Abstract
Accumulation of advanced glycation end-products (AGE) in bone alters collagen structure and function. Fluorescent AGEs are associated with fractures but less is known regarding non-fluorescent AGEs. We examined associations of carboxy-methyl-lysine (CML), with incident clinical and prevalent vertebral fractures by type 2 diabetes (T2D) status, in the Health, Aging, and Body Composition cohort of older adults. Incident clinical fractures and baseline vertebral fractures were assessed. Cox regression was used to analyze the associations between serum CML and clinical fracture incidence, and logistic regression for vertebral fracture prevalence. At baseline, mean ± standard deviation (SD) age was 73.7 ± 2.8 and 73.6 ± 2.9 years in T2D (n = 712) and non-diabetes (n = 2332), respectively. Baseline CML levels were higher in T2D than non-diabetes (893 ± 332 versus 771 ± 270 ng/mL, p < 0.0001). In multivariate models, greater CML was associated with higher risk of incident clinical fracture in T2D (hazard ratio [HR] 1.49; 95% confidence interval [CI], 1.24–1.79 per 1-SD increase in log CML) but not in non-diabetes (HR 1.03; 95% CI, 0.94–1.13; p for interaction = 0.001). This association was independent of bone mineral density (BMD), glycated hemoglobin (hemoglobin A1c), weight, weight loss, smoking, cystatin-C, and medication use. CML was not significantly associated with the odds of prevalent vertebral fractures in either group. In conclusion, higher CML levels are associated with increased risk of incident clinical fractures in T2D, independent of BMD. These results implicate CML in the pathogenesis of bone fragility in diabetes.
Keywords: FRACTURE, DIABETES, ADVANCED GLYCATION END-PRODUCTS, CARBOXY-METHYL-LYSINE, BIOMARKER
Introduction
Skeletal fragility, leading to an increased risk of fracture, is a recently recognized complication of type 2 diabetes (T2D). A recent meta-analysis shows a 33% increase in hip fracture risk in T2D.(1) High fracture risk in T2D is observed despite normal or high areal bone mineral density (BMD), and high body mass index (BMI).(2–6) This paradox implicates poor bone quality as the primary determinant of bone fragility in T2D,(6–9) but the mechanisms underlying this poor bone quality are yet to be elucidated. Putative mechanisms are manifold, likely affecting bone at multiple scales.(10–12) Low bone turnover is characteristic of diabetes. However, clinical studies have failed to demonstrate that bone turnover alone is responsible for the high fracture risk in diabetes.(13)
Alterations in bone quality in T2D may result from accumulation of advanced glycation end-products (AGEs) related to prolonged hyperglycemia and oxidative stress.(12,14–17) AGEs exert direct deleterious effects by altering the structural and functional properties of collagen, increasing stiffness, and/or reducing functional biomechanical characteristics of arteries, skin, cartilage, and bone.(12,14−19) In bone, AGE accumulation leads to generation of undesired crosslinks, which affects mineralization, material properties, and microstructure, thereby impairing biomechanical properties and reducing bone strength.(12,20,21) AGEs also exert indirect deleterious effects through their interaction with Receptor for Advanced Glycation End-products (RAGE) on the cell membrane. RAGE activation induces an inflammatory response through generation of oxygen radicals, formation of nuclear transcription factors, proinflammatory cytokines and fibrogenic growth factors, and reduction of nitric oxide formation.(12,17,22,23) Indeed, this harmful effect of AGEs is the pathogenetic mechanism underlying many complications of diabetes. In vitro, AGE accumulation can alter behavior of bone cells by decreasing osteoblast differentiation and proliferation and impairing adhesion of osteoblasts to collagen matrix, thereby inhibiting osteoblastic activity.(12,24,25) AGE accumulation also reduces bone resorption by modifying osteoclast differentiation and activity,(26) and may induce osteocyte dysfunction and apoptosis.(27)
Multiple different AGEs have been identified.(28) Pentosidine (PEN) is a widely studied fluorescent AGE that can be measured directly in blood and urine as well as bone tissue. In clinical investigations, higher levels of PEN have been associated with an increased prevalence of vertebral fractures and incidence of clinical fractures in diabetes.(29–31)
Carboxy-methyl-lysine (CML) is another well-characterized AGE that is nonfluorescent and accumulates at much higher levels in bone than PEN with aging and in diabetes.(32–34) As a nonfluorescent AGE, CML interacts differently with bone collagen and may have distinct effects on bone fragility. PEN forms intermolecular crosslinks within the organic matrix of bone, while CML has a side chain containing a negatively charged carboxyl group that can attract positively charged calcium ions, promote mineralization,(35) and link collagen and mineral in bone.(36) CML accumulation in bone is associated with reduced ability of the bone to dissipate energy,(23) making the bone potentially more vulnerable to fracture. Similar to PEN and RAGE interactions, CML-RAGE interactions have been associated with altered inflammatory response and insulin resistance.(37–39) Thus, CML may also alter bone matrix and fracture risk via its effects on bone cells and turnover. Previous studies of CML and incident hip fracture have not provided consistent results.(40,41) To clarify this association and to determine if CML is associated with higher fracture risk among T2D in particular, we examined the association of CML with incident clinical fractures and prevalent vertebral fractures in older adults with and without T2D from the Health, Aging, and Body Composition (Health ABC) Study.
