Abstract
Alcohol exposure alters the signaling of the serotoninergic system, which is involved in alcohol consumption, reward and dependence. In particular, dysregulation of serotonin receptor type 1A (5-HT1AR) is associated with alcohol intake and withdrawal-induced anxiety- like behavior in rodents. However, how ethanol regulates 5-HT1AR activity and cell surface availability remains elusive. Using neuroblastoma 2a cells (N2A) stably expressing human 5-HT1ARs tagged with hemagglutinin (HA) at the N-terminus, we found that prolonged ethanol exposure (18 hrs) reduced the basal surface levels of 5-HT1ARs in a concentration-dependent manner. This reduction is attributed to both enhanced receptor internalization and attenuated receptor recycling. Moreover, constitutive 5-HT1AR internalization in ethanol naïve cells was blocked by concanavalin A (ConA) but not nystatin, suggesting clathrin-dependent 5-HT1AR internalization. In contrast, constitutive 5-HT1AR internalization in ethanol-treated cells was blocked by nystatin but not by ConA, indicating that constitutive 5-HT1AR internalization switched from a clathrin- to a caveolin-dependent pathway. Dynasore, an inhibitor of dynamin, blocked 5-HT1AR internalization in both vehicle- and ethanol-treated cells. Furthermore, ethanol exposure enhanced the activity of dynamin I via dephosphorylation and reduced myosin Va levels, which may contribute to increased internalization and reduced recycling of 5-HT1ARs, respectively. Our findings suggest that prolonged ethanol exposure not only alters the endocytic trafficking of 5-HT1ARs but also the mechanism by which constitutive 5-HT1AR internalization occurs.
Keywords: Ethanol, 5-HT1A receptors, constitutive internalization, constitutive recycling, clathrin, caveolin
Graphical Abstract
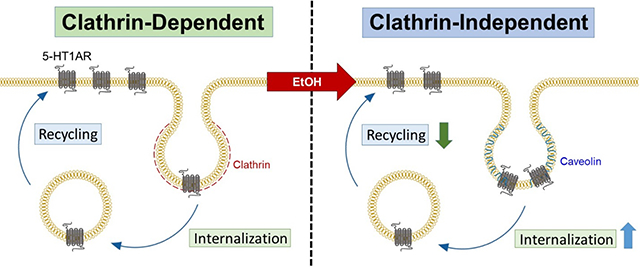
Prolonged ethanol exposure to neuroblastoma cells stably expressing hemagglutinin (HA)-tagged human serotonin type 1A (5-HT1A) receptors increases constitutive 5-HT1A receptor internalization and decreases its recycling, resulting in decreased basal surface 5-HT1A receptor levels. Moreover, ethanol exposure switches constitutive 5-HT1A receptor internalization from a clathrin-dependent to a clathrin-independent pathway. Changes in the expression and activity of proteins critically involved in endosomal trafficking may contribute to ethanol-mediated dysregulation of constitutive 5-HT1A receptor trafficking.
1. Introduction
The serotonergic system plays an important role in the pathology of alcohol use disorder (AUD) regulating alcohol consumption, preference, reward and dependence [see review in (Sari et al. 2011)]. Postmortem analysis of alcohol-dependent subjects shows increased serotonin (5-HT) turnover, and thus reduced serotonin levels, in the striatum (Kashem et al. 2016). Likewise, low levels of serotonin have been associated with enhanced ethanol intake and preference in rodents (Gongwer et al. 1989). Among the 5-HT receptors, 5-HT1A receptors (5-HT1ARs) are particularly interesting because of their distribution in the limbic system and their involvement in both alcohol intake and withdrawal-induced anxiety-like behavior (Belmer et al. 2018). The 5-HT1AR is a class A G-protein coupled receptor (GPCR) and couples to inhibitory Gαi/o proteins. When located on the dendrites of serotonergic neurons, 5-HT1ARs function as autoreceptors to inhibit 5-HT release (Hamon et al. 1988; Verge et al. 1985). When located on postsynaptic membranes of non-serotonergic neurons, 5-HT1ARs regulate the release of other neurotransmitters such as dopamine and norepinephrine (Benloucif & Galloway 1991; Schechter et al. 1990; Verge et al. 1986).
Ethanol exposure alters the availability of 5-HT1ARs on plasma membranes. For example, autoradiographic analysis of 5-HT1AR antagonist [3H]WAY-100635 on postmortem brain reveals a reduction in the density of membrane 5-HT1ARs in the perigenual anterior cingulate cortex of individuals with AUD (Storvik et al. 2009). Similarly, there is a drastic reduction (38–86%) in the binding of [3H]8-OH-DPAT, a 5-HT1AR agonist, in the frontal-parietal area of patients with AUD (Dillon et al. 1991). In contrast, chronic ethanol self-administration (12 months) without withdrawal increases 5-HT1AR levels in the posterior dentate gyrus of adult cynomolgus macaques measured by in vitro autoradiography using [3H]MPPF, a 5-HT1AR antagonist (Burnett et al. 2014). The discrepancy of these experiments is attributed to experimental variation in ethanol concentration, the duration and route of ethanol treatment, ethanol withdrawal length, and ligands used for measure of 5-HT1AR levels. To date, it is unknown whether ethanol exposure alters the availability of basal surface 5-HT1AR levels by influencing constitutive internalization, recycling, and/or degradation of 5-HT1ARs.
Upon agonist binding to 5-HT1ARs, receptors are phosphorylated and β-arrestins are recruited to the receptors to initiate receptor internalization. The internalized receptors are either recycled back to cell surface or targeted for degradation (Kumar & Chattopadhyay 2021). In addition to agonist-induced trafficking, 5-HT1ARs can undergo constitutive internalization, primarily through the clathrin-mediated pathway, and the majority of 5-HT1ARs are recycled back to plasma membranes via recycling endosomes (Kumar et al. 2019). Given that receptor trafficking influences the level of surface receptors and agonist binding, it is critical to understand how ethanol exposure regulates constitutive receptor internalization and recycling.
Previously, we reported that a single binge-like alcohol exposure to Sprague-Dawley rats (12 hr ethanol vapor exposure without withdrawal, ~200 mg/dL blood concentration levels) produced some aspects of changes that were also observed in neuroblastoma 2a (N2A) cells treated with ethanol (15–75 mM) for 18 hrs (Luessen et al. 2019). These changes include reduced arrestin expression, increased arrestin ubiquitination and an increased coupling between arrestin and an E3 ligase. Using this same ethanol treatment paradigm, we herein investigated the effect of ethanol exposure on constitutive internalization and recycling of 5-HT1ARs in N2A cells stably expressing human 5-HT1ARs. The expression and activity of key components involved in endosomal trafficking such as clathrin, dynamin I, and myosin Va were also determined following ethanol exposure.
2. Materials and Methods
The current study was not pre-registered. No randomization procedures were applied in the study. There were no pre-determined data exclusion criteria.
2.1. Materials
Ethanol was purchased from Invitrogen (cat#615101000). Concanavalin A (cat#C5275), nystatin (cat#N6261) and dynasore (cat#304448–55-3) were purchased from MilliporeSigma. All the other chemicals were purchased from MilliporeSigma or Thermo Fisher Scientific unless otherwise specified.
2.2. Cell culture
N2A cells stably expressing 5-HT1ARs with an HA-tag at the N-terminus (N2A-5-HT1ARs) were generated in our laboratory as described previously (Luessen et al. 2019). This cell line is not commonly misidentified by the International Cell Line Authentication Committee. Cells show consistent morphology and are routinely checked for mycoplasma contamination. Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco™ cat#10082147), 100 U/mL penicillin and streptomycin (Gibco™ cat#15140148). The stable expression of 5-HT1ARs was maintained in the presence of 400 μg/mL geneticin (Gibco™ cat#10131035). The cells were split every 4–5 days using 0.05% trypsin and 0.53 mM ethylenediaminetetraacetic acid (Invitrogen cat#AM9912). The passage number for experiments was between 9 and 12. No blinding procedure was used for experiments. Institutional ethics approval was not required for the present study.
2.2. Prolonged ethanol treatment to N2A-5-HT1AR cells
Cells were treated with ethanol at either 15, 30, or 75 mM concentration for 18 hrs prior to each experiment. Control cells were treated with culture medium. To maintain stable ethanol levels during the treatment, cell plates were placed within a closed polypropylene box alongside an open culture dish of a matching ethanol concentration. We previously reported a minimal loss of ethanol (3.13 ± 0.85%) over 18 hrs under this treatment condition (Luessen et al. 2019).
2.3. Immunocytochemistry of basal surface expression of 5-HT1ARs
To determine the effect of prolonged ethanol exposure on the surface expression of 5-HT1ARs, surface and intracellular receptors were labeled using different fluorophores. Briefly, cells were plated on coverslips, rinsed with cold phosphate buffered saline (PBS), and blocked with 4% normal goat serum (NGS) (Gibco™ cat#PCN5000) in PBS at 4°C for 30 min. Surface 5-HT1ARs were labeled with rabbit anti-HA (Novus Biologicals cat#NB600–363) for 60 min, followed by incubation with goat anti-rabbit Alexa Fluor 594 (Invitrogen cat#A-11012) for 45 min at 4°C. Cells were then fixed with 4% formaldehyde for 15 min and permeabilized using the blocking buffer containing 0.1% Triton x-100 for 10 min. Next, intracellular 5-HT1ARs were labeled with mouse anti-HA (MilliporeSigma, H3663) for 60 min and probed with goat anti-mouse Alexa Fluor 488 (Invitrogen cat#A-10680) for 45 min at room temperature. Coverslips were mounted using Prolong Gold mounting reagent (Invitrogen, P36930) and imaged via confocal microscopy. Surface 5-HT1AR levels were calculated as a ratio of the fluorescence intensity of Alexa Fluor 594 to that of Alexa Fluor 488 and 594 combined (surface/total 5-HT1ARs). Experiments were repeated at least three times and 15–25 cells from each group were analyzed from each experiment. Data are presented relative to the vehicle treatment.
