory of lymphoma pathogenesis, as well as for developing effective new antitumor drugs and treatment options. Various animal models of lymphoma have been reported, among which mouse models are the most popular. In the early 1970s2, a transplantable tumor was injected into CBA mice to model Gardner lymphosarcoma. The generation of adult T-cell leukaemia/lymphoma3 and human acute B-lymphoblastic leukaemia4 by xenotransplantation of primary peripheral blood mononuclear cells into combined immunodeficient mice has been recently reported. In addition to the use of immunodeficient mice, irradiation or thymectomy have also been used to optimize models5. Genetically modified mice have also been studied intensively as potential lymphoma models6, 7, 8, 9, 10.
p53 is a well-known tumor suppressor gene, and when it is knocked out in mice, it leads to spontaneous carcinogenesis11. A variety of tumors have been reported in p53− mutant mice with different genetic backgrounds. Lymphoma was observed in 47% and 53% of homogenous p53 deficient mice from the 129/Sv and BALB/c backgrounds, respectively6, 7. p53 deficient mice with mixed C57BL/6and 129/Sv backgrounds (75% C57BL/6 and 25% 129/Sv) exhibited 65–75% incidence of lymphoma6, 12. Because a high percentage of tumors from p53− mutant mice are lymphomas, p53− mutant mice are regarded as a potential model for lymphoma studies. However, its low penetrance ratio has hampered further mechanistic studies. Carcinogen-induced models have also been used to improve the incidence of lymphoma. When B6.129-Trp53 N5 heterozygous mice underwent N-methyl-N-nitrosourea (MNU) induction, 85% of mice developed lymphoma within six months8.
In the present study, a uniform lymphoma model was established by deleting the p53 gene in C57BL/6J mice. Homozygous p53− mutant mice were viable, and 93.3% developed spontaneous lymphomas from 12 to 37 weeks. Furthermore, 100% of heterozygous mice induced with 75 mg/kg of MNU developed lymphomas, and induced tumor incidence showed a dose-effect relationship with MNU. Lymphoblasts in thymic lymphomas stained positive for mouse CD3 antigen, and represented CD4+ and/or CD8+ detected by flow cytometry, indicating a T-cell lineage of the lymphoma in our model, which is similar to the mature T-cell neoplasms of human lymphoid neoplasms according to the WHO classification of tumors of hematopoietic and lymphoid tissues (revised 4th edition) in 2017.
Materials and Methods
Generation of C57BL/6J embryonic stem (ES) cells
C57BL/6J mice were maintained in a 12-hour light/12-hour dark cycle. To obtain blastocysts, female mice were induced to superovulate, and blastocysts were flushed out from uterine horns of 3.5 dpc pregnant females. Blastocysts were cultured for 5–6 days on feeder cells (mouse embryonic fibroblasts, MEFs) in 12-well plates, with the ES cell medium being changed every 1–2 days. The size of ICMs (inner cell masses) markedly increased in the culture over this period. They consisted of a central mass of stem cells and peripheral primitive endoderm-like cells. They were picked up with a mouth-controlled micropipette into a 48-well plate and then digested with 0.1% collagenase for 10–15 min, followed by 0.25% trypsin for 2–5 min. The digested ICMs were transferred onto new feeder cells for continuous culture until ES cell colonies were observed.
Mouse ES cell culture
C57BL/6J ES cells were cultured at 37 °C in a humidified incubator with 5% CO2. The cells were routinely maintained on mitotically inactivated MEFs with Knockout DMEM medium (11960-44, Gibco, Grand Island, NY14702, USA) supplemented with 15% FBS (35-081-CV, Corning, Manassas, USA), 1% MEM NEAA (10370021, Gibco, Grand Island, NY14702, USA), 1% L-glutamine (25030081, Gibco, Grand Island, NY14702, USA), 0.1% β-mercaptoethanol (21985023, Gibco, Grand Island, NY14702, USA), and 1% CHO-LiF. Mouse ES cells were passaged every 2–3 days. For routine passaging, mouse ES cells were detached by pipetting and collected by centrifugation. Then, 0.05% trypsin was added to dissociate the cell aggregates into single cells. Passaging cells in this way avoids carryover of feeders, which adversely affects mouse ES cell growth.
Karyotype analysis of ES cell lines
For karyotyping, mouse ES cells were plated onto 6-well plates at a density of 1 × 106 cells per well. One day after plating, 0.5 mg/ml colcemid (D1925, Sigma-Aldrich, St. Louis, Missouri, USA) was added to the culture and incubated for 50 min in a 37 °C water bath. Mouse ES cells were then trypsinized, fixed with a 3:1 solution of methanol and glacial acetic acid, and then spread onto glass slides. Chromosomes were stained by G-banding and karyotypes were determined using microscopy.
Generation of p53 gene knockout mice
We constructed a mouse p53 gene-targeting vector using a PGK promoter to drive the expression of a neomycin selection cassette (Neo). The targeting vector was introduced into C57BL/6J mouse ES cells by electroporation. After homologous recombination, the targeting vector replaced the p53 gene from exons 2 to 5. Neomycin-resistant ES cell colonies were selected, screened by PCR, and injected into 151 wild-type BALB/c blastocysts. ES-cell-injected blastocysts were then transferred to 14 pseudo-pregnant mice, and 8 chimeric mice were produced. Male chimeric mice were then crossed with wild-type C57BL/6J female mice to generate heterozygous p53 knockout mice.
Southern blot analysis
Genomic DNA was extracted from mouse tail biopsies and used for Southern blot analysis. After digestion with EcoRI (for the 5′ probe), the genomic DNA samples were analyzed by gel electrophoresis on a 1% agarose gel. After electrophoresis, the gel was denatured, neutralized, and blotted by capillary transfer onto a nylon membrane. The DNA membrane was fixed and hybridized with digoxigenin-labeled Southern blot hybridization probes, according to the manufacturer’s instructions (Roche, Basel, Switzerland).
Mice genotyping and RNA analysis
Tail genomic DNA was isolated using a Tissue Genomic DNA Extraction Kit (GK0121, Generay, Shanghai, China) and then, once isolated, PCR was used to verify deletion of the p53 gene. Genomic DNA of p53 deficient mice and wild-type mice were amplified with primer sets 1 (P53-WT-F, AGTTCTGCCACGTGGTTGGT; P53-WT-R, GTCTCCTGGCTCAGAGGGAG) or 2 (P53-WT-F, AGTTCTGCCACGTGGTTGGT; P53-Neo-R, CAGAGGCCACTTGTGTAGCG), with expected PCR products of 281 bp and 441 bp for wild-type and homozygous mutations, respectively. For heterozygous mutations, both bands on the agarose gel were visible.