Subjects and Methods
Study population
The Health ABC Study population consists of a healthy cohort of 3075 community-dwelling men and women (48.5% men, 41.7% black) aged 70–79 years, recruited at two centers (University of Pittsburgh, Pittsburgh, PA, USA; and University of Tennessee, Memphis, TN, USA). The Health ABC Study is a prospective study designed to investigate whether changes in body composition act as a common pathway by which age-related physiological and functional changes occur in multiple diseases.(42) Exclusion criteria included difficulty performing activities of daily living, walking ¼ mile, or climbing 10 stairs without resting. All participants gave written informed consent. The study protocol was approved by the Institutional Review Boards at the Universities of Pittsburgh and Tennessee. The baseline examination took place during 1997–1998.
Of the 3075 subjects in the cohort, 3044 participants had serum CML measurements at baseline and were included in the analytical sample. Among the 3044 subjects, 712 participants had diabetes at baseline.
Diabetes status
T2D was identified based on self-reported diagnosis of diabetes, use of hypoglycemic medications, elevated fasting glucose (≥126 mg/dL) or impaired glucose tolerance (2-hour plasma glucose during oral glucose tolerance test ≥200 mg/dL) in accordance with the American Diabetes Association criteria. Among the 712 participants with diabetes, 42 participants (5.9%) were diagnosed based on self-report alone. Except for those who reported taking insulin or oral hypoglycemic agents, all participants underwent an oral glucose tolerance test.
Serum CML
Baseline serum samples were collected after an overnight fast. Serum CML was measured using an enzyme-linked immunosorbent assay (ELISA) (AGE-CML ELISA; Microcoat, Penzberg, Germany).(33) This assay is specific and exhibits no cross-reactivity with other compounds. This assay has been validated(33,43) and has an interassay coefficient of variation of 10%.
Incident clinical fracture
Participants were queried every 6 months, by telephone or at a clinic visit, regarding occurrence of a fracture for a follow up period of up to 17.4 years. A reported fracture was verified by radiology report, except fractures of the ribs, chest/sternum, skull/face, fingers, toes, and cervical vertebra fractures. Only events adjudicated as a fracture were included. Fractures due to a pathological process, such as cancer, were excluded. If a participant had multiple fractures, only the first fracture was included. Follow-up time was defined as time to first fracture for those who fractured and overall study time for those who did not fracture.
Prevalent vertebral fracture
Lateral scout scans were obtained in a subset of participants (n = 1038) at the baseline visit to determine placement for computed tomography (CT) abdominal scans. Images were obtained in Pittsburgh using a 9800 Advantage (General Electric, Milwaukee, WI, USA) and in Memphis using a Somatom Plus 4 (Siemens, Erlangen, Germany) or a Picker PQ 2000S (Marconi Medical Systems, Cleveland, OH, USA). The CT lateral scout scans were assessed and graded for prevalent vertebral deformities by a radiologist, blinded to the diabetes status of the participants. A semiquantitative grade of 2, indicating a moderate deformity with a 25% to 40% height reduction, or grade 3, indicating a severe deformity with >40% height reduction, was defined as vertebral fracture.
Other measurements
Demographic data were self-reported by all participants. Participants were asked whether they had lost more than 5 pounds in the year before the baseline visit. Anthropometric measurements (height and weight) were obtained at the baseline study visit. BMI was expressed as weight in kilograms divided by height in meters squared (kg/m2). Areal BMD was measured at the total hip and femoral neck by dual-energy X-ray absorptiometer (DXA) (model QDR 4500A; version 9.03; Hologic Inc., Bedford, MA, USA). Participants were asked to bring prescription and over-the-counter medications used in the previous week to the baseline clinic visit. Medications were coded according to the Iowa Drug Information System (IDIS).(44) In these analyses, bisphosphonates, calcitonin, and raloxifene were grouped together as “osteoporosis medications.”
Baseline serum samples were collected after an overnight fast. At baseline, participants ingested 75 g glucose in solution (glucola) immediately after blood was drawn for fasting glucose, and a second blood sample was drawn 2 hours later for the oral glucose tolerance test. Plasma glucose and serum creatinine were measured on a Johnson and Johnson Vitros 950 analyzer (Ortho-Clinical Diagnostics; Johnson and Johnson, Rochester, NY, USA) at the Laboratory of Clinical Biochemistry at the University of Vermont. Cystatin-C, an indicator of renal function, was measured (in baseline serum stored at −70°C for an average of 6.5 years) using a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Dade Behring, Inc., Deerfield, IL, USA) on a BNII nephelometer (Dade Behring, Inc.). Intraassay and interassay coefficients of variation are 2.0% to 2.8% and 2.3% to 3.1%, respectively. Glycated hemoglobin (hemoglobin A1c) was measured using high-performance liquid chromatography (HPLC) (BioRad Variant HPLC; BioRad, Hercules, CA, USA).
Statistical analysis
Descriptive statistics were calculated and presented as mean ± standard deviation (SD) as appropriate for continuous data and as proportion for categorical variables. Chi-square test was calculated for categorical variables, and ANOVA for continuous variables to test for statistical differences between those with and without diabetes. Serum CML values were log-transformed to normalize their distributions.
Cox proportional hazards models were used to analyze the associations between log-transformed baseline serum CML and risk of clinical fractures for T2D and non-diabetes, with results presented as hazard ratios (HRs) and 95% confidence intervals (CIs) per SD increase in log CML. The risk for clinical fracture for T2D and non-diabetes was also examined across quartiles of CML, with quartile 1 serving as the reference group. CML quartile cut points were determined from the non-diabetes group and were applied to both T2D and non-diabetes. Logistic regression models were used to analyze the association between log-transformed CML levels at baseline and odds of prevalent vertebral fractures for T2D and non-diabetes, with results presented as odds ratios (ORs) and 95% CIs per SD increase in log CML. All models included age, race, sex, and clinic site; multivariate models also included current smoking status, total hip BMD, weight, weight loss of more than 5 pounds in the year before baseline, cystatin-C, A1c, and medication use (vitamin D supplements, calcium supplements, oral steroids, osteoporosis drugs, thiazide diuretics, statins, and oral estrogen). Multivariate models for T2D also included use of insulin and thiazolidinediones and diabetes duration. Interaction between CML levels and diabetes status was tested in the multivariate models to determine if the associations between CML and fracture risk differed for T2D and non-diabetes. All calculations were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). Results were considered statistically significant when p < 0.05.