Fluorescence images were captured using a Zeiss LSM 880 confocal laser scanning microscope equipped with a 60x/NA 1.4 PlanApo oil-immersion objective. Argon, DPSS, and Diode lasers were used to excite Alexa Fluor 488, Alexa Fluor 594, and DAPI signals at 488, 561, and 405 nm, respectively. All images were acquired under a similar excitation intensity, detector sensitivity, and pinhole settings to minimize cross-laser excitation and bleed-through and to allow for between-sample intensity comparisons. Images used for analysis were obtained by taking a Z-axis stack of image planes (1024 × 1024 pixels) with 2 μm steps encompassing the entire cell structure and combining image planes into a maximum intensity projection stack as described (Bonda et al. 2020; Shihan et al. 2021). The maximum fluorescence intensity was quantitated for each channel using FIJI software (NIH). Representative images were all modified using Gaussian blur for presentation purpose only.
2.4. Constitutive Internalization
The effect of prolonged ethanol exposure on constitutive internalization of 5-HT1ARs was measured using an antibody feeding technique as previously described (Luessen et al. 2019; Luessen et al. 2016). After blocking with 4% NGS for 30 min, surface 5-HT1ARs were labeled with rabbit anti-HA (Novus Biologicals cat#NB600–363) for 60 min at 4°C. Cells were then incubated at 37°C for 30 min to induce the internalization of the primary antibody-bound surface 5-HT1ARs. Internalization was terminated at 5, 15, or 30 min by washing with cold PBS and returning the cells to 4°C. The remaining primary antibody-bound surface 5-HT1ARs were probed using goat anti-rabbit Alexa Fluor 594 (Invitrogen cat#A-11012). Next, cells were fixed and permeabilized followed by probing internalized 5-HT1ARs with goat anti-rabbit Alexa Fluor 488 (Invitrogen cat#A32731TR). Images were obtained by taking a Z-axis stack of image planes using confocal microscopy as described above. The intensity of Alexa Fluor 488 and 594 was quantitated, and constitutive 5-HT1AR internalization was calculated as a ratio of fluorescence intensity of Alexa Fluor 488 over that of Alexa Fluor 594 and 488 combined (internalized/total surface 5-HT1ARs) at an indicated time. Data are presented relative to basal levels of 5-HT1ARs for each treatment condition.
To determine whether 5-HT1ARs underwent clathrin-, caveolin- or dynamin-dependent internalization, cells were treated with ConA (250 μg/mL, 1 hr at 37°C), nystatin (25 μg/ml, 30 min at 37°C), or dynasore (20 μM, 15 min at 37°C) prior to measure of receptor internalization. These experimental conditions were chosen based on previous publications (Luessen et al. 2016; Jones et al. 2012; Gao et al. 2019).
2.5. Constitutive Recycling
The effect of prolonged ethanol exposure on constitutive recycling of 5-HT1ARs was determined as described previously (Luessen et al. 2016). Surface 5-HT1ARs were labeled with rabbit anti-HA (Novus Biologicals cat#NB600–363) for 60 min at 4°C, followed by warming up cells to 37°C to induce receptor internalization. Any remaining, non-internalized antibody-bound 5-HT1ARs on the surface were blocked at 4°C using non-fluorescent secondary antibody goat anti-rabbit IgG-HRP (Cell Signaling Technology cat#7074P2). Cells were again warmed up to 37°C to allow internalized 5-HT1ARs to recycle back to the surface in 5, 15, or 30 min. The recycled 5-HT1ARs on the surface were probed with goat anti-rabbit Alexa Fluor 488 (Invitrogen cat#A32731TR). After fixation and permeabilization, internal (non-recycled) 5-HT1ARs were labeled using goat anti-rabbit Alexa Fluor 594 (Invitrogen cat#A-11012). Similar to internalization, the amount of recycling was calculated as a ratio of the intensity of Alexa Fluor 488 over that of Alexa Fluor 488 and 594 combined (recycled 5-HT1ARs/total surface 5-HT1ARs before assay) at an indicated time. Data are presented relative to basal 5-HT1AR levels for each treatment condition.
2.6. The co-localization of Rab proteins with 5-HT1ARs
To determine whether prolonged ethanol exposure altered the endocytic fate of 5-HT1ARs, the co-localizations of 5-HT1ARs with Rab4, Rab5, Rab11 and LAMP1 were determined by confocal microscopy. Briefly, following fixation and permeabilization, cells were incubated with either rabbit anti-HA tag (Novus Biologicals cat#NB600–363) or mouse anti-HA tag (MilliporeSigma cat#H3663) along with an antibody against Rab4 (mouse anti-Rab4A, Santa-Cruz cat#sc-312), Rab5 (mouse anti-Rab5, Santa-Cruz cat#sc-46692), Rab11 (rabbit anti-Rab11, Santa-Cruz cat#sc-9020), or LAMP1 (mouse anti-LAMP1, Thermo Fisher Scientific cat#MA1–164) for 60 min at room temperature. Then, secondary antibody goat anti-mouse Alexa Fluor 488 (Invitrogen cat#A-10680), goat anti-rabbit Alexa Fluor 488 (Invitrogen cat#A32731TR), goat anti-rabbit Alexa Fluor 594 (Invitrogen cat#A-11012), or goat anti-mouse Alexa Fluor 594 (Invitrogen cat#A-11005) was applied accordingly. The co-localizations of Rab proteins with 5-HT1ARs were quantified in a z-stack using the Coloc2 plugin in FIJI software (NIH) as previously described (Luessen et al. 2016; Luessen et al. 2019). The co-localization between Rab proteins and 5-HT1ARs was illustrated as yellow overlapped pixels. To determine the spatial overlap between Rab proteins and 5-HT1ARs, the background was removed and the intensity of each fluorescence signal was processed by thresholding. This method allows the conversion of an image from color to grayscale into a binary image so that images can be easily analyzed. Pearson’s correlation coefficients were generated for individual cells and averaged across cells for each group.
2.7. Biotinylation of surface 5-HT1ARs
As a complementary approach to immunofluorescence labeling of surface 5-HT1ARs, surface 5-HT1AR levels were also examined by surface biotinylation as previously described (Chen et al. 2013). Briefly, cells were washed with cold PBS and then incubated with non-membrane permeable sulfo-NHS-SS-biotin (1.5 mg/mL, Thermo Fisher Scientific cat#21331) in PBS/Ca/Mg for 90 min at 4°C. Excess biotin was quenched with 0.1 M glycine in PBS at 4°C for 15 min. Next, cells were solubilized using a buffer containing 50 mM Tris-base, 150 mM NaCl, and 1% Triton X-100 and protease and phosphatase inhibitor cocktails (MilliporeSigma cat#PPC1010). Biotinylated proteins were pulled down by streptavidin beads (Santa-Cruz, sc-2003) overnight at 4°C. Biotinylated proteins were then eluted from the beads with the SDS-loading buffer containing dithiothreitol (MilliporeSigma cat#10197777001). Total protein concentrations were measured using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific cat#23227).
Total and biotinylated proteins were separated by 10% SDS-PAGE gel electrophoresis and then transferred to nitrocellulose membranes (Thermo Fisher Scientific cat#88018). Membranes were blocked with 5% non-fat milk in Tris-buffered saline supplemented with 1% Tween-20. The 5-HT1ARs were probed with rabbit anti-HA (Novus Biologicals cat#NB600–363) at 4°C overnight followed by incubation with goat anti-rabbit IgG-HRP (Cell Signaling, cat#7074S). Proteins were visualized using North2Sourth Chemiluminescence Substrate Kit (Thermo Fisher Scientific cat#17295) or SuperSignal West Pico PLUS Chemiluminescence Substrate Kit (Thermo Fisher Scientific cat#34577). The signals were captured using a Bio-Rad Chemi Doc-Touch system. The density of each band was quantified using FIJI software. The amount of biotinylated 5-HT1ARs was determined as a ratio of biotinylated 5-HT1ARs to total 5-HT1ARs as we previously described for biotinylation of other membrane proteins (Chen et al. 2013; Furman et al. 2009; Chen et al. 2009; Chen et al. 2007). The intensity of total 5-HT1ARs is normalized to that of GAPDH (Cell Signaling cat#5174), an internal control. Data are presented as relative to the vehicle treatment for each condition.
2.8. Western blotting
Western blot was performed to measure the total levels of 5-HT1ARs, Rab proteins, clathrin, myosin V, phospho-dynamin I, and dynamin I in vehicle and ethanol-treated cells. Cells were lysed with a solubilization buffer containing protease and phosphatase inhibitor cocktails (MilliporeSigma cat#PPC1010). Equal amounts of proteins were loaded in 10% or 12% SDS-PAGE gels for gel electrophoresis and then transferred to nitrocellulose membranes. The following primary antibodies were used: rabbit anti-HA (Novus Biologicals cat# NB600–363), mouse anti-Rab4A (Santa-Cruz cat# sc-312), mouse anti-Rab5 (Santa-Cruz cat#sc-46692), rabbit anti-Rab11 (Santa-Cruz cat# sc-9020), mouse anti-clathrin heavy chain (Santa-Cruz cat#sc-12734), mouse anti-myosin Va (Santa-Cruz cat# sc-365986), mouse anti-phospho-dynamin-I (DHSB cat#3D3), and rabbit anti-dynamin-I (Invitrogen cat#pa1–660). The secondary antibodies including goat anti-mouse IgG-HRP (Invitrogen cat# G-21040) and goat anti-rabbit IgG-HRP (Cell Signaling cat# cat#7074S) were applied accordingly. The band intensity was quantified using FIJI software and the relative intensity of each band was normalized to that of GAPDH. Data are presented as relative to the vehicle treatment for each condition.
2.9. Statistical Analysis
GraphPad Prism 9 (Graphpad, San Diego, CA) was used for data plotting and analysis. No sample size calculations were performed and the number of repeat for each type of experiment was determined based on previous publications (Luessen et al. 2019; Luessen et al. 2016; Chen et al. 2013; Furman et al. 2009). Data were not analyzed for normality prior to statistical analyses. Non-parametric analyses were not performed. All data are presented as mean ± SEM. Differences in 5-HT1AR surface expression, total protein expression and the co-localization were determined using a one-way analysis of variance (ANOVA). A two-way ANOVA was applied to examine group differences in the time course of constitutive internalization and recycling. A post hoc Bonferroni test was performed when necessary. A value of p ≤ 0.05 was considered statistically significant. Statistical significance is denoted as *p<0.05, **p<0.01, ***p<0.001. For all figures, outliers in each data set were determined using Grubb’s test when p<0.05. One outlier was removed from Figure 5E.
