Abstract
The existing coverage criteria for noninvasive ventilation (NIV) do not recognize the benefits of early initiation of NIV for those with thoracic restrictive disorders and do not address the unique needs for daytime support as the patients progress to ventilator dependence. This document summarizes the work of the thoracic restrictive disorder Technical Expert Panel working group. The most pressing current coverage barriers identified were: (1) delays in implementing NIV treatment; (2) lack of coverage for many nonprogressive neuromuscular diseases; and (3) lack of clear policy indications for home mechanical ventilation (HMV) support in thoracic restrictive disorders. To best address these issues, we make the following key recommendations: (1) given the need to encourage early initiation of NIV with bilevel positive airway pressure devices, we recommend that symptoms be considered as a reason to initiate therapy even at mildly reduced FVCs; (2) broaden CO2 measurements to include surrogates such as transcutaneous, end-tidal, or venous blood gas; (3) expand the diagnostic category to include phrenic nerve injuries and disorders of central drive; (4) allow a bilevel positive airway pressure device to be advanced to an HMV when the vital capacity is < 30% or to address severe daytime respiratory symptoms; and (5) provide additional HMV when the patient is ventilator dependent with use > 18 h per day. Adoption of these proposed recommendations would result in the right device, at the right time, for the right type of patients with thoracic restrictive disorders.
Key Words: neuromuscular, noninvasive, ventilations
Abbreviations: ALS, amyotrophic lateral sclerosis; BPAP, bilevel positive airway pressure; CMS, Centers for Medicare & Medicaid Services; EtPco2, end-tidal CO2; FSS, frequent and substantial servicing; HMV, home mechanical ventilation; MIP, maximal inspiratory pressure; NCD, national coverage determination; NIV, noninvasive ventilation; NMD, neuromuscular disease; ONMAP, Optimal NIV Medicare Access Promotion; TcCO2, transcutaneous CO2; TRD, thoracic restrictive disorder; VC, vital capacity
Note to the Reader: The current document is one of a series produced by a Technical Expert Panel (TEP), whose purpose was to propose changes to Centers for Medicare & Medicaid Services national coverage determinations for the use of noninvasive ventilation and home mechanical ventilation, which were formulated in 1998. Specifically, the TEP proposed changes to national coverage determinations for thoracic restrictive disorders (neuromuscular disease), COPD, hypoventilation syndromes, central sleep apnea, and OSA. The background, makeup of the TEP, and key recommendations are highlighted in an Executive Summary. CHEST, the American Association of Respiratory Care, the American Academy of Sleep Medicine, and the American Thoracic Society formed the “Optimal NIV Medicare Access Promotion (ONMAP)” to provide processes to obtain the “right device for the right patient at the right time.” More details and rationale for the proposed changes are available in the companion documents.
Thoracic restrictive disorders (TRDs) are characterized by restrictive respiratory physiology due to weakness from neuromuscular diseases (NMDs) and/or chest wall deformity. TRDs often lead to disturbed sleep architecture, sleep hypoventilation, and ultimately daytime hypoventilation.1,2 The leading causes of death and major morbidity in these diseases are respiratory infection and respiratory failure.3 Classes of disease leading to TRD chronic respiratory failure include defects in generation of respiratory drive (eg, central congenital hypoventilation syndrome), upper airway weakness or instability (eg, amyotrophic lateral sclerosis [ALS]), weakness of the diaphragm and other respiratory muscles (eg, muscular dystrophies, ALS, diaphragmatic paralysis), or thoracic cage deformities (eg, severe scoliosis).
Background
Initially, noninvasive ventilation (NIV) was used to treat TRDs, starting during the polio epidemics of the mid-twentieth century in the form of negative pressure ventilation with the “iron lung,” and from that time on reduction in mortality and improvement of quality of life have been universally shown.4 Patients with TRD require NIV support for anywhere from a few hours to a substantial portion of the day, using a mask at night and a mouthpiece during the day. At this time (2021), daytime mouthpiece ventilation can only be realistically supplied by home mechanical ventilation (HMV) (not bilevel positive airway pressure [BPAP]), illustrating why many patients with TRD will use HMV (not BPAP devices) via noninvasive interfaces.
Based on recommendations of a consensus conference of experts organized in 1998 by the American College of Chest Physicians and the National Association of Directors of Respiratory Care,5 Medicare developed an national coverage determination (NCD) for coverage of noninvasive respiratory support of individuals with TRD. At that time, limited data were available to develop the coverage rules. A significant increase in data is now available and should be used to drive coverage decisions (https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=33800).
