Abstract
Introduction
This study assessed the construct validity and clinical utility of the National Alzheimer's Coordinating Center Lewy Body Dementia (LBD) Module, consisting of the Speeded Attention and Noise Pareidolia Tasks.
Methods
Participants included 459 older adults diagnosed as cognitively normal (n = 202), or with non‐amnestic mild cognitive impairment (n = 61), amnestic mild cognitive impairment (n = 96), Alzheimer's disease dementia (n = 44), or LBD (n = 56).
Results
Speeded Attention demonstrated strong convergent validity and moderate discriminant validity when compared to established neuropsychological tests. Noise Pareidolia demonstrated strong discriminant validity, but limited convergent validity. Noise Pareidolia scores were significantly lower in those with reported hallucinations, delusions, or REM sleep behavior disorder symptoms. LBD Module tests discriminated well between cognitively normal adults and those with LBD.
Discussion
The LBD Module demonstrates promising construct validity and clinical utility, which support its use across research and clinical settings.
Keywords: Alzheimer's disease dementia, Lewy body dementia, Lewy body disease, neuropsychology, psychometrics
1. BACKGROUND
Lewy body dementia (LBD) accounts for a significant number of dementia cases in older adults, 1 , 2 yet is frequently misdiagnosed as Alzheimer's disease dementia (AD) due to overlapping clinical and pathological characteristics. 3 , 4 More complex features of LBD, such as fluctuating cognition, have also been notoriously difficult to measure in a clinical setting. 5 , 6 , 7 To date, few measures exist to reliably discriminate LBD from cognitively normal participants or those with other neurodegenerative dementias.
In 2017, the National Alzheimer's Coordinating Center (NACC) implemented a new set of procedures and measures to assess physical, neuropsychiatric, and cognitive symptoms associated with LBD. 8 Included in this module are two neuropsychological tests. The Speeded Attention Task, an adaptation of the Stroop Color and Word Test, 9 measures an individual's executive control and selective attention. 10 The Stroop paradigm is considered a gold standard measure of executive functioning and selective attention, domains shown to be deficient in patients with LBD compared to those with frontotemporal dementia and to cognitively healthy older adults. 11 The Noise Pareidolia Task‐Short Form examines how well an individual can correctly identify a human face among visual “noise.” 12 The test targets visuospatial integration characteristically deficient in those with LBD. 12 , 13 While these tests reportedly measure underlying cognitive constructs affected by LBD using previously established neuropsychological paradigms, normative data and validity of the LBD Module are not yet available, limiting their use in research settings or the potential for clinical application.
This study evaluates the construct validity of the LBD Module neuropsychological tests by comparing performance between these tasks with established neuropsychological measures, agnostic to participant diagnosis. Additionally, we provide preliminary normative data by age group and across diagnostic groups, and assess the ability of the Speeded Attention and Noise Pareidolia Tasks to discriminate between clinical groups of interest.
2. METHODS
2.1. Participants
Data were drawn from six Alzheimer's Disease Research Centers (ADRCs) between April 2017 and March 2020. All ADRCs maintain a longitudinal cohort through which participants complete regular evaluations (usually approximately annually) that include a standard cognitive battery, neurologic examination, and clinical interview. Participants in this study also completed the LBD Module neuropsychological tests (the Speeded Attention Task and the Noise Pareidolia Task). Only first administrations for each participant were included in analyses. All participants provided informed consent at their parent institutions.
NACC diagnostic procedures have been described in detail in previous publications; 14 briefly, ADRCs utilize standardized criteria to diagnose individuals as cognitively normal or with Mild Cognitive Impairment (MCI 15 ), AD, 16 LBD, 17 , 18 or other dementias. Although the LBD module raw scores were viewable, they were not reviewed for diagnostic decisions.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional methods (eg, PubMed). Few neuropsychological measures currently exist to specifically assess cognitive deficits characteristic of Lewy Body Dementia (LBD) or to differentiate LBD from other dementias.
Interpretation: We provide preliminary evidence of the construct validity of the Speeded Attention and Noise Pareidolia tests of the National Alzheimer's Coordinating Center (NACC) LBD Module. Performance on these measures appears to differ in clinically meaningful ways across diagnostic groups. Furthermore, the combined LBD Module scores demonstrate promising sensitivity and specificity for distinguishing between various clinical groups.
Future directions: Future studies must validate the NACC LBD Module neuropsychological tests using larger, equally‐sized samples of impaired individuals. Further evidence of convergent and discriminant validity requires comparing these tests to alternate “gold standard” neuropsychological measures not available in this investigation. Assessment of the sensitivity and specificity of these measures to detect neuroanatomically defined LBD is also recommended.
