Abstract
Introduction
Slower mobility is associated with mild cognitive impairment (MCI) and dementia. We examined the interaction of endurance with gait speed on prevalent MCI and dementia.
Methods
Cross‐sectional multinomial regression in the ARIC cohort (n = 2844 participants; 71 to 94 years; 44% men; 18% Black persons) with cognitive status (normal/MCI/dementia), 4 m gait speed, and endurance (2 minute walk [2MW]).
Results
Faster gait speed (up to but not above 1 m/s) and better 2MW were separately associated with lower dementia risk. Good performance in both (2MW = 200 m, gait speed = 1.2 m/s) was associated with 99% lower dementia (Relative Prevalence Ratio [RPR] = 0.01 [95% CI: 0.0 to 0.06]) and 73% lower MCI, RPR = 0.27 (0.15 to 0.48) compared to poor performance in both (2MW = 100 m, gait speed = 0.8 m/s). Models incorporating a gait speed‐by‐2MW interaction term outperformed gait speed‐only models (P < .001).
Discussion
Gait speed relationships with dementia diminish at faster gait speeds. Combining endurance with gait speed may yield more sensitive markers of MCI and dementia than gait speed alone.
Keywords: cognition, dementia, endurance, mild cognitive impairment, walking speed
1. INTRODUCTION
Nearly 6 million people living with dementia in the United States required nearly $257 billion worth of informal caregiving in 2020 and an estimated $355 billion in health care costs in 2020. 1 Globally, the number of people living with dementia increased from ≈20 million to nearly 44 million from 1990 to 2016. 2 Dementia and mild cognitive impairment (MCI), an intermediary classification between normal cognition and dementia, have limited treatments with only modest benefits, fueling interest in promoting healthy cognitive aging earlier in life to prevent neurodegeneration. 3 Earlier recognition of at‐risk persons is required for more effective prevention and treatment.
Gait speed slowing may be a marker of elevated risk for dementia 4 , 5 and is an inexpensive and quick measure to obtain. However, slower gait speed is associated with existing cerebrovascular and even β‐amyloid neuropathology, 4 , 6 potentially limiting its utility for early identification and risk mitigation. Conversely, ceiling effects of gait speed could limit use of gait speed as an early marker of cognitive impairment risk. People with good physical function and near‐normal gait speeds might not be recognized as being at risk until more substantial brain pathology has accrued. Additionally, performance‐based measures of physical function, including short walking tests, may not adequately distinguish a gradient of function among older adults with higher physical function abilities. 7 As a result, the ceiling effects of gait speed could similarly limit use of gait speed as an early marker of cognitive impairment risk. While some studies suggest that subtle changes in gait detected with electronic walkways could predict cognitive outcomes earlier than gait speed in adults with good physical function, 8 such assessments are limited by expertise and expense requirements. A gap exists regarding early physical markers of cognitive risk, particularly for older adults with better physical performance who have not yet manifested slowing of gait.
A growing body of literature suggests endurance may be a novel physical performance measure that could have protective associations with brain health. For example, better endurance is associated with larger brain volumes, better cognitive and vascular function, 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 and with healthier cardiovascular risk profiles, 18 which are, in turn, associated with less cognitive decline and lower risk of incident dementia. 19 , 20 Endurance training improves vascular health metrics 16 , 17 associated with brain health, and is linked to better cerebral functional connectivity 21 and cerebral white matter. 22 , 23 An important characteristic of endurance is its ability to distinguish gradients of physical function performance among older adults with normal to near‐normal gait speeds and no reported mobility difficulty. 24 However, most studies examining the association of endurance with cognitive outcomes lack systematic assessments of dementia 10 , 12 and MCI 25 and have limited generalizability. 12 , 26 , 27 , 28 Furthermore, we are not aware of any studies that examined the combined relationships of gait speed and endurance to cognitive outcomes or of studies that reported potential ceiling effects of gait speed relationships with cognitive outcomes. Therefore, we examined the following hypotheses: (1) gait speed relationships with cognitive outcomes would be attenuated at faster gait speeds; (2) better endurance would be associated with better cognitive outcomes; and (3) combining information on gait speed and endurance would be more informative of cognitive status than either measure alone, especially for those with faster gait speeds.
RESEARCH‐IN‐CONTEXT
Systematic review: We reviewed the literature using PubMed, Google Scholar, and references in identified articles. Cited literature suggests gait speed predicts mild cognitive impairment (MCI) and dementia, potentially due to covert brain pathology which could limit mitigation interventions. Earlier, low cost, noninvasive markers of MCI and dementia risk are needed, especially among higher functioning adults with good gait.
Interpretation: Better endurance was associated with better cognition and lower odds of dementia and MCI, regardless of gait speed. Combining endurance and gait speed yielded a clinically meaningful and statistically better fit for all outcomes than gait speed‐only models.
Future directions: Gait speed and endurance may represent a novel endurance‐motor interface that provides information regarding MCI and dementia risk. We discuss evidence for cardiovascular health‐related mechanisms linking endurance to cognitive outcomes. The predictive utility of combining gait and endurance measures for MCI/dementia risk merits further study.