Figure 5.
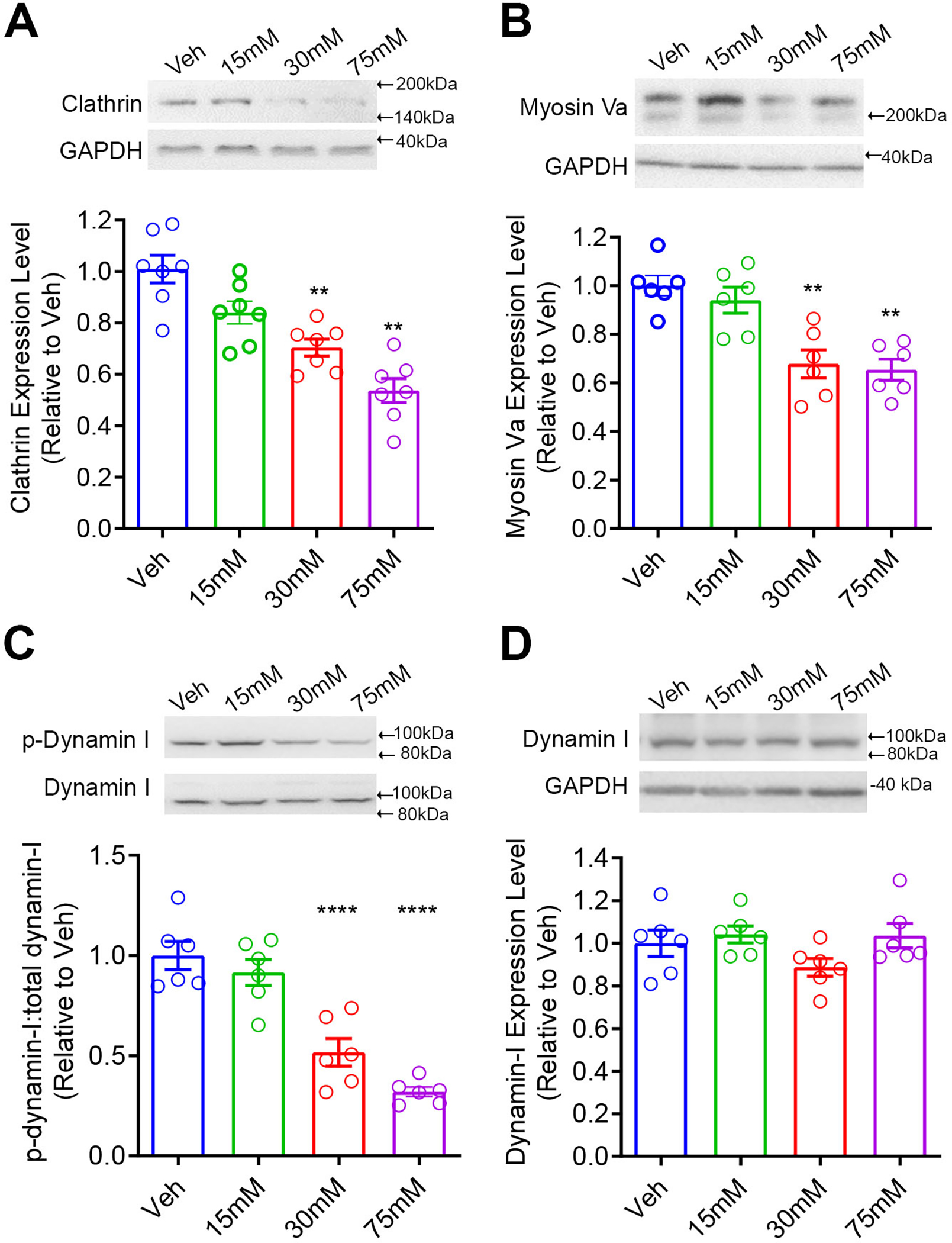
Prolonged ethanol exposure changes the expression and activity of proteins involved in endosomal internalization and recycling. The levels of clathrin (A) and myosin Va (C) were reduced in cells treated with 30 mM and 75 mM ethanol when compared to vehicle (Veh) treatment. (B) Ethanol reduced dynamin I phosphorylation and had no effect on total dynamin I in cells treated with 30 mM and 75 mM ethanol when compared to Veh. N=6 replicates per group. ** p<0.01, ****p<0.0001 vs. Veh
3. Results
3.1. Ethanol exposure reduced surface levels of 5-HT1ARs
To examine the effect of prolonged ethanol exposure on 5-HT1AR surface expression, immunocytochemistry was performed 18 hrs after treatment with ethanol (15, 30, or 75 mM) or vehicle. Surface and intracellular 5-HT1ARs were immunolabeled with Alexa Fluor 594 (red) and 488 (green), respectively (Fig. 1A). The surface 5-HT1AR level was calculated as a ratio of surface fluorescence intensity (red) to that of the total fluorescence intensity (red + green) for each cell and presented as relative to the vehicle treatment (Fig. 1B). A one-way ANOVA revealed a significant main effect of ethanol concentration on surface 5-HT1AR levels, F (3, 182) = 28, p<0.01. Post hoc Bonferroni test showed a significant reduction in surface 5-HT1AR levels in cells treated with 30 mM (p<0.05, N=47 cells) and 75 mM (p<0.001, N=42 cells) ethanol when compared to vehicle treatment (N=49 cells). However, treatment with 15 mM ethanol (N=50 cells) did not alter surface 5-HT1AR levels.
Figure 1.
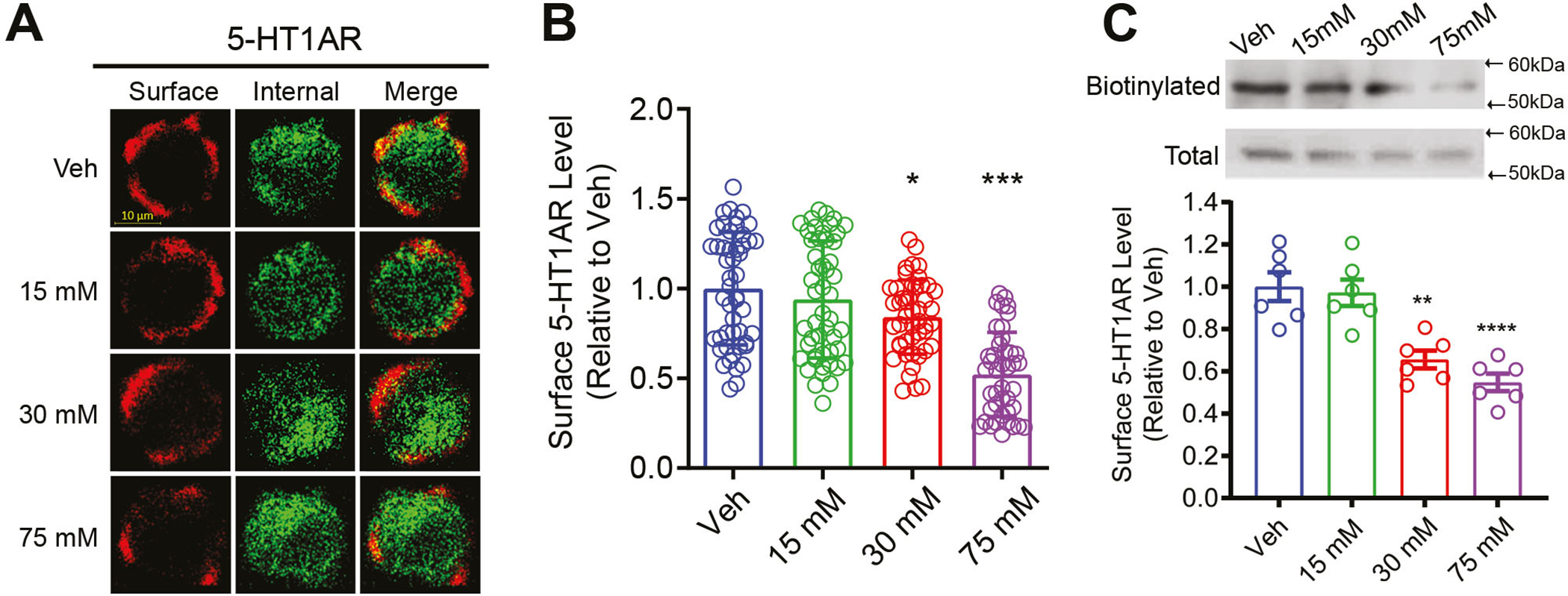
Prolonged ethanol exposure reduced surface 5-HT1AR expression in N2A cells. (A) Representative immunofluorescence images of surface and intracellular 5-HT1ARs in vehicle- (Veh) and ethanol-treated cells. (B) Quantification of surface 5-HT1ARs in cells treated with Veh (N=49 cells), 15 mM (N=50 cells), 30 mM (N=47 cells) or 75 mM (N=42 cells) ethanol assessed by immunocytochemistry. Surface 5-HT1ARs were determined by a ratio of surface to total 5-HT1ARs and presented as relative to Veh. Ethanol exposure dose-dependently reduced surface 5-HT1AR levels. (C) Upper panel: representative western blot images for biotinylated surface and total 5-HT1ARs; lower panel: quantification of biotinylated surface 5-HT1AR levels in Veh- and ethanol-treated cells. Surface 5-HT1AR levels were normalized to total 5-HT1AR levels and represented as relative to Veh (N=6 replicates per group). Ethanol exposure significantly reduced surface 5-HT1AR level in a dose-dependent manner. * p<0.01, ***p<0.001 vs. Veh
Surface biotinylation was performed to further confirm the results observed from the immunocytochemistry assay. Surface 5-HT1ARs were biotinylated with non-membrane permeable sulfo-NHS-SS-biotin. The density of biotinylated 5-HT1ARs was normalized to total levels of 5-HT1ARs. Data are presented as relative to the vehicle treatment (Fig. 1C). A one-way ANOVA indicated a significant main effect of ethanol concentration on surface 5-HT1AR levels, F (3, 20) = 16.98, p<0.01. Post hoc Bonferroni test further showed a significant reduction of surface 5-HT1AR levels in cells treated with 30 mM (p=0.0014, N=6 replicates) and 75 mM (p<0. 0001, N=6 replicates) ethanol when compared to the vehicle treatment. Treatment with 15 mM ethanol (N=6 replicates) did not change surface 5-H1AR levels. Because ethanol exposure did not alter the total 5-HT1AR levels at any of the concentrations tested (Fig. S1), the reduction in surface receptor levels is likely due to a change in intracellular trafficking of 5-HT1ARs to different endosomes.