Tissues were dissected and immediately immersed in RNA stabilization reagent (AM7021, Invitrogen, Vilnius, Lithuania) and stored at −80 °C. Total RNA was extracted from individual tissues using TRIzol (15596018, Invitrogen, Vilnius, Lithuania) and quantified using a spectrophotometer at 260 nm. Random hexamers were used to prime reverse-transcription reactions with Superscript III (12594100, Invitrogen, Vilnius, Lithuania). Real-time quantitative PCR was performed using an ABI 7300 Real Time PCR System with SYBR Green I reagent (RR82lr, Takara Bio Inc., Dalian, China). The primer set for qPCR analysis was p53 F1, 5′-CCCCTGTCATCTTTTGTCCCT-3′ and p53 R1, 5′-AGCTGGCAGAATAGCTTATTGAG-3′.
Spontaneous malignant lymphoma in p53−/− mice
Thirty p53−/− mice were maintained for the observation of spontaneous tumors. All mice were observed and palpated daily, and the clinical signs were recorded. The moribund mice were euthanized and necropsied immediately. Dead mice were necropsied immediately or kept at a low temperature and necropsied the next day. Tissues chosen for histopathological examination included the adrenal gland, aorta, brain (forebrain, midbrain, and hindbrain), cecum, colon, duodenum, epididymis (male only), oesophagus, eyes, femur with bone marrow, Harderian glands, heart, ileum, jejunum, gall bladder, kidneys, liver, lung with bronchi, lymph nodes (mesenteric and mandibular), mammary gland (female only), optic nerves, ovaries (female only), oviduct (female only), pancreas, pituitary, prostate (male only), rectum, salivary gland, sciatic nerve, seminal vesicles (male only), skeletal muscle (thigh), skin and subcutis, spinal cord (cervical, thoracic, and lumbar), spleen, sternum with bone marrow, stomach, testis (male only), thymus, thyroid and parathyroid glands, tongue, trachea, tumors/masses, urinary bladder, uterus with cervix (female only), vagina (female only), and gross lesions.
MNU induced malignant lymphoma in p53+/− mice
Thirty C57BL/6J wild-type mice and 50 p53+/− 7-week-old deficient mice (half male and half female) were grouped and administered 37.5 mg/kg or 75 mg/kg MNU or citrate buffer as a control. MNU was dissolved in citrate buffered saline and adjusted to pH 4.5 before a single dose administration to animals by intraperitoneal injection on day 1, followed by a six-month observation period. All mice were observed twice daily, and clinical signs were recorded once daily. Body weights were recorded at least once before treatment and weekly after the treatment. After 12 weeks of treatment, the mice were palpated weekly until the end of the study. All surviving mice administered MNU were sacrificed and necropsied at the end of 26 weeks.
Immunohistochemical analysis and flow cytometry
Mouse tissues and masses were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned (5 μm). For histopathological examination, the tissue sections were stained with hematoxylin and eosin.
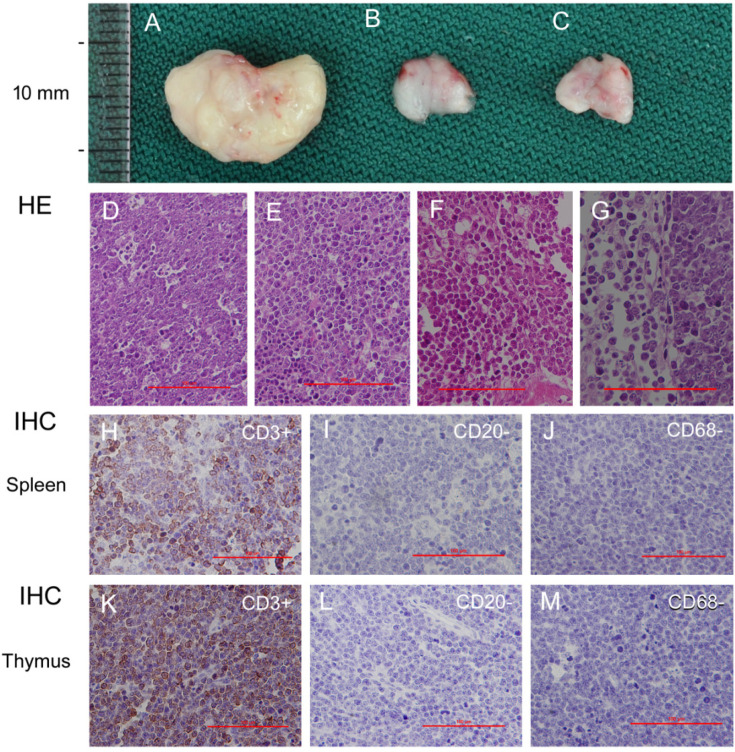
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded sections of the thymus from five animals per sex with thymic malignant lymphoma in the MNU-treated group and from two control males and two control females. Antibodies directed against CD3 (T lymphocyte marker), CD20 (B lymphocyte marker), and CD68 (macrophage marker) were used to classify the lineage of neoplastic cells in the thymus. Healthy thymic and thymic malignant lymphoma sections stained for CD3, CD20, and CD68 were pretreated by incubation at 96 °C in citrate buffer (Zhongshan Golden Bridge Biocompany, Beijing, China) at pH 6 in a microwave oven for 10 min. Sections stained for CD3 were incubated with anti-CD3 antibody (clone LN10, Zhongshan Golden Bridge Biocompany, Beijing, China) at a 1:150 dilution overnight at 4 °C after blocking with normal goat serum for 60 min at 37 °C. The sections stained for CD20 were incubated with anti-CD20 antibody (clone EP7, Zhongshan Golden Bridge Biocompany, Beijing, China) at a dilution of 1:200 overnight at 4 °C after blocking with normal goat serum for 60 min at 37 °C. The sections stained for CD68 were incubated with anti-CD68 antibody (clone PG-M1, Zhongshan Golden Bridge Biocompany, Beijing, China) at a dilution of 1:200 overnight at 4 °C after blocking with normal goat serum for 60 min at 37 °C. CD3, CD20, and CD68 immunoreactivity was detected using a biotinylated rabbit anti-rat secondary antibody followed by an avidin-biotin-horseradish peroxidase complex and visualized with diaminobenzidine. All immunohistochemical sections were counterstained with hematoxylin, dehydrated in graded concentrations of ethanol, and coverslipped using a permanent mounting medium.
To detect CD4 and CD8 positive cells, the enlarged thymus glands and spleens of p53−/− mice over six months (which, according to previous experiments, usually indicates lymphoma has occurred) were collected. BD FACS Calibur (Becton Dickinson, Franklin Lakes, NJ) was then used to perform multicolor cytometric analysis. Erythrocytes in the thymus and spleen cells were depleted by treatment with lysing solution (BD Pharmingen, San Diego, CA). Anti-mouse CD4 and CD8 monoclonal antibodies were purchased from BD Pharmingen.
Statistical analysis
Fisher’s exact test was used to analyze tumor incidence data, and the Fisher least significant difference test was used to analyze body and organ weights. The statistical analysis results of hematological data are shown as the mean ± standard deviation (SD) or one-way analysis of variance (ANOVA). SPSS version 19.0, was used to analyze the results. Dunnett’s parametric test was used for multiple comparisons between groups. Statistical significance was set at p<0.05. p<0.01 was considered highly significant.