2.2. Procedures
2.2.1. Speeded Attention Task
The Speeded Attention Task involves three consecutive trials. During the Word trial, participants are given 45 seconds to read aloud a list of colors (green, red, or blue) as quickly and accurately as possible. During the Color trial, participants are shown a list of characters (ie, “XXXX”) printed in three different colors of ink (green, red, or blue), and have 45 seconds to name them correctly, in order. The Color‐Word trial, also known as the interference trial, requires the participant to correctly name the color of ink in which an incongruent word is printed (ie, the word “GREEN” printed in red ink). Participants must correctly read as many items as possible within 45 seconds. Errors must be corrected immediately on all trials. There is no maximum score for any of the above trials.
2.2.2. Noise Pareidolia Task
The NACC version of the Noise Pareidolia Task includes 20 black and white images, some of which include a face while others contain only random noise patterns. The participant must estimate both whether a face is identifiable in the image, and if present, where the face is located (by correctly pointing at the location) in order to receive credit. The participant may also make a pareidolic or illusory error, in which they identify a face in an image in which no face exists. The resulting scores include the number of faces correctly identified (maximum = 7), the number of noise images correctly identified (maximum = 13), pareidolic errors (equivalent to the inverse of noise correct score, maximum = 13), and total score (sum of face and noise correct scores; max = 20).
2.2.3. Clinical LBD symptoms
NACC participants complete multiple assessments of symptoms, though the specific measures vary as a function of participant characteristics. These may include the Neuropsychiatric Inventory Questionnaire and the standard and LBD‐specific clinician interviews and neurologic examinations. For the purpose of this study, we summarized across multiple measures to determine the presence or absence of clinical symptoms commonly associated with LBD. Given the presence of anosagnosia among many cognitively disordered patients, we considered both the participant self‐report of symptoms, as well as reports from the co‐participant (an individual well‐known to the participant). The presence of hallucinations was marked as “present” if either the participant or the co‐participant reported that the participant has auditory or visual hallucinations on formal measures or clinician/neurologic interviews. Similarly, delusions and rapid eye movement (REM) sleep behavior disorder symptoms were coded as present if the participant or co‐participant reported these symptoms on the questionnaires or interviews administered during the evaluation.
2.3. Data analysis
2.3.1. Aim 1: construct validation
First, we assessed whether performance on tasks within the LBD module neuropsychological measures was consistent, indicating an underlying shared “LBD‐deficient” construct. As there was a significant relationship between age, education, and several LBD Module scores, we calculated partial correlations between the three raw trial scores of the Speeded Attention Task (Word, Color, and Color‐Word) and the two independent scores of the Noise Pareidolia Task (Faces Correct, Pareidolic Errors), controlling for these factors.
To evaluate convergent validity of the Speeded Attention Task, we calculated partial correlations between Word, Color, and Color‐Word raw scores and the Trail‐Making Test (TMT 19 ), an established measure of processing speed and executive functioning. Specifically, we compared against raw TMT‐A and B completion times and number of errors, as well as the B:A time ratio (TMT‐B time divided by TMT‐A time, representing a purer measure of executive “cost”), controlling for age and education. It was expected that all Speeded Attention scores would be negatively associated with performance on TMT‐A, and that the Color‐Word score in particular would be negatively associated with performance on TMT‐B and the B:A ratio because of the underlying executive burden of these tasks.
Noise Pareidolia measures visual integration and discrimination; therefore, to assess convergent validity, we calculated partial correlations between Faces Correct and Noise Correct scores and performance on the Benson Complex Figure Test (BCFT 19 ) Copy trial. BCFT Copy requires participants to copy a relatively complex figure. We expected moderate positive relationships between Noise Pareidolia scores and BCFT Copy scores.
To evaluate discriminant validity of both LBD Module neuropsychological tests, we calculated partial correlations between the Speeded Attention and Noise Pareidolia measures and performance on a confrontation naming test, the Multilingual Naming Test (MINT 20 ). Bonferroni corrections for multiple comparisons were implemented for each of the aforementioned sets of comparisons.
Some research has suggested that the Noise Pareidolia test may assess visual discrimination or misperceptions similar to those thought to underlie visual hallucinations in LBD. 21 Therefore, we also evaluated whether the presence of clinical symptoms of LBD (ie, self‐ or informant‐reported hallucinations, delusions, and REM sleep behavior disorder symptoms) was associated with lower scores on Noise Pareidolia Task using one‐way analysis of variance (ANOVA).