HIGHLIGHT
Known gait speed relations to MCI and dementia were limited to gait speeds <1m/s
Endurance relations to MCI/dementia were robust across the endurance range
Endurance relations to MCI/dementia persisted even at gait speeds >1 m/s
MCI/dementia prediction by low‐cost endurance and gait speed metrics merits study
2. METHODS
2.1. Study population and setting
The Atherosclerosis Risk in Communities (ARIC) study is a closed cohort study that recruited 15,792 participants 45 to 64 years‐old from 1987 to 1989 at four US field centers (Washington County, MD; Forsyth County, NC; Jackson, MS; and suburban Minneapolis, MN) to investigate the epidemiology of atherosclerosis from midlife. 20 After 45 years of follow up, which included three triennial examinations (1990 to 1992, 1993 to 1995, 1996 to 1998) and the fifth exam (2011 to 2013), living participants were invited to a sixth exam (Visit 6, 2015 to 2017) which marked the first functional endurance assessment and the index exam for this analysis. Of the 4003 Visit 6 participants (ages 71 to 94 years), nine Asian and two Native American participants were excluded due to small numbers. The population of interest included participants who could complete a 4‐m walk and an endurance task which was a fast‐paced 2‐minute walk (2MW). Among the remaining 3992 Black and White participants, 635 (16%) were missing gait speed and 513 (13%) did not complete the 2MW, (Appendix B‐Figure B1, in the Supporting Information), leaving 2844 participants. Institutional review boards of the participating institutions approved study protocols. All participants provided written informed consent.
2.2. Cognition and cognitive status
Cognitive status adjudication in ARIC has been described elsewhere. 29 , 30 Briefly, ARIC participants were administered the delayed word recall test, letter fluency test, and a digit symbol substitution test since the ARIC 2nd exam (midlife). At the fifth and sixth exams, participants underwent a more comprehensive neuropsychological battery assessing memory, language, and executive function/processing speed. Tests included the delayed word recall, letter fluency, digit symbol substitution test, animal naming, logical memory immediate and delayed recall, Trail making test parts A and B, WAIS‐R digits span backward, Boston naming test, incidental learning from the Wechsler Memory Scale‐III, and the Mini‐Mental State Examination. Scores were standardized with average z‐scores constructed by cognitive domain and overall. All participants with previous brain imaging, participants with evidence of cognitive decline or cognitive test z‐scores of <−1.5, and a random sample with normal cognitive scores and no cognitive decline were additionally administered the Clinical Dementia Rating (CDR, informant and participant) form and the Functional Activities Questionnaire (FAQ). A physician and a neuropsychologist independently classified cognitive status as normal (no cognitive decline, all cognitive domain test z‐scores ≥−1.5 SD, and CDR sum of boxes [SB] = 0); MCI (cognitive decline plus at least one cognitive domain z‐score <−1.5 SD, CDR‐SB 0.5 to 3, and FAQ ≤ 5), or dementia (cognitive decline plus ≥ 2 cognitive domain z‐scores <−1.5 SD, CDR‐SB > 3 and FAQ > 5), according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM‐5). 29
2.3. Functional endurance
The 2MW has been validated as a reliable (test‐retest intraclass correlation coefficient [ICC] = 0.89) measure of endurance, 31 , 32 is highly correlated with the clinical 6MW(r = 0.968), and is the recommended measure of endurance in the NIH Toolbox. 31 , 32 Participants were asked to “walk as fast as you can without running” for 2 minutes around a 50‐foot course. 31 A greater distance indicates better endurance, with 42.5 m considered the minimal detectable change. 31 Exclusion criteria included need for a walking aid, use of lower extremity immobilizing devices, crutches or casts, resting heart rate >110 beats per minute, systolic blood pressure >180 mmHg, or diastolic blood pressure >120 mmHg. For illustrations, we considered a 2MW distance of 100 m as “poor,” 150 m as “fair,” and 200 m as “good,” using differences that exceeded the minimal detectable change.
2.4. Gait speed
Usual pace 4 m gait speed (m/second) is a reliable (ICC = 0.7) 32 , 33 and valid measure predictive of incident disability, dementia, mortality, and other adverse outcomes 34 , 35 , 36 ; it is considered a “sixth vital sign” for older adults and is recommended by the NIH Toolbox for locomotion assessments in older adults. 32 Gait speed thresholds vary depending on the outcome of interest with 0.8 and 1.0 m/s commonly used; 0.8 m/s was reported as the average gait speed for persons at the median life expectancy in a meta‐analysis of gait speed relationships with mortality in older adults, 36 as the minimum to permit community ambulation, 35 and has been proposed to define “slow gait” in older adults. 35 , 36 , 37 , 38 Gait speeds of 1 m/s or faster are considered markers of healthy aging and higher survival. 36 , 39 We defined gait speed thresholds for descriptive purposes as poor <0.8 m/s, fair 0.8 to <1 m/s, and good ≥1 m/s.
2.5. Covariates
Participants self‐reported age, race, sex, and education at baseline; other covariates were collected at Visit 6. A race‐site variable simultaneously described study site (MN/MD/MS/NC) and race (Black/White), for example, MN‐White. Education was trichotomized (<12 years; 12 years/12‐year equivalent/vocational training; any college). Self‐reported smoking and alcohol use were dichotomized (current or never/former). Body mass index (BMI) was defined as (weight [kilograms])/(height [m] 2 ). We defined diabetes mellitus as a fasting glucose of ≥126 mg/dL, nonfasting glucose of ≥200 mg/dL, or taking glucose‐lowering medications. Hypertension was defined as a systolic blood pressure of ≥140 mmHg, a diastolic blood pressure of ≥90 mmHg, or use of anti‐hypertensive medications. ApoEε4 was genotyped using TaqMan assay (Applied Biosystems, Foster City, CA) and dichotomized as any/no ApoEε4 allele. Prevalent stroke and heart failure were self‐reported.
