Abstract
To assess the dynamics of human papillomavirus (HPV) serology, we analyzed HPV6-,11-,16-,18-, and 45 antibodies in infants during the first 36 months of their life. Serial serum samples of 276/327 mother–child pairs were collected at baseline (mothers) and at months 1, 2, 6, 12, 24 and 36 (offspring), and tested for HPVL1-antibodies using the GST-L1 assay. Concordance between maternal and infant HPV-antibody levels remained high until month-6 (p < = 0.001), indicating maternal antibody transfer. At 1 month, 40–62% of the infants tested seropositive to any of the 5 HPV-types. Between 1–3 years of age, 53% (58/109) of the children born to HPV-seronegative mothers tested HPV-seropositive. Times to positive seroconversion varied between13.4 and 18.7 months, and times to negative seroconversion (decay) between 8.5 and 9.9 months. Significant independent predictors of infants’ seroconversion to LR-HPV were hand warts and mother’s history of oral warts and seroconversion to LR-HPV. No predictors of seroconversion to HR-HPV were identified. Maternal HPV-IgG-antibodies are transferred to her offspring and remain detectable for 6 months, corroborating the IgG molecule’s half-life. Seroconversion to HPV-genotypes 6, 11, 16 and 18 was confirmed among children born to HPV-seronegative mothers, implicating an immune response to these HPV-genotypes during early infancy.
Subject terms: Immunology, Medical research
Introduction
Human papillomavirus (HPV)-infection has been traditionally considered a sexually transmitted infection (STI). However, HPV DNA has been found in oral and genital samples of neonates and young children as well as in the placenta and cord blood, suggesting the possibility of maternal transmission1–6. Meta-analysis on 3128 mother–child pairs showed that children of HPV-positive mothers were 33% more likely to be HPV-positive than children born to HPV-negative mothers5. Another systematic review confirmed intrauterine HPV-transmission, with a pooled frequency of 4.9% for antenatal vertical HPV transmission6.
Only few studies have focused on HPV-serology in young children and on maternal HPV-antibodies in early infancy7–11. During the era of HPV-vaccinations, it is important to understand the dynamics of maternal HPV-immunization and its impact on the mother-to-child HPV-transmission and HPV-burden in young children.
As a part of the prospective Finnish Family HPV-Study, we evaluated the dynamics of HPV-type-specific antibodies in the offspring, with special reference to maternal HPV-antibodies and infants’ seroconversion to the L1 protein of HPV 6/11/16/18/45 during the first 3 years of their life.
Methods
Study design and population
The Finnish Family HPV-Study (FFHPV) is a longitudinal cohort designed to explore the dynamics of HPV-infection in the enrolled mothers, fathers and their offspring. Between 1998–2001, a total of 327 pregnant (3rd trimester) women and their spouses were enrolled at Maternity Unit of Turku University Hospital, (Finland), as previously described1,12. The study design was approved by the Research Ethics Committee of Turku University Hospital (#3/1998), with subsequent amendments (#2/2006 and 45/180/2010), and all methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all parents who agreed to participate in the study. The mothers’ demographic data as well as their oral and genital HPV-status at baseline have been detailed elsewhere1,12–15.
HPV-serology
Blood samples were taken from all 327 pregnant women at baseline, and at 12-, 24- and 36-month follow-up (FU) visits, as described earlier13. Only the baseline HPV-serology of the mothers was included in the present analysis. From the infants, the blood samples were taken at 1-, 2-, 6-,12-, 24- and 36-month FU visits (all tested children irrespective of the mother’s serostatus), including 232, 237, 263, 272, 268 and 243 children, respectively. The first blood sample of the newborn was taken from the head, while the subsequent samples were drawn from the arm bend. The actual timing (mean months and range) of the FU visits was as follows: 1.2 (0.5–1.9), 2.2 (1.6–3.8), 6.4 (5.2–9.4), 12.6 (10.4–15.5), 24.8 (21.4–32.9), and 36.9 (34.1–47.5) months, respectively.
After collection, the blood samples were centrifuged at 2400 rpm for 10 min (Sorvall GLC-2, DuPont Instrument, Newtown, Connecticut, USA), the serum was divided into three aliquots, and stored first at − 20 °C for no longer than 1 week, and then at − 70 °C until delivered to the German Cancer Research Center (DKFZ), Heidelberg, Germany for serological analysis.
Antibodies to the major capsid protein L1 of HPV types 6/11/16/18/45 were analyzed by multiplex HPV serology, based on glutathione S-transferase fusion protein capture to fluorescent beads, as previously described16. Sera were scored as positive when the antigen-specific median fluorescence intensity (MFI) values were higher than the cut-off level of 200 MFI for the L1 antigen of individual HPV-genotypes13,16. Seroconversion event at any FU visit was recorded if both of the following conditions apply: (1) at least twofold increase of the previous MFI-value, and reaching at least the cut-off level (MFI 200) (2) any MFI-value exceeding the 200 MFI cut-off level as compared with the baseline status (i.e., the 1-month visit). Similarly, antibody decay was defined by the same two conditions in reverse order: (1) at least twofold decrease of the previous MFI-value, and (2) fall of the MFI-value below the single cut-off level of 200 MFI.
Statistical analysis
Scatterplots were generated to show the correlation between the maternal and infant serum HPV antibody-levels. The bivariate correlation of the HPV-antibody-levels between the maternal sera (baseline) and her offspring sera taken at 1-, 6-, and 12-month visit was tested using the Spearman r correlation coefficients, separately for the five HPV types.
Frequency tables were analyzed using the χ2-test, with the likelihood ratio or Fisher's exact test for categorical variables. Differences in the means of continuous variables were analyzed using Mann–Whitney’s test or Kruskal–Wallis’s test for two and multiple independent samples, respectively. Univariate survival analysis for serological outcomes (seroconversion, decay) was based on the Kaplan–Meier method, with log-rank statistics.
Co-variates of seroprevalance and seroconversion were analysed using generalized estimating equation (GEE) model, stratified by the five HPV types. In this analysis, we assumed that HPV-seroconversion depends on time since the previous sample, and a time variable was included as a covariate in these GEE models15, We entered into multivariate GEE models several covariates previously confirmed or implicated as risk factors of HPV-infections in our cohort12,14,15. SPSS® for windows, version 26 (IBM Corp. Armonk, NY) and STATA (STATA/SE 15.1, Stata Corp., College Station, TX, USA) were used, all tests being performed as two-sided, with p < 0.05 as the level of significance.