2.3.2. Aim 2: clinical utility
We compared diagnostic group performance on the Speeded Attention and Noise Pareidolia Tasks, controlling for age and education, using a multivariate analysis of covariance test (MANCOVA), followed by multivariate analysis of variance (MANOVA) with Games‐Howell nonparametric post hoc tests (given significant heterogeneity of variances between groups).
To assess the clinical utility of the LBD Module, a series of discriminant function analyses (DFA) were completed. Independent scores from the Speeded Attention (Word, Color, and Color‐Word) and Noise Pareidolia subtests (Faces Correct, Pareidolic Errors) were used to discriminate between (1) cognitively normal versus impaired (ie, any diagnosis) older adults; (2) cognitively normal older adults and those with LBD; and (3) individuals diagnosed with AD versus LBD.
In addition, for clinical reference, we evaluated preliminary normative data for each of the Speeded Attention and Noise Pareidolia raw scores, stratified by age (<65 years, 65 to 74 years, 75 to 85 years, 85 years and above) in the supplemental data of this paper.
3. RESULTS
3.1. Sample characteristics
The sample included 459 individuals from five diagnostic groups: cognitively normal older adults (CN; n = 202), and individuals diagnosed with non‐amnestic MCI (naMCI; n = 61), amnestic MCI (aMCI; n = 96), AD (n = 44), and LBD (n = 56). Demographic characteristics of the sample are summarized in Table 1. The total sample was approximately 54% female and 86% White. Age ranged from 52 to 99 years, with an average age of 73. One‐way ANOVA with Games‐Howell post hoc comparisons revealed that the AD group was significantly older than the naMCI group. Education ranged from completion of eighth grade to a doctoral or equivalent advanced degree (20 years or more), with average education of 16 years; although group statistics revealed a significant difference in education by diagnosis, post hoc comparisons did not. In regards to clinical LBD symptoms, 8% of the total sample (or their co‐participants) endorsed delusions (CN: 1%; naMCI: 8%; aMCI: 4%; AD: 9%; LBD: 38%). Eleven percent reported auditory or visual hallucinations (CN: 0.5%; naMCI: 8%; aMCI: 6%; AD: 5%; LBD: 63%). Eighteen percent endorsed REM sleep behavior disorder symptoms (CN: 3.5%; naMCI: 30%; aMCI: 16%; AD: 5%; LBD: 70%). As expected, there were significant group differences in the frequency of endorsed delusions (Fisher Exact = 60.15, P < .001), hallucinations (Fisher Exact = 126.10, P < .001), and REM sleep behavior disorder symptoms (Fisher Exact = 122.81, P < .001), largely driven by the relatively higher presence of these symptoms in the naMCI and LBD groups.
TABLE 1.
Sample demographic characteristics
| CN (n = 202) | naMCI (n = 61) | aMCI (n = 96) | AD (n = 44) | LBD (n = 56) | P | ||
|---|---|---|---|---|---|---|---|
| Age | M (SD) | 72.52 (6.88) | 71.02 (7.35) | 74.35 (8.84) | 75.86 (8.43) | 72.49 (6.75) | .006* |
| Education | M (SD) | 16.53 (2.38) | 15.75 (2.51) | 15.85 (2.58) | 15.73 (2.65) | 16.78 (2.57) | .019* |
| Sex | Female (%) | 142 (70%) | 31 (51%) | 45 (47%) | 20 (46%) | 8 (14%) | <.001* |
| Race | White (%) | 180 (89%) | 53 (87%) | 71 (74%) | 39 (89%) | 53 (94%) | .008* |
| Black (%) | 20 (10%) | 8 (13%) | 23 (24%) | 5 (11%) | 2 (4%) | ||
| Other (%) | 2 (1%) | 0 (0%) | 2 (2%) | 0 (0%) | 1 (2%) |
*P < .05.
Abbreviations: CN, Cognitively normal; naMCI, Non‐Amnestic Mild Cognitive Impairment; aMCI, Amnestic Mild Cognitive Impairment; AD, Alzheimer's Disease Dementia; LBD, Lewy Body Dementia.
3.2. Aim 1: construct validation
Analyses revealed moderate, significant positive relationships among Speeded Attention scores and Noise Pareidolia Faces Correct score (r p = .308 to .453, all Ps < .001), and moderate, significant negative relationships among Speeded Attention scores and Noise Pareidolia Pareidolic Errors (r p = −.200 to −.346, all Ps < .001), after controlling for age and education.
