Abstract
Helicobacter pylori infections are responsible for tremendous morbidity and mortality worldwide, leading to efforts to eradicate the organism. However, the effectiveness of antimicrobial therapy has been undermined by the progressive development of antimicrobial resistance. Treatments and treatment guidelines have been based on traditional pairwise meta-analyses of randomized controlled trials. More recently, network meta-analyses have also been utilized in an attempt to provide useful information to the clinician regarding which therapies appear best and which to avoid as the least efficacious. However, both forms of meta-analysis have been undermined by the same problems including the poor quality of the clinical trials using unoptimized regimens and incomparable comparisons related to marked geographic and ethnic genotypic and phenotypic heterogeneity. In addition, the comparator regimens often consist of invalid strawman comparisons. New approaches concerning H. pylori treatment and analysis of therapies are needed. H. pylori therapies should be based on antimicrobial stewardship, as in other infectious diseases. This approach requires the use of only optimized therapies proven to be reliably highly effective in the local population (e.g., a cure rate of ≥90%) for both the study and the comparator regimens. Meta-analyses should be restricted to regimens that meet these criteria and must take into account the presence of marked geographic and host genetic and phenotypic heterogeneity. In addition, to provide clinically relevant results, treatment outcomes should focus on, and present, actual cure rates in addition to odd ratios.
Keywords: Helicobacter pylori; treatment; antimicrobial therapy, cure rates; randomized control trial; meta-analysis; network meta-analysis; heterogeneity; strawman comparisons
Introduction
Helicobacter pylori (H. pylori) infection is of worldwide concern as it has been recognized as the main cause of gastritis, peptic ulcer disease, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer. In 2015, H. pylori gastritis was formally recognized as an infectious disease leading to the recommendation that all patients should receive treatment.1, 2 In 2020 the Taipei Global consensus emphasized the role of H. pylori eradication (cure) in accomplishing the goal of reducing or eliminating deaths from gastric cancer.3 Before H. pylori gastritis was defined as an infectious disease, cure was called “eradication”. An effectively treated infectious disease is defined as cured, whereas elimination of the organism from a region or country is defined as eradicated (i.e., the patient’s tuberculosis was cured or tuberculosis has been eradicated from Japan) 4.
Cure of an H. pylori infection requires effective treatment. However, despite more than a quarter of a century and many consensus conferences providing updated treatment recommendations, overall, treatment success remains poor compared to other infectious diseases 5. A wide variety of regimens contain an antisecretory drug and most often combinations of 3 or 4 drugs containing amoxicillin, clarithromycin, metronidazole, a fluoroquinolone, rifabutin, bismuth, or tetracycline. An attempt to overcome resistance led to the introduction of 4 drug therapies containing a PPI, amoxicillin, clarithromycin, and metronidazole called sequential, concomitant, hybrid, and reverse hybrid therapy. Each of these combinations contains at least one unnecessary antibiotic resulting in the annual use of thousands of kilograms of unneeded antibiotics/million treatments 6, 7. The recent introduction of the potassium competing acid blockers (P-CAB), (e.g., vonoprazan) has rekindled interest in therapies containing amoxicillin, especially dual therapy 8.
The realization that H. pylori gastritis was fundamentally an infectious rather than a typical gastroenterology disease, led to the realization that the current approach to development and assessment of therapy for H. pylori deviates from other infectious diseases which utilize the principles of antimicrobial stewardship focusing on optimization of therapy to reliably achieve high cure rates and prevent resistance to the antimicrobials used 9-11. Another difference is that with infectious diseases, the focus is on absolute cure rates rather than relative differences in the outcome of randomized controlled trials (RCTs) or meta-analyses of what are primarily overall poorly performing empiric therapies 7-9. This is also reflected in guidelines for H. pylori therapy which largely rely on analyses of RCTs and relevant pairwise meta-analyses of unoptimized therapies and are largely independent of the dominant effects of important modifiers of the outcome, especially antimicrobial resistance.
Pairwise (aka, classical) meta-analyses are particularly useful for identifying and quantifying outcome modifiers for a therapy but are limited by the large number of available therapeutic interventions. Network meta-analysis (NWM), in contrast, is an evidence synthesis tool for comparing RCTs with multiple treatments 11-13 that can incorporate both direct and indirect evidence in a collection of RCTs and provide information concerning the relative effects of all therapeutic interventions competing for a similar outcome. Theoretically, NWM would be extremely useful for assessing the multitude of H. pylori therapies and assist in guideline development and clinical decision-making. The aim of this commentary is to critically examine the approach to the development and analysis of H. pylori therapy with the goal of improving clinical decision-making and future treatment guidelines.
How H. pylori therapy came to differ from other infectious diseases
Traditionally antibiotics are developed, tested, and optimized for the treatment of susceptible infections. As such, by the time the antibiotic is submitted for FDA approval, the details of how to reliably achieve high cure rates are both known and utilized. After approval, new uses and approaches to therapy continue to be explored, and incremental improvement in optimization and utilization of the antibiotic is accomplished and incorporated. Many H. pylori therapies were developed by physicians using available drugs, and the regimens were never optimized nor submitted to regulatory bodies for approval. The impetus for experimentation has often been a response to a decline in the effectiveness of the currently preferred therapy. Development usually consisted of administering combinations of antibiotics and antisecretory drugs based on the physician’s ideas and biases regarding therapy details (e.g., drugs, doses, durations, etc.). The new regimen was then compared to a locally available therapy that had become increasingly ineffective, usually because of increased antimicrobial resistance. The results are then published while few, if any, systematic attempts were made to understand the critical factors or to improve (optimize) the new therapies or to understand their weaknesses. This approach is exemplified by the development of sequential therapy, which involved thousands of patients. Sequential therapy was repeatedly compared to the combination it was designed to replace (i.e., clarithromycin triple therapy) 12. Continued success depended on use in a region with a high prevalence of clarithromycin resistance and a low prevalence of metronidazole resistance. The early studies resulted in what initially appeared to be a spectacular success but were followed by equally spectacular failures when the regimen was used in a geographic area with different resistance patterns 13. Importantly, hundreds if not thousands of patients received the locally ineffective and poorly performing clarithromycin triple therapy as controls. The focus on comparative studies using unoptimized therapies against proven locally poorly performing “strawman” therapies (see below) to show “superiority” rather than investigating effects of resistance, dosing, duration, etc. resulted in the popularization of regimens containing unneeded antibiotics such as concomitant, hybrid, and reverse hybrid therapies. The effectiveness of these multi-antibiotic-containing regimens is primarily based on the hope that the infection will be susceptible to at least one of the antibiotics administered.