Barriers to Clinical Care in TRD
Although NIV improves both quality and length of life, there are substantial barriers to the optimal clinical care of patients with TRD, which is discussed in the following sections.
Barrier #1: Delays in Implementing NIV Treatment
There are three major causes of implementation delay for NIV:
-
1.
Failure to acknowledge symptoms as a major component of coverage.
-
2.
Difficult-to-measure and excessively stringent functional criteria (eg, spirometry, respiratory muscle pressure measurements, oxygen/CO2 assessment). Measurement of vital capacity (VC), maximal inspiratory pressure (MIP), and a measure of arterial blood CO2 level are the measures currently mandated by the Centers for Medicare & Medicaid Services (CMS) policies.
-
3.
Lack of patient access to laboratories to undergo functional measurements.
Patients with TRDs have limited access to care for hypoventilation because of the numerous hurdles that our standard health care models present. Just getting to the clinic may present a challenge due to the need for specialized transport. Once at the facility, obtaining spirometry testing is another challenge as many pulmonary function laboratories use plethysmographic (“body box”)-based systems that cannot accommodate a wheelchair and will not allow for supine measurements. A potential solution for this lack of access is home-based spirometry testing.6,7
Barrier #2: Lack of Coverage for Many Nonprogressive NMDs
Although some nonprogressive TRDs have received positive coverage decisions, there are several nonprogressive NMDs that result in hypercapnic respiratory failure (eg, phrenic nerve/diaphragm disorders, spinal cord injury, quadriplegia). The situation regarding lack of coverage may also force additional diagnostic testing and delay of needed therapy in symptomatic patients who fall above the qualifying thresholds, or in those who cannot perform the required testing because of features of the disease (eg, bulbar symptoms in cerebral palsy).
Barrier #3: Lack of Clear Policy Indications for HMV Support in TRD
The system of providing home-based ventilation support for those with NMD should, in most cases, start with the use of a BPAP with a backup rate. However, an HMV may be indicated initially for some individuals with TRD due primarily to diaphragm failure, especially if their disease is progressing rapidly or they present at a late stage in the course of their disease. Presently, there are no clear, specific clinical indicators to satisfy CMS coverage criteria for the HMV. Some practitioners prescribe an HMV as the initial device for other reasons, as summarized in Table 1. The frequent and substantial servicing (FSS) plan means that there are never-ending rental payments for the HMV, which is the only mechanism to fund home-based respiratory therapy. In addition, it is now well established that symptomatic patients with NMD need NIV, but the BPAP criteria are so outdated and challenging to fulfill that physicians simply give up and prescribe an HMV. The extra cost is outweighed to avoid the burden of the BPAP criterion.
Table 1.
Reasons Cited for Selection of HMV Over BPAP Devices
|
|
|
|
|
|
ALS = amyotrophic lateral sclerosis; BPAP = bilevel positive airway pressure; HMV = home mechanical ventilation; NIV = noninvasive ventilation; NMD = neuromuscular disease; RAD = respiratory assist device.
These reasons seem completely appropriate. However, it is likely that there is both a purely optional use of HMV in early progressive TRDs and a subsequent necessity in more advanced disease in which NIV daytime support with a portable HMV is indicated.
Current Evidence/Clinical Consensus Practice Guidelines
Over the last 20 years (particularly in the treatment of ALS), earlier treatment with NIV has been shown to improve outcomes. In a randomized controlled trial, Bourke et al8 showed that survial and quality of life improved in the NIV treatment group. Patients were started on NIV based on the symptom of orthopnea, with an average FVC of 56%. This study was followed by a retrospective study of patients with ALS who were started on “early NIV” (FVC > 65%) and a standard group (FVC < 65%).9 In addition to reduced FVC, patients were enrolled based on symptoms, specifically dyspnea, orthopnea, and/or fragmented sleep. Those in the early NIV group were found to have up to 1 year of prolonged life compared with the standard group. A retrospective cohort analysis also supports the use of “early NIV” in ALS prior to deterioration in VC. At an FVC < 80% with symptoms, adding NIV was associated with an additional 7-month survival time.10 This increased to approximately 11 months with improved adherence to NIV therapy.