3.2. Ethanol exposure increased constitutive internalization of 5-HT1ARs
We next determined whether reduced surface 5-HT1AR levels in ethanol-treated cells resulted from increased constitutive internalization of receptors using a previously established protocol (Luessen et al. 2016). The amount of internalization was calculated as the fluorescence intensity of internalized 5-HT1ARs (green) over the intensity of total 5-HT1ARs (red + green) at a specified time (see Figs. 2A & 2B for representative images). For each treatment group, data are presented as relative to their own baseline (Fig. 2C, N=46–52 cells/time point). A two-way ANOVA (concentration x time) revealed significant main effects of ethanol concentration, F (3,589) = 23.7, p<0.01; and time, F (2,589) = 11.5, p<0.01 on receptor internalization. Post hoc Bonferroni test showed that 30 mM ethanol treatment increased constitutive 5-HT1AR internalization at 30 min (p<0.05), whereas 75 mM ethanol increased 5-HT1AR internalizations at 5 min (p<0.05), 15 min (p<0.0001) and 30 min (p<0.0001) when compared to the vehicle treatment. However, 15 mM ethanol treatment did not change receptor internalization.
Figure 2.
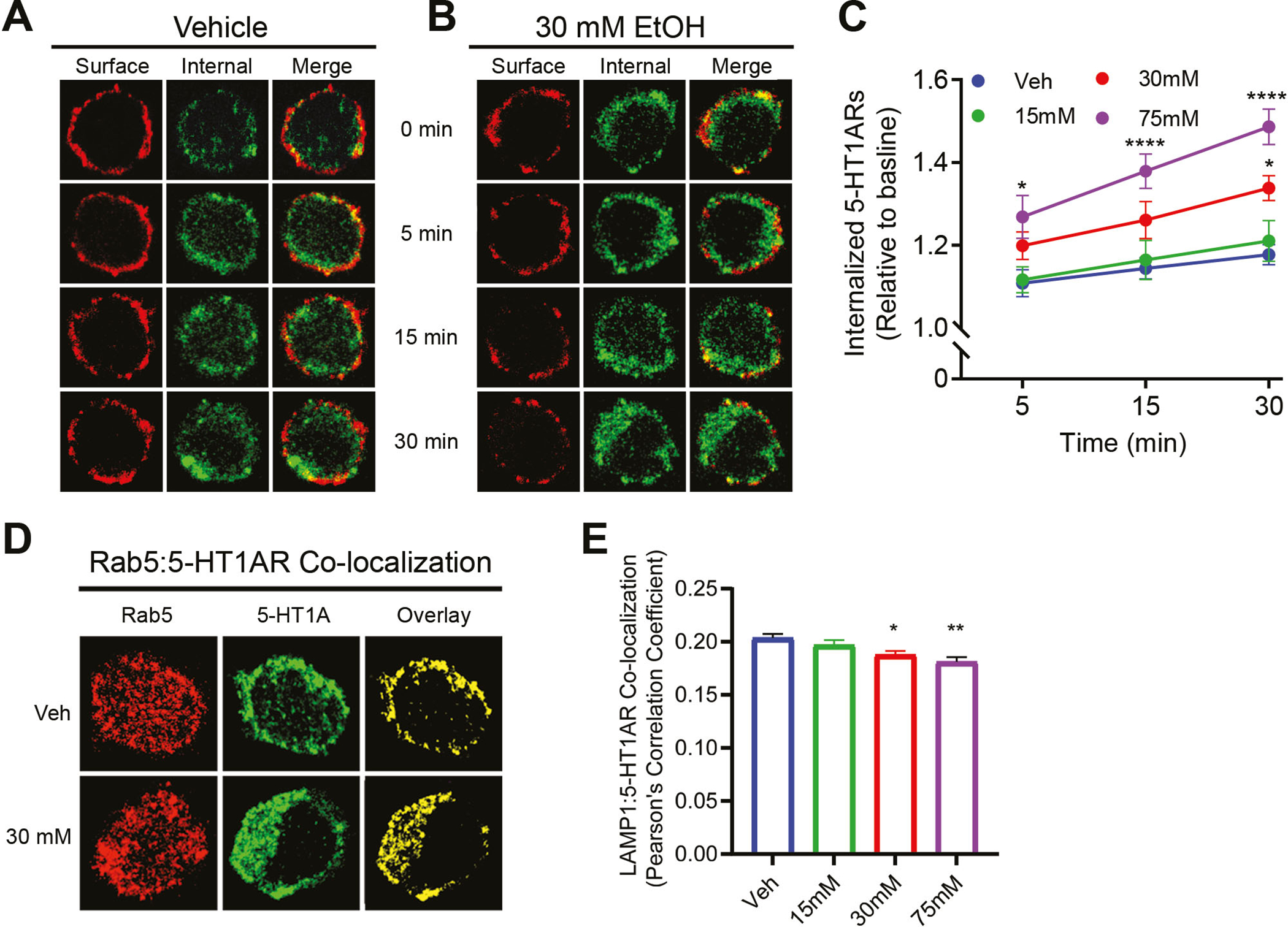
Prolonged ethanol exposure increased constitutive 5-HT1AR internalization in a dose-dependent manner. (A) and (B) Representative images of surface and internalized 5-HT1ARs in Veh and 30 mM ethanol-treated cells across time. (C) Quantification of constitutive 5-HT1AR internalization across time. Ethanol dose-dependently increased constitutive 5-HT1AR internalization. For the vehicle group, the number of cells analyzed for 5 min, 15 min and 30 min is 55, 53 and 58, respectively. For the 15 mM ethanol-treated group, the number of cells analyzed for 5 min, 15 min and 30 min is 46, 50 and 46, respectively. For the 30 mM ethanol-treated group, the number of cells analyzed for 5 min, 15 min and 30 min is 50, 46 and 52, respectively. For the 75 mM ethanol-treated group, the number of cells analyzed for 5 min, 15 min and 30 min is 47, 49 and 49, respectively. (D) Representative confocal images for Rab5, 5-HT1ARs and their co-localization in Veh and 30 mM EtOH-treated cells. (E) Quantification of Rab5 and 5-HT1ARs co-localization following treatment with vehicle (N=57 cells) or ethanol at 15 mM (N=54 cells), 30 mM (N=50 cells) and 75 mM (N=55 cells). There was a dose-dependent increase in the co-localization. *p<0.05, ** p<0.01, ****p<0.0001 vs. Veh
We next examined the co-localization of 5-HT1ARs with Rab5 to further confirm that ethanol exposure increased receptor internalization. Rab5, a common marker for early endosomes, regulates early endosome-mediated sorting of intracellular cargos into recycling and degradation compartments (Nielsen et al. 1999). The co-localization between 5-HT1ARs (green) and Rab5 (red) was calculated based on the number of spatially overlapped yellow pixels (Figs. 2D) followed by conversion into a Pearson’s correlation coefficient using FIJI ImageJ software (Fig. 2E). A one-way ANOVA indicated a significant main effect of ethanol concentration on co-localization, F (3, 212) = 9.250, p<0.0001. Post hoc Bonferroni test indicated a significant increase in the co-localization between 5-HT1ARs and Rab5 in cells treated with ethanol at 30 mM (p<0.01, N=50 cells) and 75 mM (p<0.0001, N=55 cells) when compared to the vehicle treatment (N=57 cells). Because ethanol did not change the total levels of 5-HT1ARs (Fig. S1) and Rab5 (Fig. S2A), the increased co-localization was due to a greater amount of 5-HT1ARs compartmentalized in Rab5-positive endosomes.
3.3. Ethanol exposure induced the clathrin-independent, constitutive internalization of 5-HT1AR
The 5-HT1ARs can undergo the clathrin-dependent internalization in LLC-CPK1 cells (Bouaziz et al. 2014). Herein, we demonstrated in N2A cells that constitutive internalization of 5-HT1ARs in ethanol naïve cells was also clathrin- and dynamin-dependent because both ConA and dynasore treatment diminished 5-HT1AR internalization. ConA binds to surface glycoproteins and prevents protein mobility within the membrane bilayers, resulting in blockade of clathrin-mediated GPCR internalization (Luttrell et al. 1997). Dynasore is an inhibitor of dynamin GTPase activity and blocks dynamin-dependent endocytosis (Macia et al. 2006). A two-way ANOVA indicated that ConA treatment (N=61 cells) abolished constitutive 5-HT1AR internalization when compared to the vehicle treatment (N=120 cells) in ethanol naïve cells (p<0.0001); in contrast, ConA treatment (N=54 cells) had no effect on 5-HT1AR internalization in cells treated with 30 mM ethanol (N=100 cells). Dynasore treatment prevented receptor internalization in both vehicle-and ethanol-treated cells (N=65 cells/group).
To investigate whether 5-HT1ARs in ethanol-treated cells underwent the caveolin-dependent internalization, cells were treated with nystatin, which is a sterol-binding agent that disassembles caveolae and cholesterol in the membranes and thus impairs the caveolin-dependent internalization (Rothberg et al. 1992). Nystatin treatment prevented constitutive 5-HT1AR internalization in ethanol-treated cells (N=100 cells and N=54 cells for vehicle and nystatin treatment, respectively) but had no effect in ethanol-naïve cells (Fig. 3, N=120 cells and N=54 cells for vehicle and nystatin treatment, respectively). These data suggest that prolonged ethanol exposure changed constitutive internalization of 5-HT1ARs from a clathrin-dependent to a caveolin-dependent pathway.
Figure 3.