One of the impediments to the efficient development of therapy is that publication in medical journals generally requires a comparator other than the theoretical optimal of 100% cured. This has limited the use of pilot studies to test ideas and instead requires larger sample sizes because of the requirement that the study design includes a detailed analysis of the sample size calculation based on expected cure rates. The focus on comparative difference rather than actual cure rates has encouraged the use of strawman comparators (see below). At the same time, there has been no requirement to provide details regarding the factors known to significantly effect the outcome, especially the prevalence of resistance. This led to H. pylori being unique in that the anticipated cure rate with the comparator, and often the study drug, were often below what would generally be considered acceptable. Similarly, the majority of studies chosen for meta-analyses have cure rates for both therapies that are unacceptably low. This problem has been obscured by the focus on the comparative difference being assessed as odd ratios rather than focus on the actual cure rates.
Strawman comparative studies
A strawman is defined as an intentionally misrepresented proposition that is set up because it is easier to defeat than an opponent's real argument (Oxford) or as a weak or imaginary opposition (such as an argument or adversary) set up only to be easily confuted (Merriam-Webster) 14. The development of sequential therapy provides an excellent example of how this approach resulted in strawman comparisons. By the year 2000 comparisons of data from more than 53,000 patients showed the cure rate of standard triple therapy had declined to the point where it was 80% or less and that new therapies were required 15, 16. At that time in Italy the average cure rate was approximately 75% and continued to drop over the next decade 17. The presence of a strawman comparator can usually be identified by a review of the sample size estimation showing that treatment success was essentially guaranteed. An example from a sequential trial is: “it was chosen to detect a difference of 9% in the eradication rate between the standard 7-day (assumed to have an eradication rate of 80%) and the new 10-day (estimated to have an eradication rate of 89%) regimen, with a power of 0.90 and a significance level of 0.01 (P<0.010, two-sided) 18. The actual cure rates from this trial were (intention-to-treat: 92% vs. 74%, P < 0.0001 and per-protocol: 95% vs. 77%, P < 0.0001). The ethics of strawman comparisons has been questioned based on the fact that subjects were not given truly informed consent (i.e., not informed that one of the therapies was already proven to produce clinically unacceptable results) 19. The main issue with strawman comparisons is the lack of clinical equipoise (i.e., there is good reason to doubt the assumption that there is not one 'better' intervention present) 20. Because strawman comparisons are biased, their inclusion into meta-analyses results in biased results.
Comparative studies, meta-analyses and guideline development in infectious diseases.
In infectious diseases, meta-analyses are primarily used to assess and optimize the details of disease and therapeutic strategies rather than to compare therapies. Some examples include investigation of the determinants of inappropriate empirical antibiotic treatment21, examination of treatment outcomes for problems such as multidrug-resistant Mycobacterium tuberculosis infections22, exploring the difference in favorable clinical outcomes with short-course antibiotic treatment of community-acquired pneumonia 23, asking whether carbapenems and tigecycline provide similar outcomes for the treatment of complicated intra-abdominal infections 24. Infectious disease guidelines are also practical and recognize the need to be locally based. For example, guidelines from Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia (CAP) in Adults include: 1. Locally adapted guidelines should be implemented to improve the process of care variables and relevant clinical outcomes; (Strong recommendation; level I evidence.) 2. CAP guidelines should address a comprehensive set of elements in the process of care rather than a single element in isolation; (Strong recommendation; level III evidence.) 3. Development of local CAP guidelines should be directed toward improvement in specific and clinically relevant outcomes; (Moderate recommendation; level III evidence.)”. Importantly, they note that “A major goal of therapy is the eradication of the infecting organism, with the resultant resolution of clinical disease. As such, antimicrobials are a mainstay of treatment. Appropriate drug selection is dependent on the causative pathogen and its antibiotic susceptibility. Other factors for consideration of specific antimicrobials include pharmacokinetics/pharmacodynamics, compliance, safety, and cost.”25
Comparative trials in infectious diseases are infrequently needed because the recommended therapies have been optimized to reliably achieve high cure rates. Most comparative trials are done in relation to FDA approval and are either non-inferiority or superiority trials. The published FDA guidance for H. pylori is from 2009 and still lists a requirement for the presence of a peptic ulcer. The most relevant infectious disease guidance is related to uncomplicated urinary tract infections 26. That guideline states: “In general, sponsors should use an active comparator that is considered standard of care for treatment of uncomplicated urinary tract infection (uUTI) in the United States for this indication. The active comparator generally should be approved by the FDA for the treatment of uUTI. When evaluating the current standard of care, we consider recommendations by authoritative scientific bodies (e.g., Infectious Diseases Society of America) based on clinical evidence and other reliable information that reflects current clinical practice. For a non-inferiority trial, it is important that the analysis population includes only patients for whom the bacterial pathogen is fully susceptible to the active control drug on in vitro susceptibility testing 27.
How to choose a reliably highly effective H. pylori therapy?