Results
Generation of C57BL/6J mouse ES cells
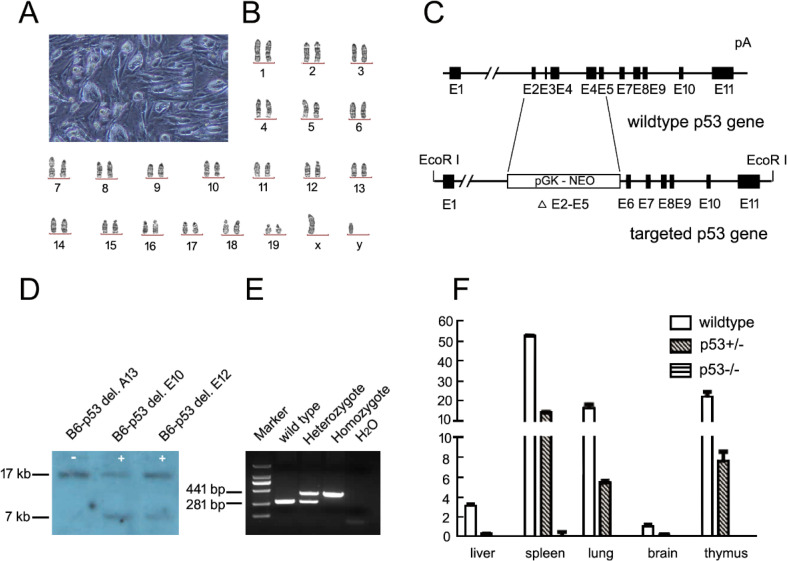
Although most ES cell targeting has been performed using ES cells of the 129/Sv background, an ES cell line isolated from the C57BL/6J inbred strain has the advantage of a much purer background and therefore no need for reciprocal backcrossing13. In this study, we used an ES cell line (Fig. 1A) established from C57BL/6J mice to generate knockout mice. We examined the karyotype of ES cells and confirmed that they were male (XY), with a normal number of chromosomes (Fig. 1B). This ES cell line has been reported to have high germline transmission efficiency. Using this highly efficient and hereditary stable C57BL/6J ES cell line, we generated p53 gene knockout mice using ES cell-based gene targeting.
Fig. 1.
Establishment and verification of a p53 gene knockout mouse model. A. Image of p53 gene targeted ES cells on a C57BL/6J background. B. Karyotype of targeted ES cells. C. Schematic representation of p53 gene knockout construction; exons 2–5 were replaced by a Neo cassette. D. Southern blotting analyses of mouse tail DNA. Genomic DNA was extracted from ES cells and used for Southern blotting analysis. After digestion with EcoRI (for the 5′ probe), the probe hybridized to a 17 kb fragment from the wild-type allele (+) and a 7 kb fragment from the p53 gene knockout allele (−). E. PCR genotyping analysis of wild type, heterozygous, and homozygous p53 gene knockout mice. The expected sizes of PCR products are 281 bp for the wild-type allele and 561 bp for the knockout allele. F. Relative real-time PCR analysis of the p53 gene mRNA in the liver, spleen, lung, brain, and thymus of wild-type, heterozygous, and homozygous p53 gene knockout mice. Values are shown as the mean ± SD for three independent experiments and were normalized to the corresponding GAPDH levels.
Generation of p53Δ2-5/+ mice
A targeting vector containing a phosphoglycerate kinase (PGK) promoter driving a Neo cassette was generated. The Neo cassette replaced exon 2 to exon 5 of the p53 gene, which accounted for approximately 40% of the coding region (Fig. 1C). The p53 targeting vector was electroporated into the established C57BL/6J ES cell line, and ES cells were screened using G418. G418 resistant colonies were further confirmed by Southern blotting. The 5ʹ probe hybridized to an EcoRI fragment of approximately 17 kb from the wild-type p53 allele, whereas induction of the PGK-Neo cassette in a mutant allele contained another EcoRI site that yielded a 7-kb fragment (Fig. 1D). Four heterozygous ES cell clones underwent blastocyst injection. A total of 151 wild-type BALB/c blastocysts were injected, and 24 chimeric mice were produced. Male chimeric mice were crossed with C57BL/6J female mice and 37 F1 mice with black fur, and 48 mice with white fur were obtained. Some F1 male and female heterozygous mice were further intercrossed to generate homozygous p53 knockout mice (Fig. 1E).
Deletion of exons 2 to 5 theoretically abolished p53 transcription. We checked p53 expression to prove that the p53Δ2-5/+ allele was a null allele. We analyzed total RNA derived from the liver, spleen, lungs, brain, and thymus. Heterozygous mice exhibited approximately half the normal p53 gene expression observed in wild-type mice, whereas homozygous mice did not express p53 mRNA at all. These results were confirmed by two independent pairs of primers, one pair located in exons 4 and 5, and the other located in exons 9 and 10 (Fig. 1F). This model is named B6-Trp53tm1DAMR/NIFDC, and is referred to as p53−/− in this study.
High frequency of spontaneous lymphoma in p53−/− mice
According to Knudson’s two-hit hypothesis14, p53−/− mice of various genetic backgrounds develop spontaneous tumors much earlier than p53+/− mice6, 7, 12, 15. As shown in Supplementary Fig. 1A, p53−/− mice indeed exhibited an accelerated rate of tumorigenesis compared with heterozygous and wild-type mice. Approximately 50% of p53−/− mice were moribund and euthanized for necropsy before 26 weeks, and all died before 32 weeks. However, all wild-type and 90% of p53+/− mice survived until the end of the study.