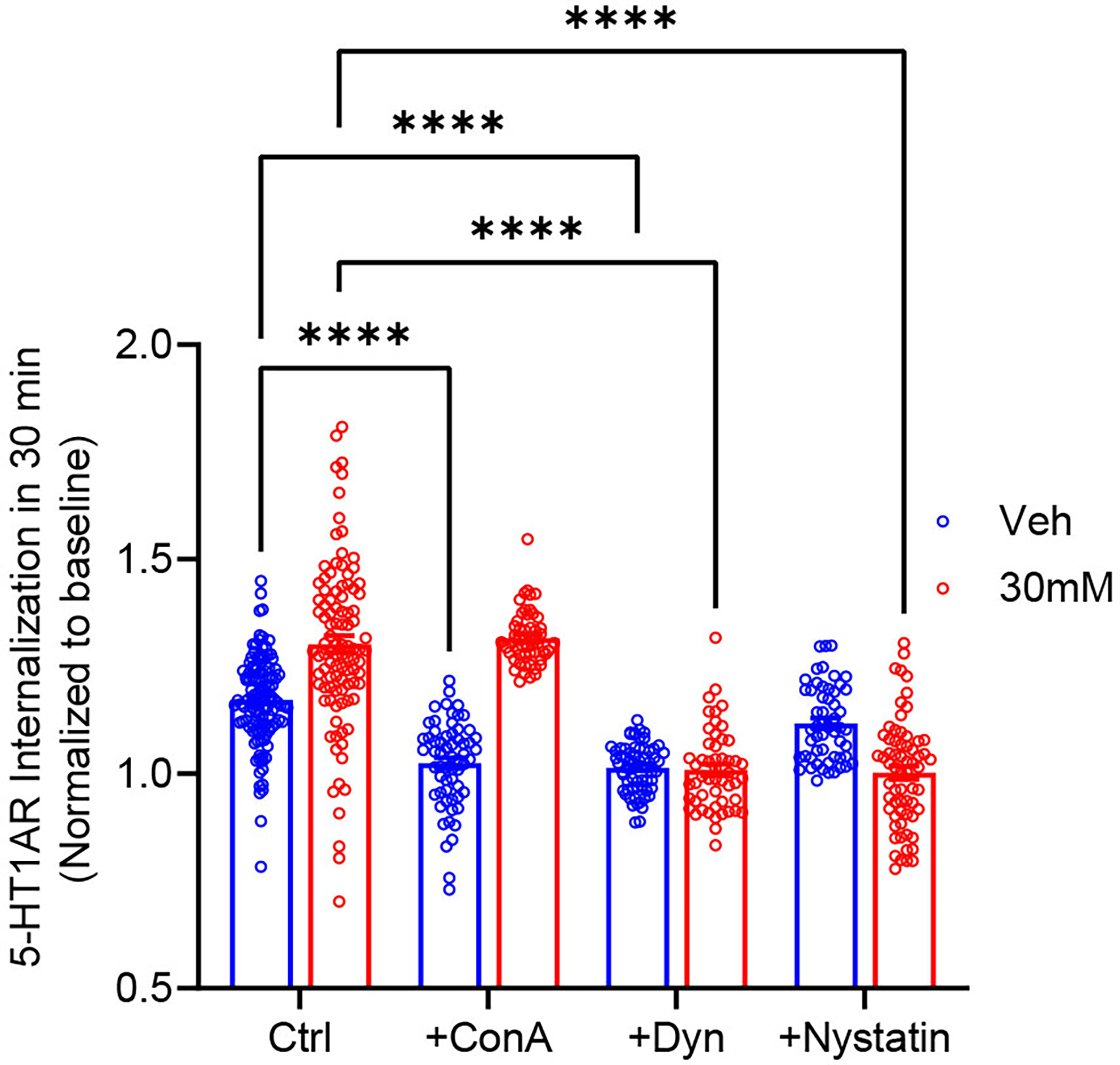
Prolonged ethanol exposure shifted constitutive 5-HT1AR internalization from a clathrin-dependent to a caveolin-dependent pathway. The constitutive 5-HT1AR internalization in 30 mM ethanol-treated cells (N=100 cells) was greater when compared to the vehicle treatment (N=120 cells). Pretreatment with concanavalin A (ConA, 250 μg/ml, 1hr at 37°C) blocked receptor internalization in Veh-treated cells (N=61 cells) but not in 30 mM ethanol-treated cells (N=54 cells). Nystatin treatment (25 μg/ml, 30 min at 37°C) diminished receptor internalization in 30 mM ethanol-treated cells (N=65 cells) and had no effect in Veh-treated cells (N=54 cells). Dynasore treatment (20 μM, 15 min at 37°C) abolished constitutive internalization in both Veh- (N=65 cells) and 30 mM ethanol-treated cells (N=52 cells). ****p<0.0001 vs. Veh
3.4. Ethanol exposure decreased constitutive recycling of 5-HT1ARs
Next, we examined whether the reduction in surface 5-HT1AR levels was attributed to altered receptor recycling. The constitutive recycling of 5-HT1ARs was measured using a similar procedure for constitutive internalization. The internalized, recycled 5-HT1ARs were labeled with Alexa Flour 488 (green) while the internalized, non-recycled, 5-HT1ARs were labeled with Alexa Flour 594 (red). Representative images are shown in Figs. 4A & 4B. The amount of receptor recycling was calculated as the intensity of recycled 5-HT1ARs (green) over total intensity of recycled and non-recycled (red + green) at a specified time, and normalized to its baseline for each treatment group (Fig. 4C, N=43–69 cells/time point). A two-way ANOVA revealed significant main effects of ethanol concentration, F (3, 867) = 68.4, p<0.01; time, F (3, 867) = 107, p<0.01; and a significant interaction effect, F (9, 867) = 9.02, p<0.01, on receptor recycling. Post hoc Bonferroni analysis indicated a significant reduction in receptor recycling in cells treated with 30 mM and 75 mM ethanol at all tested time points when compared to the vehicle treatment.
Figure 4.
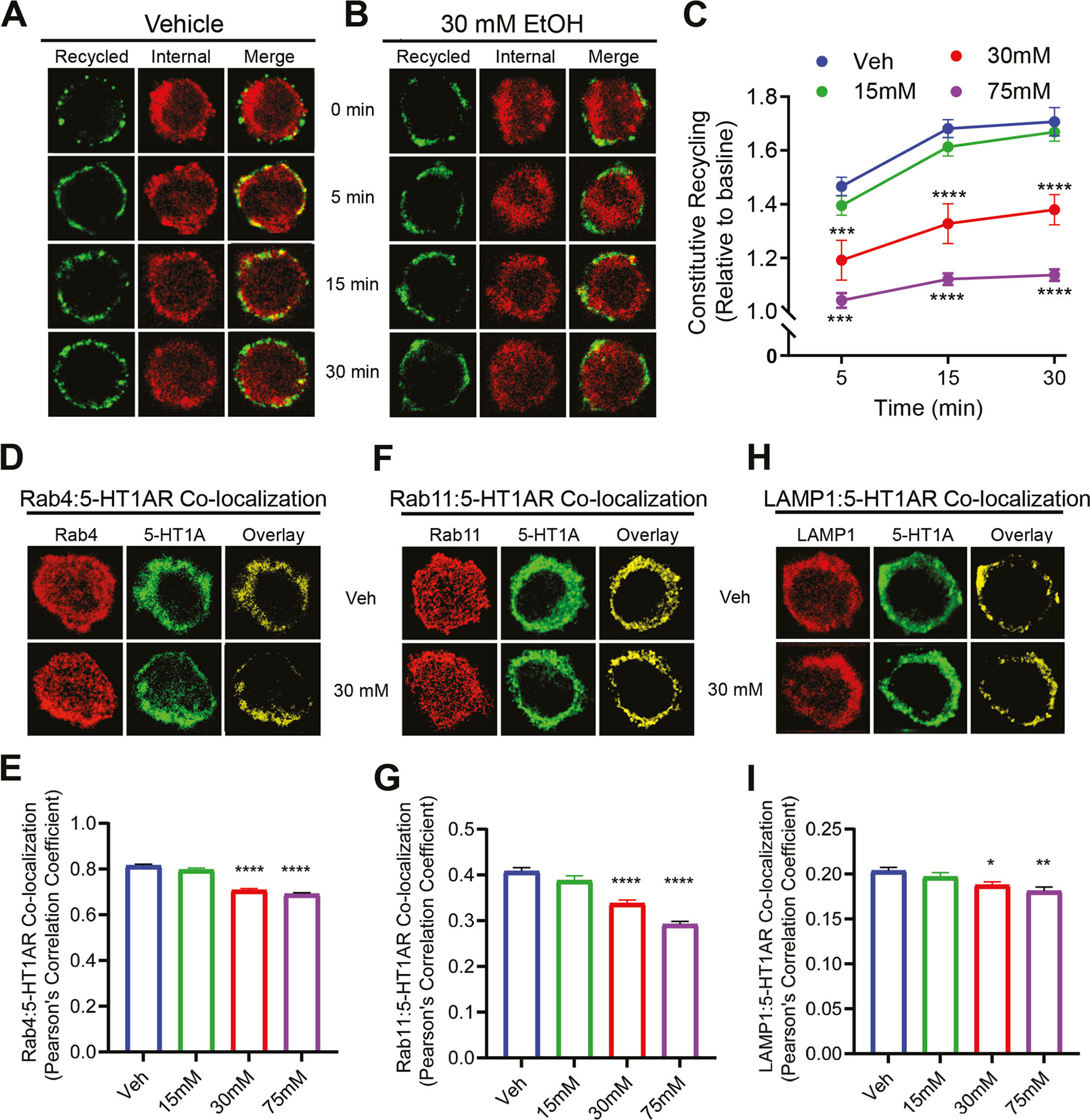
Prolonged ethanol exposure dose-dependently attenuated 5-HT1AR recycling. (A) and (B) Representative images of recycled and total internalized receptors from vehicle (Veh)- and ethanol-treated cells. (C) Quantitation of internalized and recycled 5-HT1ARs across time. Ethanol treatment (30 mM and 75 mM) significantly attenuated 5-HT1AR recycling when compared to Veh. For the Veh group, the number of cells analyzed for 5 min, 15 min and 30 min is 43, 60 and 53, respectively. For the 15 mM ethanol-treated group, the number of cells analyzed for 5 min, 15 min and 30 min is 59, 49 and 56, respectively. For the 30 mM ethanol-treated group, the number of cells analyzed for 5 min, 15 min and 30 min is 51, 62 and 49, respectively. For the 75 mM ethanol-treated group, the number of cells analyzed for 5 min, 15 min and 30 min is 46, 55 and 52, respectively. Representative confocal images for 5-HT1AR co-localization with Rab4 (D), Rab11 (F) and LAMP1 (H). (E) Quantification of 5-HT1AR co-localization with Rab4 in cells treated with Veh (N=54 cells) or ethanol at 15 mM (N=51 cells), 30 mM (N=52 cells) and 75 mM (N=50 cells). (G) Quantification of 5-HT1AR co-localization with Rab11 in cells treated with Veh (N=60 cells) or ethanol at 15 mM (N=56 cells), 30 mM (N=52 cells) and 75 mM (N=57 cells). (I) Quantification of 5-HT1AR co-localization with LAMP1 in cells treated with Veh (N=53 cells) or ethanol at 15 mM (N=52 cells), 30 mM (N=57 cells) and 75 mM (N=48 cells). Ethanol decreased co-localization of 5-HT1ARs with Rab4, Rab11 and LAMP1 at 30 mM and 75 mM doses when compared to Veh. *p<0.05, **p<0.01, ****p<0.001 vs. Veh
To further confirm that prolonged ethanol treatment attenuated 5-HT1AR recycling, we determined the co-localization of 5-HT1ARs (green) with Rab4 (red) and Rab11 (red), markers for fast recycling and slow recycling endosomes, respectively (Seachrist & Ferguson 2003). The co-localization was calculated as the number of spatially overlapped yellow pixels (Figs. 4D & 4F) followed by the conversion into a Pearson’s co-localization coefficient (Figs. 4E & 4G). A one-way ANOVA revealed a significant main effect of ethanol concentration on the co-localization of 5-HT1ARs with Rab4, F (3, 203) = 108.1, p < 0.0001; and with Rab11A, F (3, 221) = 44.95, p < 0.0001. Post hoc Bonferroni analysis showed a significant decrease in the co-localization of 5-HT1ARs with Rab4 and Rab11 at both 30 mM (p<0.05, N=52 cells) and 75 mM (p<0.01, N=52–57 cells) ethanol concentrations when compared to the vehicle treatment (N=54–60 cells). Prolonged ethanol exposure did not change the total levels of Rab4 and Rab11 proteins (Figs. S2B & S2C) nor total 5-HT1AR levels (Fig. S1), suggesting that the decreased co-localization resulted from a less amount of 5-HT1ARs in both Rab4- and Rab11-positive recycling endosomes.
