Abstract
Introduction
Self‐reported word‐finding difficulties are among the most frequent complaints in cognitively normal (CN) older adults. However, the clinical significance is still debated.
Methods
We selected 239 CN from the Alzheimer's Disease Neuroimaging Initiative database who had completed the Everyday Cognition (ECog) questionnaire, as well as a lumbar puncture for amyloid beta (Aβ) and magnetic resonance imaging.
Results
Word‐finding complaints, with a few other memory items, were significantly more severe compared to all other cognitive complaints. Ecog‐Lang1 (Forgetting names of objects) severity significantly predicted Aβ levels in CN, even when controlling for general cognitive complaint, demographic, and psychological variables. Individuals with high Ecog‐Lang1 complaints showed atrophy in the left fusiform gyrus and the left rolandic operculum compared to CN with low complaints.
Discussion
Overall, our results support the fact that word‐finding complaints should be taken seriously. They have the potential to identify CN at risk of AD and support the need to include other cognitive domains in the investigation of subjective cognitive decline.
Keywords: aging, Alzheimer's disease, language, subjective cognitive decline, subjective complaints, word finding
1. BACKGROUND
The amnestic form of Alzheimer's disease (AD), in which episodic memory disturbances are predominant, is the most common form of the disease. 1 Consequently, self‐reported memory complaints among older adults have received considerable attention in recent years as a possible early marker of the disease. 2 , 3 This interest spawns, in part, from the fact that AD has a long prodromal phase during which patients are either asymptomatic or have subtle cognitive complaints. 4 Interestingly, subjective memory complaints in cognitively normal (CN) older individuals have been associated with the presence of amyloid 2 and brain atrophy. 3
Apart from memory deficits, a decline related to language performance, and most specifically word‐finding difficulties, is also considered to be a very early sign of AD. 5 , 6 , 7 Several studies have shown that language and more specifically word‐finding complaints are among the most frequent complaints in CN older adults. 8 , 9 , 10 In daily life, these word‐finding complaints can manifest in different ways, such as forgetting the names of objects or people and having difficulties finding words in conversations. Even if the prevalence of word‐finding complaints in healthy aging is elevated, the clinical significance of self‐reported word‐finding difficulties is still undetermined. General language complaints, which combine expressive (word‐finding but other functions such as the ability to communicate thoughts efficiently, for example) and receptive (comprehending language) abilities, have been investigated with conflicting results. On one hand, some studies suggest that language complaints might not be the most relevant predictors of dementia: they might not be associated with AD pathology (amyloid beta [Aβ] and tau positron emission tomography [PET]) 11 or enhanced risk of converting to mild cognitive impairment (MCI). 12 On the other hand, a conflicting literature shows that higher language complaints are in fact associated with cerebrospinal fluid (CSF) Aβ. 13 , 14 , 15 In the only study investigating word‐finding complaints specifically, Condret‐Santi et al. found that subjective language complaints did not predict conversion to dementia in CN older adults. 8
No studies have investigated whether subjective word‐finding complaints, specifically, can be considered an early indication of AD‐related neuropathological and brain changes. In previous studies, subjective word‐finding complaints assessment has been somewhat limited by considering language as a unitary domain or using a single yes–no question. Also, sociodemographic and/or psychological factors might have an impact on self‐reported cognitive complaints. For instance, cognitive complaints were found to be correlated with sex and advanced age; women and older individuals display a higher prevalence of subjective cognitive complaints. 16 Language complaints, more specifically, have been related to depressive and anxiety‐related symptoms. 10 , 17 Consequently, determining if word‐finding difficulties are truly related to predictors of dementia might require accounting for these factors.
The present study aims at: (1) characterizing the frequency and severity of word‐finding complaints in CN older adults compared to other language and cognitive domains, (2) identifying sociodemographic and psychological characteristics associated with word‐finding complaints in CN, (3) determining if word‐finding complaints can predict CSF Aβ levels, and (4) comparing gray matter (GM) volume in CN with varying levels of word‐finding complaints. Based on the literature, we posit that (1) word‐finding difficulties will be among the most frequent and severe cognitive complaints among CN; (2) age, sex, education, depression, and anxiety will correlate with more severe word‐finding complaints; (3) subjective word‐finding complaints will predict pathological levels of CSF Aβ (even controlling for sociodemographic and psychological variables); and (4) CN with significant word‐finding complaints will present GM atrophy in brain regions that are associated with naming abilities and that are usually damaged in AD patients.
RESEARCH IN CONTEXT
Systematic review: We reviewed the literature using traditional sources (e.g., PubMed). While the prevalence of word‐finding complaints in healthy aging is elevated, outcomes regarding the clinical significance of these difficulties are conflictual.
Interpretation: Our findings suggest that word‐finding complaints are among the most severe in healthy aging. They are associated with lower cerebrospinal fluid amyloid beta levels and atrophy in the left fusiform gyrus and the left rolandic operculum, which highlights their clinical importance.