Ethics approval
Approval was granted by the Research Ethics Committee of Turku University Hospital (#3/1998), with subsequent amendments (#2/2006 and 45/180/2010).
Consent to participate
Informed consent (in written) was obtained from all mothers- and fathers-to-come who agreed to participate in the FFHPV cohort.
Consent for publication
All authors approved the final version of the manuscript for submission.
Results
Demographic and clinical data of the mothers and their offspring at birth
Altogether, 45/327 (13.8%) mothers were carriers of genital HR-HPV, 47 (14.4%) had HR-HPV DNA in their baseline oral samples, whereas LR-HPVs were detected in both genital and oral samples of 8 (2.4%) mothers. Only 39 (11.9%) of the mothers had ASCUS or worse Pap-smear cytology. Altogether, 144 (44%) were current or past smokers, and 84.2% had started smoking before the age of 17 years. The majority (55.9%) had their sexual debut between 14–16 years of age. Seventy one mothers (21.7%) reported only 1–2 life-time sexual partners, while 60 (18.3%) had more than 10. Of the users of oral contraceptives (OC), 40.8% had initiated their use between 14–16 years of age, while 24 (7.3%) had never used OC. Altogether, 266/327 (81.3%) mothers reported no history of STI, while 80 (24.5%) mothers reported ever having genital warts. History of oral warts was rare (eight cases only), but skin warts were common (n = 166; 50.8%).
None of the mothers included in the FFHPV cohort had received the HPV vaccine. At baseline, the median age of the mothers-to-come was 26 years, (range 18–46), with a median gestational age of 40 weeks (range 31.6–42.5). Of all deliveries, 255 (77.6%) were vaginal and 74 (22.4%) caesarean (i.e. 327 mothers delivered 329 babies of whom two were twins). Altogether, 58% of the infants were first-born; 155 (48%) boys and 172 (52%) girls (two missing data in the record). Their median birth weight was 3.580 g (range 1.750–4.950 g). The mean time from the membrane rupture to the start of delivery was 4.1 h (range 0–53 h).
HPV-seroprevalence of the mothers
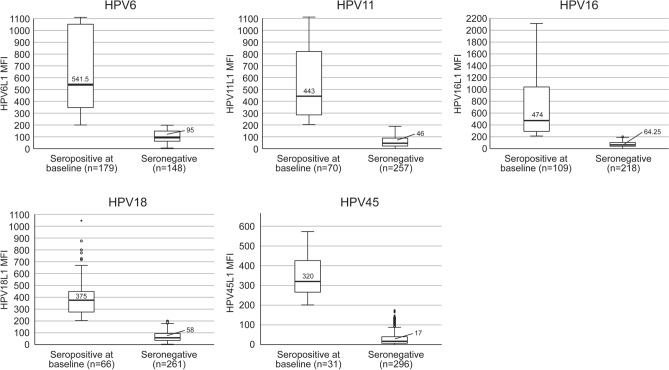
Figure 1 illustrates the mean IgG-antibody-levels to HPV 6/11/16/18/45 genotypes of the mothers at their 3rd trimester of pregnancy (baseline visit), classified as seropositive with 200 MFI cut-off (all HPV-types). HPV6-seropositivity was the most common (54.7%), followed by HPV16 (33.3%), HPV11 (21.4%), HPV18 (20.2%) and HPV45 (9.5%). The highest mean MFI-values were detected for HPV6 and HPV16 antibodies; 541 MFI and 474 MFI, respectively, with a range from 200 to over 1100 and 2100, respectively.
Figure 1.
Mothers’ seropositivity at 3rd trimester by HPV type. HPV seropositivity to HPV6, HPV 11, HPV16, HPV18 and HPV45 in the pregnant mothers (n = 327) at their third trimester. The medium fluorescence (MFI) value of 200 or more was the cut-off for the seropositivity of the respect HPV types analyzed. The mean and the range of MFI in HPV-seropositive and HPV-seronegative mothers are given in the columns. None of the mothers had been vaccinated for HPV. MFI Mean fluorescence intensity, n number of the mothers stratified by HPV-seropositivity.
HPV antibodies of the infants during their first 36 months
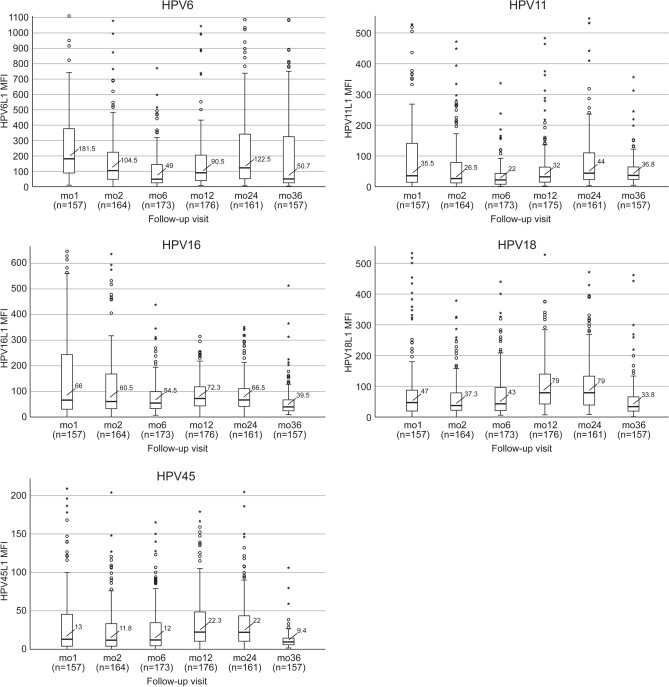
Figure 2 summarizes the median levels and range of HPV6/11/16/18/45 antibodies in the infants born to seropositive mothers (n = 179; any HPV type > 200 MFI). All infants born to the mothers so defined had measurable IgG- antibodies to the respective maternal HPV-serotype at the age of 1 month. However, only part of these infants had HPV6/11/16/18/45 antibody levels that exceeded the 200MFI cut-off for seropositivity: 45.8%, 18.4%, 30.5%, 12.1% and 5.7%, respectively. At the age of 1 month, the highest MFI mean value was found for HPV6 (MFI 181), followed by HPV16 (MFI 66), and HPV18, HPV11 and HPV45, all showing substantial variation (Fig. 2).