Next, we examined the co-localization of 5-HT1ARs with LAMP1, a marker for lysosomes, to measure whether 5-HT1ARs underwent degradation (Figs. 4H & I). A one-way ANOVA indicated a significant main effect of ethanol concentration on the co-localization, F (3, 206) = 6.66, p<0.001. Post hoc Bonferroni test showed a significant decrease in receptor co-localization with LAMP1 at 30 mM (p<0.05, N=57) and 75 mM (p<0.01, N=48) when compared to the vehicle treatment (N=53).
3.5. Ethanol exposure reduced the levels of clathrin and myosin V
Because clathrin and myosin V are involved in vesicle endocytosis and exocytosis, respectively, we performed western blot to assess their expression levels. We are particularly interested in myosin Va subtype as it is a vesicle transporting motor protein highly associated with exocytosis activity in neurons (Mercer et al. 1991). A one-way ANOVA revealed a significant main effect of ethanol concentration on the levels of clathrin, F (3, 24) = 19.81, p<0.01. Post hoc Bonferroni analysis revealed an unexpected, significant decrease in clathrin levels in cells treated with 30 mM (p <0.01) and 75 mM (p<0.01) ethanol when compared to the vehicle treatment (Fig. 5A, N=7 replicates/group). A one-way ANOVA indicated a significant main effect of ethanol concentration on myosin Va expression levels, F (3, 20) = 12.82, p<0.01. Post hoc Bonferroni analysis showed a significant reduction in myosin Va levels in cells treated with ethanol at concentrations of 30 mM (p <0.01) and 75 mM (p <0.01) (Fig. 5B, N=6 replicates/group) when compared to the vehicle treatment.
3.6. Ethanol exposure inhibited dynamin I phosphorylation
Dynamins are a family of GTPases involved in the trafficking of both early endosomes and recycling endosomes. The isoform dynamin I plays an important role in the clathrin-mediated internalization in neurons. Upon phosphorylation, dynamin I is localized in the cytoplasm; in contrast, dephosphorylate dynamin I (active) is localized on the membranes for membrane fission (McClure & Robinson 1996). We measured the levels of phosphorylated (p-dynamin I) and the total dynamin I in both vehicle- and ethanol-treated cells (Fig. 5C). The p-dynamin I was normalized by the total dynamin I and presented as relative to the vehicle treatment. A one-way ANOVA revealed a significant main effect of ethanol concentration on dynamin I phosphorylation, F (3, 20) = 28.86, p<0.01. Post hoc Bonferroni analysis showed a significant reduction in p-dynamin I levels in cells treated with 30 mM (p<0.01) and 75 mM (p<0.01) ethanol when compared to the vehicle treatment (N=6 replicates/group), indicating that there was a greater amount of active (non-phosphorylated) dynamin I located on the membranes to promote receptor internalization. There was no change in the levels of the total dynamin I between groups (Fig. 5D, N=6 replicates/group).
4. Discussion
The present study showed that prolonged ethanol exposure reduced basal surface levels of 5-HT1ARs by accelerating constitutive receptor internalization and attenuating constitutive receptor recycling in N2A cells. Significantly, ethanol exposure switched constitutive internalization of 5-HT1ARs from a clathrin-dependent to a caveolin-dependent pathway. Moreover, ethanol increased active dynamin I levels and decreased myosin Va levels, which may be associated with increased internalization and reduced recycling of 5-HT1ARs, respectively.
Our data demonstrate that 5-HT1ARs underwent constitutive trafficking in the presence and absence of ethanol in N2A cells, which is consistent with observation of constitutive 5-HT1AR internalization and recycling in LLC-PK1 cells and primary cultures of rat serotonergic raphe and hippocampal neurons (Bouaziz et al. 2014). The co-localizations of 5-HT1ARs with Rab4/Rab11/LAMP1 proteins indicate that internalized receptors are targeted for both recycling and degradation. A significant finding of the current study is that prolonged ethanol exposure increased the internalization and attenuated the recycling of 5-HT1ARs at 30 mM and 75 mM concentrations resulting in decreased basal levels of surface 5-HT1ARs. To date, there is no report of ethanol modulation of constitutive internalization and recycling of any GPCR; however, effects of ethanol on the surface availability of GPCRs including 5-HT1ARs have been observed (Dillon et al. 1991; Burnett et al. 2014). Our data suggest that ethanol impacts receptor surface expression by disrupting endocytic trafficking between plasma membranes and endosomes in a concentration dependent manner. This modulation is likely dependent on treatment duration. It has been reported that short-term ethanol treatment (≤ 2 hr) increased surface levels of dopamine transporters (DAT) in human neuroblastoma SK-N-SH cells and this increase is associated with increased reuptake activity of DAT (Riherd et al. 2008). Moreover, chronic ethanol exposure results in hypersensitization of 5-HT1ARs in mice (Kelai et al. 2008). This sensitization may serve as a compensatory mechanism for reduced basal 5-HT1AR surface expression in order to maintain serotoninergic signaling. As such, the altered 5-HT1AR trafficking in the present study may lead to increased 5-HT1AR activity. However, it has been shown that ethanol application to membranes modulates the coupling between G-proteins and 5-HT1ARs in a concentration-dependent manner (Harikumar & Chattopadhyay 2000; Harikumar & Chattopadhyay 1998). Future studies show examine the relationship between 5-HT1AR trafficking and receptor function.
The second significant finding is that prolonged ethanol exposure increased constitutive 5-HT1AR internalization in a clathrin-independent manner. The 5-HT1ARs undergo constitutive internalization through clathrin-mediated endocytosis shown in LLC-PK1 cells as a high concentration of sucrose (0.35 M), an inhibitor of clathrin-coated pits-mediated endocytosis, diminishes constitutive 5-HT1AR internalization (Bouaziz et al. 2014). In agreement, we herein also report that constitutive 5-HT1AR internalization in ethanol-naïve N2A cells was reduced by treatment with ConA and dynasore, inhibitors of clathrin- and dynamin-mediated endocytosis. Interestingly, ethanol-mediated increase in constitutive 5-HT1AR internalization was blocked by dynasore but not by ConA, suggesting a clathrin-independent endocytotic pathway. To determine whether caveolin-mediated endocytosis is involved in 5-HT1AR internalization, we incubated cells with nystatin, a blocker of caveolin-dependent endocytosis, and found that nystatin prevented constitutive 5-HT1AR internalization in ethanol-treated cells but had no effect in vehicle-treated cells. These data suggest that ethanol treatment shifted constitutive 5-HT1AR internalization from a clathrin-dependent to a caveolin-dependent pathway. Although the exact molecular mechanism for this switch is unknown, it may be related to ethanol modulation of lipid composition on the membranes. Ethanol exposure has been shown to alter the content of lipid species including polyunsaturated fatty acid (Duffy et al. 1991), ceramide (Godfrey et al. 2015) and cholesterol (Saito et al. 2007) on cell membranes. The compartmental localization of 5-HT1ARs on plasma membranes is influenced by lipid content including cholesterol and sphingolipids (Kumar & Chattopadhyay 2020; Kumar & Chattopadhyay 2021; Jafurulla et al. 2008; Ganguly et al. 2011). As such, 5-HT1ARs may not be properly inserted and positioned on plasma membranes and therefore are susceptible to internalization. Alternatively, ethanol may have redistributed 5-HT1ARs to caveolin-enriched microdomains on the membranes and these receptors are subject to greater internalization under ethanol treatment. Interestingly, high concentrations of ethanol, especially 75 mM ethanol, appears to induce clusters of receptors non-homogenously on plasma membranes when compared to the vehicle treatment (Fig.1A) and these clusters are presumably cholesterol-rich domains, further supporting the notion that ethanol exposure may place more receptors in cholesterol-enriched domains. Future studies should investigate compartmentalization of 5-HT1ARs in lipid raft and non-raft microdomains to confirm this presumption. It is worth noting that there is a small but notable overlap between the two fluorophores for surface and intracellular 5-HT1AR immunolabeling. One potential explanation is that 5-HT1ARs located in the cytoskeleton is in a close proximity to surface 5-HT1ARs, resulting in fluorescent signal overlapping. Lastly, dynasore exerts both dynamin-dependent and dynamin-independent effects. In addition to inhibit the GTPase activity of dynamin, dynasore can also disrupt lipid rafts via a dynamin-independent mechanism (Preta et al. 2015); therefore, dynasore treatment in our experiments could affect both clathrin- and caveolin-dependent receptor internalization.