Future directions: To further establish the impact of word‐finding complaints in healthy aging, future longitudinal studies should investigate the relationship between continuous word finding complaints and conversion to mild cognitive impairment or dementia. Furthermore, word‐finding complaints should not be neglected in studies of cognitive complaints or subjective cognitive decline.
2. METHODS
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. For up‐to‐date information, see www.adni‐info.org.
2.1. Participants
In the general ADNI inclusion criteria, subjects are deemed CN if they present: (1) a Mini‐Mental State Examination (MMSE) scores between 24 and 30; (2) a Clinical Dementia Rating (CDR) score of 0; (3) above education‐adjusted scores on the delayed Paragraph Recall task from the Wechsler Memory Scale Logical Memory II; (4) no significant impairment in cognitive functions or activities of daily living.
Study‐specific inclusion criteria include (1) having available self‐rated Everyday Cognition (ECog) questionnaire; and (2) CSF AD biomarkers. Written informed consent was obtained from all participants.
2.2. Clinical assessment
The severity and frequency of word‐finding difficulties were assessed using the self‐reported ECog questionnaire. The ECog 18 is a validated 39 item‐scale probing an individual's subjective complaints through six cognitive dimensions: memory (eight items), language (nine items), visuospatial (seven items), planning (five items), organization (six items), and divided attention (four items). Responses range from 1 to 4 (1 = no change or actually performs better than 10 years ago; 2 = occasionally performs the task worse than 10 years ago but not all of the time; 3 = consistently performs the task a little worse than 10 years ago; 4 = performs the task much worse than 10 years ago). Within the language dimension, two items are related to word‐finding difficulties, namely Ecog‐Lang1 (“Forgetting the names of objects”) and Ecog‐Lang3 (“Finding the right words to use in a conversation”). These two items represent our main variables of interest.
Individuals enrolled in ADNI were also required to perform a plethora of clinical tests. The present study particularly considers two of them: Geriatric Depression Scale (GDS 19 ) to assess depression‐related symptoms, and Neuropsychiatric Inventory Examination 20 to assess anxiety‐related symptoms (anxiety item).
2.3. Biomarkers collection
Aβ concentration was considered the main biological proxy for probable AD dementia. The complete descriptions of the collection, transportation, and analyses protocols are provided in the ADNI procedural manual at www.adni‐info.org.
2.4. MRI acquisition and preprocessing
All subjects underwent the standardized MRI protocol of ADNI as described at http://www.loni.ucla.edu/ADNI/Research/Cores/index.shtml.
Image pre‐processing was performed using CAT12 (http://www.neuro.uni‐jena.de/cat/), which is an extension of SPM12 (Statistical Parametric Mapping 12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), running on MATLAB (Mathworks). All T1‐weighted images were corrected for bias (field inhomogeneities and noise). They were then segmented into GM, white matter, and CSF, 21 and spatially normalized and modulated. After preprocessing, all scans passed a visual check for artefacts and the automated CAT12 quality check protocol. The modulated and normalized GM images were then smoothed with a Gaussian kernel of 8 mm full width half‐maximum.
2.5. Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics 26 and R version 3.6. First, to compare the severity of Ecog‐Lang1 and Ecog‐Lang3 to the severity of all other cognitive complaints, a one‐way repeated measures analysis of variance (ANOVA) with custom contrasts was carried. A Bonferroni‐corrected statistical threshold of P ≤ .00066 was used for the 76 comparisons. Second, to identify demographic and psychological correlates of word‐finding complaints in CN, Spearman rank correlations were used to establish the degree of association between word‐finding complaint items (as ordinal variables) and selected demographic (age, sex, education) and psychological (depression‐related symptoms, anxiety‐related symptoms) variables. Third, we aimed to determine whether word‐finding complaints can predict CSF Aβ levels. One‐way ANOVA tests were performed to determine whether statistically significant differences were observable in CSF Aβ concentration between CN in each word‐finding complaint's levels. Multiple regression models were also fitted to analyze the ability of word‐finding complaints to predict CSF Aβ levels accounting for other covariates (demographic and psychological factors). Fourth, we aimed to compare GM volume in CN with varying levels of word‐finding complaints. The voxel‐based morphometry analysis was performed on smoothed GM images. Two one‐way ANOVAs were performed to compare groups of CN according to their level of word‐finding complaints (one for Ecog‐Lang1 and one for Ecog‐Lang3). Contrasts were set to compare GM volume among three groups: CN with no word‐finding complaint, CN with occasional word‐finding complaint, and CN reporting consistent or much worse word‐finding difficulties. Age, sex, handedness, total intracranial volume, and scanner were included as nuisance covariates. Whole‐brain statistical analyses were conducted at a statistical threshold of P < .05 family‐wise error (FWE), corrected for multiple comparisons was used.