Results
Baseline characteristics of the 3044 study participants with CML data are presented in Table 1. Mean age was 73.7 ± 2.8 in T2D (n = 712) and 73.6 ± 2.9 years in non-diabetes (n = 2332). Mean BMD at total hip and femoral neck was higher in T2D. Baseline CML levels were significantly higher in T2D than non-diabetes (893 ± 332 versus 771 ± 270 ng/ml, respectively, p < 0.0001). Among T2D, there was a significant positive correlation of CML with A1c, diabetes duration, and cystatin-C. Among non-diabetes, higher CML levels were associated with lower weight and higher cystatin-C. Among both T2D and non-diabetes, mean CML was significantly higher for men than for women. There was no association of CML with age or BMD for T2D or non-diabetes: the correlation between log CML and total hip BMD was r = 0.004 for T2D and r = −0.019 for non-diabetes.
Table 1.
Characteristics of Older Adults by Diabetes Status (Health, Aging, and Body Composition Study)
| Diabetes |
|||
|---|---|---|---|
| Characteristics | No | Yes | p |
|
| |||
| n | 2332 | 712 | |
| Age at baseline (years) | 73.6 ± 2.9 | 73.7 ± 2.8 | 0.67 |
| Sex (% men) | 46.7 | 54.6 | 0.0002 |
| Race (% white) | 61.7 | 47.8 | <0.0001 |
| Clinic site (% Memphis) | 50.2 | 50.4 | 0.92 |
| BMI (kg/m2) | 26.9 ± 4.7 | 29.0 ± 4.8 | <0.0001 |
| Weight (kg) | 74.3 ± 14.8 | 80.8 ± 14.9 | <0.0001 |
| Lost 5+ pounds in previous 12 months (% yes) | 30.5 | 46.5 | <0.0001 |
| Cystatin-C (mg/L) | 1.03 ± 0.25 | 1.08 ± 0.30 | 0.0006 |
| Current smoker (% yes) | 10.8 | 8.6 | 0.09 |
| Calcium supplement use (% yes) | 20.0 | 12.0 | <0.0001 |
| Vitamin D supplement use (% yes) | 9.6 | 4.6 | <0.0001 |
| Oral steroid use (% yes) | 2.1 | 2.7 | 0.37 |
| Oral estrogen use (women only) (% yes) | 12.1 | 9.1 | 0.03 |
| Thiazide diuretic use (% yes) | 17.2 | 21.2 | 0.01 |
| Statin use (% yes) | 12.4 | 14.9 | 0.08 |
| Osteoporosis drug use (% yes)a | 4.3 | 2.5 | 0.03 |
| A1c (%) | 6.0 ± 0.5 | 7.6 ± 1.6 | <0.0001 |
| CML (ng/mL)b | 771.1 ± 269.9 | 893.2 ± 332.3 | <0.0001 |
| Baseline BMD (g/cm2) | |||
| Hip total | 0.87 ± 0.17 | 0.94 ± 0.17 | <0.0001 |
| Femoral neck | 0.73 ± 0.14 | 0.79 ± 0.14 | <0.0001 |
| Prevalent vertebral fracturec | |||
| Moderate/severe (SQ grade ≥ 2) (% with at least one deformity) | 3.3 | 2.2 | 0.30 |
| Clinical fracture (% with at least one fracture) | 21.8 | 19.1 | 0.12 |
| In participants with diabetes only | |||
| Insulin use (% yes) | 16.2 | ||
| Thiazolidinedione use (% yes) | 1.8 | ||
| Oral hypoglycemic use (% yes) | 39.1 | ||
| Diabetes duration (years) | 8.6 ± 11.6 | ||
Data are expressed as mean ± SD or percentage.
Includes bisphosphonates, calcitonin, and raloxifene.
Geometric means based on log-transformed CML.
Lateral scout data available in a subset of 1038 participants.
Fracture risk
Incident clinical fractures occurred in 136 T2D participants over a mean follow-up of 9.6 ± 5.1 years and in 509 non-diabetes participants over a mean of 10.9 ± 5.2 years. In models adjusted for age, race, sex, and clinic site, each 1-SD increase in log CML increased the risk of incident clinical fracture by 45% among T2D (HR 1.45; 95% CI, 1.22–1.73; p < 0.0001); there was no evidence of increased risk among non-diabetes (HR 1.07; 95% CI, 0.98–1.16; p = 0.16; p value for interaction = 0.004). After adjustment for additional covariates (weight, weight loss, smoking, A1c, total hip BMD, cystatin-C, diabetes duration, and medications [vitamin D supplements, calcium supplements, oral steroids, osteoporosis drugs, thiazide diuretics, statins, oral estrogen and, use of insulin and thiazolidinediones]), log CML remained associated with clinical fracture risk among T2D (HR 1.49; 95% CI, 1.24–1.79; p < 0.0001) but not among non-diabetes (HR 1.03; 95% CI, 0.94–1.13; p = 0.50; p value for interaction = 0.001; see Table 2).
Table 2.