Ethanol-induced acceleration in constitutive 5-HT1AR internalization may be mediated by increased availability of active dynamin I. Dynamin regulates plasma membrane fission for both clathrin- and caveolin-mediated endocytosis [see review in (Sandvig et al. 2018)]. Dynamin assembles into polymers on the necks of budding membranes in cells, undergoes GTP-dependent conformational changes, and thus catalyzes membrane fission. The dynamin I is solely expressed in the brain and is critical for receptor endocytosis and vesicle infusion to membranes [see review in (Smillie & Cousin 2005)]. In intact synaptosomes prepared from the whole rat brain, dynamin I is present in both the cytosol and vesicle membranes; however, phosphorylated dynamin I is restricted to the cytosol (Liu et al. 1994). Moreover, it appears that dephosphorylated dynamin I (active) traffics to the membranes to facilitate membrane fission (McClure & Robinson 1996). In the present study, we found that ethanol exposure reduced phosphorylation of dynamin I; thus, it is tempting to speculate that ethanol exposure led to a greater distribution of active dynamin I on the membranes, which in turn contributed to increased constitutive endocytosis of 5-HT1ARs. Dynamin I can be phosphorylated by protein kinase C (PKC) and cyclin-dependent kinase 5 (Cdk5), and dephosphorylated by calcineurin (Smillie & Cousin 2005). It has been shown in vitro that ethanol treatment inhibits calcineurin phosphatase activity (Ohashi et al. 2004). Moreover, prolonged exposure to ethanol decreases PKC activity in the hippocampus and the cortex of rat brain (Battaini et al. 1989). Therefore, it is possible that prolonged ethanol exposure disrupts the balance between phosphorylation and dephosphorylation of dynamin I by decreasing the activity of PKC and Cdk5 and/or increasing the activity of calcineurin, resulting in decreased phosphorylation of dynamin I and increased 5-HT1AR internalization. Given that ethanol exposure reduced the total expression levels of clathrin and caveolin (not shown), it would be interesting to further investigate whether our observations are applicable to other GPCRs under ethanol treatment.
The third important finding is that prolonged ethanol exposure attenuated 5-HT1AR recycling. This conclusion was further confirmed by the observation that that there was a significant less amount of receptors in the fast recycling (Rab4-positive) and slow recycling (Rab11-positive) endosomes in cells treated with ethanol (30 and 75 mM). Although little information is known about the impact of ethanol exposure on GPCR recycling, short-term exposure (2–30 min) to ethanol (100 mM) in HEK293 cells increases the surface levels of DAT by accelerating the rate of DAT recycling (Methner & Mayfield 2010). These data suggest that prolonged ethanol exposure can disrupt the receptor/transporter recycling machinery and thus influence their surface expression and function. The actin and microtubule cytoskeleton along with motor proteins (e.g. myosin) play a critical role in surface receptor recycling. Myosins, a superfamily of motor proteins, are involved in the transport of cargos over long distances within the cells. Myosin V has three different isoforms (Va, Vb and Vc) that display differential tissue-specific expression patterns. Among the myosin V isoforms, myosin Va is highly expressed in the brain (Mercer et al. 1991). Myosin Va binds to actin and Rab11 and tethers endosomes at the cell periphery for local recycling (Provance et al. 2004; Provance et al. 2008). It has been shown that myosin Va can regulate the trafficking of receptors from recycling endosomes to the plasma membranes including AMPA receptors (Correia et al. 2008) and tropomyosin receptor kinase B (Sui et al. 2015). Herein we found that prolonged ethanol exposure reduced the levels of myosin Va, which likely contributes to the reduced 5-HT1AR recycling.
Lastly, it is intriguing that ethanol exposure (e.g. 30 mM and 75 mM) increased the accumulation of 5-HT1ARs in Rab5-positive early endosomes whereas decreased the accumulation in both Rab4/Rab11-positive recycling endosomes and in LAMP1-positive lysosomes. The 5-HT1ARs seemed to be retained in early endosomes (sorting endosomes) and were not properly sorted for recycling and degradation. It is possible that ethanol exposure causes a deficit in the fission of recycling endosomes derived from the early sorting endosomes, resulting in less recycling of receptors. Moreover, ethanol exposure may disrupt the maturation of early endosomes into later endosomes, leading to impairment of receptor degradation. It is necessary to investigate how ethanol dysregulates components involved in early endosome maturation and plasma membrane fission so that we can have a better understanding of ethanol-mediated disruption of endocytic trafficking of 5-HT1ARs.
To summarize, we show here that prolonged ethanol exposure reduced basal surface 5-HT1AR levels by increasing receptor internalization and decreasing receptor recycling, which is accompanied by reduced expression of clathrin and myosin Va and increased activity of dynamin I. The constitutive 5-HT1AR internalization is switched from a clathrin- to a caveolin-dependent pathway under the influence of ethanol. Whether our findings are applicable to trafficking of other GPCRs warrants further investigation. It is worth noting that the concentrations (15–75 mM) used in the current study proximate the blood alcohol levels (70–345 mg/dL) in humans following binge ethanol consumption (Dolganiuc & Szabo 2009; Dubowski 1980). These concentrations produce symptoms ranging from disruption of attention and coordination to sedation and decreased response to stimuli. Therefore, our findings on 5-HT1AR trafficking may underlie these clinical behavioral manifestations in response to acute ethanol exposure noted in humans.
Supplementary Material
Acknowledgements
The authors would like to thank the Wake Forest Microscopic Imaging Core Facility. This work was supported in part by National Institute of Drug Abuse R01DA042862 (RC) and National Institute of General Medicine R15GM139107 (KR). Jonte Roberts is supported by a NIDA training grant T32DA041349. Debora Luessen was supported by F31AA025532 and NIAAA training grant T32AA007565.
Abbreviations
- 5-HT
serotonin
- 5-HT1AR
serotonin receptor type 1A
- AUD
alcohol use disorder
- conA
concanavalin A
- DAT
dopamine transporter
- GPCR
G-protein coupled receptor
- N2A cells
neuroblastoma 2a cells
- NGS
normal goat serum
- PBS
phosphate buffered saline
- Veh
vehicle
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interests with the content of this article.
--Human subjects --
Involves human subjects:
If yes: Informed consent & ethics approval achieved:
=> if yes, please ensure that the info “Informed consent was achieved for all subjects, and the experiments were approved by the local ethics committee.” is included in the Methods.
ARRIVE guidelines have been followed:
Yes
=> if it is a Review or Editorial, skip complete sentence => if No, include a statement in the “Conflict of interest disclosure” section: “ARRIVE guidelines were not followed for the following reason:
“
(edit phrasing to form a complete sentence as necessary).
=> if Yes, insert in the “Conflict of interest disclosure” section:
“All experiments were conducted in compliance with the ARRIVE guidelines.” unless it is a Review or Editorial
Conflicts of interest: None
=> if ‘none’, insert “The authors have no conflict of interest to declare.”