Relationships between LBD neuropsychological test scores and established neuropsychological measures are summarized in Table 2. All Speeded Attention scores were moderately to strongly negatively associated with TMT‐A time and TMT‐B time, demonstrating strong convergent validity. While no Speeded Attention scores were associated with errors on TMT‐A, Color and Color‐Word scores were negatively associated with errors on the more complex TMT‐B trial. Color‐Word, the score we expected to be most executively loaded, was also negatively associated with the TMT B:A ratio. Speeded Attention scores demonstrated small (albeit significant) positive relationships with performance on the MINT naming test, providing evidence for discrimination from measurement of confrontation naming and broader language abilities.
TABLE 2.
Partial correlations between LBD Module neuropsychological task scores and established neuropsychological measures in the total sample, controlling for age and education
| TMT‐A | TMT‐A Errors | TMT‐B | TMT‐B Errors | TMT A:B Ratio | MINT | Benton Copy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | r | P | |
| SA—Word | −.378 | <.001* | −.048 | .333 | −.326 | <.001* | −.145 | .006 | −.071 | .171 | .208 | <.001* | ||
| SA—Color | −.425 | <.001* | −.042 | .387 | −.392 | <.001* | −.217 | <.001* | −.132 | .008 | .245 | <.001* | ||
| SA—Color‐Word | −.559 | <.001* | −.086 | .080 | −.445 | <.001* | −.229 | <.001* | −.232 | <.001* | .236 | <.001* | ||
| NP—Faces Correct | .097 | .042 | .407 | <.001* | ||||||||||
| NP—Pareidolic Errors | −.092 | .053 | −.363 | <.001* | ||||||||||
*P < .001 (Bonferroni‐corrected)
Abbreviations: TMT, Trail‐Making Test; MINT, Multilingual Naming Test; SA, Speeded Attention Task; NP, Noise Pareidolia Task.
Noise Pareidolia scores shared a moderate positive relationship with BCFT Copy, suggesting adequate convergent validity. Similarly, analyses revealed nonsignificant partial correlations between Noise Pareidolia and MINT scores, supporting adequate discriminant validity. Additionally, across diagnoses, Noise Pareidolia performance was significantly poorer in individuals with self‐ or informant‐reported delusions (Faces Correct: F (1) = 4.93, P = .027; Pareidolic Errors: F (1) = 5.17, P = .023), hallucinations (Faces Correct: F (1) = 47.72, P < .001; Pareidolic Errors: F (1) = 49.41, P < .001), or REM sleep behavior disorder symptoms (Faces Correct: F (1) = 37.26, P < .001; Pareidolic Errors: F (1) = 46.20, P < .001).
3.3. Aim 2: clinical utility
Normative data for each of the Speeded Attention and Noise Pareidolia scores are provided, stratified by age (Table 3) or diagnosis (Table 4). We found significant diagnostic group differences on the Word (F (4) = 44.43, P < .001, Partial η2 = .307), Color (F (4) = 58.05, P < .001, Partial η2 = .367), and Color‐Word (F (4) = 57.29, P < .001, Partial η2 = .367) trials of the Speeded Attention Task, with large effect sizes (Figures 1A‐1C). For both Word (all Ps < .03) and Color (all Ps < .001) scores, cognitively normal older adults significantly outperformed all other diagnostic groups, while individuals with LBD significantly underperformed relative to all other diagnostic groups. In regards to Color‐Word score, cognitively normal older adults significantly outperformed all other diagnostic groups. Individuals with naMCI and aMCI performed similarly, and outperformed the two dementia groups, who performed similarly (all Ps < .001).
TABLE 3.
Normative data for the LBD Module neuropsychological tasks, stratified by age
| Age Group (years) | ||||
|---|---|---|---|---|
| ≤65 (n = 61) | 65–74 (n = 222) | 75–84 (n = 129) | 85 ± (n = 35) | |
| Speeded Attention—Word | 77.55 (21.17) | 79.41 (19.64) | 78.03 (18.48) | 71.15 (15.49) |
| Speeded Attention—Color | 56.97 (16.86) | 58.01 (16.58) | 54.93 (15.37) | 47.69 (14.64) |
| Speeded Attention—Color‐Word | 29.70 (11.03) | 29.08 (11.92) | 23.24 (11.07) | 21.72 (9.92) |
| Noise Pareidolia—Faces Correct | 6.75 (0.54) | 6.77 (0.76) | 6.71 (0.81) | 6.69 (0.76) |
| Noise Pareidolia—Noise Correct | 12.52 (1.07) | 12.16 (1.88) | 11.95 (2.28) | 11.97 (2.67) |
| Noise Pareidolia—Pareidolic Errors | 0.48 (1.07) | 0.88 (1.99) | 1.05 (2.28) | 1.06 (2.75) |
| Noise Pareidolia ‐ Total Score | 19.30 (1.22) | 18.89 (2.56) | 18.67 (2.59) | 18.66 (3.17) |
TABLE 4.