Risk of Incident Clinical Fracture per SD Increase in log CML
| Parameter | Diabetes |
Non-diabetes |
p for interaction |
|---|---|---|---|
| HR (95% CI) (n = 712) | HR (95% CI) (n = 2332) | ||
|
| |||
| Number of participants with at least one fracture | n = 136 | n = 509 | |
| Minimally adjusted | 1.45 (1.22–1.73) | 1.07 (0.98–1.16) | 0.004 |
| Multivariate adjusted | 1.49 (1.24–1.79) | 1.03 (0.94–1.13) | 0.001 |
All models include age, race, sex, and clinic site. Multivariate models also include current smoking status, total hip BMD, weight, weight loss of 5+ pounds in year before baseline, cystatin-C, A1c, and medication use (vitamin D supplements, calcium supplements, oral steroids, osteoporosis drugs, thiazide diuretics, statins, oral estrogen, and, in models with diabetes participants, use of insulin and thiazolidinediones, and diabetes duration).
Bolded HR values signifies increased risk of incident clinical fracture in T2D.
The risk of incident clinical fracture by CML quartile is displayed in Fig. 1. Among T2D, the fully adjusted risk of fracture was at least 2.3 times higher for those in the top three CML quartiles compared to those in the lowest quartile. Among non-diabetes, there was no difference in fracture risk for the lower three CML quartiles, but the risk was slightly higher for those in the top CML quartile compared to those in the lowest (HR 1.25; 95% CI, 0.96–1.63; p = 0.09).
Fig 1.
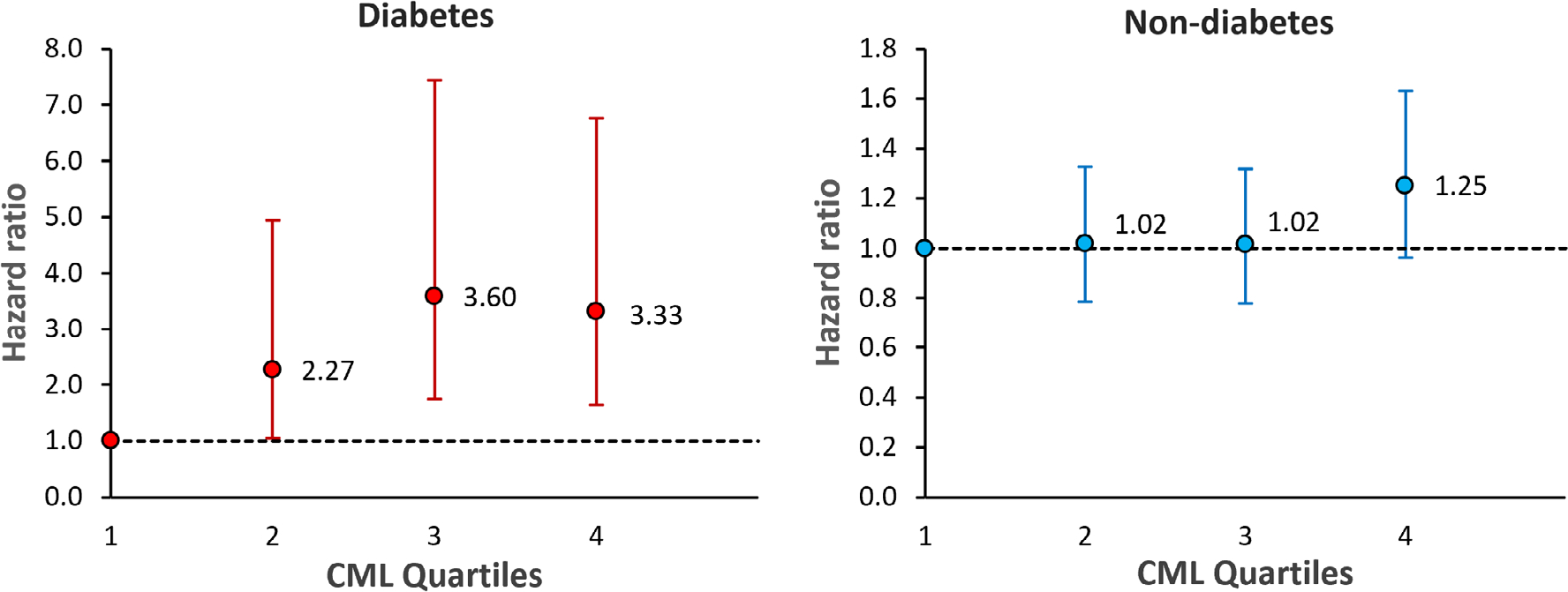
Risk of incident clinical fracture by CML quartile. CML quartile 1 (CML < 576 ng/mL, n = 97 diabetes, n = 553 non-diabetes) was the reference group for comparisons with quartile 2 (CML 576–691 ng/mL, n = 107 diabetes, n = 547 non-diabetes), quartile 3 (CML 691–849 ng/mL, n = 154 diabetes, n = 555 non-diabetes), and quartile 4 (CML ≥849 ng/mL, n = 275 diabetes, n = 558 non-diabetes). Quartile cut points determined from the non-diabetes group. The vertical bars denote 95% confidence intervals. Models include age, race, sex, clinic site, current smoking status, total hip BMD, weight, weight loss of 5+ pounds in year before baseline, cystatin-C, A1c, and medication use (vitamin D supplements, calcium supplements, oral steroids, osteoporosis drugs, thiazide diuretics, statins, oral estrogen and, in models with diabetes participants, use of insulin and thiazolidinediones, and diabetes duration).