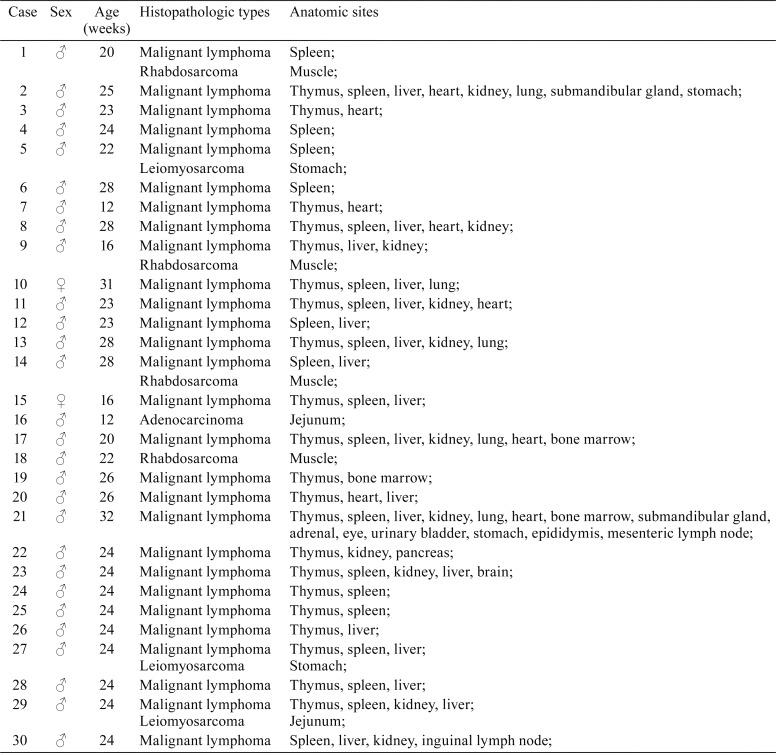
Necropsy revealed obvious thymus enlargement in 23 out of 30 mice. Microscopic observation revealed that among the p53−/− homozygotes, the most frequently observed tumor was malignant lymphoma, affecting 28 of 30 tumor-bearing mice. Of the remaining two mice, one had rhabdosarcoma and the other had adenocarcinoma (Table 1). The tumor frequencies of malignant lymphoma, rhabdosarcoma, leiomyosarcoma, and adenocarcinoma were 93.3%, 13.3%, 10%, and 3.3%, respectively (Supplementary Fig. 1B). These data imply that this model is susceptible to tumor development. Of the 30 mice, 22 (73.3%) developed lymphoma, 3 had both lymphoma and rhabdosarcoma, and 3 animals had both lymphoma and leiomyosarcoma. The relative frequency of lymphoma occurrence was 92.5%. The second most common tumor was rhabdosarcoma, accounting for 3.7% of all tumors (Supplementary Fig. 1C). The tumor spectrum was quite different from that observed in p53 knockout mice with different genetic backgrounds. Donehower reported that 10 types of tumors were observed6, 11, while only four types of tumors were found in B6J homozygotes in this study, indicating that p53−/− mice with a C57BL/6J background predominantly develop lymphoma.
Table 1. Spontaneous Tumors in p53-deficient Homozygous (p53−/−) Mice.
The anatomic sites of tumors included the spleen, thymus, liver, kidney, heart, lung, and stomach. As expected, malignant lymphoma occurred mainly in the spleen and thymus, at a rate of 70%, indicating that the spleen and thymus were the primary organs of lymphoma in this model. In addition, lymphoma was frequently observed in the bone marrow, lymph nodes, liver, kidney, and heart (Supplementary Fig. 1D). Rhabdosarcoma was observed in the muscle, adenocarcinoma was found in the jejunum, and two mice had leiomyosarcoma in the stomach.
Generation of a lymphoma model by MNU induction in p53+/− mice
Since p53−/− mice with a C57BL/6 background had a higher occurrence of lymphoma (93.3%) than other strains6, 12, 16, we wanted to generate a lymphoma model with higher consistency and earlier occurrence. Genotoxic carcinogens are generally referred to as initiating agents of tumorigenesis because they damage DNA and induce mutations in key target genes, which is thought to be the initial event leading to cancer development17. MNU is a widely used positive genotoxic carcinogen for p53 deficient mice and other transgenic mice in carcinogenicity studies8, 18, 19. Therefore, we used MNU to shorten the lymphoma occurrence time and establish an efficient tumor model.
Wild-type, p53+/−, and p53−/− mice were administered 37.5 mg/kg of MNU dissolved in citrate buffered saline (adjusted to pH 4.5) by a single intraperitoneal injection. All p53−/− mice died within one week of injection, while p53+/− and wild-type mice survived until the end of the experiments (Supplementary Fig. 2A). Since tumors usually do not develop within a week, only wild-type and p53+/− mice were observed. The incidence of lymphoma was 65% in p53+/− mice and 10% in wild-type mice (Supplementary Fig. 2B, p<0.05). Except for malignant lymphoma, no other types of tumors were observed (Supplementary Fig. 2C). The lymphoma frequencies in different organs are shown in Supplementary Fig. 2D, and higher incidences of tumors were observed in the thymus and spleen.
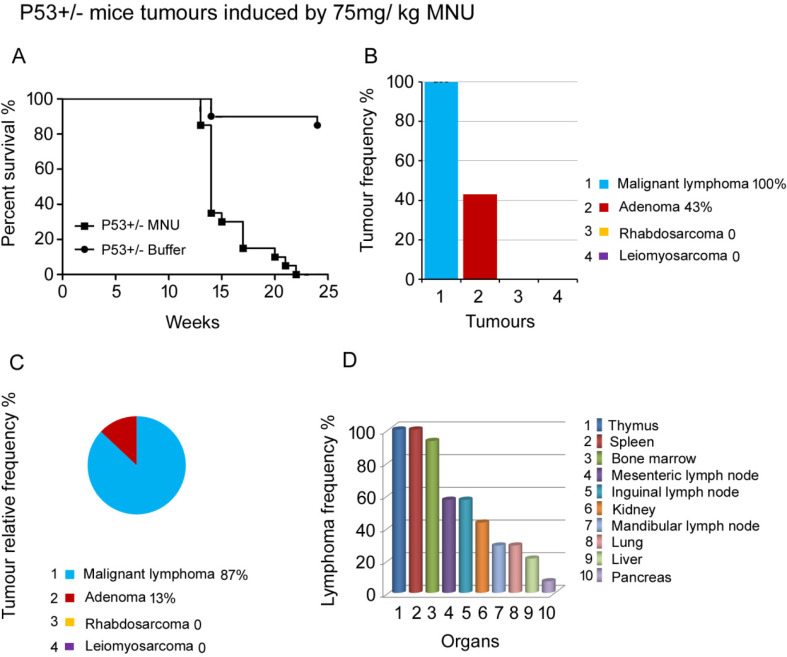
Fig. 2.
Lymphoma occurrence in p53 deficient heterozygous mice induced by 75 mg/kg of MNU. A. Survival curve of p53+/− mice administered 75 mg/kg of MNU. Most animals died between 13–17 weeks after MNU administration. B. The tumor profile and incidence of p53+/− mice. Lymphoma and adenoma were observed. Notably, the incidence of lymphoma was 100%. C. Relative tumor frequency of lymphoma, adenoma, rhabdosarcoma and leiomyosarcoma. D. The malignant lymphoma frequency in various organs. 14 p53+/− mice were used in this experiment.
To further test the tumor incidence and the uniformity of the model, a group of p53+/− mice was administered 75 mg/kg MNU by a single intraperitoneal injection. The onset of tumorigenesis in these mice was much earlier than that in p53+/− mice administered 37.5 mg/kg MNU. Because most animals died between 13 and 17 weeks, other surviving animals were sacrificed by 23 weeks (Fig. 2A). The incidence of lymphoma was 65% in 37.5 mg/kg MNU) p53+/− mice, whereas the incidence of lymphoma was 100% in 75 mg/kg MNU p53+/− mice. This indicates that a higher concentration of MNU resulted in a higher incidence of lymphoma.
Microscopy revealed that malignant lymphoma was the most predominant tumor, occurring in 100% of mice at the end of the experiment. The second most common tumor was adenoma, with a rate of 43% (Fig. 2B). Unlike other spontaneous tumor models, rhabdosarcoma and leiomyosarcoma were not observed. Figure 2C shows the tumor distribution in this animal group; as expected, the most common tumor was lymphoma (87%), and the proportion of adenomas was 13%.