Average performance on Speeded Attention and Noise Pareidolia Tasks by diagnosis
| CN (n = 202) | naMCI (n = 1) | aMCI (n = 96) | AD (n = 44) | LBD (n = 56) | P | |
|---|---|---|---|---|---|---|
| SA—Word | 86.04 (13.93) | 76.47 (14.47) | 79.93 (15.61) | 70.30 (19.86) | 51.16 (22.91) | <.001 |
| SA—Color | 64.79 (11.63) | 54.67 (11.97) | 54.35 (14.76) | 46.05 (15.69) | 33.27 (13.74) | <.001 |
| SA—Color‐Word | 33.27 (9.10) | 25.21 (9.12) | 26.05 (10.20) | 15.29 (10.09) | 13.70 (9.40) | <.001 |
| NP—Faces Correct | 6.94 (0.24) | 6.92 (0.28) | 6.79 (0.57) | 6.59 (0.73) | 5.77 (1.63) | <.001 |
| NP—Noise Correct | 12.55 (1.08) | 12.32 (1.25) | 12.34 (1.52) | 12.32 (1.53) | 9.56 (1.53) | <.001 |
| NP—Pareidolic Errors | 0.45 (1.08) | 0.68 (1.25) | 0.77 (1.90) | 0.68 (1.54) | 3.44 (4.04) | <.001 |
| NP—Total Score | 19.50 (1.10) | 19.24 (1.33) | 19.13 (1.76) | 18.93 (1.82) | 15.15 (5.10) | <.001 |
Abbreviations: CN, Cognitively normal; naMCI , non‐amnestic mild cognitive impairment; aMCI , amnestic mild cognitive impairment; AD , Alzheimer's Disease Dementia; LBD, Lewy Body Dementia; SA , Speeded Attention; NP, Noise Pareidolia.
FIGURE 1.
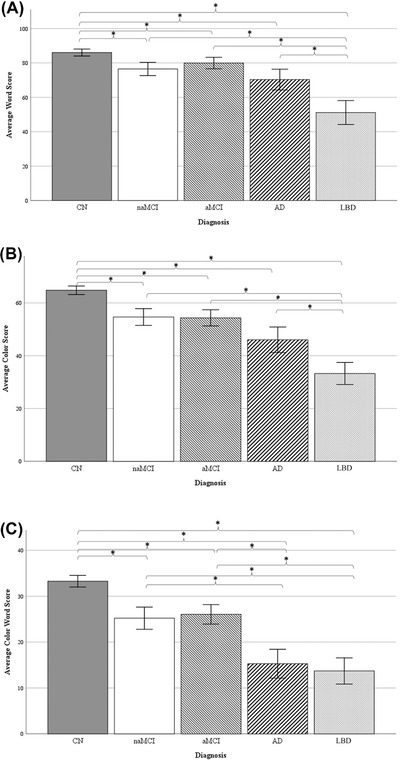
Average Speeded Attention Task scores by diagnosis, for the (A) Word, (B) Color, and (C) Color‐Word trials. Error bars reflect 95% confidence intervals. Abbreviations: CN, Cognitively normal; naMCI , non‐amnestic mild cognitive impairment; aMCI , amnestic mild cognitive impairment; AD , Alzheimer's disease dementia; LBD, Lewy body dementia. *P < .05
We also found significant diagnostic group differences on the Noise Pareidolia Faces Correct (F (4) = 31.68, P < .001, Partial η2 = .224), Noise Correct (F (4) = 28.14, P < .001, Partial η2 = .204), and Pareidolic Errors (F (4) = 28.14, P < .001, Partial η2 = .204) scores, with medium effect sizes (Figures 2A‐2B). Cognitively normal older adults significantly outperformed both dementia groups (both Ps < .03) on Faces Correct, but performed similarly to both MCI groups. The LBD group performed significantly worse than all other groups on Faces Correct (all Ps < .02). In regards to Noise Correct scores, the LBD group performed significantly worse compared to all other diagnostic groups (all Ps < .001), who performed similarly.