2.6. Statistical analyses
We used multivariable linear regression to estimate associations between predictors and continuous cognitive factor scores, multivariable logistic regression to estimate odds ratios (OR) for dichotomous cognitive outcomes (dementia vs non‐dementia), and multivariable multinomial regression to estimate relative prevalence ratios (RPR) for trichotomous cognitive outcomes (dementia, MCI, normal). Gait speed and 2MW were modeled as continuous variables. Lowess smoothers assessed non‐linear predictor‐outcome associations, from which linear splines for gait speed, with statistically supported knots at 1 m/s, were incorporated. Nonlinearities were not supported for 2MW. Three final predictor models, all adjusted for the covariates described above, were examined for all outcomes: (1) a gait speed‐only model with a linear spline at 1 m/s; (2) a linear 2MW‐only model; (3) a combined model using gait speed (with a linear spline at 1 m/s), linear 2MW and a gait speed‐by‐2MW interaction term (Gait‐x‐2MW). Full specifications for all regression models and additional modeling details are provided in the supplemental statistical appendix (Appendix A).
In addition to regression association parameters, we used reclassification techniques 40 to further illustrate information gained from adding 2MW to gait speed‐only models for dementia. Net reclassification improvement (NRI), which is the increase in correct classification of outcomes from one model (Gait‐speed only) compared to another (Gait‐speed+2MW), was estimated separately for dementia and non‐dementia participants. There are no established thresholds for NRI examinations of MCI and dementia. We considered ≥5% (moderate risk) and ≥10% (high risk) as predictive risk thresholds since these were useful in relation to the risk percentiles and prevalence estimates of MCI and dementia. We also developed an interactive figure to compare NRIs using alternative thresholds (Appendix B‐Figure B2).
We illustrate relationships of gait speed, 2MW and cognitive status using probability plots and scatterplots of gait speed and 2MW with joint distribution median contour lines across cognitive status categories. The joint distribution (of Gait‐speed, 2MW) is a three‐dimensional surface describing the probability of observing gait speed and 2MW values simultaneously. Median contour lines are two‐dimensional projections of the three‐dimensional surface showing boundaries in which 50% of the (Gait‐speed, 2MW) datapoints lie inside the lines.
Likelihood ratio (LR) tests, Akaike information criterion (AIC), Hosmer‐Lemeshow and area under the curve (AUC) diagnostics assessed model fit. Sensitivity analyses for missing 2MW data incorporated probabilities of cognitive outcomes from gait speed models for participants missing 2MW as a comparison to the primary results. These yielded similar results.
3. RESULTS
Participants (n = 2844, mean age 79; 44% men; 18% Black persons, 18% with MCI, 4% with dementia) with poor gait speed (Table 1) and those who did not complete the 2MW (Appendix B‐Table B.1) were older, more likely to be women and Black persons, have lower education, higher BMI, more comorbidity, and poorer cognition. Participants with poor gait compared to good gait speeds walked shorter 2MW distances, and those not completing the 2MW had slower gait speeds than those who completed the 2MW.
TABLE 1.
Characteristics of participants completing gait speed overall and by gait speed categories
| Gait speed | ||||
|---|---|---|---|---|
| Total | Poor (<0.8 m/s) | Fair (0.8 to <1 m/s) | Good (≥1 m/s) | |
| n = 2844 | n = 596 (21.0%) | n = 1218 (42.8%) | n = 1030 (36.2%) | |
| Age, years, mean (SD) | 78.9 (4.4) | 79.9 (4.6) | 79.2 (4.5) | 77.8 (3.9) |
| Men, No. (%) | 1251 (44%) | 176 (30%) | 519 (43%) | 556 (54%) |
| Black, No. (%) | 523 (18%) | 185 (31%) | 227 (19%) | 111 (11%) |
| Education (≥12 years), No. (%) | 2579 (91%) | 503 (85%) | 1093 (90%) | 983 (95%) |
| BMI (kg/m2), mean (SD) | 27.8 (4.8) | 29.4 (5.5) | 27.8 (4.7) | 27.0 (4.3) |
| Current alcohol user, No. (%) | 1518 (54%) | 242 (41%) | 636 (52%) | 640 (62%) |
| Current smoker, No. (%) | 181 (6%) | 43 (7%) | 81 (7%) | 57 (6%) |
| Hypertension, No. (%) | 2170 (78%) | 512 (87%) | 934 (78%) | 724 (72%) |
| Diabetes, No. (%) | 593 (21%) | 170 (29%) | 273 (23%) | 150 (15%) |
| Stroke, No. (%) | 93 (3%) | 27 (5%) | 44 (4%) | 22 (2%) |
| Heart disease, No. (%) | 411 (15%) | 96 (17%) | 171 (14%) | 144 (14%) |
| Any ApoEε4 allele, No. (%) | 774 (33%) | 176 (37%) | 335 (33%) | 263 (31%) |
| 2Minute walk, m, mean (SD) | 137.9 (27.9) | 109.3 (22.0) | 135.0 (21.6) | 158.0 (20.8) |
| MMSE (0‐30), mean (SD) | 28.1 (2.3) | 27.5 (2.8) | 28.1 (2.2) | 28.5 (1.9) |
| Mild cognitive impairment | 512 (18%) | 143 (24%) | 202 (17%) | 167 (16%) |
| Dementia | 106 (4%) | 41 (7%) | 43 (4%) | 22 (2%) |
Abbreviations: SD, standard deviation; BMI, body mass index; MMSE, Mini‐Mental State Examination.
3.1. Associations of gait speed and 2MW separately with cognitive scores
In the Gait‐speed‐only model, each standard deviation (SD, 0.2 m/s) faster gait speed up to 1 m/s was associated with a 0.21 better standardized global cognitive score (95% Confidence Interval: 0.16, 0.26); associations were not supported for gait speed ≥1 m/s, −0.001 (−0.06, 0.06). The non‐linearity was statistically supported (P < .001, Figure 1A). Cognition was linearly associated with 2MW across the 2MW spectrum in the 2MW‐only model; each SD higher 2MW (30 m) was associated with a 0.15 (0.12, 0.18) better standardized global cognition (Figure 1B). The combined Gait‐speed+2MW model improved model fit over Gait‐speed‐alone and 2MW‐alone models (lowest AIC, LR tests P < .001; Appendix B‐Table B.2)
FIGURE 1.