We further investigated the incidence of lymphoma in different organs. Tumors were mainly present in the hematolymphoid system, including the thymus (100%), spleen (100%), bone marrow (93%), mesenteric lymph nodes (57%), inguinal lymph nodes (57%), and mandibular lymph nodes (29%). Metastatic lymphoma was found in some organs, such as the kidneys, lungs, and liver (Fig. 2D). In contrast to the 37.5 mg/kg MNU group (Supplementary Fig. 2D), the hematolymphoid systems of animals in the 75 mg/kg group showed high numbers of lesions, and the tumor incidence increased from 65% to 100%. With the advantages of easy sampling and high rate of lymphomagenesis, the thymus and spleen can be used as the most appropriate target organs for the study of mechanisms of lymphomagenesis.
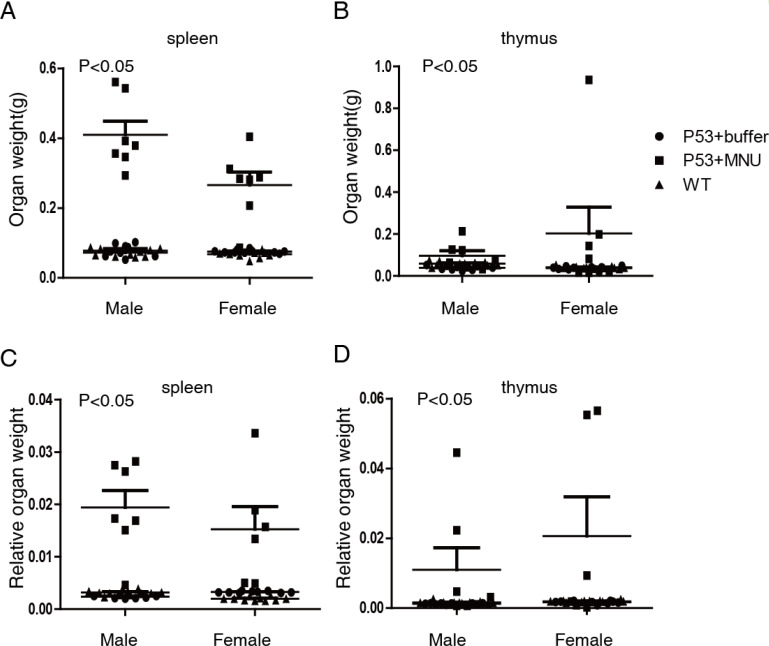
Organ enlargement was frequently observed during gross pathological examination (Supplementary Table 1), with enlargement of the thymus gland and spleen being more common than of other organs. To further investigate, the weights of the heart, spleen, lung, kidney, brain, thymus, salivary gland, adrenal gland, ovaries, and uterus were measured. Figure 3 shows that the absolute weight and relative organ weight of the spleen and thymus were higher in the 75 mg/kg MNU group than in the control group (p<0.05).
Fig. 3.
Weight and relative weight of spleen and thymus of mice treated with or without 75 mg/kg MNU. A. Spleen weight of WT, p53+/− mice treated with or without MNU. B. Thymus weight of WT, p53+/− mice treated with or without MNU. C. Relative weight (organ weight/body weight) of spleen (C) and thymus (D) of WT, p53+/− mice treated with or without MNU (n=7 per group).
T-cell lineage of the malignant lymphomas of the thymus and spleen
To determine the cell origin of the malignant lymphomas, we performed immunohistochemical staining of sections from five male and five female MNU-treated p53+/− mice (Fig. 4A–G) as well as four p53−/− mice diagnosed with spontaneous thymus and spleen malignant lymphoma using antibodies directed against CD3 (T lymphocyte marker), CD20 (B lymphocyte marker), and CD68 (macrophage marker). All neoplastic cells in the thymus and spleen malignant lymphoma sections from animals with induced or spontaneous malignant lymphoma were positive for CD3 (Fig. 4H, 6K) and negative for CD20 (Fig. 4I, 4L) and CD68 (Fig. 4J, 4M), indicating that the malignant lymphomas were of T-cell lineage, as is consistent with previous reports8, 15. To further confirm the cell origin, whole thymic and splenic cells were isolated for the flow cytometry assay. CD4+ and CD8+ cells were observed in the thymus and spleen of all animals, regardless of lymphoma incidence or p53 mutation, but CD4/CD8 double positive cells were mainly found in the thymus (Supplementary Fig. 3A, B). Meanwhile, only differentiated CD4+ or CD8+ populations were found in the spleen (Supplementary Fig. 3C, D). The ratios of CD4+, CD8+, double positive, and double negative cell populations were different in p53−/− mice with thymic lymphoma, representing 29.2%, 15%, 31%, and 24% of total lymphocytes, respectively, compared with 12.3%, 6%, 74%, and 5.3% in wild-type mice, respectively (Supplementary Fig. 3E), perhaps indicating that lymphomagenesis occurred after the formation of CD4/CD8+ T lymphocytes20. These observations are different from those of Donehower et al.6, where both B and T lymphomas were detected. This might be due to the different genetic backgrounds of the mice used in the studies.
Fig. 4.
Histopathological and immunohistochemical results of 75 mg/kg MNU induced lymphomas in p53+/− gene deficient mice. A. An enlarged thymus from p53+/− mice administered 75 mg/kg MNU. B. Thymus from p53+/− mice administrated citrate buffer (control). C. Normal thymus from wild type B6 mice. D–G. Photomicrograph of the spleen, thymus, bone marrow and mesenteric lymph node. D–G. Magnification is ×100, bar=100 μm. H–J. Photomicrographs of spleen lymphomas stained using CD3, CD20, and CD68. H. The spleen lymphoma stained positive for CD3 (T lymphocyte marker). I. The spleen lymphoma stained negative for CD20 (B lymphocyte marker). J. The spleen lymphoma stained negative for CD68 (macrophage marker). K–M. Photomicrographs of thymus lymphomas stained using CD3, CD20, and CD68. K. The thymus lymphoma stained positive for CD3. L. The thymus lymphoma stained negative for CD20. M. The thymus lymphoma stained negative for CD68. H–M. Magnification is ×200, bar=100 μm.