Based on the aforementioned data and consensus expert opinion, many international professional society guidelines have strongly advised earlier intervention with NIV ventilatory support (Table 2).4,11, 12, 13, 14, 15, 16, 17, 18 The more up-to-date international statements significantly expand criteria for NIV compared with the current CMS coverage policies. The US policies have remained in line with the CMS criterion because of the funding limitations of the current system. A very important and consistent message from these international guidelines is that patient symptoms play a crucial role in determining application of NIV in individuals with TRD.
Table 2.
International Guidelines and Criteria for Initiation of Assisted Ventilation
| Source | Diagnosis | Criteria for Assisted Ventilation (Any of the Following) |
||||
|---|---|---|---|---|---|---|
| Symptoms/Signsa | Spirometry | Respiratory Muscle Pressure | Awake/Daytime Hypoventilation/Oxygenation | Nocturnal/Sleep Hypoventilation/Oxygenation | ||
| AAN16 | MND | Orthopnea | FVC < 50% | MIP < –60 cm H2O SNIP < –40 cm H2O |
Nocturnal oximetry < 90% for 1 cumulative minute | |
| AFM13 | NMD | VC < 50% | Paco2 > 45 mm Hg | Nocturnal hypercapnia | ||
| Symptoms indicative of hypoventilation (eg, morning headaches, fatigue) and nocturnal hypoxemia: Sao2 < 88% for ≥ 5 consecutive minutes or < 90% for > 5% of nocturnal time | ||||||
| AHRQ4 | NMD/RTD | FVC < 40% in RTD FVC < 50% in NMD |
MIP < –60 cm H2O | Paco2> 45 mm Hg | O2 saturation < 88% for ≥ 5 consecutive minutes | |
| ASA/TSANZ11 | NMD | VC < 50% | MIP < 40% | Paco2 ≥ 45 mm Hg | Sao2 < 90% for > 2 consecutive minutes Sao2 < 90% for > 5% of night Etco2/Tcco2 > 50 mm Hg for > 50% of sleep Etco2/Tcco2 rise > 8 mm Hg above wake |
|
| Symptoms of significant nocturnal obstructive or hypopneic events (obtain PSG for history suggestive of sleep-disordered breathing or FVC < 40%, base excess > 4 mM on ABG or erect/supine fall in VC ≥ 25%) | ||||||
| CTS18 | MND | Orthopnea | FVC < 50% | SNIP < – 40 cm H2O MIP < –40 cm H2O |
Arterial or capillary pco2 > 45 mm Hg | |
| Symptoms AND FVC sitting or supine < 80% AND SNIP < –50 cm H2O or MIP < –65 male subjects or < –55 female subjects | ||||||
| Symptoms and nocturnal O2 saturation < 90% for > 5% of sleep time or < 88% for ≥ 5 consecutive minutes, or ≥ 10 mm Hg increase in Tcco2 during sleep | ||||||
| DMD Care Considerations Working Group14 |
DMD | Signs or symptoms | FVC < 50% | MIP < –60 cm H2O | CO2 (Etco2/Tcco2, arterial venous or capillary) > 45 mm Hg Awake baseline Spo2< 95% |
Etco2/Tcco2 > 50 mm Hg for ≥ 2% of sleep time Increased Etco2/Tcco2 of 10 mm Hg above awake baseline for ≥ 2% of sleep time, Spo2 ≤ 88% or less for ≥ 2% of sleep time or ≥ 5 continuous minutes AHI ≥ 5 events/h |
| German Society for Pneumology17 | NMD/RTD | Symptoms and rapid significant VC decline Symptoms and Paco2 ≥ 45 mm Hg |
||||
| Symptoms and either nocturnal Paco2 ≥ 50 mm Hg or Tcco2 rise ≥ 10 mm Hg (obtain PSG with Tcco2 when VC < 70%) | ||||||
| NICE12 | MND | VC < 50% | SNIP/MIP < –40 cm H2O Decrease in SNIP/MIP > 10 cm H2O per 3 months |
|||
| Symptoms or signs (especially orthopnea) with VC < 80% or SNIP/MIP < –65 cm H2O in men and < 55 cm H2O in women | ||||||
MIP and SNIP are negative and “<“ reads as “worse than.” AAN = American Academy of Neurology; ABG = arterial blood gas; AFM = Association Française contre les Myopathies; AHI = apnea-hypopnea index; AHRQ = Agency for Healthcare Research and Quality (US); ASA/TSANZ = Australasian Sleep Association/Thoracic Society of Australia and New Zealand; CTS = Canadian Thoracic Society; DMD = Duchenne muscular dystrophy; EFNS = European Federation of Neurological Societies; Etco2 = end-tidal CO2; MIP = maximal inspiratory pressure; MND = motor neuron disease; NICE = National Institute for Health Care and Excellence (UK); NMD = neuromuscular disease; PSG = polysomnogram; RTD = restrictive thoracic disorders; Sao2 = arterial oxygen saturation; SNIP = sniff nasal inspiratory pressure; Spo2 = arterial oxygen saturation by pulse oximetry; Tcco2 = transcutaneous CO2; TRD = thoracic restrictive disorders; VC = vital capacity.