3. RESULTS
3.1. Description of participants
In total, 239 CN were included in the present study (Table 1). The sample was 47.3% male and 52.7% female; mean age was 73.1 years. Participants were highly educated (16.7 years of education on average). CN were cognitively unimpaired at baseline (average MMSE of 29.1). Over a follow‐up period of 2 years, 219 participants remained cognitively unimpaired, 17 participants converted to amnestic MCI, and 3 participants converted to amnestic AD.
TABLE 1.
Demographic and psychological characteristics of the sample
| CN older adults Mean ± SD | Range | |
|---|---|---|
| N | 239 | – |
| Male/Female (%) | 47.3/52.7 | – |
| Age | 73.1 ± 6.1 | 56.0–89.0 |
| Years of education | 16.7 ± 2.5 | 8.0–20.0 |
| CSF amyloid beta (pg/mL) | 197.5 ± 50.1 | 82.7–303.0 |
| ECog total score | 54.5 ± 13.0 | 39.0–102.0 |
| Geriatric Depression Scale (GDS) | 0.8 ± 1.1 | .0–6.0 |
| Anxiety item (Neuropsychiatric Inventory Examination) | 0.1 ± 0.7 | .0–8.0 |
| Mini‐Mental State Examination (MMSE) | 29.1 ± 1.2 | 24.0–30.0 |
Abbreviations: CN, cognitively normal; CSF, cerebrospinal fluid; ECog, Everyday Cognition questionnaire; SD, standard deviation.
3.2. Severity and frequency of word‐finding complaints in CN
Figure 1 presents the most severe cognitive complaints among our sample of 239 CN. Comparing the severity level of Ecog‐Lang1 (“Forgetting the names of objects”) to all the other cognitive complaints, we found that it was equivalent to four other memory complaints (“Remembering a few shopping items without a list,” “Recalling conversations a few days later,” “Repeating stories and or questions,” “Remembering I have already told someone something”), but significantly higher than all other cognitive complaints (P ≤ .00066 Bonferroni‐corrected). Ecog‐Lang3 (“Finding the right words to use in a conversation”) was also significantly higher than most of the other cognitive complaints (P ≤ .00066 Bonferroni‐corrected) and equivalent to three other memory complaints (“Remembering a few shopping items without a list,” “Remembering where I have placed objects,” “Remembering I have already told someone something”). Conversely, no other cognitive complaint was significantly higher than the two word‐finding complaints.
FIGURE 1.
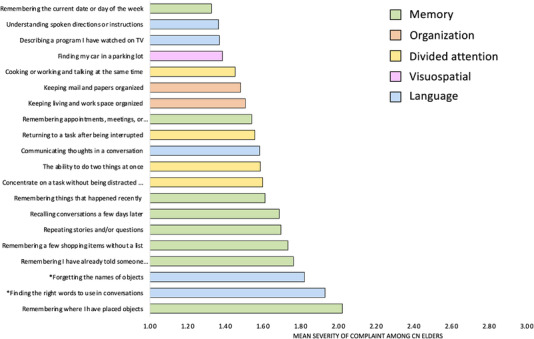
Levels of severity of cognitive complaints among cognitively normal older adults (20 top items out of the 39 Everyday Cognition questionnaire items)
In terms of frequency of word‐finding complaints, while most CN report no change (36.0%) or being occasionally worse (47.2%) at forgetting the names of objects (Ecog‐Lang1), 15.5% report being consistently a little worse and 1.3% report being consistently much worse. In terms of finding the right words to use in conversation (Ecog‐Lang3), 31.0% report no change, 50.6% report being occasionally worse, 13.0% report being consistently a little worse, and 5.4% report being consistently much worse. The distribution of responses per level of complaint for all the ECog items is presented in Table SI. The most frequent subjective complaints across the 39 items of the ECog, considering the percent of CN who scored either 3 or 4, were the word‐finding complaints (16%, 18%) as well as on two memory‐related items (“Remembering a few shopping items without a list”: 18%; “Remembering where I have placed objects”: 26%). Due to low response rates for the fourth complaint level (4 = performs the task much worse than 10 years ago), the third and fourth response options were merged for all other analyses.
3.3. Demographic and psychological correlates of word‐finding complaints in CN
Results for the Spearman rank correlations are presented in Table 2. Ecog‐Lang1 was not significantly related with any demographic (age, sex, years of education) or psychological variable (depression‐related symptoms, anxiety‐related symptoms). Ecog‐Lang3 was significantly negatively associated with years of education (rho = –.154; P ≤ .001) and positively associated with depression‐related symptoms (rho = .274; P ≤ .001). There was a significant but moderate positive correlation between Ecog‐Lang1 and Ecog‐Lang3 (rho = 0.468; P ≤ .001), which suggests that although they are related to some degree, they also capture different facets of word‐finding complaints. Ecog‐Lang1 and Ecog‐Lang3 were therefore considered separately for the remaining analyses.
TABLE 2.