The ultimate key to an excellent and valid result is to account for as many effect modifiers (defined as variables or characteristics that modify the observed relative effects) as possible 28. Modifiers and confounders that can affect the outcome of H. pylori therapy include: a) factors related directly to the regimens such as: doses used, duration of therapy, formulations, administration in relation to meals, the potency of the antisecretory adjuvant, etc., b) host factors, especially those that affect the metabolism of the administered drugs (e.g., CYP 2C19 polymorphisms) and c) factors that affect the host’s ability to secret acid (e.g., size, race, parietal cell mass, presence and severity/extent of corpus gastritis, etc.). One of the most important factors is the presence of antimicrobial resistance. The effect of these different factors can be assessed globally by the cure rate achieved in adherent patients with susceptible infections, which are the criteria used to define when a regimen has been optimized (i.e., to reliably achieve a cure rate of ≥95%). The tremendous variability among H. pylori and humans dictates that therapies be optimized for each target population. In practice, whether the optimization has been done or is necessary can be determined by the cure rate with the recommended regimen (i.e., does it achieve ≥95% cure rates). In practice, the only consistent feature of most therapies is their name (e.g., 7-day clarithromycin triple therapy) as the actual constitutions often vary significantly (e.g., with 40 mg of pantoprazole or of rabeprazole or 9 vs. 72 mg omeprazole equivalent PPI). All of the details of a highly successful therapy may be important, ranging from the manufacturer of the drugs to the duration of therapy. Among populations, the greatest differences are between Western and Eastern Asian populations. In general, Eastern Asian’s have a higher proportion of slow PPI metabolizers, smaller parietal cells masses and a higher frequency of corpus gastritis which together makes antimicrobial therapy more effective 29-32.
The importance of clinical relevance
Meta-analyses focus on differences often assessed as odd ratios, whereas clinicians are most interested in outcomes. The important outcome clinically is the cure rate which may, in turn, be influenced by a variety of factors, including adherence, duration, frequency of drug administration, antimicrobial resistance, etc. This concept of clinical relevance is often considered under the heading of minimal clinically important difference 33. The first step is to define whether the result (i.e., cure rate) is clinically acceptable. The second is to define what difference matters in clinical care. Most would agree that a cure rate below 80% is unacceptable unless there are extenuating circumstances (e.g., presence of poly-antibiotic resistance). As our understanding of how to achieve high cure rates, the minimum cure rate has risen. For example, the cut-off for an acceptable therapy of ≥90% with cure rates between 85% to 89% being denoted as borderline acceptable, ≥90% to<95% as good, and ≥95% as excellent 7. The ultimate goal is to be able to reliably achieve a cure rate ≥95% 6, 7.
Pairwise meta-analyses and network meta-analyses
For valid pairwise meta-analyses and network meta-analyses (NWM) some rules that have to be considered are suggested in Tables 1 and 2. Traditional pairwise meta-analyses and NWM are designed to analyze H. pylori treatment trials based on treatment outcome comparisons. In this process, the authors select the criteria for the therapies to be compared. However, in H. pylori treatment trials these criteria are not uniform, which has allowed authors to choose trials with clinically unacceptable outcomes as well as those using strawman comparators. As illustrated below, this enables meta-analyses to achieve impressive odds ratios despite having clinically unacceptable cure rates (i.e., results with little, if any, clinical relevance in terms of assisting the clinician in choosing which therapy is best for their patients) 34, 35. Solutions to this conundrum include restricting studies to those with prespecified cure rates above a cut-off (e.g., 80% or 85%) and emphasizing the cure rates in the tables, results, and discussion. In the past, many studies only showed the actual cure rates in supplementary tables 34, whereas emphasizing the cure rates throughout the manuscript provides details about clinical relevance 36.
Table 1.
Suggested rules for a valid H. pylori pairwise or network meta-analyses
|
|
|
|
|
|
Table 2.
Suggested details to be reported for each therapy used in a meta-analysis.
|
|
|
|
|
|
|
A traditional pairwise meta-analysis is often considered as a Holy Grail that provides reliable comparisons and recommendations regarding the use of different H. pylori therapies. However, since the earliest day of meta-analysis, critics have been concerned with making incomparable comparisons leading to the development of methods to assess statistical heterogeneity and account for effect modifiers 28, 37-39. In reality, meta-analyses are often unable to provide clinically meaningful analyses of the majority of current and past treatment trials in part because few trials provide data regarding critically important outcome modifiers. Meta-analyses provide no tools to produce meaningful results from arbitrary empiric therapies with strawman comparators. Most meta-analyses are a holdover or relic from earlier times when H. pylori was considered a typical Gastroenterology rather than an Infectious Disease. Gastroenterology diseases differ in many fundamental ways from the typical infectious disease. The major differences are that a cure (100% success) is possible in infectious disease and there is no placebo response to therapy. The presence of a placebo response requires the use of a comparator (i.e., a placebo and/or another therapy whose outcome is proven to be clearly and reliably superior to placebo). An example in Gastroenterology is irritable bowel syndrome, where a placebo and an active comparator are often required. The placebo is needed to confirm that the active comparator is superior to the placebo in the population tested. In contrast, with H. pylori the lack of a placebo response and the ability to cure most or all of the infected individuals permits the comparator even to be the theoretical and sought after, cure rate of 100%.
H. pylori was initially saddled with randomized controlled trials and relevant pairwise meta-analyses because the treatment outcome was assessed with regard to the healing of peptic ulcers. Peptic ulcer healing is associated with a strong placebo response as the ulcers heal and recur spontaneously. Initially, the focus on peptic ulcers rather than cure of the infection required a comparator (e.g., a histamine 2 receptor antagonist or PPI) to judge whether treating H. pylori was beneficial 40, 41. The focus on ulcers was reflected in the low cure rates of the antimicrobial regimens approved for human use 40, 42. For example, the US pivotal trial of rabeprazole-clarithromycin triple therapy did not evaluate the optimal treatment duration of 14 days and instead tested for 3-, 7- and 10-days. Cure rates of 27%, 77% and 78%, respectively, were achieved 42. The comparator, 10-day omeprazole triple therapy, achieved a cure rate of 73%. The duration of 7 days was chosen because it had the advantage of being shorter than its competitors. Clearly, by 2003, the date of the study, both 7 and 10-day clarithromycin triple therapies were no longer effective in the US. In that study, approximately 10% of strains were clarithromycin resistant. Similarly, because cure rates were not the primary outcome measure, the 3-in-1 bismuth quadruple therapy, Pylera®, was also able to choose a 10-day duration for marketing advantage over the 14-day duration, Helidic®.