=> else insert info unless it is already included
Open Science Badges
No, I am not interested to achieve Open Science Badge(s) => if yes, please see Comments from the Journal for further information => if no, no information needs to be included in the manuscript
Data available statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Battaini F, Del Vesco R, Govoni S and Trabucchi M (1989) Chronic alcohol intake modifies phorbol ester binding in selected rat brain areas. Alcohol 6, 169–172. [DOI] [PubMed] [Google Scholar]
- Belmer A, Patkar OL, Lanoue V and Bartlett SE (2018) 5-HT1A receptor-dependent modulation of emotional and neurogenic deficits elicited by prolonged consumption of alcohol. Scientific reports 8, 2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S and Galloway MP (1991) Facilitation of dopamine release in vivo by serotonin agonists: studies with microdialysis. Eur J Pharmacol 200, 1–8. [DOI] [PubMed] [Google Scholar]
- Bonda U, Jaeschke A, Lighterness A, Baldwin J, Werner C, De-Juan-Pardo EM and Bray LJ (2020) 3D Quantification of Vascular-Like Structures in z Stack Confocal Images. STAR Protoc 1, 100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz E, Emerit MB, Vodjdani G, Gautheron V, Hamon M, Darmon M and Masson J (2014) Neuronal phenotype dependency of agonist-induced internalization of the 5-HT(1A) serotonin receptor. J Neurosci 34, 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett EJ, Grant KA, Davenport AT, Hemby SE and Friedman DP (2014) The effects of chronic ethanol self-administration on hippocampal 5-HT1A receptors in monkeys. Drug and alcohol dependence 136, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Daining CP, Sun H, Fraser R, Stokes SL, Leitges M and Gnegy ME (2013) Protein kinase Cbeta is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. J Neurochem 125, 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Furman CA, Zhang M, Kim MN, Gereau R. W. t., Leitges M and Gnegy ME (2009) Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J Pharmacol Exp Ther 328, 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhang M, Park S and Gnegy ME (2007) C57BL/6J mice show greater amphetamine-induced locomotor activation and dopamine efflux in the striatum than 129S2/SvHsd mice. Pharmacology, biochemistry, and behavior 87, 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M and Esteban JA (2008) Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nature neuroscience 11, 457–466. [DOI] [PubMed] [Google Scholar]
- Dillon KA, Gross-Isseroff R, Israeli M and Biegon A (1991) Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain research 554, 56–64. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A and Szabo G (2009) In vitro and in vivo models of acute alcohol exposure. World J Gastroenterol 15, 1168–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowski KM (1980) Alcohol determination in the clinical laboratory. Am J Clin Pathol 74, 747–750. [DOI] [PubMed] [Google Scholar]
- Duffy O, Menez JF, Floch HH and Leonard BE (1991) Changes in whole brain membranes of rats following pre- and post-natal exposure to ethanol. Alcohol Alcohol 26, 605–613. [DOI] [PubMed] [Google Scholar]
- Furman CA, Chen R, Guptaroy B, Zhang M, Holz RW and Gnegy M (2009) Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live-cell imaging using total internal reflection fluorescence microscopy. J Neurosci 29, 3328–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Paila YD and Chattopadhyay A (2011) Metabolic depletion of sphingolipids enhances the mobility of the human serotonin1A receptor. Biochem Biophys Res Commun 411, 180–184. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang X, Zhao M, Liu E, Duan M, Guan Z, Guo Y and Zhang M (2019) Entry of Challenge Virus Standard (CVS) −11 into N2a cells via a clathrin-mediated, cholesterol-, dynamin-, pH-dependent endocytic pathway. Virol J 16, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey J, Jeanguenin L, Castro N et al. (2015) Chronic Voluntary Ethanol Consumption Induces Favorable Ceramide Profiles in Selectively Bred Alcohol-Preferring (P) Rats. PLoS One 10, e0139012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongwer MA, Murphy JM, McBride WJ, Lumeng L and Li TK (1989) Regional brain contents of serotonin, dopamine and their metabolites in the selectively bred high- and low-alcohol drinking lines of rats. Alcohol 6, 317–320. [DOI] [PubMed] [Google Scholar]
- Hamon M, Fattaccini CM, Adrien J, Gallissot MC, Martin P and Gozlan H (1988) Alterations of central serotonin and dopamine turnover in rats treated with ipsapirone and other 5-hydroxytryptamine1A agonists with potential anxiolytic properties. J Pharmacol Exp Ther 246, 745–752. [PubMed] [Google Scholar]
- Harikumar KG and Chattopadhyay A (1998) Modulation of agonist and antagonist interactions in serotonin 1A receptors by alcohols. FEBS letters 438, 96–100. [DOI] [PubMed] [Google Scholar]
- Harikumar KG and Chattopadhyay A (2000) Effect of alcohols on G-protein coupling of serotonin(1A) receptors from bovine hippocampus. Brain research bulletin 52, 597–601. [DOI] [PubMed] [Google Scholar]
- Jafurulla M, Pucadyil TJ and Chattopadhyay A (2008) Effect of sphingomyelinase treatment on ligand binding activity of human serotonin1A receptors. Biochimica et biophysica acta 1778, 2022–2025. [DOI] [PubMed] [Google Scholar]
- Jones KT, Zhen J and Reith ME (2012) Importance of cholesterol in dopamine transporter function. J Neurochem 123, 700–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashem MA, Ahmed S, Sultana N, Ahmed EU, Pickford R, Rae C, Sery O, McGregor IS and Balcar VJ (2016) Metabolomics of Neurotransmitters and Related Metabolites in Post-Mortem Tissue from the Dorsal and Ventral Striatum of Alcoholic Human Brain. Neurochemical research 41, 385–397. [DOI] [PubMed] [Google Scholar]
- Kelai S, Renoir T, Chouchana L, Saurini F, Hanoun N, Hamon M and Lanfumey L (2008) Chronic voluntary ethanol intake hypersensitizes 5-HT(1A) autoreceptors in C57BL/6J mice. J Neurochem 107, 1660–1670. [DOI] [PubMed] [Google Scholar]
- Kumar GA and Chattopadhyay A (2020) Statin-Induced Chronic Cholesterol Depletion Switches GPCR Endocytosis and Trafficking: Insights from the Serotonin1A Receptor. ACS chemical neuroscience 11, 453–465. [DOI] [PubMed] [Google Scholar]
- Kumar GA and Chattopadhyay A (2021) Membrane cholesterol regulates endocytosis and trafficking of the serotonin1A receptor: Insights from acute cholesterol depletion. Biochim Biophys Acta Mol Cell Biol Lipids 1866, 158882. [DOI] [PubMed] [Google Scholar]
- Kumar GA, Sarkar P, Jafurulla M, Singh SP, Srinivas G, Pande G and Chattopadhyay A (2019) Exploring Endocytosis and Intracellular Trafficking of the Human Serotonin1A Receptor. Biochemistry 58, 2628–2641. [DOI] [PubMed] [Google Scholar]
- Liu JP, Powell KA, Sudhof TC and Robinson PJ (1994) Dynamin I is a Ca(2+)-sensitive phospholipid-binding protein with very high affinity for protein kinase C. J Biol Chem 269, 21043–21050. [PubMed] [Google Scholar]
- Luessen DJ, Hinshaw TP, Sun H, Howlett AC, Marrs G, McCool BA and Chen R (2016) RGS2 modulates the activity and internalization of dopamine D2 receptors in neuroblastoma N2A cells. Neuropharmacology 110, 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luessen DJ, Sun H, McGinnis MM, Hagstrom M, Marrs G, McCool BA and Chen R (2019) Acute ethanol exposure reduces serotonin receptor 1A internalization by increasing ubiquitination and degradation of beta-arrestin2. J Biol Chem 294, 14068–14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Daaka Y, Della Rocca GJ and Lefkowitz RJ (1997) G protein-coupled receptors mediate two functionally distinct pathways of tyrosine phosphorylation in rat 1a fibroblasts. Shc phosphorylation and receptor endocytosis correlate with activation of Erk kinases. J Biol Chem 272, 31648–31656. [DOI] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C and Kirchhausen T (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10, 839–850. [DOI] [PubMed] [Google Scholar]
- McClure SJ and Robinson PJ (1996) Dynamin, endocytosis and intracellular signalling (review). Mol Membr Biol 13, 189–215. [DOI] [PubMed] [Google Scholar]
- Mercer JA, Seperack PK, Strobel MC, Copeland NG and Jenkins NA (1991) Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature 349, 709–713. [DOI] [PubMed] [Google Scholar]
- Methner DN and Mayfield RD (2010) Ethanol alters endosomal recycling of human dopamine transporters. J Biol Chem 285, 10310–10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Severin F, Backer JM, Hyman AA and Zerial M (1999) Rab5 regulates motility of early endosomes on microtubules. Nature cell biology 1, 376–382. [DOI] [PubMed] [Google Scholar]
- Ohashi I, Pohoreki R, Morita K and Stemmer PM (2004) Alcohols increase calmodulin affinity for Ca2+ and decrease target affinity for calmodulin. Biochimica et biophysica acta 1691, 161–167. [DOI] [PubMed] [Google Scholar]
- Preta G, Cronin JG and Sheldon IM (2015) Dynasore - not just a dynamin inhibitor. Cell Commun Signal 13, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provance DW Jr., Addison EJ, Wood PR, Chen DZ, Silan CM and Mercer JA (2008) Myosin-Vb functions as a dynamic tether for peripheral endocytic compartments during transferrin trafficking. BMC Cell Biol 9, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provance DW Jr., Gourley CR, Silan CM, Cameron LC, Shokat KM, Goldenring JR, Shah K, Gillespie PG and Mercer JA (2004) Chemical-genetic inhibition of a sensitized mutant myosin Vb demonstrates a role in peripheral-pericentriolar membrane traffic. Proc Natl Acad Sci U S A 101, 1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riherd DN, Galindo DG, Krause LR and Mayfield RD (2008) Ethanol potentiates dopamine uptake and increases cell surface distribution of dopamine transporters expressed in SK-N-SH and HEK-293 cells. Alcohol 42, 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR and Anderson RG (1992) Caveolin, a protein component of caveolae membrane coats. Cell 68, 673–682. [DOI] [PubMed] [Google Scholar]
- Saito M, Chakraborty G, Mao RF, Wang R, Cooper TB, Vadasz C and Saito M (2007) Ethanol alters lipid profiles and phosphorylation status of AMP-activated protein kinase in the neonatal mouse brain. J Neurochem 103, 1208–1218. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Kavaliauskiene S and Skotland T (2018) Clathrin-independent endocytosis: an increasing degree of complexity. Histochem Cell Biol 150, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Johnson VR and Weedman JM (2011) Role of the serotonergic system in alcohol dependence: from animal models to clinics. Progress in molecular biology and translational science 98, 401–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter LE, Bolanos FJ, Gozlan H, Lanfumey L, Haj-Dahmane S, Laporte AM, Fattaccini CM and Hamon M (1990) Alterations of central serotoninergic and dopaminergic neurotransmission in rats chronically treated with ipsapirone: biochemical and electrophysiological studies. J Pharmacol Exp Ther 255, 1335–1347. [PubMed] [Google Scholar]
- Seachrist JL and Ferguson SS (2003) Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci 74, 225–235. [DOI] [PubMed] [Google Scholar]
- Shihan MH, Novo SG, Le Marchand SJ, Wang Y and Duncan MK (2021) A simple method for quantitating confocal fluorescent images. Biochem Biophys Rep 25, 100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie KJ and Cousin MA (2005) Dynamin I phosphorylation and the control of synaptic vesicle endocytosis. Biochem Soc Symp, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storvik M, Hakkinen M, Tupala E and Tiihonen J (2009) 5-HT(1A) receptors in the frontal cortical brain areas in Cloninger type 1 and 2 alcoholics measured by whole-hemisphere autoradiography. Alcohol Alcohol 44, 2–7. [DOI] [PubMed] [Google Scholar]
- Sui WH, Huang SH, Wang J, Chen Q, Liu T and Chen ZY (2015) Myosin Va mediates BDNF-induced postendocytic recycling of full-length TrkB and its translocation into dendritic spines. J Cell Sci 128, 1108–1122. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Marcinkiewicz M, Patey A, el Mestikawy S, Gozlan H and Hamon M (1986) Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci 6, 3474–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge D, Daval G, Patey A, Gozlan H, el Mestikawy S and Hamon M (1985) Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. Eur J Pharmacol 113, 463–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