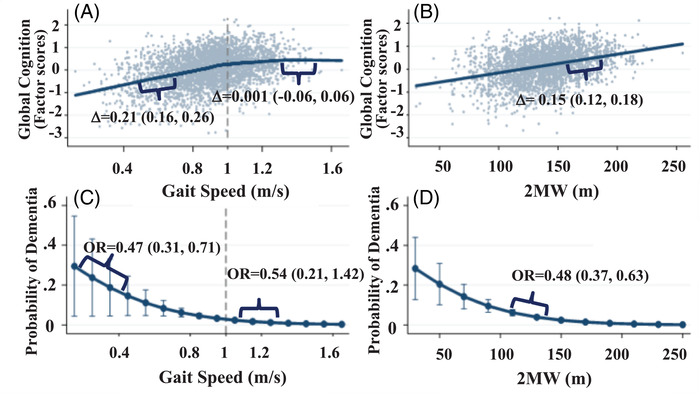
Separate models showing relations of cognition (Factor scores) with (A) gait speed and (B) 2 minute walk distance. Panels C and D show probabilities of dementia associated with (C) gait speed and with (D) 2 minute walk distance
3.2. Model performance in reclassification of dementia versus non‐dementia
In separate models, faster gait speed up to 1 m/s was associated with lower odds of dementia (Figure 1C), and 2MW distance was associated with lower odds of dementia. (Figure 1D). The combined Gait‐speed+2MW model improved model fit over Gait‐speed‐alone and 2MW‐alone models (lowest AIC, LR tests P < .001; Appendix B‐Table B2).
The combined model improved net reclassification over Gait‐speed‐only models (Table 2) among participants with dementia. Specifically, the combined model upward reclassified 17.4% of participants with dementia into medium or high risk compared to the gait speed‐only model and downgraded 3.5% into a lower risk category, yielding a dementia case NRI of 13.95% (95% CI: 4.56%, 23.35%) (Table 2A).
TABLE 2.
Risk reclassification of participants with (2A) and without (2B) dementia comparing non‐linear gait speed model to model with non‐linear gait speed and 2 minute walk
| (2A) Participants with Dementia | |||||
|---|---|---|---|---|---|
| Combined Gait speed + 2MW model | |||||
| Low risk | Med risk | High risk | Total | ||
| Gait speed only model | Low Risk | 20 (23%) | 10 (12%) | 1 (1%) | 31 (36%) |
| Med Risk | 2 (2%) | 12 (14%) | 4 (5%) | 18 (21%) | |
| High Risk | 0 (0%) | 1 (1%) | 36 (42%) | 37 (43%) | |
| Total | 22 (26%) | 23 (27%) | 41 (48%) | 86 (100%) | |
| Dementia NRI = (17.44% upgraded) – (3.49% downgraded) = 13.95% (95% CI: 4.56%, 23.35%) | |||||
| (2B) Participants without Dementia | |||||
|---|---|---|---|---|---|
| Combined Gait speed + 2MW model | |||||
| Low risk | Med risk | High risk | Total | ||
| Gait speed only model | Low Risk | 1604 (76%) | 63 (3%) | 10 (0%) | 1677 (80%) |
| Med Risk | 68 (3%) | 141 (7%) | 51 (2%) | 260 (12%) | |
| High Risk | 8 (0%) | 26 (1%) | 129 (6%) | 163 (8%) | |
| Total | 1680 (80%) | 230 (11%) | 190 (9%) | 2100 (100%) | |
Abbreviations: NRI, net reclassification improvement; 2MW, Two Minute Walk.
Non‐Dementia NRI = (4.86% downgraded) – (5.90% upgraded) = ‐1.05% (95% CI: ‐2.48%, 0.38%).
 Improved risk classification with Full Model.
Improved risk classification with Full Model.
 Inferior risk classification with Full Model.
Inferior risk classification with Full Model.
Values represent No., (%).
Models were adjusted for age, sex, education, race‐site, smoking status, alcohol use, BMI, diabetes, hypertension, stroke, heart failure, and APOEe4.
Note: Similar improvements in risk reclassification were seen when comparing the combined gait speed+2MW model with gait speed alone using a linear gait speed term (Model 1).
Risk Categories: Low: Probability(Dementia) < 5%, Med: 5 % < Probability(Dementia) < 10%, High: 10 % < Probability(Dementia).
Scatterplots of dementia probabilities from the combined model and the non‐linear gait speed only model are shown for individual participants; a QR code accesses an interactive figure illustrating NRIs using alternative dementia risk thresholds (Appendix‐B‐Figure B2).
3.3. Associations with cognitive status: (dementia, MCI, normal cognition)
Separate Models: In the Gait‐speed‐only multinomial cognitive status model, each SD faster gait speed up to 1 m/s was associated with 61% lower risk of dementia versus normal cognitive status, RPR = 0.39 (0.26, 0.59), and 50% lower risk of MCI versus normal, RPR = 0.50 (0.40, 0.63; Appendix B‐Figure B3). In the 2MW‐only model, each SD higher 2MW was associated with a 57% lower risk of dementia versus normal, RPR = 0.43 (0.33, 0.57; Appendix B‐Figure B3) and 34% lower risk of MCI versus normal cognitive status RPR = 0.66 (0.58, 0.76).