Figure 2.
Antibody-levels to HPV6, HPV 11, HPV16, HPV18 and HPV45 in offspring born to seropositive mothers defined by MFI 200 or more for any HPV type. In total 179 of the 327 pregnant mothers were seropositive to HPV6, HPV 11, HPV16, HPV18 or HPV45 L1 protein (cut-off, any HPV type > 200 MFI). The mean HPV antibody levels and the range in their offspring are given as columns stratified by HPV serotypes and follow-up visits. The number of tested offspring (= n) of the seropositive mothers are given in parenthesis under the horizontal axis at the different follow-up visits (mo = month). Unfortunately, blood samples were not available from all infants at all different time points, and this is the reason why the numbers are not unanimous.
During the first 6 months, HPV6 and 11 antibody levels waned but started increasing again until the 24-month FU-visit. Similarly, the antibody-levels of the HR-HPVs remained relatively stable during the first 6 months and started to increase at the 12- and 24-month FU-visits followed by a decline at the 36-month FU visit.
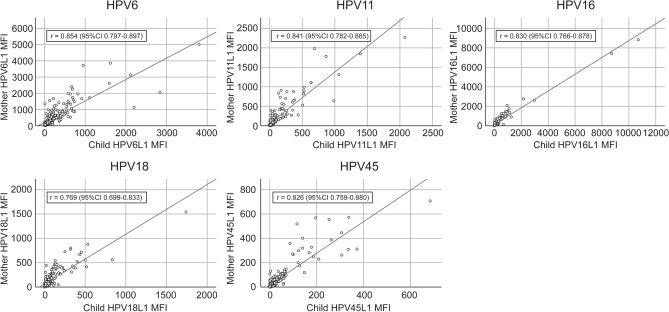
Mothers’ baseline HPV antibody levels had an almost perfect correlation with the respective antibody levels of their offspring at the 1-month FU, Spearman correlation coefficient (r) varying between 0.769 (HPV18) and 0.854 (HPV6) (Fig. 3). This correlation to infants’ serology remained highly significant (p < 0.001) for all HPV types at the 6-month FU (p < 0.001): HPV6 (r = 0.266), HPV11 (r = 0.344), HPV16 (r = 0.321), HPV18 (r = 0.220) and HPV45 (r = 0.259). At the age of 12 months, this correlation still persisted for HPV11 (r = 0.214, p < 0.001), HPV18 (r = 0.132, p = 0.030) and HPV45 (r = 0.169, p = 0.005) antibodies, whereas the infants’ HPV6 (r = 0.070, p = 0.250) and HPV16 antibodies (r = 0.100, p = 0.100) had lost their significant correlation with the baseline maternal antibody levels.
Figure 3.
Correlation of antibody levels between the mothers—offspring pairs by HPV type. Correlation of individual HPV L1 serum antibody levels (MFI values) between pregnant mothers at 3rd trimester and offspring aged 1 month. None of the mothers had been vaccinated for HPV. Lines represent linear regression analyses; r Spearman coefficient, MFI Mean fluorescence intensity, CI confidence interval.
HPV-seroprevalence of the offspring born to HPV-seropositive mothers
Table 1 summarizes the HPV type-specific seroprevalence and median MFI antibody-levels of seropositive infants born to mothers who were seropositive (≥ 200 MFI) to the same HPV-genotypes. Seroprevalence of the children is given as the number and (percentage) of seropositive (≥ 200MFI) offspring out of total number of tested offspring at each time point. At the age of 1 month, 40 to 62% of the infants were seropositive to any of the tested HPV-genotypes. During the FU, HPV6-seropositive children far outnumbered the children seropositive to the other HPV-genotypes. Infants’ HPV-seropositivity was at lowest at the 6-month FU-visit for all HPV types: 19.3%, 3.8%, 9.8% and 14.3% for HPV6, HPV11, HPV16 and HPV18, respectively, and seropositivity to HPV45 was non-existent from this point on. At the age of 2 years, 38.6% of the children were still seropositive for HPV6, and 14.9% were positive for HPV16 and HPV18, each. At the age of 3 years, 43 children were still HPV6-seropositive while only four and one were seropositive to HPV16 and HPV18, respectively.
Table 1.
Concordance of HPV type-specific (HPV6, 11, 16, 18, 45) seropositivity between the mothers and their offspring.
| Age of the infant | Offspring testing seropositive to the same HPV type as their mothers* | ||||
|---|---|---|---|---|---|
| HPV6 n = 179* |
HPV11 n = 70* |
HPV16 n = 109* |
HPV18 n = 66* |
HPV45 n = 31* |
|
|
aNumber and (percentage) of seropositive offspring / total number of tested offspring at each time point bMedian MFI (range) |
|||||
| One month |
72/134 (53.7)a 606 (201–3798)b |
29/53 (54.7) 530 (203–2076) |
47/76 (61.8) 538 (201–10,695) |
19/47 (40.4) 455 (212–1740) |
9/21 (42.9) 341 (209–684) |
| Two months |
42/132 (31.8) 547(202–2023) |
17/51 (33.3) 267 (207–1470) |
31/86 (36.0) 518 (206–6698) |
12/54 (22.2) 273 (207–1094) |
4/23 (17.4) 290 (204–446) |
| Six months |
27/140 (19.3) 319 (209–4180) |
2/53 (3.8) 287 (238–337) |
9/92 (9.8) 269 (207–1577) |
8/56 (14.3) 277 (200–401) |
0/24 |
| 12 months |
37/145 (25.5) 850 (201–3167) |
5/56 (8.9) 312 (207–585) |
10/89 (11.2) 232 (208–255) |
13/55 (23.6) 290 (210–652) |
0/22 |
| 24 months |
51/132 (38.6) 877 (207–3110) |
4/49 (8.2) 312 (207–585) |
13/87(14.9) 420 (201–13,799) |
7/47 (14.9) 279(209–1125) |
0/22 |
| 36 months |
43/128 (33.6) 692 (205–2373) |
0/47 |
4/83 (4.8) 2 (203–365) |
1/46 (2.2) 1225 |
0/19 |
The seropositivity cut-off in the mother–child pairs ≥ 200 MFI.