Dyspnea, tachypnea, orthopnea, disturbed sleep due to nocturnal desaturation/arousals, including frequent nocturnal awakenings or difficult arousal, awakenings with dyspnea and tachycardia, or frequent nightmares, daytime fatigue, difficulty concentrating, daytime hypersomnolence, use of auxiliary respiratory muscles at rest, and paradoxical respiration.
Functional Measures of Neuromuscular Weakness
There has been significant advancement in the understandings of the best measures of respiratory function in the individual with TRD.
Spirometry
VC is a significant predictor of survival, sleep-disordered breathing, nocturnal hypoventilation, and daytime hypercapnia.19 Studies confirm the importance of VC as a predictor of survival in ALS and Duchenne muscular dystrophy.20,21 The CMS coverage criteria of an FVC < 50% predicted as the minimal finding for initiation of NIV is clearly too restrictive. The policies fail to consider those who are symptomatic with a lesser reduction in their FVC. Although FVC is the most commonly used measurement, slow VC may be easier to perform and is equivalent to FVC.22,23
Measures of Respiratory Muscle Strength
MIP predicts survival, has been used as an end point of clinical trials, and is a sensitive predictor of nocturnal hypoxemia.24,25 The sniff nasal inspiratory pressure is an alternative to MIP to assess respiratory muscle weakness. Whereas MIP assesses global inspiratory muscle strength, sniff nasal inspiratory pressure assesses diaphragm weakness and can be easier to perform for those with bulbar dysfunction.26,27
Gas Exchange Measures
Elevation in Pco2 is accepted universally as the hallmark of hypoventilation, and the arterial blood gas is the standard for measurement. Daytime hypercapnia predicts benefit from NIV8 and is a sensitive predictor of sleep hypoventilation.28 Arterial blood gas sampling is the only measure currently recognized by the CMS policies for measurement of gas exchange. The venous blood gas Pco2, end-tidal CO2 (EtPco2), and transcutaneous CO2 (Tcco2) are measures that have been used successfully as surrogates for Paco2. The Tcco2 measurements have been shown to track closely with arterial blood gases.29 The venous blood gas can track with Pco2 on arterial blood gas but are known to exceed the value by approximately 5 mm Hg.30 Lastly, EtPco2 has been commonly used in sleep laboratories in patients with NMD. Although this method is known to frequently report false-negative findings when evaluating these patients for hypoventilation, false-positive findings are not an issue. The ease of use and low cost suggest that EtPco2 will continue to be frequently used with confidence and that elevated values reliably identify hypoventilation.31
Nocturnal hypoventilation without diurnal hypercapnia is a strong predictor of daytime respiratory failure within 12 to 24 months.32 Nocturnal hypercapnia is defined by a mean Tcco2 > 50 mm Hg or as an increase in Tcco2 ≥ 10 mm Hg to a value > 50 mm Hg for ≥ 10 min.33 In a randomized study, initiation of NIV in ALS for nocturnal oxygen desaturation (oxygen saturation by pulse oximetry < 90% for 1 cumulative minute) improved quality of life compared with initiation at a more conventional FVC < 50%.34 Furthermore, successful correction of nocturnal desaturation with NIV in patients with ALS improved survival.35
Optimal NIV Medicare Access Promotion (ONMAP): New Policies for TRDs
Proposed Solutions to Barrier #1: Delays in Implementing NIV Treatment
Our proposal for the revision of CMS NCD and related policies for NIV initiation is shown in Table 3. It incorporates a strong emphasis on patient symptoms combined with more appropriate “cutoffs” for functional measurements. In addition, we propose the use of alternative physiological measurements that may be easier to access.
Table 3.