Spearman's rank correlations between word‐finding complaints and demographic/psychological variables
| Sex | Age | Years of schooling | Geriatric Depression Scale (GDS) | Anxiety item (NPI) | |
|---|---|---|---|---|---|
| Forgetting the names of objects (Ecog‐Lang1) | –0.008 | 0.106 | –0.037 | 0.12 | 0.066 |
| Finding the right words to use in conversations (Ecog‐Lang3) | 0.063 | –0.031 | –.154b | .274a | 0.068 |
Correlation is significant at the 0.01 level (2‐tailed).
Correlation is significant at the 0.05 level (2‐tailed).
Abbreviations: ECog, Everyday Cognition questionnaire; NPI, Neuropsychiatric Inventory.
3.4. CSF Aβ levels according to word‐finding complaints severity levels
A statistically significant difference in CSF Aβ was found depending on the complaint severity of Ecog‐Lang1 (F[2,236] = 5.044, P = .007; Figure 2A). The mean CSF Aβ level was 206.1 ± 50.9 for CN with no Ecog‐Lang1 word‐finding complaint, 198.6 ± 48.6 with occasional Ecog‐Lang1 complaint, and 176.3 ± 47.7 for CN who reported consistent or much worst Ecog‐Lang1 complaint. The post hoc test showed that the CN who reported having consistently or much more difficulty remembering the names of objects (severity levels 3 and 4 combined) had significantly lower levels of CSF Aβ compared to the first (P = .005) and second level of complaint (P = .039).
FIGURE 2.
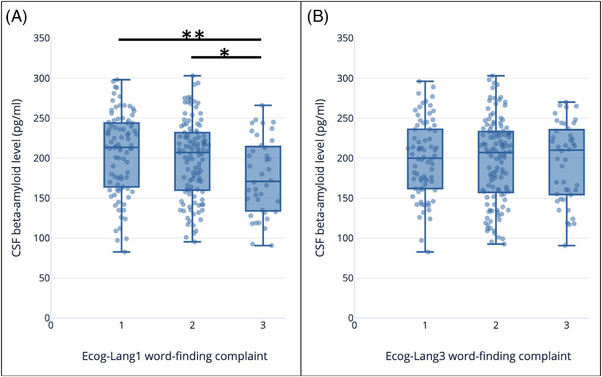
Amyloid beta levels (pg/mL) according to severity of (A) Ecog‐Lang1 complaints (Forgetting the names of objects) (B) Ecog‐Lang3 complaints (Finding the right words to use in conversations). The values represent: 1 = no change or actually performs better than 10 years ago; 2 = occasionally performs the task worse than 10 years ago but not all of the time; 3 = consistently performs the task a little worse than 10 years ago or performs the task much worse than 10 years ago. CSF, cerebrospinal fluid; ECog, Everyday Cognition questionnaire
Considering the second Ecog‐Lang3, CSF Aβ levels did not differ significantly depending on the severity levels of the complaint (P = .903; Figure 2B).
3.5. Relation between word‐finding complaints and CSF Aβ levels controlling for demographic and psychological factors
The results of our univariate analyses showed that the subjective complaint Ecog‐Lang1 is related to CSF Aβ levels in CN. To increase confidence in these findings, we extended our previous analyses by including relevant control variables. Table 3 presents results of multiple regression models in which CSF Aβ concentration is the dependent variable. The first model only included the predictor of interest (Ecog‐Lang1), entered as a categorical variable. The second model was comprised of the total score on the ECog (without Ecog‐Lang1 and Ecog‐Lang3), to assess if word‐finding complaints are specifically associated with AD biomarkers, controlling for the overall degree of subjective cognitive complaints. Finally, a third model was fitted to control for demographic and psychological variables (sex, age, years of education, depression‐related symptoms, and anxiety‐related symptoms). When all these covariates were included in the model, the most severe level of complaint predicted a statistically significant decrease of 26.906 pg/mL in CSF Aβ concentrations. Interestingly, total score on the ECog (without items Ecog‐Lang1 and Ecog‐Lang3) did not significantly predict CSF Aβ levels (B = –0.025; P = .936).
TABLE 3.
Regression analysis of predictors of CSF Aβ levels (Ecog‐Lang1 as the main IV)
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Ecog‐Lang 1(1/3) | Ref | Ref | Ref |
| Ecog‐Lang 1(2/3) | –7.501 | –8.048 | –5.106 |
| (7.052) | (7.301) | (7.184) | |
| Ecog‐Lang 1(3/3) | –29.816** | –31.555** | –26.906* |
| (9.431) | (11.106) | (10.902) | |
| ECog total score (without Lang1//Lang3) | .093 | –.013 | |
| (.312) | (.318) | ||
| Sex | –15.885* | ||
| (6.606) | |||
| Age | –1.652** | ||
| (.526) | |||
| Years of education | 2.022 | ||
| (1.311) | |||
| Geriatric Depression Scale (GDS) | 2.191 | ||
| (2.927) | |||
| Anxiety score | –4.037 | ||
| (4.509) | |||
| Constant | 206.071*** | 201.899*** | 315.152*** |
| (5.314) | (14.978) | (50.761) |
Note: Standard errors in parentheses.