*Numbers of mothers seropositive to HPV6 (n = 179), HPV11 (n = 70), HPV16 (n = 109), HPV18 (n = 66) and HPV45 (n = 31).
aNumbers and (%) of the offspring testing seropositive to the same HPV type as their mothers/the total number of offspring tested at each time point. As an example of HPV6, 179 mothers (out of 327) tested seropositive to HPV6, but only 134 offspring of these mothers were serologically tested at 1-month; 132 at 2-month, 140 at 6-month, 145 at 12-month, 132 at 24-month and 128 at 36-month follow-up visit. The other HPV types follow the same pattern of presentation.
bMedian MFI values and (range).
Altogether, 113 mothers had antibodies to more than a single HPV-type (≥ MFI 200). In total, 42 children born to these 113 mothers had also HPV-antibodies to multiple types. As a reference, there were 105 mothers classified seropositive to a single HPV-type only. Interestingly, only one offspring born to these “single-type seropositive” mothers had antibodies to multiple HPV-types.
HPV-seroprevalence of the offspring born to HPV-seronegative mothers
To identify the infants’ seroconversion caused by an acquired HPV-infection during the 3-year FU, we evaluated the infants born to HPV-seronegative (< 200 MFI) mothers. Altogether, 53% (n = 58) of the infants born to 109 HPV-seronegative mothers did seroconvert to HPV by the age of 3 years, peaking at the age of 24 months (Table 2). HPV6-seropositivity was most prevalent among the children aged 1 year, and HPV6-seroconversion rate increased by age. Similarly, the mean levels of HPV6 antibodies were higher (> 1100 MFI at 12 and 24 months; 642 MFI at 36 months) than found for any other tested HPV-types. At the age of 3 years, 23 (4.7%) HPV-seropositive children were identified of whom six were seropositive to HPV6 or HPV18 at all three FU visits.
Table 2.
Human papillomavirus (HPV) seroprevalence to HPV-genotype 6, 11, 16, 18 and 45 in the offspring at the age of 12-, 24, and 36 months born to the mothers seronegative for the respective HPV-genotype.
| Follow up visit | 12 months | 24 months | 36 months | |||
|---|---|---|---|---|---|---|
| HPV antibodies in sera | Seropositive infants (n) mean MFI ± SD |
Infants seropositive for the same HPV type at two visits (n) | Infants seropositive for the same HPV type at three visits (n) | Total number of HPV-seropositive offspring at the 12-, 24-, and 36-month FU-visits born to 109 HPV-seronegative mothers | ||
| HPV6 |
9 1152 ± 1033 |
10 1167 ± 954 |
16 642 ± 364 |
10 | 3 | 19 |
| HPV11 |
7 498 ± 330 |
12 360 ± 150 |
3 293 ± 57 |
3 | 19 | |
| HPV16 |
3 276 ± 66 |
5 397 ± 252 |
1 549 |
3 | 6 | |
| HPV18 |
4 475 ± 260 |
14 417 ± 233 |
3 624 ± 445 |
2 | 3 | 13 |
| HPV45 | 0 |
1 561 |
0 | 1 | ||
| Total HPV-sero-positivity at the given age | 23 | 42 | 23 | 18 | 6 | In total, 53% (58/109) |
The cut-off for seropositivity was ≥ 200 MFI.
Vaginal delivery was statistically significantly (p = 0.048), associated with infants’ seropositivity at the age of 2 years. The first-born children had significantly lower HPV6-antibody levels as compared with the non-first-born children at the age of 1 year (p = 0.0001) and 2 years (p = 0.023), as did HPV11-antibodies at 1 year (p = 0.0001).
Outcome of HPV serology in children during the first 36 months of life
In Kaplan–Meier analysis for seroconversion (Fig. 4, upper panel), 46.1% of the infants showed seroconversion to HPV6, 14.1% converted to HPV11-, 10.3% to HPV16-, 13.8% to HPV18- and 1.6% to HPV45- L1-antigens (p = 0.0001). The mean (95% CI) seroconversion times for HPV6/11/16/18/45 were 18.7 months (16.8–20.6), 17.9 months (15.5–20.2), 17.3 months (13.7–20.8), 15.5 months (12.5–18.4), and 13.4 months (5.0–21.8), respectively (p = 0.639).
Figure 4.

Cumulative seroconversion and antibody decay by HPV type in offspring during the first 3 years of life. Kaplan–Meier analysis showing 3 years cumulative antibody seroconversion (upper panel) and decay (lower panel) for HPV6, HPV 11, HPV16, HPV18 and HPV45 antibodies.
Figure 4 (lower panel) shows the Kaplan–Meier analysis for the cumulative decay of HPV antibodies in these infants without making any difference whether antibodies are of maternal origin or newly acquired by the infants. During the median FU-time, decay of HPV6/11/16/18/45-antibodies was observed in 15.5%, 5.2%, 10.6%, 5.2% and 2.6% of the infants, respectively (log-rank p = 0.0001). The mean (95% CI) times for antibody decay were as follows: 9.9 months (6.0–13.8), 8.5 months (4.5–12.5), 9.8 months (6.5–13.1), 9.1 months (2.3–15.9), and 4.0 months (1.2–6.8), for HPV6, HPV11, HPV16, HPV18 and HPV45, respectively (p = 0.054).
To explore the possible differences in HPV antibody decay or seroconversion between the infant antibodies of maternal origin and those newly acquired by the infants, we made a new Kaplan –Meier analysis of the infants stratified by their mother’s HPV-serostatus (HPV-seronegative or –positive). There was nearly no decay of the HPV-antibodies in infants born to HPV-seronegative mothers. The differences between infants born to seronegative and seropositive mothers was most obvious for HPV6 and HPV16 antibody decay. Due to the limited number of subjects, however, these differences were not statistically significant (HPV6 p < 0.195, HPV11 p < 0.995, HPV16 p < 0.334, HPV18 p < 0.417, HPV45 p < 0.267). Importantly, the serostatus of the mother for the individual HPV-types had no effect on infant’s seroconversion to the respective HPV-type.