FIGURE 2.
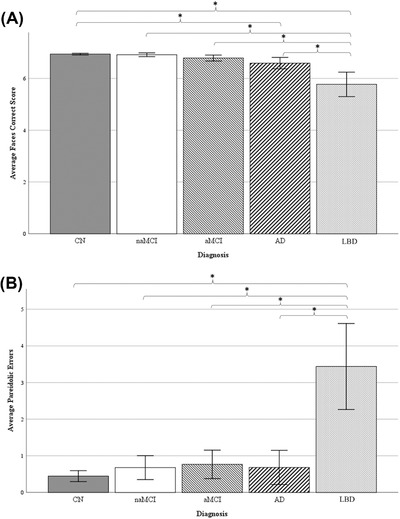
Average (A) Faces Correct and (B) Noise Correct scores on the Noise Pareidolia Task, by diagnosis. Error bars reflect 95% confidence intervals. Abbreviations: CN , Cognitively normal; naMCI, non‐amnestic mild cognitive impairment; aMCI , amnestic mild cognitive impairment; AD , Alzheimer's disease dementia; LBD, Lewy body dementia. *P < .05
DFAs revealed that the Speeded Attention and Noise Pareidolia Tasks significantly differentiated between those who were cognitively normal versus those with a cognitive diagnosis (Wilks’ λ = 0.715, P < .001; sensitivity = 68.9%; specificity = 74.8%) and accounted for 28.5% of the variance. These tests also significantly differentiated between those who were cognitively healthy and those with an LBD diagnosis (Wilks’ λ = 0.411, P < .001; sensitivity = 99.5%; specificity = 70.5%), accounting for 59.0% of the variance. Finally, we evaluated the ability of these measures to distinguish between AD and LBD; the resulting model was significant (Wilks’ λ = 0.758, P < .001; sensitivity = 74.4%; specificity = 61.4%), accounting for 24.1% of the variance
4. DISCUSSION
The current study provides data regarding the validity and clinical utility of the NACC LBD Module neuropsychological tests. These analyses support the potential for these measures to assess deficiencies seen across older adults, as well as weaknesses specific to the LBD clinical phenotype. Our findings demonstrate that the Speeded Attention and Noise Pareidolia Tasks show good convergent and discriminant validity when compared to established neuropsychological measures. It has been hypothesized that the visual hallucinations in LBD may represent misperceptions of real visual stimuli due to poor visual integration. 21 Consistent with prior investigations into this hypothesis, 12 , 13 , 22 we found that both Noise Pareidolia scores were significantly lower in those with hallucinations, as well as other clinical features of LBD.
Regarding clinical comparisons, this study is the first to provide normative data for the NACC LBD Module neuropsychological tests, and data by diagnostic group, for clinical reference. Though preliminary, our results suggest clinically relevant patterns of diagnostic group differences on these tasks. On the Speeded Attention Word and Color subtests, all impaired groups performed worse than cognitively healthy controls; however, individuals with LBD performed significantly worse than all impaired groups. On the Color‐Word task, we saw a clinical “gradient” of performance, with cognitively normal older adults outperforming both MCI groups, who in turn outperformed both dementia groups. Given that executive dysfunction is a prominent feature of both LBD and AD, these findings are not unexpected. We also found evidence of distinctly different performance on the Noise Pareidolia subtests; individuals with LBD performed worse than all other groups on both Faces Correct and Pareidolic Errors. Additional evidence for the clinical utility of the LBD Module arises from the discriminant function analyses. The LBD Module was particularly good at distinguishing between CN and LBD, with excellent sensitivity and specificity acceptable for research settings (≈.70). Furthermore, the battery neared but did not reach cut‐offs for use in research for distinguishing between cognitively normal older adults and those who are impaired (all diagnoses), as well as between those with AD and LBD due to insufficient specificity. Collectively, these findings suggest that the LBD Module neuropsychological tests demonstrate promise at distinguishing even among those with clinical diagnoses.
Our findings fit with a larger literature suggesting that the Stroop paradigm may accurately distinguish LBD from other groups, including AD 23 , 24 with LBD and/or Parkinson's disease dementia (PDD) performing most poorly on these tests. Patients with LBD may also decline on Stroop tasks more rapidly over time compared to those with AD or other diagnoses. 25 Furthermore, our findings are commensurate with longitudinal literature demonstrating that individuals in the MCI stage who exhibit poorer attention and visuoperceptual abilities are more likely to progress to LBD diagnosis as opposed to AD. 26 One study reported a progression rate of naMCI (with attentional and executive impairments) to LBD as 20% per year, and to AD as 2% per year 27 .