P < .05
P < .01
P < .001.
Abbreviations: Aβ, amyloid beta; CSF, cerebrospinal fluid; ECog, Everyday Cognition questionnaire; IV, instrumental variable.
3.6. Comparison of GM atrophy between individuals with varying levels of word‐finding complaints
Considering Ecog‐Lang1, CN reporting consistent or much worse word‐finding difficulties had significant GM atrophy in the left fusiform gyrus (x = –29, y = –50, z = –12) and the left rolandic operculum (x = –44, y = –8, z = 11) compared to CN without word‐finding complaints (P < .05 FWE; Figure 3). The reverse contrast did not show any significant difference. CN with occasional word‐finding complaints did not significantly differ from either of the other groups. Whole‐brain GM volume did not differ between the three groups (GM volume in for Ecog‐Lang 1 “No change (1)” = 563.4 mm3; “Occasionally worse (2)” = 554.3 mm3; “Consistently a little worse or much worse (3)” = 555.8 mm3; F[2, 232] = 0.845, P = .431).
FIGURE 3.
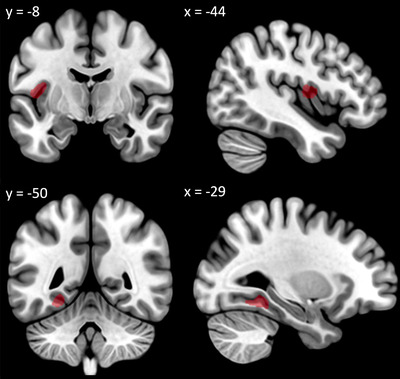
Decreased gray matter volume in participants with significant Ecog‐Lang1 word‐finding complaint (Forgetting the names of objects) versus participants with no Ecog‐Lang1 word‐finding complaint (significant at P < .05 family‐wise error corrected controlling for age, sex, handedness, scanner, total intracranial volume, presented at P < .001 uncorrected for display). ECog, Everyday Cognition questionnaire
Considering Ecog‐Lang3, GM volume did not differ significantly between groups with different levels of complaint.
3.7. Post hoc analyses separating Aβ– and Aβ+ CN
Given the observed relationship between Ecog‐Lang1 and CSF Aβ (Sections 3.4 and 3.5), we wanted to determine whether our results were dependent on the Aβ status of the participants. Therefore, we divided our CN sample between Aβ negative (Aβ–) and Aβ positive (Aβ+) based on a CSF Aβ cut‐off that was previously established in the ADNI sample (Aβ+ if inferior or equal to 192pg/mL). 22 Based on this criterion, 136 participants were Aβ– and 103 participants were Aβ+.
We first ran Spearman rank correlations to establish the demographic and psychological correlates of word‐finding complaints in each group. In Aβ– CN, in addition to replicating the finding that Ecog‐Lang3 was significantly negatively associated with years of education (rho = –.239; P = .005). and positively associated with depression‐related symptoms (rho = .376; P = .001), we found that Ecog‐Lang1 was also positively associated with depression‐related symptoms (rho = .211; P = .014). In Aβ+ CN, no significant correlation was found.
We then ran two neuroimaging analyses to compare GM volumes between (1) Aβ– CN according to their Ecog‐Lang1 severity levels and (2) Aβ+ CN according to their Ecog‐Lang1 severity levels. These two analyses did not reveal any significant region using a similar threshold (P < .05 FWE), most likely due to lower statistical power. Nonetheless, using an uncorrected threshold (P < .001 uncorrected), similar results were observed in both groups (Aβ– CN and Aβ+ CN): CN reporting consistent or much worse word‐finding difficulties presented lower GM volumes in the left fusiform gyrus and the left rolandic operculum, compared to CN without word‐finding complaint.
4. DISCUSSION
In this study, we demonstrated that word‐finding complaints are among the most frequent and severe in CN. In this population, forgetting the name of objects was relatively independent of main sociodemographic or psychological characteristics. Furthermore, forgetting the name of objects significantly predicted lower levels of CSF Aβ, even when controlling for sociodemographic variables, psychological measures, and total cognitive complaints. Finally, CN who report consistently forgetting the names of objects show GM atrophy in the left fusiform gyrus and left rolandic operculum, compared to CN who don't report this phenomenon or who only report it occasionally. Our results highlight the importance of word‐finding complaints for the early screening of older adults at higher risk of developing AD.