Predictors of the offspring’s seroconversion during the follow-up
Several maternal covariates such as age, Pap-smear cytology, smoking, age at the sexual depute, mother’s genital or oral warts, HPV DNA status at the 3rd trimester (Table 3) were included in the GEE-modelling in addition to pertinent covariates of the child to identify the predictors of the offspring’s seroconversion during the 3-year follow-up. In univariate GEE, mother’s seroconversion to LR-HPV predicted infant’s seroconversion to LR-HPV (p = 0.014; OR = 1.9, 95% CI 1.14–3.13) (Table 3). When adjusted for the other covariates, mother’s history of oral warts (p = 0.0001; OR = 55.9; 95% CI 6.74–463.08) and infant’s hand warts at the age of 3 years (p = 002; OR = 42.8, CI 4.15–440.76) were significant independent predictors of infant’s seroconversion to LR-HPV. In contrast, no significant predictors for infants’ seroconversion to HR-HPVs were disclosed by either univariate or multivariate GEE.
Table 3.
Predictors of seroconversion of the infants to LR-HPV (HPV 6, 11) and HR-HPV (HPV 16, 18, 45) L1 antigens during the follow-up in time-dependent GEE modelling1 run in univariate model and as adjusted for other covariates.
| Covariates | Seroconversion to LR-HPV (HPV6 L1 and/or HPV 11L1)* | Seroconversion to HR-HPV (HPV 16L1, HPV 18L1 and/or HPV 45L1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude OR | 95%CI | Significance | **Adjusted OR | 95%CI | Significance | Crude OR | 95%CI | Significance | @Adjusted OR | 95%CI | Significance | |
| Age (categorical) of mother | 1.2 | 0.54–2.70 | 0.650 | 0.2 | 0.02–2.36 | 0.196 | 2.6 | 0.91–7.42 | 0.074 | 1.6 | 0.27–9.61 | 0.609 |
| Genital HR-HPV DNA status of mother2 | 0.7 | 0.46–1.09 | 0.121 | 1.0 | 0.98–1.00 | 0.082 | 1.2 | 0.74–2.07 | 0.421 | 1.2 | 0.54–2.58 | 0.686 |
| Oral HR-HPV DNA status of mother2 | 1.1 | 0.84–1.55 | 0.413 | 1.0 | 1.00–1.00 | 0.996 | 1.2 | 0.82–1.69 | 0.371 | 1.1 | 0.66–1.98 | 0.640 |
| Baseline Pap cytology of mother (ASCUS cut-off) | 0.9 | 0.60–1.41 | 0.693 | 1.0 | 0.99–1.00 | 0.428 | 1.3 | 0.81–2.22 | 0.249 | 1.5 | 0.62–3.59 | 0.366 |
| Seroconversion to LR-HPV of mother (no conversion; ref) | 1.9 | 1.14–3.13 | 0.014 | 1.8 | 0.60–5.24 | 0.301 | 1.7 | 0.96–3.00 | 0.066 | 1.3 | 0.53–2.94 | 0.607 |
| Smoking status of mother | 1.1 | 0.79–1.40 | 0.741 | 0.9 | 0.36–2.31 | 0.842 | 1.2 | 0.68–1.99 | 0.585 | 1.1 | 0.51–2.47 | 0.769 |
| Mother’s history of genital warts (no history; ref) | 1.2 | 0.72–2.12 | 0.438 | 0.5 | 0.17–1.42 | 0.188 | 0.7 | 0.40–1.27 | 0.245 | 0.5 | 0.20–1.08 | 0.074 |
| Mother’s history of oral warts (no history; ref) | 7.5 | 0.88–63.23 | 0.065 | 55.9 | 6.74–463.08 | 0.0001 | 1.1 | 0.20–5.66 | 0.943 | 1.3 | 0.14–11.75 | 0.818 |
| Mother’s history of skin warts (no history; ref) | 1.2 | 0.89–1.54 | 0.253 | 1.3 | 0.89–2.05 | 0.162 | 0.8 | 0.57–1.09 | 0.154 | 0.9 | 0.60–1.29 | 0.500 |
| Sex of infant | 1.0 | 0.62–1.54 | 0.910 | 0.7 | 0.27–1.67 | 0.389 | 1.1 | 0.63–1.78 | 0.816 | 1.0 | 0.47–2.27 | 0.927 |
| Mode of delivery | 1.0 | 0.57–1.85 | 0.927 | 2.4 | 0.73–7.91 | 0.148 | 0.7 | 0.32–1.41 | 0.324 | 0.6 | 0.20–1.59 | 0.280 |
| Genital HR-HPV DNA status of infant2 | 0.8 | 0.61–1.05 | 0.112 | 1.0 | 0.99–1.00 | 0.482 | 0.9 | 0.66–1.19 | 0.418 | 0.9 | 0.53–1.54 | 0.706 |
| Oral HR-HPV DNA status of infant2 | 1.0 | 0.75–1.27 | 0.844 | 1.0 | 1.00–1.01 | 0.140 | 0.9 | 0.71–1.23 | 0.644 | 0.8 | 0.46–1.57 | 0.602 |
| Atopia | 1.1 | 0.57–2.14 | 0.769 | 0.9 | 0.63–1.33 | 0.630 | 0.6 | 0.30–1.24 | 0.172 | NCF | NCF | NCF |
| Allergy | 1.0 | 0.77–1.29 | 0.985 | 1.4 | 0.53–3.73 | 0.500 | 0.7 | 0.46–1.12 | 0.149 | NCF | NCF | NCF |
| Oral warts at 36 months | NC | NC | NC | NC | NC | NC | NC | NC | NC | NCF | NCF | NCF |
| Genital warts at 36 months | NC | NC | NC | NC | NC | NC | NC | NC | NC | NCF | NCF | NCF |
| Hand warts at 36 months | 5.4 | 0.61–47.33 | 0.128 | 42.8 | 4.15–440.76 | 0.002 | 2.6 | 0.51–13.50 | 0.247 | NCF | NCF | NCF |
Statistically significant values are marked as bolded.