List of Indications for Initiation of a BPAP Device
| Any single criterion sufficient to initiate BPAP device in TRD. |
|
|
|
|
|
|
|
|
|
|
|
|
|
ABG = arterial blood gas; ALS = amyotrophic lateral sclerosis; BPAP = bilevel positive airway pressure; Etco2 = end-tidal CO2; HST = home sleep test; MIP = maximal inspiratory pressure; NIV = noninvasive ventilation; PSG = polysomnogram; SNIP = sniff nasal inspiratory pressure; Tcco2 = transcutaneous CO2; TRD = thoracic restrictive disorder; VBG = venous blood gas; VC = vital capacity.
Proposed Solutions to Barrier # 2: Lack of Coverage for Many Nonprogressive NMDs
A revised CMS policy should allow coverage of TRDs that are nonprogressive including (but not limited to) phrenic nerve injury, spinal cord injury, cerebral palsy, multiple sclerosis, spina bifida, and congenital central hypoventilation syndrome as categorized in Table 4.
Table 4.
Diagnostic Groups Contained in Updated TRD Category
|
|
|
|
|
|
|
|
|
|
TRD = thoracic restrictive disorder.
Proposed Solution Barrier #3: Clear NCD Indications for HMV Support in TRDs
We propose that an updated CMS policy for NIV in TRDs incorporates indications such as those noted in Tables 5 and 6 for coverage of HMV used either in transition from BPAP to HMV or as the initial NIV device.
Table 5.
Finding Needed to Advance to HMV Following Nocturnal Use of BPAP (Any)
|
|
|
|
|
BPAP = bilevel positive airway pressure; HMV = home mechanical ventilation; NIV = noninvasive ventilation; VC = vital capacity.
Table 6.
Findings Needed to Start With HMV as the Initial NIV Device in TRDs (Both)
|
|
BPAP = bilevel positive airway pressure; HMV = home mechanical ventilation; NIV = noninvasive ventilation; TRDs = thoracic restrictive disorders.
Summary of Recommendations
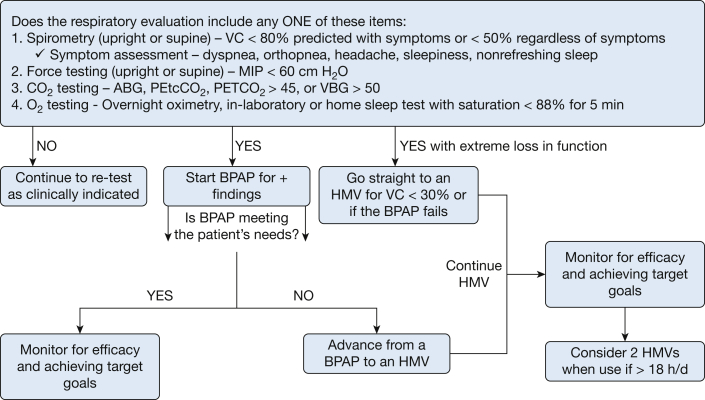
We propose that an updated CMS NCD policy for NIV in TRDs incorporate a clear pathway for coverage of HMV, as noted in Figure 1.
Figure 1.
Suggested initiation and monitoring of noninvasive ventilation therapy in thoracic restrictive disorder. ABG = arterial blood gas; BPAP = bilevel positive airway pressure; HMV = home mechanical ventilator; maximal inspiratory pressure; O2 = oxygen; PETCO2 = end-tidal CO2;VBG = venous blood gas; VC = vital capacity.
Final Issues for Consideration
-
1.
Frequent and Substantial Servicing
Patients with TRDs face chronic respiratory failure that is both debilitating and potentially deadly. Malfunction of a BPAP device in this patient population can lead to very significant and dangerous clinical situations. Although we strongly support the use of certain BPAP devices in TRDs, the fact that BPAP payment is a capped rental and does not include FSS results in potentially dangerous situations for patients with TRDs. We recommend the FSS provision of the statute apply to BPAP or HMV in the comparatively narrow TRD population.
-
2.
Evaluate efficacy and compliance of BPAP and HMV
Following the initiation of BPAP, efficacy and compliance should be closely monitored. The compliance of the BPAP and the efficacy of HMV should be evaluated.36 These patients are the most vulnerable of all the other TEP categories and should be given every opportunity to adapt to NIV.
Use outside the sleep period suggests that portability and daytime mouthpiece ventilation are being used. Typically, it is assumed that the overnight sleep time does not last > 9.5 h, and once use is > 10 h, it should be assumed that at least some daytime use is needed outside of normal sleep. The utilization of NIV using HMV should be closely monitored for adherence and efficacy. Any daytime use of mouthpiece or mask ventilation suggests that the HMV should continue. This could be shown by finding either the total use >10 h per day or use of NIV setting > 2 h during daytime (Table 7).