As with prior studies using pareidolia paradigms, 12 we found that participants with LBD underperformed other groups on the Noise Pareidolia tests—and particularly the Pareidolic Errors score. Commensurate with the Speeded Attention results, we found that participants with naMCI performed most consistently with those with LBD, followed by those with AD. Again, the strong effect size of these comparisons indicates that an increased sample size in each diagnostic group may improve future ability to detect group differences. Prior literature has demonstrated that similar noise pareidolia tests demonstrate limited sensitivity (60%) but strong specificity (92%) in distinguishing LBD from AD; however, when combined with other pareidolia paradigms (ie, scene pareidolia), sensitivity for distinguishing the two dementias improves to a clinically acceptable level of 82%. Consistent with our own preliminary findings in the ADRC sample, both sensitivity and specificity of a noise pareidolia task to distinguish between cognitively normal older adults and those with LBD is 85%, 12 Overall, our findings correspond with the larger literature pointing to the promise of the Speeded Attention and Noise Pareidolia LBD neuropsychological module as important indicators of general attentional and perceptual abilities, psychiatric functioning, and early cognitive changes specific to LBD.
As previously mentioned, these data are preliminary, and while critical for the implementation of these measures across ADRCs and other research or clinical settings, cannot fully substantiate the psychometric properties of the tasks. The current ADRC protocol recommends administration of the LBD Module neuropsychological tasks primarily to participants suspected of having LBD or PDD, with the exception of a few ADRCs which implement the module more broadly. Our sample sizes are therefore both unequal, and for the LBD group, small; while statistical analyses demonstrate medium to large effect sizes even between these groups, a larger, more diverse dataset would provide the opportunity for greater acuity using receiver operating characteristic analyses to determine clinical cutoffs. Additionally, the sample is predominantly White, contains unequal representation of men and women across diagnostic groups, and includes individuals with a Bachelor's‐level educational attainment on average, limiting our ability to conduct finer‐grained comparisons in normative data. Furthermore, as the tests are given over multiple annual visits, our ability to assess test‐retest reliability will improve as more data are acquired. We were also limited somewhat by the standard batteries administered across ADRCs. While the universal batteries are a tremendous strength in regards to data collection and sharing across ADRCs, the inability to compare against other common or gold standard neuropsychological tests not included in these batteries is limited. Future studies may consider integrating other cognitive measures of visual discrimination as a means of validating the noise pareidolia test in particular.
CONFLICTS OF INTEREST
None.
ACKNOWLEDGEMENTS
Data for this study were provided in part by the National Alzheimer's Coordinating Centers (NACC). The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA‐funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG062428‐01 (PI James Leverenz, MD) P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P30 AG062421‐01 (PI Bradley Hyman, MD, PhD), P30 AG062422‐01 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P30 AG062429‐01(PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P30 AG062715‐01 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD). Additional data were gathered from the Michigan Alzheimer's Disease Research Center (MADRC), which is funded by the National Institute on Aging (P30 AG053760).
Rahman‐Filipiak A, Sadaghiyani S, Davis K, et al. Validation of the National Alzheimer's Coordinating Center (NACC) Lewy Body Disease Module neuropsychological tests. Alzheimer's Dement. 2022;14:e12279. 10.1002/dad2.12279
REFERENCES
- 1. Sanford AM. Lewy body dementia. Clin Geriatr Med. 2018;34:603‐615. 10.1016/j.cger.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 2. Yousaf T, Dervenoulas G, Valkimadi PE, Politis M. Neuroimaging in Lewy body dementia. J Neurol. 2019;266:1‐26. 10.1007/s00415-018-8892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goodwin R. Case series of dementia with Lewy bodies: consensus criteria in practice. Australas Psychiatry. 2020;28:80‐83. 10.1177/1039856219891649. [DOI] [PubMed] [Google Scholar]
- 4. Rizzo G, Arcuti S, Copetti M, et al. Accuracy of clinical diagnosis of dementia with Lewy bodies: a systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry. 2018;89:358. 10.1136/jnnp-2017-316844. [DOI] [PubMed] [Google Scholar]