*Binary outcome (Conversion/No conversion) detected during the 36-mo follow-up (Cut-off 200 MFI, and > 2 × increase of Ab titers in two subsequent samples); 1Results obtained from time-dependent GEE with logit link for binary outcomes clustered by child ID number; **adjusted for all other covariates in the model; 2High-Risk (HR)-HPV detected at any follow-up (FU) visit;
OR, odds ratio; CI, confidence interval; NC, not computable, no relevant cases; NCF, not computable due to failure to achieve convergence.
Discussion
This is the largest prospective study examining HR- and LR–HPV-serology in infants during the first 3 years of life. Clearly, IgG antibodies to the major capsid protein (L1) of HPV6, HPV11, HPV16, HPV18 and HPV45 are vertically transferred from the mother to her offspring, and concordance between maternal and newborn HPV-antibody levels remains high during the first 6 months after delivery. Importantly, HPV-seroconversion among children born to HPV-seronegative mothers is common.
Multiplex serology assay used in our study (also known as GST-L1 assay) is a commonly used method validated in several seroepidemiological studies on HPV, and thus appropriate for the present study. As determined from the natural history studies on HPV infection, GST-L1 seems to be s a good measure of cumulative immunity induced by the natural HPV infection but not a reliable marker of immune protection, because the assay does not distinguish between neutralizing and non-neutralizing antibodies17. HPV vaccine immunogenicity has been typically assessed by three different methods: (1) the virus like particle (VLP)-based enzyme-linked immunosorbent assay (VLP-ELISA), (2) competitive Luminex immunoassay (cLIA), and (3) secreted alkaline phosphatase-based pseudovirus neutralization assay (SEAPNA)18. The comparison between these four different assays is problematic as they do not measure equivalent aspects of the immune response and their seropositivity cut-offs are not calibrated against each other. Much of the observed lack of agreement can be explained by differences between the seropositivity cut-offs used by the different assays. The results of the available assays generally correlate well particularly in specimens with high antibody levels18,19.
Our results are in line with the few previous studies, even if performed with different assays, confirming close HPV-type-specific concordance between maternal and newborn antibodies, irrespective of the methods used for (i) newborn blood sampling (cord blood, dried blood spot or serum) and/or (ii) for HPV-antibody testing7,8,11,17. Important to note is that the mothers included in the prior studies referred here are non-HPV vaccinated, similarly as the mothers of our Finnish Family HPV Study cohort. Kawana and coworkers were the first to report the presence of neutralizing antibodies against HPV6 in sera of the infants born to mothers (n = 2) with HPV6-positive genital warts7. Maternal sera at delivery and at 5-week postpartum contained IgG- and IgM-antibodies reactive to HPV6L1/L2 capsids. The titers of the neutralizing HPV6 IgG-antibodies in cord sera were equivalent to those of maternal sera but no IgM-antibodies were detectable7.
Heim and coworkers analyzed HPV-antibody-levels in 104 mother-newborn-pairs using VLPs as the antigen8. Maternal IgG-positivity rates to HPV6, HPV11, HPV16, HPV18 and HPV31 in the newborn sera were 23.1%, 2.9%, 8.7%, 5.8%, and 9.6%, respectively8. IgM-HPV-antibodies were detected in 19% of the mothers, but not in newborns, except three who showed a weak IgM-immunoreactivity for HPV11 and HPV16 without any HPV IgG-antibodies. Similarly, Smith and coworkers analyzed 333 mother-newborn pairs reporting very high type-specific concordance for HPV16, HPV18, HPV31 and HPV33 antibodies, in the range of 96–97%20. Our results confirmed that the concordance of HPV-type-specific antibodies in mother-infant pairs remained high at least for 6 months. All infants born to HPV-seropositive mothers had measurable HPV-antibody levels at the age of 1 month, but these HPV-antibody levels did not reach the cut-off level of HPV-seropositivity (≥ 200 MFI) in around 50% of the tested infants (range from 38 to 60% for HPV16 and HPV18, respectively as given in Table 1).). This indicates a gradual waning of the HPV-antibodies starting at the age of 1 month and continuing until the age of 6 months.
Recently, Zahreddine and coworkers detected maternal antibodies in 58 newborns, of whom 23 were followed for their HPV-antibody dynamics during the first 24 months of life11. As might be expected, four mothers who had been vaccinated against HPV6/11/16/18 had the highest antibody levels, as did their offspring. These data corroborate with the recent two studies on maternal transfer of HPV-antibodies into the cord blood after vaccination with the 4-valent or 9-valent HPV vaccines21,22. These authors also described that newborns seropositive at birth cleared their HPV-antibodies (below the 200 MFI cut-off) in 80% and 100% by the 12- and 24-month FU, respectively11. However, measurable HPV-antibody levels (< 200 MFI) for HPV6/11/16/18 were detectable still in 24 and 15 children aged 1 and 2 years, respectively11. The significance of these low-titer HPV-antibodies, also found in the present study, remains unknown. In their series, the median clearance time for HPV6 antibodies was 6 months while antibodies to HPV11, HPV16 and HPV18 all cleared within 12 months11, similar as observed in the present study (Fig. 4). Our larger cohort and longer FU time might explain the minor dissimilarities (for HPV6 and HPV16) between these two studies.
Several studies on other pathogens highlight that maternal antibodies can be vertically transferred to the fetus and subsequently protect the neonate against the respective infection23. Maternal (IgG) immunoglobulins are transported across the placental membranes during pregnancy by an active, FcRn receptor-mediated process23. With the continuation of pregnancy, FcRn expression and trans-placental transport increase, peaking during the last 4 weeks of gestation. Thus, the 3rd trimester of pregnancy may serve as the optimal time to test for pathogen-specific antibodies. High maternal antibody levels are the most predictive factors of trans-placental antibody-transfer and maternal antibody concentrations in the infant23. Our results showed that nearly half of the infants born to seropositive mothers had HPV-antibody levels below the 200 MFI cut-off, while some had 10–80 times higher levels. This clearly suggests that there is a difference in the efficacy of the maternal antibody transfer related to maternal antibody concentrations24. Further studies are needed to understand the role of vertically transferred HPV-antibodies in protecting the infant from maternal HPV-infections either before birth or at early infancy.