Prevalent vertebral fractures
Among the subset of 1038 participants with scout data, 2.8% (11 T2D and 18 non-diabetes participants) had a prevalent vertebral fracture. In models adjusted for age, race, sex, and clinic site, each 1-SD increase in log CML increased the odds of prevalent vertebral fracture by 51% among those with diabetes, but this association did not reach statistical significance (OR 1.51; 95% CI, 0.82–2.79; p = 0.19); there was no association among those without diabetes (OR 0.79; 95% CI, 0.52–1.19; p = 0.27; p value for interaction = 0.09). After adjustment for additional covariates, the association between CML and prevalent vertebral fracture strengthened among T2D but was not statistically significant (OR 2.23; 95% CI, 0.90–5.50; p = 0.08). Adjustment for additional covariates had little impact on the estimated association among non-diabetes (OR 0.75; 95% CI, 0.48–1.19; p = 0.22; p value for interaction = 0.06; see Table 3).
Table 3.
Risk of Prevalent Vertebral Fracture per SD Increase in log CML Among 1038 Participants With Lateral Scout Data
| Parameter | Diabetes |
Non-diabetes |
p for interaction |
|---|---|---|---|
| OR (95% CI) (n = 493) | OR (95% CI) (n = 545) | ||
|
| |||
| Number of participants with moderate or severe vertebral deformity | n = 11 | n = 18 | |
| Minimally adjusted | 1.51 (0.82–2.79) | 0.79 (0.52–1.19) | 0.09 |
| Multivariate adjusted | 2.23 (0.90–5.50) | 0.75 (0.48–1.19) | 0.06 |
All models include age, race, sex and clinic site. Multivariate models also include current smoking status, total hip BMD, weight, weight loss of 5+ pounds in year before baseline, cystatin-C, A1c, and medication use (vitamin D supplements, calcium supplements, oral steroids, osteoporosis drugs, thiazide diuretics, statins, oral estrogen and, in models with diabetes participants, use of insulin and thiazolidinediones, and diabetes duration).
Discussion
We found that greater CML levels are associated with increased risk of incident clinical fractures in diabetes, independent of BMD and other risk factors for osteoporotic fracture. Evidence on circulating AGEs and bone outcomes has emerged in recent years and is inconclusive thus far. Previous clinical studies have reported the contribution of PEN, an AGE with fluorescent properties, to fracture risk.(29–31,45) In the Health ABC Study, PEN was associated with incident clinical fracture only in those with diabetes.(31) In the Os des Femmes de Lyon (OFELY) cohort of healthy postmenopausal women, there was no association between PEN and incident fracture in the adjusted models.(46) Our study finding demonstrates the clinical relevance of circulating levels of CML, a nonfluorescent AGE, as a biomarker of fracture risk in diabetes.
CML, a nonfluorescent AGE adduct, has been demonstrated to exceed accumulation of other AGEs in various tissues including but not limited to bone.(23,27,28,32–34) CML is found in substantial amounts in collagenous tissues, including skin, vasculature, and bone, where it has been associated with poor mechanical performance of the tissues. For example, in the Health ABC Study, an association between serum CML and greater arterial stiffness has been reported.(47) In the current study, we observed significantly higher serum CML levels in those with diabetes versus non-diabetes in this cohort. Our finding of an association of increased incident fracture risk in diabetes with greater serum CML levels is therefore consistent with the hypothesis that AGE accumulation has a negative effect on skeleton, yielding inferior bone biomechanical properties and lower bone strength. After adjusting for potential confounders, greater CML levels tended to be associated with prevalent vertebral fractures, though it did not reach statistical significance (p = 0.07). This could be a function of the small number of prevalent vertebral fractures in this study. Studies on vertebral fracture risk in T2D have yielded conflicting results, with some studies showing that this risk is not significantly higher in T2D,(48,49) whereas studies based on clinical and morphometric assessments found an increased risk of vertebral fracture in T2D.(50–52) To this end, a recent large-scale population analysis showed diabetes is associated with a higher risk of all types of fractures.(2) In this Health ABC cohort, an increased risk of incident clinical and prevalent vertebral fractures with higher urine PEN levels has been reported; however, there was no difference in PEN levels between those with and without diabetes.(31)
Ex vivo studies have demonstrated increased deposition of fluorescent and nonfluorescent AGEs in bone samples from subjects with diabetes.(16,21,23,34,53) Although Piccoli and colleagues(53) showed that total fluorescent AGE content was associated with microarchitectural deficits in T2D, no differences in biomechanical properties were found in bones of those with diabetes and controls. This finding is in agreement with the mechanistic role of AGEs as moderators underlying the bone fragility in diabetes. An additional explanation would be the lack on nonfluorescent AGE measurement in the study by Piccoli and colleagues.(53) Nonfluorescent AGEs interact differently with bone collagen and may have distinct effects on bone fragility, as discussed previously (under Introduction). In addition to altering the bone matrix and fracture risk via effects on bone cells and turnover, CML accumulation in bone has been associated with reduced toughness, indicating that CML may reduce the ability of the bone to dissipate energy, making the bone potentially more vulnerable to fracture. Further work is, however, required to understand the mechanistic basis of CML impact on bone fragility.