4.1. Word‐finding complaints are among the most frequent and severe in CN
Our results show that word‐findings complaints were as severe and frequent as other episodic memory complaints, and significantly more severe and frequent than all the other cognitive complaints (from the organization, divided attention, and visuospatial domains). Approximately 50% of CN report occasional word‐finding difficulties and 15% to 18% report consistent word‐finding difficulties. These results suggest that CN are highly sensitive to word‐finding changes, possibly due to the frequent use of language in daily life and the potential impacts of word‐finding problems on social life. They are consistent with previous studies showing the elevated prevalence of such complaints. 8 , 9 , 10 The current study adds to this literature by directly comparing word‐finding complaints with complaints from other cognitive domains, therefore providing a complete profile of cognitive complaints in CN. Finally, word‐finding complaints were significantly more severe than other types of language complaints, which highlight the importance of investigating word‐finding complaints in isolation in CN. Otherwise, language complaints in CN would most likely be underestimated.
4.2. Forgetting the name of objects is relatively independent of sociodemographic or psychological characteristics in CN
We were also interested in understanding if there are sociodemographic and/or psychological profiles that are associated with elders who are more likely to report experiencing word‐finding difficulties. Our results suggest that age and sex are not related to word‐findings complaints, contrary to previous studies suggesting that women and older individuals displayed higher prevalence of global subjective cognitive complaints. 16 For the remaining characteristics, we obtained different associations depending on the word‐finding complaint investigated (Ecog‐Lang1 or Ecog‐Lang3) and the Aβ status (Aβ+ and Aβ–). Ecog‐Lang1 was not related to education, depression‐, or anxiety‐related symptoms in Aβ+, but was related to depression‐related symptoms in Aβ– CN. Ecog‐Lang3 was modestly correlated with education (only in Aβ– CN) and depression‐related symptoms, but not with anxiety‐related symptoms. Overall, this suggests that in Aβ+ CN, word‐finding complaints cannot be attributed to external factors such as demographic factors or psychological symptoms, and are therefore most likely related to the Aβ status. In Aβ– CN, word‐finding complaints might be less specific and more multifactorial. Our results also suggest that Ecog‐Lang1 is more independent from demographic and psychological factors than Ecog‐Lang3. Previous studies have also showed an association of subjective language complaints with psychological symptoms, although these studies did not consider Aβ status. 10 , 17
4.3. Word‐finding complaints can help identify individuals with signs of AD pathology
Our study suggests that these complaints could serve to identify individuals presenting signs of AD pathology and who are at higher risk to develop AD dementia, before they show any objective signs of cognitive decline. In CN, greater self‐reported difficulties to find the name of objects significantly predicted lower levels of CSF Aβ. Individuals with greater word‐finding complaints also presented lower GM volumes in the left fusiform gyrus and the left rolandic operculum. Together, these results suggest that word‐finding complaints reported by CN can be the expression of early signs of AD pathology. This was specifically true for Ecog‐Lang1, and not Ecog‐Lang3. This can inform clinicians and researchers on the best way to interrogate patients about their word‐finding difficulties: forgetting the names of objects appears very specific and is associated with AD biomarkers and brain atrophy, versus finding the right words to use in conversations, which might be more multifactorial and is not predictive of such indicators.
Our results confirm that these complaints are worrying in older adults. Interestingly, CN who reported consistent or much worse Ecog‐Lang1 complaints presented, on average, a level of CSF Aβ that is below the established cutoff of ≤192 pg/mL (percentage of participants who are below that threshold in each Ecog‐Lang1 complaint level “No change (1)” = 37.2%; “Occasionally worse (2)” = 41.6%; “Consistently a little worse or much worse (3)” = 60%). 22 This is consistent with previous studies reporting an association between general language complaints and CSF Aβ in CN, 13 , 14 , 15 further showing the relevance of word‐finding complaints specifically. Even though Condret‐Santi et al. 8 found no increased risk of dementia in CN with word‐finding complaints, they only assessed word‐finding complaints using a yes or no question. By investigating word‐finding complaints using a continuous scale, we show that occasional word‐finding difficulties is not associated with AD biomarkers or atrophy, while consistent word‐finding difficulties is.
Our study is also the first to show that individuals with greater word‐finding complaints present more brain atrophy compared to those with no or lower word‐finding complaints. This result was observed in the left fusiform gyrus and the left rolandic operculum, both regions implicated in language functions. 23 , 24 , 25 , 26 Our post hoc analyses showed that this effect was not driven by either Aβ– CN and Aβ+ CN. Interestingly, the left fusiform gyrus is atrophied 27 and is implicated in naming 23 , 24 , 25 and semantic 28 impairments in AD patients. Interestingly, Pravatà et al. 23 found that one year before an AD diagnosis, MCI patients with naming difficulties showed greater GM loss in the left fusiform gyrus than MCI patients without naming difficulties. Our study suggests that these brain changes could happen even earlier, at a stage when individuals don't present any objective cognitive impairment yet.