In 2012, RedHill Biopharm presented a white paper to the FDA requesting that the FDA reconsider the mandate that subjects for H. pylori testing be required to have peptic ulcer disease. This request was based on the concept that H. pylori was a serious infectious disease responsible for atrophic gastritis, peptic ulcer disease, and gastric cancer and that its treatment was an end unto itself. Their clinical trials on rifabutin triple therapy were approved based only on the presence of an H. pylori infection. The comparator was high dose PPI and amoxicillin which were also components of the main therapy that allowed examination regarding whether rifabutin played a critical role in treatment outcome). Most recently, the FDA outcome assessment for vonoprazan-containing therapies was defined as the effectiveness in susceptible infections 43. Comparators included the dual therapy vonoprazan plus amoxicillin with or without clarithromycin and a comparatively low dose PPI clarithromycin, amoxicillin dual therapy. The FDA also does not require that the therapies submitted for approval have been optimized. This is an important weakness as it allowed less than optimal regimens to become dominant and remains a major barrier to ensuring that available therapies are universally reliably highly effective. In some countries, only the approved combinations are available or can be prescribed (e.g., Pylera® in Europe). This is another residual effect of the period when H. pylori infection was considered a gastrointestinal disease rather than the gastrointestinal manifestation of an infection.
Although traditional pair-wise meta-analyses form the basis for most guidelines, the method does not allow comparative effectiveness all of the available therapeutic interventions competing for a similar result. This possibility is provided by NWM, which is an evidence synthesis tool for comparing RCTs with multiple treatments that incorporates both direct and indirect evidence from a collection of RCTs 10-12. This approach provides information concerning the relative effects of all therapeutic interventions competing for a similar result. In addition, NWM, through the construction of surfaces under cumulative ranking (SUCRA) - based efficacy ranking league matrices, allow the study of the comparative efficacies of all the regimens included in the NWM. In this way, information can be extracted concerning the best and least efficacious treatments. The two major assumptions of a network meta-analysis are: transitivity and consistency. Transitivity means no systematic differences between the available comparisons other than the treatments being compared 37. Consistency is the statistical manifestation of transitivity to the data and means that both direct and indirect evidence agree 44. The results of NWM have not yet been involved in the development of guidelines concerning H. pylori treatment.
Recently, two robust NWM were published in the top-tier Gastroenterology journals, Gut and Gastroenterology 32, 33 and were highlighted as must-read articles. The first study 32 published in 2017 presented “evidence-based hierarchy for the effectiveness of 17 eradication regimens according to clarithromycin resistant rate. The findings were that overall sequential therapy for 14-days presented the highest eradication rates (OR=3.74, 95% CI = 2.37 to 5.96) and that sequential (OR=6.53, 95% CI = 3.23 to 13.63) and hybrid therapy for 10 or more days (OR=2.85, 95% CI = 1.58 to 5.37), appeared the most effective therapies in areas with high and low clarithromycin resistance respectively”. The period covered was from 2005 to 2016 and included data from 117 trials, with 32, 9853 patients from 30 different countries and 17 different therapies. All studies were compared with 7-day clarithromycin triple therapy. The actual cure rates in low and high clarithromycin resistance countries are shown in Figure 1 and Supplementary Tables 1, 2. Among the low clarithromycin resistance regions, sequential therapy ≤10-days achieved borderline acceptable cure rates (i.e., 86%). The 14-day sequential and the ≤10-day hybrid therapy achieved good results (e.g., 90.7% and 92% cure rates). Importantly, despite the high odds rations, the majority of studies produced unacceptably low cure rates; less than 11% achieved a cure rate of 90%, mostly from Taiwan or Italy, emphasizing the importance of geography as a variable. Detailed data for the 7-day clarithromycin comparator were not provided.
Figure 1.
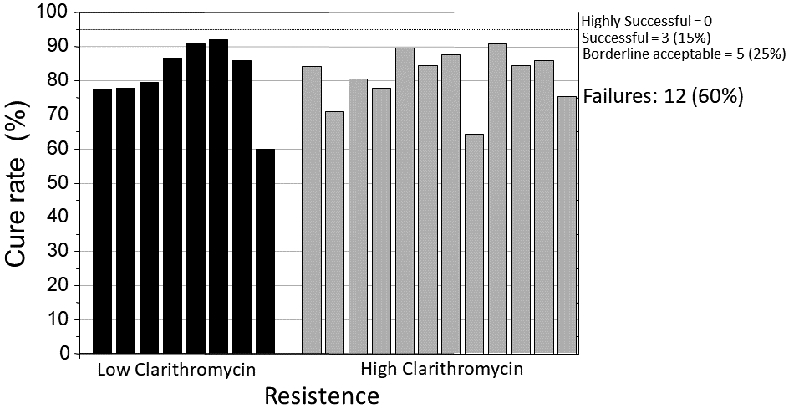
Cure rates according to clarithromycin resistance (data from Ref. 32 with permission).
The second NWM was published in 202135 and consisted of 68 randomized trials with at least 7-day treatment duration in all arms; 1 arm included patients allocated to clarithromycin-containing triple treatment (triple therapy) and also contained at least 50 patients per arm. In this NWM there were 92 paired comparisons with a total of 22,975 patients. Eight first-line therapies were compared against clarithromycin triple therapy. The therapies studied were: an amoxicillin dual therapy, bismuth quadruple therapy, sequential therapy, levofloxacin triple therapy, non-bismuth quadruple therapy (concomitant therapy), reverse hybrid therapy and vonoprazan triple therapy. The data were analyzed overall and by region classified as West, East Asia, or West Asia. The conclusion was among first-line empiric treatments of H pylori infection, vonoprazan triple therapy and reverse hybrid therapy achieved cure rates of >90%; levofloxacin triple therapy achieved the highest cure rates (e.g., >88.5%) in Western countries. Standard triple therapy was the least efficacious regimen (Figure 2). However, the data for vonoprazan triple therapy came from only 3 studies from Japan with an average cure rate of 91.3% (95% CI 88.5 - 93.8) and the data regarding reverse hybrid therapy data was from only one study (Taiwan) with a cure rate of 93.6% (95% CI 90.4 - 96.8). The levofloxacin data came from 11 studies 5 (45%) of which had cure rates below 80%; none had a cure rate of 90% or greater. Only 6 had a cure rate >85% and only achieved borderline acceptable results (Figure 3). The mean (95% CI) 7-day clarithromycin comparator cure rate was 75.7 (74.9–76.4) (Figure 4) and in 76.4% of the studies the cure rate was below 80%.