Our study findings differ from previous clinical investigations of CML that have focused only upon hip fractures.(40,41) Barzilay and colleagues(40) reported that increasing levels of CML are associated with increased hip fracture risk in older adults and found no between group differences in a subset analysis of participants with and without diabetes. In contrast to our findings of increased fracture risk with greater CML levels in diabetes, Lamb and colleagues(41) identified a nonlinear (U-shaped) association between CML and incident hip fracture risk in older men with and without diabetes. The study by Lamb and colleagues(41) did not include BMD or A1c assessment. Further, hip fractures in both these studies were ascertained through hospital discharge codes.(40,41) In the present study, only fractures with radiographic adjudication were included. There was no significant relationship between hip BMD and serum CML concentrations observed in our study. This finding is consistent with reports by Barzilay and colleagues(40) and Nakano and colleagues(54) and indicates that this association between CML and fracture is likely through bone quality and not bone quantity. However, the study by Nakano and colleagues(54) also reported a significant negative correlation between lumbar spine BMD and serum CML levels, suggesting that the relationship of CML with BMD may be site-specific.
There was no evidence of an association between CML and fracture risk in the non-diabetes group in the present study. This finding contrasts with reports of an increased risk of hip fracture with higher concentrations of serum CML in older adults with and without diabetes,(40,41) and an association between tissue level CML accumulation in femoral cortical bone and its fracture properties.(23) Similar to our study finding, Nakano and colleagues(54) also reported no independent association of CML with the presence of vertebral fracture in women without diabetes. These differences suggest that, under T2D, CML may accumulate early or in greater amount due to increased hyperglycemia and oxidative stress,(10,14–17) as well as higher surface to volume ratio of cancellous bone tissue(55) that occupies large proportion of vertebrae and contributes to its load bearing and biomechanical properties. Such early or enhanced CML accumulation in vertebral cancellous in T2D would manifest itself as alterations in vertebral bone microstructure, mineralization, and/or material properties that impair biomechanical properties and reduce vertebral strength.
A significant positive correlation was observed between CML and A1c, as expected, indicating that CML accumulation increases with severity of T2D. Glycemic control has been shown to predict fracture,(56–59) and CML accumulation may be one of the mechanisms through which glycemic control affects bone. Thus, in examining the association between CML and fracture, glycemic control is a potential confounder. However, multivariate models in our study showed an association between serum CML and fracture even after adjusting for glycemic control. Because AGEs accumulate with prolonged hyperglycemia, diabetes duration is also a potential confounder. Adjusting the models further for diabetes duration had little effect on the relationship between CML and fracture.
Increased fracture risk in diabetes is also related to medications used to treat diabetes (thiazolidinediones, history of insulin use).(60,61) In the present study, multivariate regression analysis revealed that serum CML levels were associated with the presence of incident clinical fractures independent of insulin or thiazolidinediones, or other known risk factors for osteoporosis such as steroids, smoking, and weight loss. Similarly, reduced renal function has been shown to predict fracture.(62,63) In this study, CML levels directly correlated with cystatin C, a marker of renal function. The relationship between CML and incident fracture persisted after adjusting for renal function and other risk factors for fragility fracture.
The strengths of our study include a well-defined cohort and a long duration of follow up. Diabetes was well characterized in this cohort. Serum CML levels were measured at baseline before incident fractures were ascertained. We also considered covariables that are known confounders (smoking, bone mineral density, weight, weight loss, and glycemic control). A limitation of this study is the lack of a direct measurement of CML in bone collagen. The direct assessment of AGEs in bone tissue is often not possible because it requires an invasive procedure. Serum CML levels are influenced by CML from sites other than the skeleton including muscle, skin, cartilage, and adipose tissue. Increased levels of CMLs and other AGEs in muscle may contribute to sarcopenia, which in turn may increase fracture risk. We were not able to consider this potential pathway separately. We were not able to adjust for PEN levels. Thus, we could not assess whether CML is independently associated with fracture or whether it is primarily a marker for overall AGE content. Prevalent vertebral fractures were assessed from CT lateral scout scans, possibly leading to an underestimation of vertebral deformities in comparison to traditional spine radiographs. Such underassessment would likely be nondifferential with respect to CML levels, which would tend to attenuate any association between CML and fracture toward the null. We did not assess individual sites, such as hip, for individual fractures. As with any observational study, we cannot rule out the presence of confounding due to residual effects or unknown confounders. This was a study of older adults, aged 70 to 79 years, and the findings may not be generalizable to other age groups.
In conclusion, higher levels of serum CML, a non-crosslinking AGE, are associated with increased incident fracture risk in T2D. This finding extends previous reports that AGEs contribute to the inferior bone quality in T2D. The mechanisms underlying this contribution of CML to bone fragility in diabetes are unclear and require further investigations, but the association is independent of glycemic control.
Acknowledgments
This work was supported by the National Institutes of Health (NIH): National Institute on Aging (NIA) (contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; and grants R01-AG028050 [PI: Elsa S. Strotmeyer]; R01-AG17482 [PI: Beata Lecka-Czernik], R01-AG-02-7012 [PI: RDS] and R56-AG-02-0618 [PI: DV]); National Institute of Nursing Research (grant R01-NR012459 [PIs: Steven M. Albert, June R. Lunney]). This research was supported in part by the Intramural Research Program of the NIH, NIA. Data availability: The data that support the findings of this study are available from NIA. Data are available from the authors with the permission of NIA.
Authors’ roles: RD conceived the study idea. RD and AVS designed the study; analysis was conducted by SKE; RD, AVS, and SKE contributed to interpretation of the data. RD wrote the original draft. RD, SKE, DV, RDS, and AVS contributed to writing-review and editing. All authors provided critical review throughout the development and approved the final draft of the manuscript for publication. All authors agree to be responsible for the content of this work.
Conflict of Interest
RD has served as a scientific advisory board member/consultant for Ultragenyx and Radius Health. SKE, DV, and RDS have no disclosures. AVS has participated in a scientific advisory board for Amgen and has received a research grant from Hologic.