-
3.
Second Device for Safety at Home
Table 7.
Evaluation of NIV in TRDs
|
|
|
|
|
|
|
|
BPAP = bilevel positive airway pressure; HMV = home mechanical ventilation; NIV = noninvasive ventilation; TRDs = thoracic restrictive disorders.
When patients progress to the use of HMV for > 18 h a day, it strongly suggests that the patient is essentially ventilator dependent and should have a second device. At least one of the two devices should be portable so that wheelchair attachment and mobility are possible. These patients are at risk for hospital admission, injury, or even mortality if they have only one device and it fails. This is consistent with our current model in-home NIV care for those with NMD who require an HMV.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
∗ONMAP Technical Expert Panel members: Overall Chairs: Peter C. Gay, MD, FCCP, and Robert L. Owens, MD. Thoracic Restrictive Disorders: Chair/Co-Chair—Lisa F. Wolfe, MD, FCCP, and Joshua O. Benditt, MD, FCCP. Thoracic Restrictive Disorders Committee Members—Loutfi S. Aboussouan, MD, FCCP, John M. Coleman III, MD, and Dean R. Hess, PhD, RRT. COPD: Chair/Co-chair—Nicholas S. Hill, MD, FCCP, and Gerard J. Criner, MD, FCCP. COPD Committee Members—Richard D. Branson, MSc, RRT, Bartolome R. Celli, MD, FCCP, Neil R. MacIntyre, MD, FCCP, and Amen Sergew, MD. Central Sleep Apnea: Chair/Co-Chair—Timothy I. Morgenthaler, MD, and Atul Malhotra, MD, FCCP. Central Sleep Apnea Committee Members—Richard B. Berry, MD, Karin G. Johnson, MD, and Marc I. Raphaelson, MD. Hypoventilation: Chair/Co-chair—Babak Mokhlesi, MD, FCCP, and Christine H. Won, MD. Hypoventilation Committee Members—Bernardo J. Selim, MD, FCCP, Barry J. Make, MD, FCCP, and Bernie Y. Sunwoo, MBBS. OSA: Chair/Co-chair—Nancy A. Collop, MD, Master FCCP, and Susheel P. Patil, MD, PhD. OSA Committee Members—Alejandro D. Chediak, MD, FCCP, Eric J. Olson, MD, and Kunwar Praveen Vohra, MD, FCCP.
Contributor Information
Lisa F. Wolfe, Email: lwolfe@northwestern.edu.
ONMAP Technical Expert Panel:
Peter C. Gay, Robert L. Owens, Lisa F. Wolfe, Joshua O. Benditt, Loutfi S. Aboussouan, John M. Coleman, III, Dean R. Hess, Nicholas S. Hill, Gerard J. Criner, Richard D. Branson, Bartolome R. Celli, Neil R. MacIntyre, Amen Sergew, Timothy I. Morgenthaler, Atul Malhotra, Richard B. Berry, Karin G. Johnson, Marc I. Raphaelson, Babak Mokhlesi, Christine H. Won, Bernardo J. Selim, Barry J. Make, Bernie Y. Sunwoo, Nancy A. Collop, Susheel P. Patil, Alejandro D. Chediak, Eric J. Olson, and Kunwar Praveen Vohra
References
- 1.Perrin C., Unterborn J.N., Ambrosio C.D., Hill N.S. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve. 2004;29(1):5–27. doi: 10.1002/mus.10487. [DOI] [PubMed] [Google Scholar]
- 2.Fermin A.M., Afzal U., Culebras A. Sleep in neuromuscular diseases. Sleep Med Clin. 2016;11(1):53–64. doi: 10.1016/j.jsmc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Bergofsky E.H. Respiratory failure in disorders of the thoracic cage. Am Rev Respir Dis. 1979;119(4):643–669. doi: 10.1164/arrd.1979.119.4.643. [DOI] [PubMed] [Google Scholar]
- 4.Wilson M., Wang Z., Dobler C., et al. Noninvasive positive pressure ventilation in the home. Project ID: PULT0717. Prepared by the Mayo Clinic Evidence-Based Practice Center under Contract No. HHSA290201500013I_HHSA29032004T. Rockville, MD: Agency for Healthcare Research and Quality. https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/ta/hmv/hmv-ta-fullreport.pdf
- 5.Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest. 1999;116(2):521–534. doi: 10.1378/chest.116.2.521. [DOI] [PubMed] [Google Scholar]