- 5. Gomperts SN. Lewy body dementias: dementia with Lewy bodies and Parkinson disease dementia. Continuum (Minneap Minn). 2016;22:435‐463. 10.1212/CON.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oda H, Yamamoto Y, Maeda K. The neuropsychological profile in dementia with Lewy bodies and Alzheimer's disease. Int J Geriatr Psychiatry. 2009;24:125‐131. 10.1002/gps.2078. [DOI] [PubMed] [Google Scholar]
- 7. Van Dyk K, Towns S, Tatarina O, et al. Assessing fluctuating cognition in dementia diagnosis: interrater reliability of the clinician assessment of fluctuation. Am J Alzheimers Dis Other Demen. 2016;31:137‐143. 10.1177/1533317515603359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Alzheimer's Coordinating Center . Lewy‐body Dementia Module (Version 3.0). August 2017. Accessed December 23, 2021. https://files.alz.washington.edu/documentation/lbd3‐np‐worksheets.pdf
- 9. Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Stoelting Co; 1978. [Google Scholar]
- 10. Naber M, Vedder A, Brown SB, Nieuwenhuis S. Speed and lateral inhibition of stimulus processing contribute to individual differences in Stroop‐Task performance. Front Psychol. 2016;7:822. 10.3389/fpsyg.2016.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johns EK, Phillips NA, Belleville S, et al. Executive functions in frontotemporal dementia and Lewy body dementia. Neuropsychology. 2009;23:765‐777. 10.1037/a0016792. [DOI] [PubMed] [Google Scholar]
- 12. Mamiya Y, Nishio Y, Watanabe H, Shimomura T, Iizuka O, Mori E. The pareidolia test: a simple neuropsychological test measuring visual hallucination‐like illusions. PLoS One. 2016;11:e0154713. 10.1371/journal.pone.0154713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yokoi K, Nishio Y, Uchiyama M, et al. Hallucinators find meaning in noises: pareidolic illusions in dementia with Lewy bodies. Neuropsychologia. 2014;56:245‐254. 10.1016/j.neuropsychologia.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 14. Rahman‐Filipiak AM, Giordani B, Heidebrink J, Bhaumik A, Hampstead BM. Self‐ and informant‐reported memory complaints: frequency and severity in cognitively intact individuals and those with mild cognitive impairments and neurodegenerative dementias. J Alzheimers Dis. 2018;65:1011‐1027. 10.3233/JAD-180083.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270‐279. 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263‐269. 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Litvan I, Bhatia KP, Burn DJ, et al. SIC Task Force appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord. 2003;18:467‐486. 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 18. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89: 88‐100. 10.1212/wnl.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91‐101. 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stasenko A, Jacobs DM, Salmon DP, Gollan TH. The Multilingual Naming Test (MINT) as a measure of picture naming ability in Alzheimer's disease. J Int Nueropsychol Soc. 2019;25:821‐833. 10.1017/S1355617719000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ballard C, McKeith I, Harrison R, et al. A detailed phenomenological comparison of complex visual hallucinations in dementia with Lewy bodies and Alzheimer's disease. Int Psychogeriatr. 1997;9:381‐388. 10.1017/S1041610297004523. [DOI] [PubMed] [Google Scholar]
- 22. Sasai‐Sakuma T, Nishio Y, Yokoi K, Mori E, Inoue Y. Pareidolias in REM sleep behavior disorder: a possible predictive marker of Lewy body diseases?. Sleep. 2017;40. zsw045. 10.1093/sleep/zsw045. [DOI] [PubMed] [Google Scholar]
- 23. Calderon J, Perry RJ, Erzinclioglu SW, Berrios GE, Dening TR, Hodges JR. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;70:157‐164. 10.1136/jnnp.70.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park KW, Kim HS, Cheon SM, Cha JK, Kim SH, Kim JW. Dementia with Lewy bodies versus Alzheimer's disease and Parkinson's disease dementia: a comparison of cognitive profiles. J Clin Neurol. 2011;7:19‐24. 10.3988/jcn.2011.7.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Breitve MH, Chwiszczuk LJ, Brønnick K, et al. A longitudinal study of neurocognition in dementia with Lewy bodies compared to Alzheimer's disease. Front Neurol. 2018;9:124. 10.3389/fneur.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoon JH, Kim M, Moon SY, Yong SW, Hong JM. Olfactory function and neuropsychological profile to differentiate dementia with Lewy bodies from Alzheimer's disease in patients with mild cognitive impairment: a 5‐year follow‐up study. J Neurol Sci. 2015;355:174‐179. 10.1016/j.jns.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 27. Ferman TJ, Smith GE, Kantarci K, et al. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology. 2013;81:2032‐2038. 10.1212/01.wnl.0000436942.55281.47. [DOI] [PMC free article] [PubMed] [Google Scholar]