4.4. Word‐finding complaints should be taken seriously and considered with other cognitive complaints in the screening of prodromal AD
Cognitive complaints are at the core of the diagnosis of subjective cognitive decline (SCD) and MCI. 4 , 29 Although these diagnostic criteria consider complaints from any cognitive domains, a majority of studies on these populations have focused on episodic memory complaints. 4 It has been reported that most SCD clinical tools focus on memory. 30 More precisely, when considering all SCD assessment tools, 59% of items are related to memory while only 8% are related to language. 30 Although our study was not specifically designed to investigate SCD, it suggests that word‐finding complaints are central in older adults. For this reason, word‐finding complaints should be considered in the definition and screening of individuals with SCD.
4.5. Limitations and future studies
First, the correlations between psychological symptoms and word‐finding complaints could be affected by the fact that severe depression‐related symptoms are an exclusion criterion for the ADNI cohort. Furthermore, anxiety‐related symptoms were not measured extensively (only with one item of the Neuropsychiatric Inventory). Thus, future studies should investigate word‐finding complaints in a more representative sample and with a more detailed anxiety questionnaire.
Second, the number of CN who eventually converted to amnestic MCI or AD was too limited to allow statistical analyses on this variable. Future longitudinal studies with larger sample size and longer follow‐ups should investigate the relationship between continuous word‐finding complaints and conversion to MCI or dementia in CN.
Finally, recent studies have shown that findings from clinic‐based cohorts (such as the ADNI cohort) may not generalize to the community. In fact, recent studies comparing the ADNI cohort to community‐based samples have shown that the associations among risk factors, biomarkers, and neuroimaging outcomes can differ in these two types of samples. 31 , 32 This might be due to the fact that ADNI participants are on average more likely to be male, highly educated, apolipoprotein ε4 positive and less cognitively impaired. 32 Therefore, the results from our study should be confirmed in a community‐based sample.
5. CONCLUSION
Contrary to previous studies suggesting that word‐finding complaints are not worrying in older adults, our study suggests that these complaints should be taken seriously. This study highlights the importance of investigating word‐finding subjective complaints in CN for the screening of AD, on top of other consistently investigated complaints such as memory‐related complaints. This investigation should be at least specific to forgetting the names of objects and conducted using a continuous scale. Our results also advocate for the inclusion of word‐finding complaints in the investigation of SCD.
CONFLICTS OF INTEREST
MM and SS have no conflicts of interest to report. In the past 36 months, SMB has received payment from Université de Montréal for presentations.
Supporting information
Supporting information
ACKNOWLEDGMENTS
MM is supported by postdoctoral funding from Fonds de Recherche en Santé du Québec (FRQS) and Canadian Health Institutes of Research (CIHR). SS has no funding to report. SMB is supported by research funding from Fonds de Recherche en Santé du Québec (FRQS), Heart and Stroke Foundation of Canada, Canadian Institutes for Health Research (CIHR), and Alzheimer Society of Canada. Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH‐12‐2‐0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Montembeault M, Stijelja S, Brambati SM, For the Alzheimer's Disease Neuroimaging Initiative . Self‐reported word‐finding complaints are associated with cerebrospinal fluid amyloid beta and atrophy in cognitively normal older adults. Alzheimer's Dement. 2022;14:e12274. 10.1002/dad2.12274
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wpcontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
REFERENCES
- 1. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amariglio RE, Buckley RF, Mormino EC, et al. Amyloid‐associated increases in longitudinal report of subjective cognitive complaints. Alzheimers Dement (N Y). 2018;4:444‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peter J, Scheef L, Abdulkadir A, et al. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimer's Dement. 2014;10:99‐108. [DOI] [PubMed] [Google Scholar]
- 4. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimer's Dement: J Alzheimer's Assoc. 2014;10:844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taler V, Phillips NA. Language performance in Alzheimer's disease and mild cognitive impairment: a comparative review. J Clin Exp Neuropsychol. 2008;30:501‐556. [DOI] [PubMed] [Google Scholar]
- 6. Montembeault M, Brambati SM, Joubert S, et al. Naming unique entities in the semantic variant of primary progressive aphasia and Alzheimer's disease: towards a better understanding of the semantic impairment. Neuropsychologia. 2017;95:11‐20. [DOI] [PubMed] [Google Scholar]
- 7. Slegers A, Filiou RP, Montembeault M, Brambati SM. Connected speech features from picture description in Alzheimer's disease: a systematic review. J Alzheimer's Dis: JAD. 2018;65:519‐542. [DOI] [PubMed] [Google Scholar]