Footnotes
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4466.
Data availability statement
The data that support the findings of this study are available from the NIA. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of NIA.
References
- 1.Vilaca T, Schini M, Harnan S, et al. The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: a systematic review and meta-analysis update. Bone. 2020;137:115457. [DOI] [PubMed] [Google Scholar]
- 2.Ha J, Jeong C, Han KD, et al. Comparison of fracture risk between type 1 and type 2 diabetes: a comprehensive real-world data. Osteoporos Int. 2021. Published online ahead of print 31 July 31, 2021. 10.1007/s00198-021-06032-z. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305(21):2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giangregorio LM, Leslie WD, Lix LM, et al. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res. 2012;27(2): 301–308 [Published correction appears in J Bone Miner Res. 2017;32 (11):2319.]. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson AG, Sundh D, Johansson L, et al. Type 2 diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women: a population-based study. J Bone Miner Res. 2017;32(5):1062–1071. [DOI] [PubMed] [Google Scholar]
- 7.Farr JN, Khosla S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone. 2016;82:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patsch JM, Burghardt AJ, Yap SP, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28(2):313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhaliwal R, Rosen CJ. Type 2 diabetes and aging: a not so sweet scenario for bone. Horm Metab Res. 2016;48(11):771–778. [DOI] [PubMed] [Google Scholar]
- 10.Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int. 2014;25(7): 1969–1973. [DOI] [PubMed] [Google Scholar]
- 11.Ural A, Vashishth D. Hierarchical perspective of bone toughness—from molecules to fracture. Int Mater Rev. 2014;59(5):245–263. [Google Scholar]
- 12.Sihota P, Yadav RN, Dhaliwal R, et al. Investigation of mechanical, material, and compositional determinants of human trabecular bone quality in type 2 diabetes. J Clin Endocrinol Metab. 2021;106(5): e2271–e2289. [DOI] [PubMed] [Google Scholar]
- 13.Napoli N, Conte C, Eastell R, et al. Bone turnover markers do not predict fracture risk in type 2 diabetes. J Bone Miner Res. 2020;35(12): 2363–2371. [DOI] [PubMed] [Google Scholar]
- 14.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21(2):195–214. [DOI] [PubMed] [Google Scholar]
- 15.Furst JR, Bandeira LC, Fan WW, et al. Advanced glycation endproducts and bone material strength in type 2 diabetes. J Clin Endocrinol Metab. 2016;101(6):2502–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karim L, Moulton J, Van Vliet M, et al. Bone microarchitecture, biomechanical properties, and advanced glycation end-products in the proximal femur of adults with type 2 diabetes. Bone. 2018;114:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65(9):963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking and advanced glycation endproducts in aging human skeletal muscle. J Appl Physiol. 2007;103(6):2068–2076. [DOI] [PubMed] [Google Scholar]
- 19.Karim L, Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS One. 2012; 7(4):e35047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Follet H, Viguet-Carrin S, Burt-Pichat B, et al. Effects of preexisting microdamage, collagen cross-links, degree of mineralization, age, and architecture on compressive mechanical properties of elderly human vertebral trabecular bone. J Orthop Res. 2011;29(4):481–488. [DOI] [PubMed] [Google Scholar]
- 21.Hunt HB, Torres AM, Palomino PM, et al. Altered tissue composition, microarchitecture, and mechanical performance in cancellous bone from men with type 2 diabetes mellitus. J Bone Miner Res. 2019; 34(7):1191–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rashid G, Benchetrit S, Fishman D, Bernheim J. Effect of advanced glycation end-products on gene expression and synthesis of TNF-alpha and endothelial nitric oxide synthase by endothelial cells. Kidney Int. 2004;66(3):1099–1106. [DOI] [PubMed] [Google Scholar]
- 23.Thomas CJ, Cleland TP, Sroga GE, Vashishth D. Accumulation of carboxymethyl-lysine (CML) in human cortical bone. Bone. 2018; 110:128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy AD, Uemura T, Etcheverry SB, Cortizo AM. Advanced glycation endproducts interefere with integrin-mediated osteoblastic attachment to a type-I collagen matrix. Int J Biochem Cell Biol. 2004;36(5):840–848. [DOI] [PubMed] [Google Scholar]
- 25.Kume S, Kato S, Yamagishi S, et al. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res. 2005;20(9):1647–1658. [DOI] [PubMed] [Google Scholar]
- 26.Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem. 2007;282(8):5691–5703. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Zhang C, Wei W, et al. FOXO1 mediates advanced glycation end products induced mouse osteocyte-like MLO-Y4 cell apoptosis and dysfunctions. J Diabetes Res. 2019;2019:6757428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(3):1013–1019. [DOI] [PubMed] [Google Scholar]
- 30.Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T. Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab. 2008;26(1): 93–100. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz AV, Garnero P, Hillier TA, et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(7):2380–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirata K, Kubo K. Relationship between blood levels of N-carboxy-methyl-lysine and pentosidine and the severity of microangiopathy in type 2 diabetes. Endocr J. 2004;51(6):537–544. [DOI] [PubMed] [Google Scholar]
- 33.Boehm BO, Schilling S, Rosinger S, et al. Elevated serum levels of N(epsilon)-carboxymethyl-lysine, an advanced glycation end product, are associated with proliferative diabetic retinopathy and macular oedema. Diabetologia. 2004;47(8):1376–1379. [DOI] [PubMed] [Google Scholar]