Combined Model: Figure 2 and Table 3 illustrate the relationships of cognitive status with different combined levels of gait speed and 2MW. Both also depict the a priori expected non‐existence of participants with poor gait speeds and good endurance or good gait speeds and poor endurance (structural data absences). Figure 2A shows the scatterplot of gait speed and 2MW with median contours extending to the lower left quadrant (worse gait speed and 2MW) for those with dementia and extending to the upper right quadrant (better gait speed and 2MW). Large black bullets at the cross‐sections of gait speed and 2MW represent poor, fair, and good levels of each and correspond to the estimates provided in Table 3. Figure 2B shows the synergistic relationship of gait speed and 2MW. When both are poor (<0.8 m/s, 2MW = 100 m), the separation of MCI and dementia status is especially evident, and when both are fair or good, the separation of normal is most striking, but MCI and dementia separation is also clearly evident, even at gait speeds of 1 m/s or faster. The combined model with statistically supported gait speed nonlinearities at 1 m/s and the Gait‐speed‐by‐2MW interaction was superior to the Gait‐speed‐only and 2MW‐only models (lowest AIC, LR tests P < .001; Appendix B‐Table B2).
FIGURE 2.
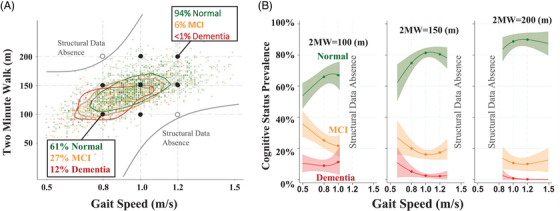
(A) Gait speed (GS) and 2 minute walk (2MW) scatterplots with median joint distribution contour lines for normal, mild cognitive impairment (MCI) and dementia status. (B) Cognitive status prevalence across gait speed for three 2MW distances
TABLE 3.
Relative prevalence ratios (RPRs) of cognitive status across varying gait speeds and two minute walk distances
| (3A) Dementia versus Normal, RPR (95% CI) P‐value | |||
|---|---|---|---|
| 2MW = 100 m | 2MW = 150 m | 2MW = 200 m | |
| GS = 0.8 m/s (poor) | ‐ref a ‐ | 0.37 (0.19, 0.71) P = 0.003 | ‐Structural data absence‐ |
| GS = 1.0 m/s (fair) | 0.75 (0.46, 1.24) P = 0.264 | 0.11 (0.05, 0.23) P < .001 | 0.02 (0.00, 0.07) P < .001 |
| GS = 1.2 m/s (good) | ‐Structural data absence‐ | 0.18 (0.07, 0.42) P < .001 | 0.01 (0.00, 0.06) P < .001 |
| (3B) Dementia versus MCI, RPR (95% CI) P‐value | |||
|---|---|---|---|
| 2MW = 100 m | 2MW = 150 m | 2MW = 200 m | |
| GS = 0.8 m/s (poor) | ‐ref a ‐ | 0.56 (0.28, 1.11) P = 0.096 | ‐Structural data absence‐ |
| GS = 1.0 m/s (fair) | 1.14 (0.68, 1.93) P = 0.615 | 0.29 (0.14, 0.63) P = 0.002 | 0.08 (0.02, 0.36) P = 0.001 |
| GS = 1.2 m/s (good) | ‐Structural data absence‐ | 0.33 (0.14, 0.80) P = 0.015 | 0.04 (0.01, 0.24) P < 0.001 |
| (3C) MCI versus Normal, RPR (95% CI) P‐value | |||
|---|---|---|---|
| 2MW = 100 m | 2MW = 150 m | 2MW = 200 m | |
| GS = 0.8 m/s (poor) | ‐ref a ‐ | 0.66 (0.47, 0.93) P = 0.018 | ‐Structural data absence‐ |
| GS = 1.0 m/s (fair) | 0.66 (0.51, 0.85) P = 0.001 | 0.38 (0.28, 0.51) P < .001 | 0.22 (0.12, 0.39) P < .001 |
| GS = 1.2 m/s (good) | ‐Structural data absence‐ | 0.54 (0.39, 0.74) P < .001 | 0.27 (0.15, 0.48) P < .001 |
Abbreviations: MCI, Mild Cognitive Impairment; GS, Gait Speed; 2MW, Two Minute Walk; m/s, meters per second.
RPRs from multinomial model of cognitive status on 2MW, interaction between 2MW and gait speed, spline terms for gait speed.
Models adjusted for age, sex, alcohol use, site‐race, education, body mass index, diabetes, hypertension, coronary heart disease, stroke, ApoEε4.
Note. Structural data absences exist in the off‐diagonals where participants with high‐GS and low‐endurance (or the reverse) were not observed for one or more outcome groups.
Marginalized adjusted reference prevalence at GS = 0.8 m/s and 2MW = 100 m were estimated at 12% Dementia, 27% MCI, and 61% Normal.
Dementia versus Normal: Compared to a referent poor gait speed (0.8 m/s) and poor 2MW (100 m), the risk of dementia versus normal cognitive status was 63% lower for those with poor gait speed (0.8 m/s) but fair 2MW (150 m), RPR = 0.37 (0.19, 0.71). Having a faster gait speed (1 m/s) with 2MW remaining poor (100 m) showed less support for dementia associations, RPR = 0.75 (0.46, 1.24). However, having good gait speed (1.2 m/s) and good 2MW (200 m) was associated with 99% lower dementia rates than having poor levels in both domains (0.8 m/s and 100 m), RPR = 0.01 (0.00, 0.06; Table 3A).