Body weight, and haematological and biochemistry analyses
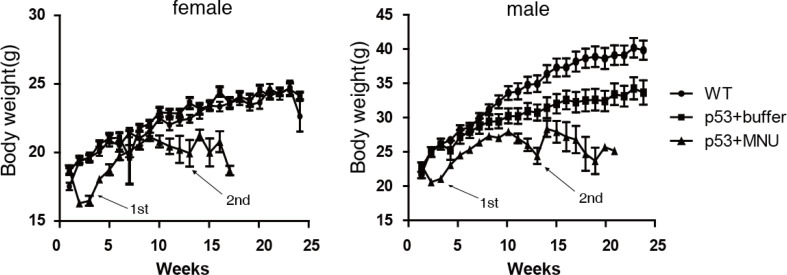
To examine the characteristics of the lymphoma model, we monitored the clinical features and body weight changes from the time of MNU administration to death or sacrifice. We also measured hematological and blood biochemical parameters at the end of 24 weeks. Clinical symptoms, such as decreased activity, hunched back, listlessness, thinness, and rapid breathing patterns were observed at approximately 12–13 weeks after the administration of MNU. None of the animals administered citrate buffer (controls) showed any clinical signs and all survived until sacrifice. Mice administered 75 mg/kg MNU exhibited significantly decreased body weights compared with those administered citrate buffer 2–3 weeks after dosing. This body weight loss may be caused by the acute toxicity of this chemical5. Although the body weight of mice administered MNU gradually recovered, it remained lower than that of the citrate buffer control animals at most time points. A second body weight decrease occurred at 12–13 weeks after dosing (Fig. 5), probably due to the progression of malignant lymphoma, which is in accordance with the time of moribund/death of many animals (Fig. 2A) and the appearance of clinical symptoms.
Fig. 5.
Dynamics of body weight in male and female mice treated with or without 75mg/kg of MNU. Troughs of body weight of mice treated with MNU were observed at 2–3 weeks and 12–13 weeks as indicated by arrows. The second trough is consistent with the moribund or death of many animals. Data are presented as the mean ± SD (n=10 per group).
In the hematological analysis, we counted the total number and calculated the relative percentages of white blood cells, neutrophilic granulocytes, lymphocytes, monocytes, eosinophils, basophils, and red blood cells. We found a greater number of neutrophilic granulocytes in the p53+/− MNU group than in the citrate buffer controls (p<0.05), while the number and relative percentage of lymphocytes did not increase significantly (p>0.05). The increase in the number of neutrophilic granulocytes might have resulted from systemic inflammatory responses occurring at the end stage of the tumor, because histopathology observed inflammatory cell infiltration in various organs. The red blood cell count, quantity of hemoglobin, and quantity of total protein decreased significantly (p<0.05) in the p53+/− MNU group. Four biochemical parameters (triglyceride, urea, total cholesterol, and calcium), had increased while serum albumin and creatinine had decreased in the MNU group at the end of the experiment, compared with the citrate buffer group (Supplementary Fig. 4).
Non-neoplastic microscopic findings in the p53+/− mouse lymphoma model
Non-neoplastic microscopic findings included adenomatous hyperplasia of the duodenum and jejunum, glandular hyperplasia of the duodenum, and retinal degeneration of the eyes (Supplementary Fig. 5). The characteristics of adenomatous hyperplasia and glandular hyperplasia included an increase in crypt length and the number of cells per crypt, lengthened villi, and an increased diameter of crypts, but without formation of the circumscribed area of the epithelium. Notably, 100% of mice administered MNU showed retinal degeneration, indicating high homogeneity in this animal model.
Discussion
Small, economical animal models have been widely used for the study of oncological mechanisms and for screening of therapeutic regimens21. An animal model with high phenotypic consistency may facilitate research on tumor pathogenesis by improving the reproducibility and success rate of experiments, and reducing the number of animals required. Here, we present a new mouse model of lymphoma established in mice with a C57BL/6J background which underwent p53 gene deletion followed by MNU administration. We found that 100% of p53+/− knockout mice administered a high dose of MNU developed lymphomas. Animal death was observed mainly from 13 to 17 weeks after MNU administration (Fig. 2), indicating that this lymphoma model has uniform tumorigenesis, predictable early onset time, and high reproducibility.
The uniform tumorigenesis of this model may result from its homogeneous genetic background. Before the establishment of the B6 ES cell line in 200722 and application of the CRISPR/Cas9 system in mammalian cells23, most genetically modified animal models were developed by targeting ES cell lines derived from 129/Sv mice, which require several generations of backcrossing with C57BL/6J to modify the genetic background. However, these mouse models still have a mixed background even after 20 generations of backcrossing. In the present study, the p53 deletion model using a C57BL/6J derived ES cell line had a pure genetic background. These mice with a homogeneous genetic background developed uniform malignant lymphomas as well as retinal degeneration (Supplementary Fig. 55).
According to somatic mutation theory, genetics and the environment (carcinogens), as well as their interactions, can drive tumorigenesis24, 25, 26. p53 is a crucial tumor suppressor gene that plays an important role in physiological processes27, 28. Furthermore, p53 gene mutation or deletion promotes tumorigenesis in humans and animal models21, 25, 29, 30 The potential for carcinogens to induce or accelerate tumor genesis has also been extensively reported8, 31, 32. In a lymphoma model33, MNU induction, decreased PTEN expression, and increased Muts homolog 2 (MSH2) expression was observed. Extensive deletion of P16INK4A was found in another lymphoma model established by UV irradiation of p53 knockout mice34. Different strategies have been used to develop lymphoma models, such as humanized mice, xenografting4, 35 or others36, 37 to mimic the effects of genetic and environmental factors on tumorigenesis. Here, we established a lymphoma model with a 4 month latent period of lymphoma, instead of a period of over 6 months.
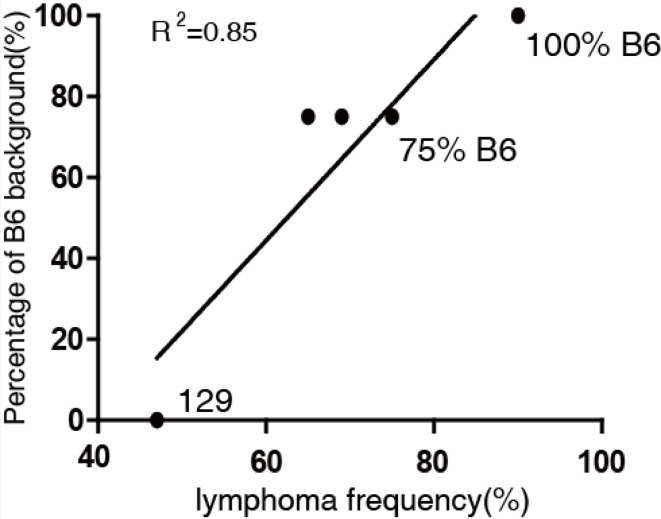
We have always expected to establish a small animal model with a high tumor incidence, focusing on lymphoma, for the study of its pathogenesis. Parameters that affect tumorigenesis in p53-deficient mice have been widely described, such as the genetic background and status of the p53 gene (homozygous or heterozygous)6, 12, 15. Various mouse strains, including D315, BALB/c7, 129/Sv6, 12, and C57BL/6J (as used in this study), have been used for p53 gene deletion models, all of which exhibit diverse tumor spectra and prominent tumor types. Supplementary Table 2 shows that the top three spontaneous tumors in D3-p53+/− mice were osteosarcoma, lymphoma, and fibrosarcoma15. The main tumor types in BALB/c background P53+/− mice were mammary carcinoma, lymphoma, and hemangiosarcoma. Because the early onset of mammary carcinoma is the most common cancer in women with Li-Fraumeni syndrome, this model has been considered a Li-Fraumeni syndrome model7. In 129/Sv mice, the major tumors were osteosarcomas and lymphomas. However, only low lymphoma frequencies (from 22% to 25%) were observed in these three mouse models. As expected, given Knudson’s two-hit hypothesis14, the profile of p53 gene deletion homozygous mice was quite different from that of heterozygotes; spontaneous lymphoma was the prominent tumor type, with frequencies of up to 65% in BALB/c mice7 and 71% in D315 mice. Interestingly, we observed that the frequency of spontaneous lymphoma was 47% in 129/Sv-p53−/− mice, 65–75% in 75% C57BL/6×25% 129-Sv mixed-background mice, (Supplementary Table 2), and 93.3% in mice with a 100% C57BL/6J background (Supplementary Fig. 1). Using linear regression, we demonstrated that the frequency of lymphoma was positively correlated with the percentage of C57BL/6J background (R2 = 0.85, Fig. 6). However, when C57BL/6J p53 heterogeneous mice were induced with 75 mg/kg of MNU, the frequency of lymphoma increased to 100% (Fig. 2), in contrast with 85% in 129/Sv mice and B6 mixed-background mice8. Previous studies have shown that the genetic background of mice may alter tumor development, but tumorigenesis is not strain-specific12, 38. Combined with our data and clinical observations39, we hypothesize that tumor genesis is strain-specific, and B6 background mice are prone to lymphomagenesis. How the B6 genetic background affects the tumor profile or incidence is still unknown.