Importantly, our results indicate that seroconversion to LR- and/or HR-HPVL1 proteins is possible already early in life, confirming the results of three previous studies9,25,26. In 1999, Mann and coworkers showed that 3/100 newborns developed HPV16-antibodies to HPV16 VLP at the age of 1 to 2 years. Two of these three children had HPV16-antibodies still detectable at the age of 5 years25. Af Geijersstam and coworkers measured serum antibodies to HPV16, HPV18 and HPV33 capsids (L1/L2 proteins) among 1031 children aged between 0–13 years26. In the age group of > 1.5–3 years (n = 181), seroprevalence for HPV16, HPV18 and HPV33 was 2.8%, 0.6% and 1.7%, respectively26. Mariais et al. (2007), however, reported higher seroprevalence rates in 37 children younger than 2 years: 5.4% to HPV16, 13.5% to HPV11, and 10.8% to HPV18 VLPs9. These data have an almost perfect match with the present results indicating that children born to HPV-seronegative mothers were seropositive to HPV16 (5.5%), HPV11 (17.4%) and HPV18L1 (11.9%) at the age of 1 to 3 years. In addition, Zahreddine and coworkers identified one child, HPV6/11-seronegative at birth, but seropositive with increasing antibody levels at 12- and 24-month FU-visits11.
Confirmed HPV-seroconversion during the first 3 years of life implicates an early exposure to HPV, i.e., a virus-producing infection capable of eliciting an immunological (antibody) response. However, we do not know asyet why some of the infants experience HPV seroconversion during these early months of their life. The two key unsolved questions are, (1) by which route has the exposure to different HPV-types taken place at early infancy? and (2) at which site has the serological response been mounted? The present study is the first to explore in more detail the predictors of HPV seroconversion at early age. The disclosed predictors (Table 3) suggest that HPV infection resulting in seroconversion at early childhood might be intra-familial, the mother being the most likely transmitter. This notion is supported by the discovery that mother’s history of oral warts (usually HPV6/11) and mother´s seroconversion to LR-HPVs are significantly associated with the offspring’s seroconversion to LR-HPV. Furthermore, we have previously demonstrated that persistent oral HR-HPV infection in children was associated with oral HPV-carriage of the mother at delivery as well as with the seroconversion of the mother to HR-HPV during the follow-up (OR = 1.60–1.92, 95% CI 1.02–2.74)27.
Taken together, the present results confirm that maternal IgG antibodies to HPVL1 ptotein are readily transferred to their offspring and remain detectable at least for 6 months, corroborating the known half-life of IgG-immunoglobulins. Furthermore, we also confirmed seroconversion to HPV-genotypes 6, 11, 16, 18 (but not HPV45) among children born to HPV-seronegative mothers, implicating an immune response to HPV-infection by these genotypes already at early infancy.
Acknowledgements
The authors express their sincere thanks to all participants in the FFHPV cohort study during the past two decades. We also thank all the members of the assistant team of the FFHPV Study for their expert technical assistance.
Author contributions
S.S., M.R. and S.G. conceptualized and designed the study. S.S., S.G. and M.R. collected the data and contributed samples. T.W. and M.P. were responsible for serological analysis. K.S. and K.L. preformed the statistical analysis. S.S. wrote the first draft of the manuscript. All authors drafted the manuscript, contributed to the revision thereof and approved the final and submitted version of the manuscript.
Funding
At different phases, the FFHPV study has received support from the Päivikki and Sakari Sohlberg Foundation, the Government Special Foundation for the Turku University Hospital, the Finnish Cancer Foundation, the Academy of Finland, the Finnish Cultural Foundation, the Turku University Foundation and the Cancer Foundations of South-Western Finland. HPV serology analyses were supported by a fellowship of the Peter and Traudl Engelhorn-Stiftung. The funders had no role in the design or conduct of the study.
Data availability
Data and material are available from the corresponding author upon reasonable request.
Code availability
Codes are available from the corresponding author upon reasonable request.
Competing interests
Tim Waterboer serves on advisory boards for Merck (MSD) Sharp & Dohme. The other authors declare that they have no competing interests to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sarkola ME, Grénman SE, Rintala MA, Syrjänen KJ, Syrjänen SM. Human papillomavirus in the placenta and umbilical cord blood. Acta Obstet. Gynecol. Scand. 2008;87:1181–1188. doi: 10.1080/00016340802468308. [DOI] [PubMed] [Google Scholar]
- 2.Rintala M, Grénman S, Puranen M, Isolauri E, Ekblad U, Kero PO, Syrjänen S. Transmission of high-risk human papillomavirus (HPV) between parents and infant: A prospective study of HPV in families in Finland. J. Clin. Microbiol. 2005;43:376–381. doi: 10.1128/JCM.43.1.376-381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trottier H, Mayrand MH, Coutlée F, Monnier P, Laporte L, Niyibizi J, et al. Human papillomavirus (HPV) perinatal transmission and risk of HPV persistence among children: Design, methods and preliminary results of the HERITAGE study. Papillomavirus Res. 2016;2:145–152. doi: 10.1016/j.pvr.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syrjänen S. Current concepts on human papillomavirus infections in children. APMIS. 2010;118:494–509. doi: 10.1111/j.1600-0463.2010.02620.x. [DOI] [PubMed] [Google Scholar]
- 5.Merckx M, Liesbeth WV, Arbyn M, Meys J, Weyers S, Temmermanet M, et al. Transmission of carcinogenic human papillomavirus types from mother to child: a meta-analysis of published studies. Eur. J. Cancer Prev. 2013;22:277–285. doi: 10.1097/CEJ.0b013e3283592c46. [DOI] [PubMed] [Google Scholar]
- 6.Zouridis A, Kalampokas T, Panoulis K, Salakos N, Deligeoroglou E. Intrauterine HPV transmission: A systematic review of the literature. Arch. Gynecol. Obstet. 2018;298:35–44. doi: 10.1007/s00404-018-4787-4. [DOI] [PubMed] [Google Scholar]