Figure 2.
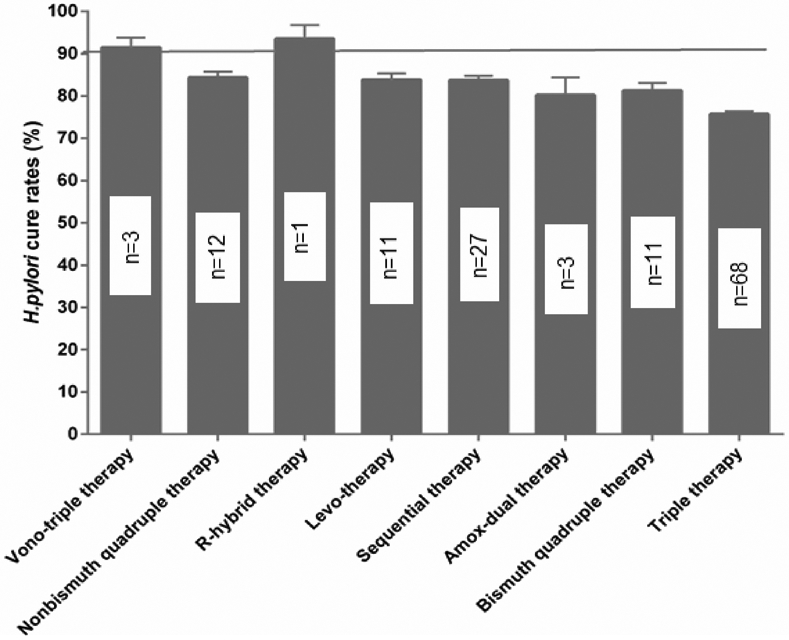
Cure rates according to regimen; n=number of RCTs (data from Ref. 33 with permission).
Figure 3.
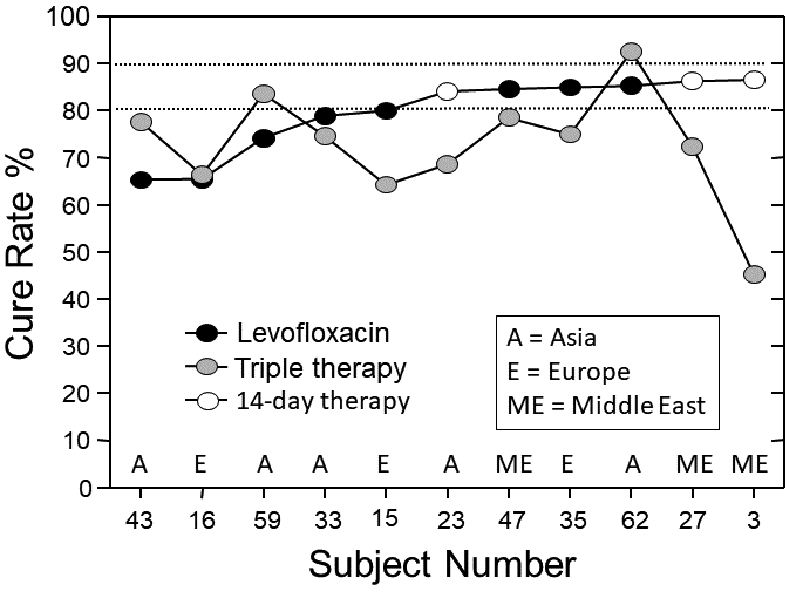
Levofloxacin cure rates, in comparison to triple therapy (data from Ref. 33 with permission).
Figure 4.
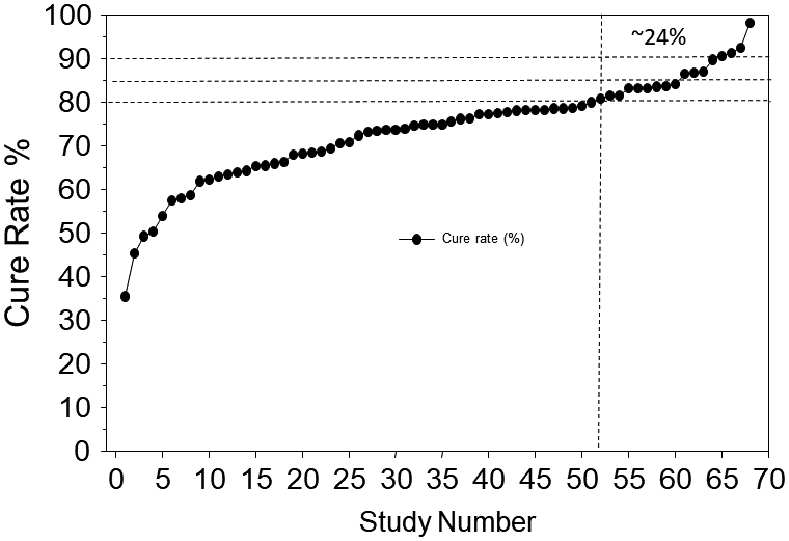
Triple therapy cure rates in RCTs involved, (data from Ref. 33, with premission).
Although vonoprazan triple therapy and hybrid therapy ranked highest, both contain at least one drug not involved in treatment success and serve only to contribute to global antimicrobial resistance 7, 45. In addition, to be limited to Japan, the cure rates with vonoprazan triple therapy have been falling as clarithromycin resistance has continued to increase. Furthermore, the high success rate with vonoprazan triple therapy was also not confirmed in the US/European trial, suggesting a geographic effect 43. As noted above, reverse hybrid also included an unnecessary antibiotic, and the data and recommendations are based on only a single study. Lastly, the levofloxacin triple therapy used was not optimized, and importantly, levofloxacin has been cited by the US and European regulatory agencies as not to be used unless there are no other options because of serious long term complications 46.
The goal of a NWM is to enhance the decision-making process regarding alternative treatments for a certain disorder in a target population by combining direct and indirect evidence from randomized clinical trials. In both published NWMs,32,33 the majority of the comparator studies used were strawman comparators, and from this point of view, it can be said that they did not provide reliable information regarding global and regional data concerning H. pylori therapies. However, the detailed NWM data, regarding the best and worse available treatment performances, is useful in current clinical decision-making both worldwide and regionally and is expected to be considered in future guidelines.