- 8. Condret‐Santi V, Barbeau EJ, Matharan F, Le Goff M, Dartigues JF, Amieva H. Prevalence of word retrieval complaint and prediction of dementia in a population‐based study of elderly subjects. Dement Geriatr Cogn Disord. 2013;35:313‐324. [DOI] [PubMed] [Google Scholar]
- 9. Kim BS, Lee MS, Kim H. Subjective language complaints: are they reflected in objective language test performance?. Commun Sci Disord. 2015;20:214‐221. [Google Scholar]
- 10. Martins IP, Mares I, Stilwell PA. How subjective are subjective language complaints. Eur J Neurol. 2012;19:666‐671. [DOI] [PubMed] [Google Scholar]
- 11. Shokouhi S, Conley AC, Baker SL, et al. The relationship between domain‐specific subjective cognitive decline and Alzheimer's pathology in normal elderly adults. Neurobiol Aging. 2019;81:22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farias ST, Lau K, Harvey D, Denny KG, Barba C, Mefford AN. Early functional limitations in cognitively normal older adults predict diagnostic conversion to mild cognitive impairment. J Am Geriatr Soc. 2017;65:1152‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. La Joie R, Perrotin A, Egret S, et al. Qualitative and quantitative assessment of self‐reported cognitive difficulties in nondemented elders: association with medical help seeking, cognitive deficits, and β‐amyloid imaging. Alzheimer's Dement (Amsterdam, Netherlands). 2016;5:23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miebach L, Wolfsgruber S, Polcher A, et al. Which features of subjective cognitive decline are related to amyloid pathology? Findings from the DELCODE study. Alzheimer's Res Ther. 2019;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valech N, Tort‐Merino A, Coll‐Padrós N, et al. Executive and language subjective cognitive decline complaints discriminate preclinical Alzheimer's disease from normal aging. J Alzheimer's Dis : JAD. 2018;61:689‐703. [DOI] [PubMed] [Google Scholar]
- 16. Park MH, Min JY, Min HY, Lee HJ, Lee DH, Song MS. Subjective memory complaints and clinical characteristics in elderly Koreans: a questionnaire survey. Int J Nurs Stud. 2007;44:1400‐1405. [DOI] [PubMed] [Google Scholar]
- 17. Balash Y, Mordechovich M, Shabtai H, Giladi N, Gurevich T, Korczyn AD. Subjective memory complaints in elders: depression, anxiety, or cognitive decline?. Acta Neurol Scand. 2013;127:344‐350. [DOI] [PubMed] [Google Scholar]
- 18. Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22:531‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent Evidence and Development of a Shorter Version. Haworth Press; 1986:165‐173. [Google Scholar]
- 20. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory. Comprehen Assess Psychopatholo Dement. 1994;44:2308. [DOI] [PubMed] [Google Scholar]
- 21. Ashburner J, Friston KJ. Voxel‐Based morphometry—the methods. Neuroimage. 2000;11:805‐821. [DOI] [PubMed] [Google Scholar]
- 22. Shaw LM, Vanderstichele H, Knapik‐Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pravatà E, Tavernier J, Parker R, Vavro H, Mintzer JE, Spampinato MV. The neural correlates of anomia in the conversion from mild cognitive impairment to Alzheimer's disease. Neuroradiology. 2015;58:59‐67. [DOI] [PubMed] [Google Scholar]
- 24. Lars F, Stefan K, Stefan T, et al. Left anterior temporal lobe sustains naming in Alzheimer's dementia and mild cognitive impairment. Curr Alzheimer's Res. 2011;8:893‐901. [DOI] [PubMed] [Google Scholar]
- 25. Melrose RJ, Campa OM, Harwood DG, Osato S, Mandelkern MA, Sultzer DL. The neural correlates of naming and fluency deficits in Alzheimer's disease: an FDG‐PET study. Int J Geriatr Psychiatry. 2009;24:885‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Triarhou LC. Cytoarchitectonics of the Rolandic operculum: morphofunctional ponderings. Brain Struct Funct. 2021;226:941‐950. [DOI] [PubMed] [Google Scholar]
- 27. Chapleau M, Aldebert J, Montembeault M, Brambati SM. Atrophy in Alzheimer's disease and semantic dementia: an ALE meta‐analysis of voxel‐based morphometry studies. J Alzheimer's Dis. 2016;54:941‐955. [DOI] [PubMed] [Google Scholar]
- 28. Zahn R, Garrard P, Talazko J, et al. Patterns of regional brain hypometabolism associated with knowledge of semantic features and categories in Alzheimer's disease. J Cogn Neurosci. 2006;18:2138‐2151. [DOI] [PubMed] [Google Scholar]
- 29. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement: J Alzheimer's Assoc. 2011;7:270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rabin LA, Smart CM, Crane PK, et al. Subjective cognitive decline in older adults: an overview of self‐report measures used across 19 international research studies. J Alzheimer's Dis: JAD. 2015;48(Suppl 1):S63‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whitwell JL, Wiste HJ, Weigand SD, et al. Comparison of imaging biomarkers in the Alzheimer's disease neuroimaging initiative and the mayo clinic study of aging. Arch Neurol. 2012;69:614‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gianattasio KZ, Bennett EE, Wei J, et al. Generalizability of findings from a clinical sample to a community‐based sample: a comparison of ADNI and ARIC. Alzheimer's Dement: J Alzheimer's Assoc. 2021;17:1265‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