Fig. 6.
Relation of lymphoma occurrence rate and B6 genetic background. R2 represents the coefficient of determination, original data and references listed in Supplementary Table 2.
In summary, a lymphoma mouse model with an entire B6 background was established for the first time, which induced tumors in 100% of specimens, focusing on lymphoma occurring in specific organs. Lymphomas in this model were CD3, CD4, and CD8 positive; and CD20 and CD68 negative, and therefore of the T cell lineage (Fig. 4, Supplementary Fig. 3), which is possibly similar to the mature T cell neoplasms of humans. Our uniform thymic malignant lymphoma model has a predictable time of occurrence: as early as 12 weeks for spontaneous lymphoma in p53−/− homozygous mice, and 13-17 weeks for induced lymphoma in p53+/− heterozygous mice. This consistency means the model may prove useful as a potential tool in lymphomagenesis studies in the future. The cause of lymphoma in humans is still unclear, but may involve many factors, such as viral infection, and genetic and environmental factors. It is postulated that p53 gene deletion might be involved in the development of lymphoma, especially T-cell lymphoma, and that MNU might have a synergistic effect in the development of lymphoma.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Supplemental Table
Acknowledgments
This work was supported by the National Major Scientific and Technological Special Project for “Significant New Drugs Development” during the thirteenth Five-Year Plan Period (SQ2018ZX090101), Academic Leaders Funded by National Institutes for Food and Drug Control (No. 2019X2) and the National Natural Science Foundation of China (Grant number: 81502396 to Xi Wu). We thank Dr. Yanan Guo, Dr. Shuya Zhou, Qin Zuo, Chenfei Wang, and Dr. Meng Wang for their technical support in this study.
References
- 1.Fahrer J, and Kaina B. Impact of DNA repair on the dose-response of colorectal cancer formation induced by dietary carcinogens. Food Chem Toxicol. 106: 583–594. 2017. [DOI] [PubMed] [Google Scholar]
- 2.Calman KC. Why are small bowel tumours rare? An experimental model. Gut. 15: 552–554. 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura D, Yoshimitsu M, Kuroki A, Hachiman M, Kamada Y, Ezinne CC, Arai A, Inoue H, Hamada H, Hayashida M, Suzuki S, Fujino S, Arima N, Arima M, Tabuchi T, Okada S, and Arima N. A new ATL xenograft model and evaluation of pyrrolidine dithiocarbamate as a potential ATL therapeutic agent. Exp Hematol. 43: 944–950. 2015. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Deng Y, Chen FL, Fang YJ, Zhang W, and Yu L. [Establishment of human acute B-lymphoblastic leukemia–NOD/SCID xenotransplant mouse model]. Zhongguo Shi Yan Xue Ye Xue Zazhi. 23: 623–626. 2015 (in Chinese). [DOI] [PubMed] [Google Scholar]
- 5.Sun-Hoffman L, and Winicov I. MNU affects mouse erythroleukemia cell differentiation at sub-cytotoxic doses. Chem Biol Interact. 100: 241–254. 1996. [DOI] [PubMed] [Google Scholar]
- 6.Donehower LA, Harvey M, Vogel H, McArthur MJ, Montgomery CA, Jr , Park SH, Thompson T, Ford RJ, and Bradley A. Effects of genetic background on tumorigenesis in p53-deficient mice. Mol Carcinog. 14: 16–22. 1995. [DOI] [PubMed] [Google Scholar]
- 7.Kuperwasser C, Hurlbut GD, Kittrell FS, Dickinson ES, Laucirica R, Medina D, Naber SP, and Jerry DJ. Development of spontaneous mammary tumors in BALB/c p53 heterozygous mice. A model for Li-Fraumeni syndrome. Am J Pathol. 157: 2151–2159. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morton D, Bailey KL, Stout CL, Weaver RJ, White KA, Lorenzen MJ, and Ball DJ. N-Methyl-N-Nitrosourea (MNU): A positive control chemical for p53+/- mouse carcinogenicity studies. Toxicol Pathol. 36: 926–931. 2008. [DOI] [PubMed] [Google Scholar]
- 9.Kelliher MA, Seldin DC, and Leder P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIalpha. EMBO J. 15: 5160–5166. 1996. [PMC free article] [PubMed] [Google Scholar]
- 10.Chervinsky DS, Lam DH, Zhao XF, Melman MP, and Aplan PD. Development and characterization of T cell leukemia cell lines established from SCL/LMO1 double transgenic mice. Leukemia. 15: 141–147. 2001. [DOI] [PubMed] [Google Scholar]
- 11.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr , Butel JS, and Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 356: 215–221. 1992. [DOI] [PubMed] [Google Scholar]
- 12.Harvey M, McArthur MJ, Montgomery CA, Jr , Bradley A, and Donehower LA. Genetic background alters the spectrum of tumors that develop in p53-deficient mice. FASEB J. 7: 938–943. 1993. [DOI] [PubMed] [Google Scholar]
- 13.Kim SU, Han YH, Lee TH, Hyun BH, Lee SH, Lee DS, and Yu DY. Effective production of microinjectable blastocysts for germ-line transmission of embryonic stem cells. Exp Anim. 53: 475–477. 2004. [DOI] [PubMed] [Google Scholar]
- 14.Knudson AG.Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 68: 820–823. 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, and Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 4: 1–7. 1994. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Tsukamoto T, Sakai H, Shirai N, Ohgaki H, Furihata C, Donehower LA, Yoshida K, and Tatematsu M. p53 knockout mice (-/-) are more susceptible than (+/-) or (+/+) mice to N-methyl-N-nitrosourea stomach carcinogenesis. Carcinogenesis. 21: 1891–1897. 2000. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, and Weinberg RA. Hallmarks of cancer: the next generation. Cell. 144: 646–674. 2011. [DOI] [PubMed] [Google Scholar]
- 18.Takaoka M, Sehata S, Maejima T, Imai T, Torii M, Satoh H, Toyosawa K, Tanakamaru ZY, Adachi T, Hisada S, Ueda M, Ogasawara H, Matsumoto M, Kobayashi K, Mutai M, and Usui T. Interlaboratory comparison of short-term carcinogenicity studies using CB6F1-rasH2 transgenic mice. Toxicol Pathol. 31: 191–199. 2003. [DOI] [PubMed] [Google Scholar]