Geographic bias
Detailed analysis of these two network meta-analyses also illustrates the problem of geographic bias. All therapy is local, and it follows that the dictum to “only use what is highly effective locally” is both sound and good advice. The presence of a geographic effect on outcome has long been recognized but has infrequently been discussed or written about. 47, 48 It has long been known that H. pylori genotypes vary geographically as does the expression of H. pylori-related diseases 49. Similarly, antimicrobial resistance, a major effector of cure rates, varies greatly both within and between geographic regions 50. Many factors that directly affect outcome of antimicrobial therapy other than resistance vary geographically, such as access to individual drugs and the quality of the drugs available both in terms of manufacturer and storage as many drugs such as PPIs are adversely affected by heat (discussed in detail in ref. 48). PPIs also different greatly in relative potency and in relation to the effect of host drug metabolizing enzymes both of which an markedly influence outcome. For example, 40 mg of pantoprazole = an omeprazole equivalent of 9 mg, whereas 40 mg of rabeprazole = 72 mg omeprazole equivalent 51. As such there is little reason to expect that meta-analyses of therapies based on trials with the same name that the actual constituents details of therapy are in fact similar. Host genotype (e.g., CYP2C19) and host phenotype (e.g., BMI, proportion with corpus gastritis) also affect outcome 29-32. For example, the differences in the prevalence of drug metabolizing enzymes, parietal cell mass, presence of corpus gastritis, body mass, etc., all have significant effects making it difficult to obtain reliable comparisons of therapies across geographic areas. An example is the high cure rates achieved with dual PPI amoxicillin therapy in Japan and some Asian countries that are rarely obtained in western countries 52-56. The onus to explain the outcome is on both the authors of an original of an optimized therapy that failed to achieve high cure results as well as on the authors of any subsequent meta-analysis that decided to include the study in their analyses.
Discussion
Although H. pylori is a worldwide concern affecting billions of people, the ideal treatment regimen(s) remain unclear despite the 30 years of therapeutic experience worldwide. By the year 2000 it was clear that the presence of resistance had greatly reduced the effectiveness of clarithromycin-containing therapies 15, 16. The prevalence of resistance to clarithromycin, metronidazole, and levofloxacin has continued to increase to the point that recent consensus statements have suggested they should no longer be used empirically 2, 3. RCTs and pairwise classic meta-analyses have been increasingly less useful in assisting health care providers in choosing the best antimicrobial therapy for their H. pylori-infected patients. Increasingly their usefulness has been compromised by technical flaws such as a) comparing unoptimized empiric therapies, b) use of invalid strawmen comparators, and c) their focus on odd ratios rather than actual cure rates. The validity of any method of comparison is based on the comparability of the groups. The major effectors in H. pylori therapy include whether the regimens are optimized to reliably achieve high cure rates (i.e., reliably ≥95% or if impossible, at least ≥90%) in an adherent patient with susceptible infections. Comparability extends to the details of the regimens critical to outcome such as a) doses, durations, frequency of administration, the relative potency of the antisecretory drug, etc), b) the local prevalence of antimicrobial resistance to the antibiotics utilized, c) geographic differences, and d) host genotypes and phenotypes in relation to drug metabolism and acid secretion.
Although theoretically, NWM should provide useful information to the clinician regarding which therapies appear best and to avoid the least efficacious treatment, they too are affected by the same problems that affect classical paired comparisons, including the poor quality of the clinical trials available for inclusion as well as the heterogeneity introduced by differences in resistance and host characteristics. Under these circumstances, new approaches concerning H. pylori treatment and analysis of therapies are needed. Towards this end, there are views supporting the notion that it is time to transition from the trial and error methodology and utilize antimicrobial stewardship, as in other infectious diseases 7. This approach requires therapy to be restricted to optimized therapies proven to be reliably highly effective in the local population (e.g., cure rate of ≥95%). Comparison should be restricted to regimens that meet strict criteria (e.g., Tables 1 and 2).
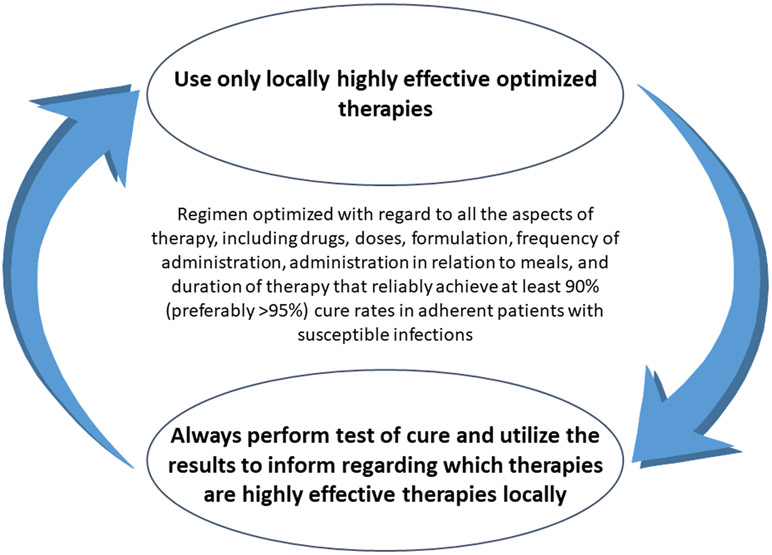
It is apparent that the current guidelines for H. pylori therapy and the approach to therapy using trial and error have failed. All highly effective antimicrobial therapy is susceptibility-based. Whether a regimen is highly effective locally can be determined either by susceptibility testing, by local clinical experience, or both 6 6, 8. The failure to utilize local clinical experience (e.g., test-of-cure data) to guide therapy (Figure 5) has resulted in empiric therapy that is most often poorly effective4, 6. Until recently, in the US clinicians were forced to rely on empiric therapy. However, lately most of the major clinical laboratories now provide culture and susceptibility testing and molecular testing is also available using stools that obviates the need for endoscopy. 57 The time for changing guidelines and practices is now.