- 6.Dilektasli A.G., Porszasz J., Casaburi R., et al. A novel spirometric measure identifies mild COPD unidentified by standard criteria. Chest. 2016;150(5):1080–1090. doi: 10.1016/j.chest.2016.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards C., Costello E., Cassidy N., Vick B., Russell A.M. Use of the patient Mpower app with home-based spirometry to monitor the symptoms and impact of fibrotic lung conditions: longitudinal observational study. JMIR Mhealth Uhealth. 2020;8(11) doi: 10.2196/16158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourke S.C., Tomlinson M., Williams T.L., Bullock R.E., Shaw P.J., Gibson G.J. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5(2):140–147. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 9.Lechtzin N., Scott Y., Busse A.M., Clawson L.L., Kimball R., Wiener C.M. Early use of non-invasive ventilation prolongs survival in subjects with ALS. Amyotroph Lateral Scler. 2007;8(3):185–188. doi: 10.1080/17482960701262392. [DOI] [PubMed] [Google Scholar]
- 10.Khamankar N., Coan G., Weaver B., Mitchell C.S. Associative increases in amyotrophic lateral sclerosis survival duration with non-invasive ventilation initiation and usage protocols. Front Neurol. 2018;9:578. doi: 10.3389/fneur.2018.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Respiratory Network Domiciliary Non-Invasive Ventilation Working Group . NSW Agency for Clinical Innovation; 2012. Domiciliary non-invasive ventilation in adult patients—a consensus statement.https://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0008/159794/ACI-NIV-guidelines.pdf [Google Scholar]
- 12.National Institute for Health Care and Excellence Motor neurone disease: assessment and management/NICE guideline. https://www.nice.org.uk/guidance/ng42 [PubMed]
- 13.Association Française contre les Myopathies-Haute Autorité de Santé Modalités pratiques de la ventilation non invasive en pression positive, au long cours, à domicile, dans les maladies neuromusculaires. https://www.has-sante.fr/jcms/c_334439/fr/modalites-pratiques-de-la-ventilation-non-invasive-en-pression-positive-au-long-cours-a-domicile-dans-les-maladies-neuromusculaires [PubMed]
- 14.Birnkrant D.J., Bushby K., Bann C.M., et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17(4):347–361. doi: 10.1016/S1474-4422(18)30025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKim D.A., Road J., Avendano M., et al. Home mechanical ventilation: a Canadian Thoracic Society clinical practice guideline. Can Respir J. 2011;18(4):197–215. doi: 10.1155/2011/139769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller R.G., Jackson C.E., Kasarskis E.J., et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73(15):1227–1233. doi: 10.1212/WNL.0b013e3181bc01a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Windisch W., Walterspacher S., Siemon K., Geiseler J., Sitter H., German Society for Pneumology Guidelines for non-invasive and invasive mechanical ventilation for treatment of chronic respiratory failure. Published by the German Society for Pneumology (DGP) Pneumologie. 2010;64(10):640–652. doi: 10.1055/s-0030-1255558. [DOI] [PubMed] [Google Scholar]
- 18.Rimmer K.P., Kaminska M., Nonoyama M., et al. Home mechanical ventilation for patients with amyotrophic lateral sclerosis: a Canadian Thoracic Society clinical practice guideline. Can J Respir Crit Care Sleep Med. 2019;3(1):9–27. [Google Scholar]
- 19.Ragette R., Mellies U., Schwake C., Voit T., Teschler H. Patterns and predictors of sleep disordered breathing in primary myopathies. Thorax. 2002;57(8):724–728. doi: 10.1136/thorax.57.8.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czaplinski A., Yen A.A., Appel S.H. Amyotrophic lateral sclerosis: early predictors of prolonged survival. J Neurol. 2006;253(11):1428–1436. doi: 10.1007/s00415-006-0226-8. [DOI] [PubMed] [Google Scholar]
- 21.Phillips M.F., Quinlivan R.C., Edwards R.H., Calverley P.M. Changes in spirometry over time as a prognostic marker in patients with Duchenne muscular dystrophy. Am J Respir Crit Care Med. 2001;164(12):2191–2194. doi: 10.1164/ajrccm.164.12.2103052. [DOI] [PubMed] [Google Scholar]
- 22.Pinto S., de Carvalho M. Comparison of slow and forced vital capacities on ability to predict survival in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(7-8):528–533. doi: 10.1080/21678421.2017.1354995. [DOI] [PubMed] [Google Scholar]