- 19.Shirai N, Tsukamoto T, Yamamoto M, Iidaka T, Sakai H, Yanai T, Masegi T, Donehower LA, and Tatematsu M. Elevated susceptibility of the p53 knockout mouse esophagus to methyl-N-amylnitrosamine carcinogenesis. Carcinogenesis. 23: 1541–1547. 2002. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Werneck MB, Wilson BG, Kim HJ, Kluk MJ, Thom CS, Wischhusen JW, Evans JA, Jesneck JL, Nguyen P, Sansam CG, Cantor H, and Roberts CW. TCR-dependent transformation of mature memory phenotype T cells in mice. J Clin Invest. 121: 3834–3845. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenzelmann Broz D, and Attardi LD. In vivo analysis of p53 tumor suppressor function using genetically engineered mouse models. Carcinogenesis. 31: 1311–1318. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keskintepe L, Norris K, Pacholczyk G, Dederscheck SM, and Eroglu A. Derivation and comparison of C57BL/6 embryonic stem cells to a widely used 129 embryonic stem cell line. Transgenic Res. 16: 751–758. 2007. [DOI] [PubMed] [Google Scholar]
- 23.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, and Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 339: 819–823. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danforth DN.Genomic changes in normal breast tissue in women at normal risk or at high risk for breast cancer. Breast Cancer (Auckl). 10: 109–146. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narayan S, Jaiswal AS, Law BK, Kamal MA, Sharma AK, and Hromas RA. Interaction between APC and Fen1 during breast carcinogenesis. DNA Repair (Amst). 41: 54–62. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnenschein C, and Soto AM. Carcinogenesis explained within the context of a theory of organisms. Prog Biophys Mol Biol. 122: 70–76. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vousden KH, and Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2: 594–604. 2002. [DOI] [PubMed] [Google Scholar]
- 28.Vousden KH, and Prives C. Blinded by the light: the growing complexity of p53. Cell. 137: 413–431. 2009. [DOI] [PubMed] [Google Scholar]
- 29.Soussi T. p53 alterations in human cancer: more questions than answers. Oncogene. 26: 2145–2156. 2007. [DOI] [PubMed] [Google Scholar]
- 30.Ooms AH, Gadd S, Gerhard DS, Smith MA, Guidry Auvil JM, Meerzaman D, Chen QR, Hsu CH, Yan C, Nguyen C, Hu Y, Ma Y, Zong Z, Mungall AJ, Moore RA, Marra MA, Huff V, Dome JS, Chi YY, Tian J, Geller JI, Mullighan CG, Ma J, Wheeler DA, Hampton OA, Walz AL, van den Heuvel-Eibrink MM, de Krijger RR, Ross N, Gastier-Foster JM, and Perlman EJ. Significance of TP53 mutation in Wilms tumors with diffuse anaplasia: a report from the Children’s Oncology Group. Clin Cancer Res. 22: 5582–5591. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calabrese EJ, and Blain RB. The Single Exposure Carcinogen Database: assessing the circumstances under which a single exposure to a carcinogen can cause cancer. Toxicol Sci. 50: 169–185. [DOI] [PubMed] [Google Scholar]
- 32Cillo AR, Kürten CHL, Tabib T, Qi Z, and Vignali DAAJI. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. 52: 183–199.e189 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huo X, Li Z, Zhang S, Li C, Guo M, Lu J, Lv J, Du X, and Chen Z. Analysis of the expression level and methylation of tumor protein p53, phosphatase and tensin homolog and mutS homolog 2 in N-methyl-N-nitrosourea-induced thymic lymphoma in C57BL/6 mice. Oncol Lett. 14: 4339–4348. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang W, Ananthaswamy HN, Muller HK, Ouhtit A, Bolshakov S, Ullrich SE, El-Naggar AK, and Kripke ML. UV irradiation augments lymphoid malignancies in mice with one functional copy of wild-type p53. Proc Natl Acad Sci USA. 98: 9790–9795. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura D, Yoshimitsu M, Kuroki A, Hachiman M, and Arima NJEh. A new ATL xenograft model and evaluation of pyrrolidine dithiocarbamate as a potential ATL therapeutic agent. Exp Hematol. 43: 944–950. 2015. [DOI] [PubMed] [Google Scholar]
- 36.Sunaoshi M, Amasaki Y, Hirano-Sakairi S, Blyth BJ, Morioka T, Kaminishi M, Shang Y, Nishimura M, Shimada Y, Tachibana A, and Kakinuma S. The effect of age at exposure on the inactivating mechanisms and relative contributions of key tumor suppressor genes in radiation-induced mouse T-cell lymphomas. Mutat Res. 779: 58–67. 2015. [DOI] [PubMed] [Google Scholar]
- 37.Laur AM, Floch P, Chambonnier L, Benejat L, Korolik V, Giese A, Dubus P, Mégraud F, Bandeira A, and Lehours P. Regulatory T cells may participate in Helicobacter pylori persistence in gastric MALT lymphoma: lessons from an animal model. Oncotarget. 7: 3394–3402. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith GS, Walford RL, and Mickey MR. Lifespan and incidence of cancer and other diseases in selected long-lived inbred mice and their F 1 hybrids. J Natl Cancer Inst. 50: 1195–1213. 1973. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Xia Y, Feng LN, Chen JR, Li HM, Cui J, Cai QQ, Sim KS, Nairismägi ML, Laurensia Y, Meah WY, Liu WS, Guo YM, Chen LZ, Feng QS, Pang CP, Chen LJ, Chew SH, Ebstein RP, Foo JN, Liu J, Ha J, Khoo LP, Chin ST, Zeng YX, Aung T, Chowbay B, Diong CP, Zhang F, Liu YH, Tang T, Tao M, Quek R, Mohamad F, Tan SY, Teh BT, Ng SB, Chng WJ, Ong CK, Okada Y, Raychaudhuri S, Lim ST, Tan W, Peng RJ, Khor CC, and Bei JX. Genetic risk of extranodal natural killer T-cell lymphoma: a genome-wide association study. Lancet Oncol. 17: 1240–1247. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.