Figure 5.
Schema to ensure that only highly effective therapies are used.
Supplementary Material
Bullet points.
Treatments and treatment guidelines have been based on traditional pair-wise meta-analyses of randomized controlled trials. More recently, network meta-analyses have also been utilized in an attempt to provide useful information to the clinician regarding which therapies appear best and which to avoid as the least efficacious.
Both traditional and network meta-analyses of H. pylori therapy have been undermined by the same problems including the poor quality of the clinical trials using unoptimized regimens and incomparable comparisons related to marked geographic and ethnic genotypic and phenotypic heterogeneity.
In order to provide meaningful data to assist clinicians to successful treat patients, H. pylori meta-analyses should be restricted to regimens that use only optimized therapies proven to be reliably highly effective in the local population (e.g., cure rate of ≥90%) for both the study and the comparator regimens.
To provide clinically relevant results concerning treatment outcomes, the analyses should focus on, and present, actual cure rates in addition to odd ratios.
Aknowledgement:
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Funding:
Dr Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center. Dr. Hernaez is supported by the Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413), Michael E. DeBakey VA Medical Center, Houston, TX.
Abbreviations used in this paper:
- NWM
network meta-analysis
- OR
odds ratio
- PPI
proton pump inhibitor
- RCT
randomized controlled trial
- R-hybrid therapy
reverse hybrid therapy
- SUCRA
surface under the cumulative ranking curve.
Footnotes
Competing interests: The author has made the following disclosures: Dr Graham is a consultant for RedHill Biopharma for antimicrobial therapies for Crohn’s disease, Phathom Pharmaceuticals regarding novel H. pylori therapies, and Otsuka Japan with regard to novel breath tests. Drs. Hernaez and Rokkas have nothing to declare.
References
- 1.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Kao JY, Kanwal F, et al. Houston Consensus Conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol 2018;16:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liou JM, Malfertheiner P, Lee YC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut 2020;69:2093–2112. [DOI] [PubMed] [Google Scholar]
- 4.Dowdle WR. The principles of disease elimination and eradication. Volume 48(SU01), 1999:23–26. [PMC free article] [PubMed] [Google Scholar]
- 5.Nyssen OP, Bordin D, Tepes B, et al. European Registry on Helicobacter pylori management (Hp-EuReg): patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2021;70:40–54. [DOI] [PubMed] [Google Scholar]
- 6.Graham DY, Liou JM. Primer for development of guidelines for Helicobacter pylori therapy using antibiotic stewardship. Clin.Gastroenterol.Hepatol. 2021;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham DY. Transitioning of Helicobacter pylori therapy from trial and error to antimicrobial stewardship. Antibiotics (Basel) 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut 2020;69:1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyar OJ, Huttner B, Schouten J, et al. What is antimicrobial stewardship? Clin Microbiol. Infect 2017;23:793–798. [DOI] [PubMed] [Google Scholar]
- 10.Core elements of antibiotic stewardship. Centers for disease control and prevention, 2019. [Google Scholar]
- 11.World Health O. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: a WHO practical toolkit. Geneva: World Health Organization, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatta L, Vakil N, Leandro G, et al. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am. J. Gastroenterol 2009;104:3069–3079. [DOI] [PubMed] [Google Scholar]
- 13.Gatta L, Vakil N, Vaira D, et al. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ 2013;347:f4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straw man. Volume 2021: Merriam-Webster.com Dictionary. [Google Scholar]
- 15.Laheij RJ, Rossum LG, Jansen JB, et al. Evaluation of treatment regimens to cure Helicobacter pylori infection- a meta-analysis. Aliment. Pharmacol. Ther 1999;13:857–864. [DOI] [PubMed] [Google Scholar]
- 16.Janssen MJ, Van Oijen AH, Verbeek AL, et al. A systematic comparison of triple therapies for treatment of Helicobacter pylori infection with proton pump inhibitor/ ranitidine bismuth citrate plus clarithromycin and either amoxicillin or a nitroimidazole. Aliment. Pharmacol. Ther 2001;15:613–624. [DOI] [PubMed] [Google Scholar]
- 17.Tursi A, Elisei W, Giorgetti G, et al. Decreasing efficacy of the standard seven-day triple therapy containing amoxycillin and clarithromycin in curing Helicobacter pylori infection in clinical setting in Italy: a 10-year follow-up study. Panminerva medica 2014;56:57–61. [PubMed] [Google Scholar]
- 18.Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment. Pharmacol. Ther 2003;17:719–726. [DOI] [PubMed] [Google Scholar]
- 19.Graham DY. Helicobacter pylori eradication therapy research: Ethical issues and description of results. Clin Gastroenterol Hepatol 2010;8:1032–1036. [DOI] [PubMed] [Google Scholar]
- 20.London AJ. Equipoise in Research: Integrating Ethics and Science in Human Research. JAMA 2017;317:525–526. [DOI] [PubMed] [Google Scholar]
- 21.Carrara E, Pfeffer I, Zusman O, et al. Determinants of inappropriate empirical antibiotic treatment: systematic review and meta-analysis. International Journal of Antimicrobial Agents 2018;51:548–553. [DOI] [PubMed] [Google Scholar]
- 22.Johnston JC, Shahidi NC, Sadatsafavi M, et al. Treatment Outcomes of Multidrug-Resistant Tuberculosis: A Systematic Review and Meta-Analysis. PLOS ONE 2009;4:e6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tansarli GS, Mylonakis E. Systematic Review and Meta-analysis of the Efficacy of Short-Course Antibiotic Treatments for Community-Acquired Pneumonia in Adults. Antimicrobial Agents and Chemotherapy 2018;62:e00635–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Liang X, Jiang J, et al. Carbapenems vs tigecycline for the treatment of complicated intra-abdominal infections: A Bayesian network meta-analysis of randomized clinical trials. Medicine 2019;98:e17436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clinical Infectious Diseases 2007;44:S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagenlehner FME, Bjerklund Johansen TE, Cai T, et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nature Reviews Urology 2020;17:586–600. [DOI] [PubMed] [Google Scholar]