- 34.Wölfel EM, Jähn-Rickert K, Schmidt FN, et al. Individuals with type 2 diabetes mellitus show dimorphic and heterogeneous patterns of loss in femoral bone quality. Bone. 2020;140:115556. [DOI] [PubMed] [Google Scholar]
- 35.Ehrlich H, Hanke T, Frolov A, et al. Modification of collagen in vitro with respect to formation of Nepsilon-carboxymethyllysine. Int J Biol Macromol. 2009;44(1):51–56. [DOI] [PubMed] [Google Scholar]
- 36.Sroga GE, Vashishth D. Controlled formation of carboxymethyllysine in bone matrix through designed glycation reaction. JBMR Plus. 2021; 5(11):e10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basta G, Lazzerini G, Massaro M, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105(7):816–822. [DOI] [PubMed] [Google Scholar]
- 38.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63(4):582–592. [DOI] [PubMed] [Google Scholar]
- 39.Gaens KH, Goossens GH, Niessen PM, et al. Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34(6):1199–1208. [DOI] [PubMed] [Google Scholar]
- 40.Barzilay JI, Bůžková P, Zieman SJ, et al. Circulating levels of carboxy-methyl-lysine (CML) are associated with hip fracture risk: the Cardiovascular Health Study. J Bone Miner Res. 2014;29(5):1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamb LS, Alfonso H, Norman PE, et al. Advanced glycation end products and esRAGE are associated with bone turnover and incidence of hip fracture in older men. J Clin Endocrinol Metab. 2018;103(11): 4224–4231 [Published correction appears in Clin Endocrinol Metab. 2019;104(1):38.]. [DOI] [PubMed] [Google Scholar]
- 42.Harris TB, Visser M, Everhart J, et al. Waist circumference and sagittal diameter reflect total body fat better than visceral fat in older men and women. The Health, Aging and Body Composition Study. Ann N Y Acad Sci. 2000;904:462–473. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Frischmann M, Kientsch-Engel R, et al. Two immunochemical assays to measure advanced glycation end-products in serum from dialysis patients. Clin Chem Lab Med. 2005;43(5):503–511. [DOI] [PubMed] [Google Scholar]
- 44.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka S, Kuroda T, Saito M, Shiraki M. Urinary pentosidine improves risk classification using fracture risk assessment tools for postmenopausal women. J Bone Miner Res. 2011;26(11):2778–2784. [DOI] [PubMed] [Google Scholar]
- 46.Gineyts E, Munoz F, Bertholon C, Sornay-Rendu E, Chapurlat R. Urinary levels of pentosidine and the risk of fracture in postmenopausal women: the OFELY study. Osteoporos Int. 2010;21(2):243–250. [DOI] [PubMed] [Google Scholar]
- 47.Semba RD, Sun K, Schwartz AV, et al. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with arterial stiffness in older adults. J Hypertens. 2015;33(4):797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Napoli N, Schwartz AV, Schafer AL, et al. Vertebral fracture risk in diabetic elderly men: the MrOS study. J Bone Miner Res. 2018;33(1): 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanley DA, Brown JP, Tenenhouse A, et al. Associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: crosssectional results from the Canadian Multicentre Osteoporosis Study. J Bone Miner Res. 2003;18(4):784–790. [DOI] [PubMed] [Google Scholar]
- 50.Holmberg AH, Johnell O, Nilsson PM, et al. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos Int. 2006;17(7):1065–1077. [DOI] [PubMed] [Google Scholar]
- 51.Vestergaard P, Rejnmark L, Mosekilde L Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005; 48(7):1292–1299. [DOI] [PubMed] [Google Scholar]
- 52.Kanazawa I, Yamaguchi T, Yamamoto M, et al. Combination of obesity with hyperglycemia is a risk factor for the presence of vertebral fractures in type 2 diabetic men. Calcif Tissue Int. 2008;83(5):324–331. [DOI] [PubMed] [Google Scholar]
- 53.Piccoli A, Cannata F, Strollo R, et al. Sclerostin regulation, microarchitecture, and advanced glycation end-products in the bone of elderly women with type 2 diabetes. J Bone Miner Res. 2020;35(12):2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakano M, Nakamura Y, Suzuki T, et al. Pentosidine and carboxymethyl-lysine associate differently with prevalent osteoporotic vertebral fracture and various bone markers. Sci Rep. 2020; 10(1):22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karim L, Tang SY, Sroga GE, Vashishth D. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos Int. 2013;24(9):2441–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmad OS, Leong A, Miller JA, et al. A Mendelian randomization study of the effect of type-2 diabetes and glycemic traits on bone mineral density. J Bone Miner Res. 2017;32(5):1072–1081. [DOI] [PubMed] [Google Scholar]
- 57.Li CI, Liu CS, Lin WY, et al. Glycated hemoglobin level and risk of hip fracture in older people with type 2 diabetes: a competing risk analysis of Taiwan Diabetes Cohort Study. J Bone Miner Res. 2015;30(7): 1338–1346. [DOI] [PubMed] [Google Scholar]
- 58.Schneider AL, Williams EK, Brancati FL, Blecker S, Coresh J, Selvin E. Diabetes and risk of fracture-related hospitalization: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2013;36(5):1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oei L, Zillikens MC, Dehghan A, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabetes Care. 2013; 36(6):1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ, Blue Mountains Eye Study. Diabetes and risk of fracture: the Blue Mountains Eye Study. Diabetes Care. 2001;24(7):1198–1203. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz AV, Sellmeyer DE, Vittinghoff E, et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006; 91(9):3349–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ensrud KE, Lui LY, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167(2): 133–139. [DOI] [PubMed] [Google Scholar]
- 63.Dukas L, Schacht E, Stähelin HB. In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int. 2005;16(12):1683–1690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the NIA. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of NIA.