- 7.Kawana K, Yasugi T, Yoshikawa H, Kawana Y, Matsumoto K, Nakagawa S, et al. Evidence for the presence of neutralizing antibodies against human papillomavirus type 6 in infants born to mothers with condyloma acuminata. Am. J. Perinatol. 2003;20:11–16. doi: 10.1055/s-2003-37949. [DOI] [PubMed] [Google Scholar]
- 8.Heim K, Hudelist G, Geier A, Szedenik H, Christensen ND, Concin N, et al. Type-specific antiviral antibodies to genital human papillomavirus types in mothers and newborns. Reprod. Sci. 2007;14:806–814. doi: 10.1177/1933719107309546. [DOI] [PubMed] [Google Scholar]
- 9.Marais DJ, Sampson CC, Urban MI, Sitas F, Wiliamson AL. The seroprevalence of IgG antibodies to human papillomavirus (HPV) types HPV-16, HPV-18, and HPV-11 capsid-antigens in mothers and their children. J. Med. Virol. 2007;79:1370–1374. doi: 10.1002/jmv.20874. [DOI] [PubMed] [Google Scholar]
- 10.Koskimaa HM, Waterboer T, Pawlita M, Grénman S, Syrjänen K, Syrjänen S. Human papillomavirus genotypes present in the oral mucosa of newborns and their concordance with maternal cervical human papillomavirus genotypes. J. Pediatr. 2012;160:837–843. doi: 10.1016/j.jpeds.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Zahreddine M, Mayrand MH, Therrien C, Trevisan A, Dagenais C, Monnier P, et al. Antibodies to human papillomavirus types 6, 11, 16 and 18: Vertical transmission and clearance in children up to two years of age. EClinicalMedicine. 2020;7(21):100334. doi: 10.1016/j.eclinm.2020.100334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rintala MA, Grenman SE, Järvenkylä ME, Syrjanen KJ, Syrjanen SM. High-risk types of human papillomavirus (HPV) DNA in oral and genital mucosa of infants during their first 3 years of life: Experience from the Finnish HPV Family Study. Clin. Infect. Dis. 2005;41:1728–1733. doi: 10.1086/498114. [DOI] [PubMed] [Google Scholar]
- 13.Syrjänen S, Waterboer T, Sarkola M, Michael K, Rintala M, Syrjänen K, et al. Dynamics of human papillomavirus serology in women followed up for 36 months after pregnancy. J. Gen. Virol. 2009;90:1515–1516. doi: 10.1099/vir.0.007823-0. [DOI] [PubMed] [Google Scholar]
- 14.Rautava J, Willberg J, Louvanto K, Wideman L, Syrjänen K, Grénman S, Syrjänen S. Prevalence, genotype distribution and persistence of human papillomavirus in oral mucosa of women: A six-year follow-up study. PLoS ONE. 2012;7:e42171. doi: 10.1371/journal.pone.0042171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louvanto K, Rintala MA, Syrjänen KJ, Grénman SE, Syrjänen SM. Incident cervical infections with high- and low-risk human papillomavirus (HPV) infections among mothers in the prospective Finnish Family HPV Study. BMC Infect. Dis. 2011;11:179. doi: 10.1186/1471-2334-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione S -transferase fusion proteins. Clin. Chem. 2005;51:1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 17.Robbins HA, Li Y, Porras C, Pawlita M, Ghosh A, Rodriguez AC, et al. Glutathione S-transferase L1 multiplex serology as a measure of cumulative infection with human papillomavirus. BMC Infect. Dis. 2014;14:120. doi: 10.1186/1471-2334-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto LA, Dillner J, Beddows S, Unger ER. Immunogenicity of HPV prophylactic vaccines: Serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32):4792–4799. doi: 10.1016/j.vaccine.2017.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbins HA, Kemp TJ, Porras C, Rodriguez AC, Schiffman M, Wacholder S, et al. Comparison of antibody responses to human papillomavirus vaccination as measured by three assays. Front. Oncol. 2014;3:328. doi: 10.3389/fonc.2013.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith EM, Parker MA, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP. Evidence for vertical transmission of HPV from mothers to infants. Infect. Dis. Obstet. Gynecol. 2010;3:326369. doi: 10.1155/2010/326369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matys K, Mallary S, Bautista O, Vuocolo S, Manalastas R, Pitisuttithum P, et al. Mother-infant transfer of anti-human papillomavirus (HPV) antibodies following vaccination with the quadrivalent HPV (type 6/11/16/18) virus-like particle vaccine. Clin. Vacc. Immunol. 2012;19:881–885. doi: 10.1128/CVI.00002-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guevara AM, Suarez E, Victoria A, Ngan HY, Lindén Hirschberg A, Fedrizziet E, et al. Maternal transfer of anti HPV 6 and 11 antibodies upon immunization with the 9-valent HPV vaccine. Hum. Vaccin. Immunother. 2019;15:141–145. doi: 10.1080/21645515.2018.1514227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albrecht KA, Arck PC. Vertically transferred immunity in neonates: Mothers, mechanisms and mediators. Front. Immunol. 2020;31(11):555. doi: 10.3389/fimmu.2020.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pou C, Nkulikiyimfura D, Henckel E, Olin A, Lakshmikanth T, Mikes J, et al. The repertoire of maternal anti-viral antibodies in human newborns. Nat. Med. 2019;25:591–596. doi: 10.1038/s41591-019-0392-8. [DOI] [PubMed] [Google Scholar]
- 25.Manns A, Strickler HD, Wiktor SZ, Pate EJ, Gray R, Waters D. Low incidence of human papillomavirus type 16 antibody seroconversion in young children. Pediatr. Infect. Dis. J. 1999;18:833–835. doi: 10.1097/00006454-199909000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Geijersstam V, Eklund C, Wang Z, Sapp M, Schiller JT, Dillner J, Dillner L. A survey of seroprevalence of human papillomavirus types 16, 18 and 33 among children. Int. J. Cancer. 1999;80:489–493. doi: 10.1002/(SICI)1097-0215(19990209)80:4<489::AID-IJC1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Syrjänen S, Rintala M, Sarkola M, Willberg J, Rautava J, Koskimaa H, et al. Oral human papillomavirus infection in children during the first 6 years of life. Emerg. Infect. Dis. 2021;3:759–766. doi: 10.3201/eid2703.202721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material are available from the corresponding author upon reasonable request.
Codes are available from the corresponding author upon reasonable request.