- 27.Uncomplicated Urinary Tract Infections: Developing Drugs for Treatment Guidance for Industry. Silver Spring, MD 2: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration, 2019:14. [Google Scholar]
- 28.Efthimiou O, Debray TPA, van Valkenhoef G, et al. GetReal in network meta-analysis: a review of the methodology. Research Synthesis Methods 2016;7:236–263. [DOI] [PubMed] [Google Scholar]
- 29.Morino Y, Sugimoto M, Nagata N, et al. Influence of Cytochrome P450 2C19 Genotype on Helicobacter pylori Proton Pump Inhibitor-Amoxicillin-Clarithromycin Eradication Therapy: A Meta-Analysis. Frontiers in Pharmacology 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugimoto M, Furuta T, Shirai N, et al. Treatment strategy to eradicate Helicobacter pylori infection: impact of pharmacogenomics-based acid inhibition regimen and alternative antibiotics. Expert Opinion on Pharmacotherapy 2007;8:2701–2717. [DOI] [PubMed] [Google Scholar]
- 31.Abdullahi M, Annibale B, Capoccia D, et al. The Eradication of Helicobacter pylori is Affected by Body Mass Index (BMI). Obesity Surgery 2008;18:1450–1454. [DOI] [PubMed] [Google Scholar]
- 32.Eto H, Suzuki S, Kusano C, et al. Impact of body size on first-line Helicobacter pylori eradication success using vonoprazan and amoxicillin dual therapy. Helicobacter 2021;26:e12788. [DOI] [PubMed] [Google Scholar]
- 33.McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA 2014;312:1342–3. [DOI] [PubMed] [Google Scholar]
- 34.Yeo YH, Shiu SI, Ho HJ, et al. First-line Helicobacter pylori eradication therapies in countries with high and low clarithromycin resistance: a systematic review and network meta-analysis. Gut 2018;67:20–27. [DOI] [PubMed] [Google Scholar]
- 35.Rokkas T, Gisbert JP, Malfertheiner P, et al. Comparative Effectiveness of Multiple Different First-Line Treatment Regimens for Helicobacter pylori Infection: A Network Meta-analysis. Gastroenterology 2021;161:495–507 e4. [DOI] [PubMed] [Google Scholar]
- 36.Guo B, Cao NW, Zhou HY, et al. Efficacy and safety of bismuth-containing quadruple treatment and concomitant treatment for first-line Helicobacter pylori eradication: A systematic review and meta-analysis. Microb Pathog 2021;152:104661. [DOI] [PubMed] [Google Scholar]
- 37.Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Internal and emergency medicine 2017;12:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3:80–97. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro S Meta-analysis/Shmeta-analysis. Am. J Epidemiol 1994;140:771–778. [DOI] [PubMed] [Google Scholar]
- 40.Hopkins RJ. Current FDA-approved treatments for Helicobacter pylori and the FDA approval process. Gastroenterology 1997;113:S126–S130. [DOI] [PubMed] [Google Scholar]
- 41.Graham DY, Lew GM, Evans DG, et al. Effect of triple therapy (antibiotics plus bismuth) on duodenal ulcer healing. A randomized controlled trial. Ann. Intern. Med 1991;115:266–269. [DOI] [PubMed] [Google Scholar]
- 42.Vakil N, Lanza F, Schwartz H, et al. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther 2004;20:99–107. [DOI] [PubMed] [Google Scholar]
- 43.Vonoprazan, 2021. [Google Scholar]
- 44.Freeman SC, Fisher D, White IR, et al. Identifying inconsistency in network meta-analysis: Is the net heat plot a reliable method? Stat Med 2019;38:5547–5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graham DY, Lu H, Shiotani A. Vonoprazan-containing Helicobacter pylori triple therapies contribution to global antimicrobial resistance. J Gastroenterol Hepatol 2021;21:1159–1163. [DOI] [PubMed] [Google Scholar]
- 46.Keller A Fluoroquinolones: ConsumerNotice.org, 2020. [Google Scholar]
- 47.Ierardi E, Giorgio F, Losurdo G, et al. How antibiotic resistances could change Helicobacter pylori treatment: A matter of geography? World journal of gastroenterology 2013;19:8168–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: The good, the bad, and the ugly. Gastroenterol Clin North Am 2015;44:537–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wirth HP, Yang M. Different Pathophysiology of Gastritis in East and West? A Western Perspective. Inflammatory Intestinal Diseases 2016;1:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savoldi A, Carrara E, Graham DY, et al. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018;155:1372–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol 2018;6:800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Furuta T, Shirai N, Xiao F, et al. High-dose rabeprazole/amoxicillin therapy as the second-line regimen after failure to eradicate H. pylori by triple therapy with the usual doses of a proton pump inhibitor, clarithromycin and amoxicillin. Hepatogastroenterology 2003;50:2274–2278. [PubMed] [Google Scholar]
- 53.Tai WC, Liang CM, Kuo CM, et al. A 14 day esomeprazole- and amoxicillin-containing high-dose dual therapy regimen achieves a high eradication rate as first-line anti-Helicobacter pylori treatment in Taiwan: a prospective randomized trial. J. Antimicrob. Chemother 2019;74:1718–1724. [DOI] [PubMed] [Google Scholar]
- 54.Shirai N, Sugimoto M, Kodaira C, et al. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. European Journal of Clinical Pharmacology 2007;63:743–749. [DOI] [PubMed] [Google Scholar]
- 55.Furuta T, Yamade M, Kagami T, et al. Dual Therapy with Vonoprazan and Amoxicillin Is as Effective as Triple Therapy with Vonoprazan, Amoxicillin and Clarithromycin for Eradication of Helicobacter pylori. Digestion 2020;1-1:743–751. [DOI] [PubMed] [Google Scholar]
- 56.Shi K, Shen Z, Zhu G, et al. Systematic review with network meta-analysis: dual therapy for high-risk bleeding peptic ulcers. BMC. Gastroenterol 2017;17:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee YC, Dore MP, Graham DY. Diagnosis and Treatment of Helicobacter pylori Infection. Annu.Rev.Med 2022;73:4.1–.12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.