- 23.Pinto S., de Carvalho M. Correlation between forced vital capacity and slow vital capacity for the assessment of respiratory involvement in amyotrophic lateral sclerosis: a prospective study. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(1-2):86–91. doi: 10.1080/21678421.2016.1249486. [DOI] [PubMed] [Google Scholar]
- 24.Gay P.C., Westbrook P.R., Daube J.R., Litchy W.J., Windebank A.J., Iverson R. Effects of alterations in pulmonary function and sleep variables on survival in patients with amyotrophic lateral sclerosis. Mayo Clin Proc. 1991;66(7):686–694. doi: 10.1016/s0025-6196(12)62080-1. [DOI] [PubMed] [Google Scholar]
- 25.Schoser B., Fong E., Geberhiwot T., et al. Maximum inspiratory pressure as a clinically meaningful trial endpoint for neuromuscular diseases: a comprehensive review of the literature. Orphanet J Rare Dis. 2017;12(1):52. doi: 10.1186/s13023-017-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefanutti D., Benoist M.R., Scheinmann P., Chaussain M., Fitting J.W. Usefulness of sniff nasal pressure in patients with neuromuscular or skeletal disorders. Am J Respir Crit Care Med. 2000;162(4 pt 1):1507–1511. doi: 10.1164/ajrccm.162.4.9910034. [DOI] [PubMed] [Google Scholar]
- 27.Chaudri M.B., Liu C., Watson L., Jefferson D., Kinnear W.J. Sniff nasal inspiratory pressure as a marker of respiratory function in motor neuron disease. Eur Respir J. 2000;15(3):539–542. doi: 10.1034/j.1399-3003.2000.15.18.x. [DOI] [PubMed] [Google Scholar]
- 28.Hukins C.A., Hillman D.R. Daytime predictors of sleep hypoventilation in Duchenne muscular dystrophy. Am J Respir Crit Care Med. 2000;161(1):166–170. doi: 10.1164/ajrccm.161.1.9901057. [DOI] [PubMed] [Google Scholar]
- 29.De Braekeleer K., Toussaint M. transcutaneous carbon dioxide measurement in adult patients with neuromuscular disorders: a quality level assessment. J Neuromuscul Dis. 2021;8(2):305–313. doi: 10.3233/JND-200516. [DOI] [PubMed] [Google Scholar]
- 30.Nassar B.S., Schmidt G.A. Estimating arterial partial pressure of carbon dioxide in ventilated patients: how valid are surrogate measures? Ann Am Thorac Soc. 2017;14(6):1005–1014. doi: 10.1513/AnnalsATS.201701-034FR. [DOI] [PubMed] [Google Scholar]
- 31.Won Y.H., Choi W.A., Lee J.W., Bach J.R., Park J., Kang S.W. Sleep transcutaneous vs. end-tidal CO2 monitoring for patients with neuromuscular disease. Am J Phys Med Rehabil. 2016;95(2):91–95. doi: 10.1097/PHM.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 32.Ward S., Chatwin M., Heather S., Simonds A.K. Randomised controlled trial of non-invasive ventilation (NIV) for nocturnal hypoventilation in neuromuscular and chest wall disease patients with daytime normocapnia. Thorax. 2005;60(12):1019–1024. doi: 10.1136/thx.2004.037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogna A., Quera Salva M.A., Prigent H., et al. Nocturnal hypoventilation in neuromuscular disease: prevalence according to different definitions issued from the literature. Sleep Breath. 2016;20(2):575–581. doi: 10.1007/s11325-015-1247-2. [DOI] [PubMed] [Google Scholar]
- 34.Carlucci A., Richard J.C., Wysocki M., Lepage E., Brochard L. Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med. 2001;163(4):874–880. doi: 10.1164/ajrccm.163.4.2006027. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Bermejo J., Morelot-Panzini C., Arnol N., et al. Prognostic value of efficiently correcting nocturnal desaturations after one month of non-invasive ventilation in amyotrophic lateral sclerosis: a retrospective monocentre observational cohort study. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(5-6):373–379. doi: 10.3109/21678421.2013.776086. [DOI] [PubMed] [Google Scholar]
- 36.Kleopa K.A., Sherman M., Neal B., Romano G.J., Heiman-Patterson T. Bipap improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci. 1999;164(1):82–88. doi: 10.1016/s0022-510x(99)00045-3. [DOI] [PubMed] [Google Scholar]