Dementia versus MCI: Faster gait speeds did not show statistically supported differences in dementia versus MCI rates with concurrent poor 2MW (100 m) (Table 3, 3B). However, faster gait was associated with lower dementia versus MCI rates at fair (150 m) and good (200 m) 2MW. Better endurance was associated with lower dementia versus MCI risk at fair and above gait speeds (≥1.0 m/s). For example, dementia was 71% less likely than MCI with fair gait speed (1.0 m/s) and fair 2MW (150 m), RPR = 0.29 (0.14, 0.63), and 96% lower with good levels in both domains (1.2 m/s and 200 m), RPR = 0.04 (0.01, 0.24), compared to those with poor levels in both domains (0.8 m/s and 100 m) (Table 3B).
MCI versus Normal: All combinations of better gait speed and endurance were associated with lower MCI versus normal cognitive status compared to having poor levels in both domains (0.8 m/s and 100 m). MCI versus normal rates were 34% lower with either fair gait (1.0 m/s) and poor 2MW (100 m) or with poor gait (0.8 m/s) and fair 2MW (150 m) (Table 3C). MCI rates were 62% lower with fair scores in both domains (1.0 m/s and 150 m), RPR = 0.38 (0.28, 0.51) and 73% lower with good scores in both domains (1.2 m/s and 200 m), RPR = 0.27 (0.15, 0.48; Table 3C).
4. DISCUSSION
Findings in this study extend existing knowledge of mobility‐cognition relationships first by illustrating ceiling effects of gait speed that may limit its utility as an early marker of dementia and MCI risk in older adults with better gait speeds. Specifically, these new findings demonstrated that gait speed relationships with cognition and cognitive status were only evident up to gait speeds of 1 m/s. Secondly, endurance tended to be associated with cognitive outcomes across the endurance and gait speed spectrums, even at normal gait speeds which were not associated with cognitive outcomes; furthermore, modeling the gait speed‐by‐2MW interaction improved net reclassification of participants with dementia compared to using gait speed or endurance alone. Interpretations were consistent across multiple cognitive outcomes including continuous cognitive scores, cognitive status classified as normal/MCI/dementia, and reclassification statistics for dementia/no dementia. Lastly, although MCI can be clinically challenging to discern from normal aging or early stages of a dementing process, our findings suggest that combining gait speed with a brief endurance measure may be a better marker of cognitive impairment than gait speed alone, especially for persons with good mobility. These findings are foundational for showing endurance measures add value to mobility (motor) measures in describing relationships with the cognitive impairment spectrum, and thus merit further study as potential combined markers of cognitive impairment risk.
Relationships of slower gait speed with poorer cognitive outcomes are well‐established, 4 , 5 likely indicative of cerebral pathology in the vasculature, macro‐ and microstructural white matter, and gray matter (via atrophy and potentially β‐amyloid deposition). 4 , 41 The current study adds to the body of research that promotes gait speed slowing as a potential early marker of cognitive risk 4 , 8 by illustrating that below‐normal gait speeds likely drive gait‐cognition relationships; because neuropathology resulting in slowed gait is thought to explain the gait speed‐cognition connection, 4 older adults who are at risk but who have not yet manifested gait speed slowing, that is, those at the higher end of motor function, may not be detected early enough for mitigation if relying only on slowed gait. The current findings also extend previous studies supporting relationships of cognition and brain health with endurance that did not evaluate the synergistic potential of endurance with gait speed. 10 , 12 , 15 , 25 , 27 The current study lays the groundwork for future studies of endurance measures, in conjunction with gait speed, as potential markers for early cognitive impairment risk detection.
Several lines of research support cardiovascular and vascular health as biologic mechanisms to explain endurance and gait relationships with cognitive outcomes. Cerebral microvascular disease is associated with gait disturbances in older adults 42 and is implicated in the development of dementia. 43 Better endurance is associated with fewer cardiovascular diseases and risk factors 18 that are also risk factors for dementia. 19 , 20 Better endurance requires a healthy vascular system to efficiently respond to increased demand for oxygen, nutrients and blood flow in muscles when performing higher endurance‐requiring tasks. 44 , 45 Similarly, a healthy cerebral vascular system supplies much needed oxygen and glucose to fuel neuronal activity, 46 with the brain consuming 20% of energy reserves. 47 Furthermore, functional endurance and fitness improve with exercise 21 , 46 , 48 and endurance training exercises improve vascular measures that are linked to dementia, including decreasing arterial stiffness 16 and increasing endothelial‐derived vasodilatation through improved nitric oxide bioavailability. 17 Endurance training is also linked to better cerebral functional connectivity 21 and white matter microstructural integrity. 22 , 23 Two studies specifically suggest that exercise and physical activity confer brain health benefits via improvements in endurance and fitness. 21 , 23 , 46 Together, these studies suggest pathways involving reduced cardiometabolic disease, improved cerebrovascular health, cerebral connectivity and microstructural integrity may explain relationships of endurance with cognitive outcomes.
Clinical implications are premature, but these novel findings advance the research conceptualization of the cognitive‐motor interface by illustrating the added importance of endurance in describing gait speed relationships with cognition and cognitive impairment. Although the data do not support recommendations for thresholds of gait speed or 2MW to identify risk, the robust relationships observed validate a need for further research on endurance‐gait relationships with cognition in attempts to move the field forward with identifying at‐risk persons. Even though the Gait‐speed‐by‐2MW model improved classification of dementia, both models still classified 23% of participants with dementia as “low risk,” indicating improvement is needed. Incorporating information from multiple systems in relation to aging phenotypes has precedent in other aging‐related syndromes, for example, frailty and the dual task cognitive‐motor paradigm. For the latter, declines in cognitive or gait speed performance while simultaneously walking and performing a cognitive task (calculations) predict progression from MCI to dementia. 49 The complementary relationship of gait speed and endurance with cognitive outcomes may similarly represent a novel endurance‐motor interface associated with poorer cognitive measures.
Some limitations and strengths warrant discussion. Limitations include the cross‐sectional design. Reverse causality is possible, but does not nullify the potential value of endurance alongside gait speed in cognitive status classification. The present findings require replication in other populations and prospective studies to determine the utility of combining these measures for MCI and dementia prediction. However, the current study addresses gaps in existing studies, most of which lack systematic cognitive examinations or information on MCI, 12 , 26 and were limited by studying healthy, primarily Caucasian populations seeking preventive care, 12 , 26 and male veterans. 27 Our study included community‐dwelling White and African American men and women with adjudicated dementia and MCI outcomes, although generalizability to other race and ethnic groups remains a gap. Chronic diseases were prevalent. ARIC lacks biomarkers to identify etiologies of dementia, although a majority likely have Alzheimer or mixed Alzheimer‐cerebrovascular disease. 29 Our approach requiring both a short (4 m) walk without an assistive device and 2MW performance measures limits generalizability to older adults with better physical performance measures who could participate in the 2MW testing. However, because the study question required measures of usual gait speed and endurance, the findings should not be biased for the population of interest: those who can complete both tasks. Lack of a formal maximal graded exercise test could be considered a limitation. However, the 2MW is a broadly accepted submaximal field‐based test among older people that has been safely implemented in a greater number of older people than a graded treadmill test, thus reducing the selection bias of healthy participants who are willing and able to perform a graded exercise test. 32 , 50 The 2MW has been validated as a reliable measure of endurance 31 , 32 and is highly correlated with the clinical 6MW(r = 0.968).
Gait speed has been promoted as a potential early marker of dementia risk, but we show that its utility may diminish at higher function. Endurance did not appear to have a ceiling effect with cognition relationships. Combining simple measures of endurance and gait speed as joint markers of brain health and cognitive impairment shows promise and merits further study. Longitudinal studies should examine MCI and dementia risk prediction using this endurance‐motor‐cognition interface.
DISCLAIMER
This article was partially prepared while Dr. Rebecca Gottesman was employed at the Johns Hopkins University School of Medicine. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
CONFLICT OF INTERESTS
Dr. Knopman served on a Data Safety Monitoring Board for the DIAN study. He serves on a Data Safety Monitoring Board for a tau therapeutic for Biogen, but receives no personal compensation. He is an investigator in a clinical trial sponsored by Lilly Pharmaceuticals and the University of Southern California. He serves as a consultant for Samus Therapeutics, Third Rock and Alzeca Biosciences but receives no personal compensation. Drs. Windham, Knopman, Gabriel, Palta, Gottesman, Griswold, and Mosley receive grant funding from the National Institutes of Health. The other authors have no conflicts of interest to disclose.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The authors thank the staff and participants of the ARIC study for their important contributions. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). Neurocognitive data were collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01‐HL70825 from the NHLBI. The authors thank the staff and participants of the ARIC study for their important contributions. S. Parker and K. Parker are supported by the Medical Student Training in Aging Research Grant through National Institute on Aging (5T35AG038027‐06) American Federation for Aging Research and the Hearin Foundation, University of Mississippi Medical Center (UMMC) Medical Student Research Program. P. Palta is supported by grant R00AG052830 from the National Institute on Aging.
Windham BG, Parker SB, Zhu X, et al. Endurance and gait speed relationships with mild cognitive impairment and dementia. Alzheimer's Dement. 2022;14:e12281. 10.1002/dad2.12281
REFERENCES
- 1. 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327‐406. https://alz‐journals.onlinelibrary.wiley.com/doi/10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 2. Collaborators GBDD. GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid‐β‐targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15(2):73‐88. [DOI] [PubMed] [Google Scholar]
- 4. Beauchet O, Annweiler C, Callisaya ML, et al. Poor gait performance and prediction of dementia: results from a meta‐analysis. J Am Med Dir Assoc. 2016;17(6):482‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68(8):929‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sullivan KJ, Ranadive R, Su D, et al. Imaging‐based indices of Neuropathology and gait speed decline in older adults: the atherosclerosis risk in communities study. Brain Imaging Behav. 2021;15(5):2387‐2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simonsick EM, Gardner AW, Poehlman ET. Assessment of physical function and exercise tolerance in older adults: reproducibility and comparability of five measures. Aging (Milano). 2000;12(4):274‐280. [DOI] [PubMed] [Google Scholar]
- 8. Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78(9):929‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barnes DE, Santos‐Modesitt W, Poelke G, et al. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med. 2013;173(9):797‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51(4):459‐465. [DOI] [PubMed] [Google Scholar]
- 11. Brown AD, McMorris CA, Longman RS, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging. 2010;31(12):2047‐2057. [DOI] [PubMed] [Google Scholar]
- 12. DeFina LF, Willis BL, Radford NB, et al. The association between midlife cardiorespiratory fitness levels and later‐life dementia: a cohort study. Ann Intern Med. 2013;158(3):162‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prakash RS, Voss MW, Erickson KI, et al. Cardiorespiratory fitness and attentional control in the aging brain. Front Hum Neurosci. 2011;4:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spartano NL, Himali JJ, Beiser AS, et al. Midlife exercise blood pressure, heart rate, and fitness relate to brain volume 2 decades later. Neurology. 2016;86(14):1313‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu N, Jacobs DR Jr, Schreiner PJ, et al. Cardiorespiratory fitness and cognitive function in middle age: the CARDIA Study. Neurology. 2014;82(15):1339‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T. Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle‐aged sedentary men. Jpn J Physiol. 2005;55(4):235‐239. [DOI] [PubMed] [Google Scholar]
- 17. DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age‐related declines in endothelium‐dependent vasodilation in healthy men. Circulation. 2000;102(12):1351‐1357. [DOI] [PubMed] [Google Scholar]
- 18. Grundy SM, Barlow CE, Farrell SW, Vega GL, Haskell WL. Cardiorespiratory fitness and metabolic risk. Am J Cardiol. 2012;109(7):988‐993. [DOI] [PubMed] [Google Scholar]
- 19. González HM, Tarraf W, Harrison K, et al. Midlife cardiovascular health and 20‐year cognitive decline: Atherosclerosis Risk in Communities Study results. Alzheimers Dement. 2018;14(5):579‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sabia S, Fayosse A, Dumurgier J, et al. Association of ideal cardiovascular health at age 50 with incidence of dementia: 25 year follow‐up of Whitehall II cohort study. BMJ. 2019;366:l4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Voss MW, Weng TB, Burzynska AZ, et al. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. NeuroImage. 2016;131:113‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voss MW, Heo S, Prakash RS, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one‐year exercise intervention. Hum Brain Mapp. 2013;34(11):2972‐2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding K, Tarumi T, Zhu DC, et al. Cardiorespiratory fitness and white matter neuronal fiber integrity in mild cognitive impairment. J Alzheimers Dis. 2018;61(2):729‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well‐functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M644‐M649. [DOI] [PubMed] [Google Scholar]
- 25. Nyberg J, Åberg MAI, Schiöler L, et al. Cardiovascular and cognitive fitness at age 18 and risk of early‐onset dementia. Brain. 2014;137(5):1514‐1523. 10.1093/brain/awu041 [DOI] [PubMed] [Google Scholar]
- 26. Liu R, Sui X, Laditka JN, et al. Cardiorespiratory fitness as a predictor of dementia mortality in men and women. Med Sci Sports Exerc. 2012;44(2):253‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Müller J, Chan K, Myers JN. Association between exercise capacity and late onset of dementia, Alzheimer disease, and cognitive impairment. Mayo Clin Proc. 2017;92(2):211‐217. [DOI] [PubMed] [Google Scholar]
- 28. Hörder H, Johansson L, Guo X, et al. Midlife cardiovascular fitness and dementia: a 44‐year longitudinal population study in women. Neurology. 2018;90(15):e1298‐e1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC‐NCS). Alzheimers Dement (Amst). 2016;2:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rawlings AM, Bandeen‐Roche K, Gross AL, et al. Factor structure of the ARIC‐NCS Neuropsychological Battery: an evaluation of invariance across vascular factors and demographic characteristics. Psychol Assess. 2016;28(12):1674‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bohannon RW, Wang YC, Gershon RC. Two‐minute walk test performance by adults 18 to 85 years: normative values, reliability, and responsiveness. Arch Phys Med Rehabil. 2015;96(3):472‐477. [DOI] [PubMed] [Google Scholar]
- 32. Reuben DB, Magasi S, McCreath HE, et al. Motor assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S65‐S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim HJ, Park I, Lee HJ, Lee O. The reliability and validity of gait speed with different walking pace and distances against general health, physical function, and chronic disease in aged adults. J Exerc Nutrition Biochem. 2016;20(3):46‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dumurgier J, Artaud F, Touraine C, et al. Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol A Biol Sci Med Sci. 2017;72(5):655‐661. [DOI] [PubMed] [Google Scholar]
- 35. Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23(2):314‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cruz‐Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well‐functioning older people‐results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675‐1680. [DOI] [PubMed] [Google Scholar]
- 40. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wennberg AMV, Lesnick TG, Schwarz CG, et al. Longitudinal association between brain amyloid‐beta and gait in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2018;73(9):1244‐1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high‐functioning older adults. J Am Geriatr Soc. 2005;53(4):649‐654. [DOI] [PubMed] [Google Scholar]
- 43. Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41(4):600‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laughlin MH, Armstrong RB. Muscle blood flow during locomotory exercise. Exerc Sport Sci Rev. 1985;13:95‐136. [PubMed] [Google Scholar]
- 45. Hall C, Figueroa A, Fernhall B, Kanaley JA. Energy expenditure of walking and running: comparison with prediction equations. Med Sci Sports Exerc. 2004;36(12):2128‐2134. [DOI] [PubMed] [Google Scholar]
- 46. Vidoni ED, Johnson DK, Morris JK, et al. Dose‐response of aerobic exercise on cognition: a community‐based, pilot randomized controlled trial. PLoS ONE. 2015;10(7):e0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte‐neuron metabolic cooperation. Cell Metab. 2011;14(6):724‐738. [DOI] [PubMed] [Google Scholar]
- 48. Zoll J, Koulmann N, Bahi L, Ventura‐Clapier R, Bigard A‐X Quantitative and qualitative adaptation of skeletal muscle mitochondria to increased physical activity. J Cell Physiol. 2003;194(2):186‐193. 10.1002/jcp.10224 [DOI] [PubMed] [Google Scholar]
- 49. Montero‐Odasso MM, Sarquis‐Adamson Y, Speechley M, et al. Association of dual‐task gait with incident dementia in mild cognitive impairment: results from the Gait and Brain Study. JAMA Neurol. 2017;74(7):857‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pettee Gabriel K, Whitaker KM, Duprez D, et al. Clinical importance of non‐participation in a maximal graded exercise test on risk of non‐fatal and fatal cardiovascular events and all‐cause mortality: CARDIA study. Prev Med. 2018;106:137‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.


