Abstract
Introduction
We sought to examine a magnetic resonance imaging (MRI)‐based marker of neurodegeneration from the AT(N) (amyloid/tau/neurodegeneration) framework among a multi‐ethnic, community‐dwelling cohort.
Methods
Community‐dwelling Mexican Americans and non‐Hispanic White adults and elders were recruited. All participants underwent comprehensive assessments including an interview, functional exam, clinical labs, informant interview, neuropsychological testing and 3T MRI of the brain. A neurodegeneration MRI meta‐region of interest (ROI) biomarker for the AT(N) framework was calculated.
Results
Data were examined from n = 1305 participants. Mexican Americans experienced N at significantly younger ages. The N biomarker was significantly associated with cognitive outcomes. N was significantly impacted by cardiovascular factors (e.g., total cholesterol, low‐density lipoprotein) among non‐Hispanic Whites whereas diabetes (glucose, HbA1c, duration of diabetes) and sociocultural (household income, acculturation) factors were strongly associated with N among Mexican Americans.
Discussion
The prevalence, progression, timing, and sequence of the AT(N) biomarkers must be examined across diverse populations.
Keywords: Alzheimer's disease, amyloid, biomarkers, diversity, Hispanic, Mexican American, mild cognitive impairment, neurodegeneration
1. BACKGROUND
The 2018 AT(N) (amyloid/tau/neurodegeneration) research framework 1 provided the field with the first‐ever biological‐based system for studying Alzheimer's disease (AD). The presence of the AT(N) framework itself represents a tremendous advancement for the field and is only possible due to the advancement of technology capable of examining the core pathological markers of amyloid (A), tau (T), and neurodegeneration (N) among living individuals rather than having to wait until autopsy. While far from clinical practice, this framework is the underlying foundation for many clinical trials as well as the therapeutic discovery and pipeline approaches for many labs globally (academic and industry). However, as noted in the framework publication itself, there is a significant need to examine each of these markers among diverse populations. 1
The representation of ethnic and racially diverse communities in the research that investigates these advanced A, T, and N biomarkers is incredibly sparse. 2 This situation is problematic because prior work in other areas demonstrates that biomarkers that guide therapeutics are impacted by race/ethnicity. As an example, BRCA mutations have been studied extensively for clinical diagnosis, treatment, and prevention of BRCA‐related cancers; however, most of these data (and clinical guidelines) are based on non‐Hispanic White populations. 3 Recent data show that race and ethnicity impact the prevalence of BRCA mutations meaning that the non‐Hispanic White–based clinical guidelines may not be appropriate. 3 , 4 , 5 Existing data also suggest that the prevalence and possible diagnostic utility and cut‐scores for pathological AD biomarkers may vary by race and ethnicity. 6 , 7 , 8 , 9 Therefore, if the A, T, and N biomarkers are to effectively guide clinical trial design (and future clinical practice), a comprehensive understanding of their prevalence, sequence, timing, and clinical impact needs to be determined across diverse populations.
According to 2019 Census Bureau data, 10 Hispanics make up the largest minority population in the United States. In fact, ≈50% of the US population growth from 2010 to 2019 was due to an increase in the Hispanic population. 11 The percentage of Hispanics aged 65 and older will triple by the year 2050 12 and this ethnic group will experience the largest increase in AD and AD‐related dementia (ADRD) diagnoses among any racial/ethnic group by 2060. 13 Approximately 65% of Hispanics in the United States are of Mexican American ethnicity; 14 however, few studies have explicitly examined mild cognitive impairment (MCI) and AD among Mexican Americans. Here we investigated N from the AT(N) framework among Mexican Americans compared to non‐Hispanic Whites.
Even though the link between magnetic resonance imaging (MRI)‐based measures of N and cognitive loss has been studied extensively, few studies have been conducted on this topic among Hispanics. Therefore, here we provide a comprehensive examination of the Jack et al. 15 ‐defined “meta ROI [regions of interest],” which comprises the entorhinal cortex, fusiform, inferior temporal, and middle temporal gyri and serves as a measure of N biomarker in the AT(N) framework. As pointed out in the AT(N) framework, 1 the N biomarker is non‐specific and likely reflective of comorbidities. Given our prior work showing that Mexican Americans: (1) are diagnosed with cognitive impairment at significantly younger ages, (2) suffer from disproportionate levels of medical comorbidities, (3) have lower rates of the apolipoprotein E (APOE) ε4 genotype, and (4) have a different proteomic profile of AD compared to non‐Hispanic Whites, we hypothesized that the MRI‐based meta‐ROI N biomarker would be different among Mexican Americans compared to non‐Hispanic Whites from the Health and Aging Brain among Latino Elders (HABLE) study.
2. METHODS
2.1. Participants & assessment
The HABLE study is an ongoing, longitudinal, community‐based project examining health disparities in MCI and AD among Hispanic, Mexican Americans. HABLE methods have been previously published 16 and are briefly outlined. Inclusion criteria for the study includes (1) self‐reported ethnicity of Mexican American or non‐Hispanic White, (2) willingness to provide blood samples, (3) capability of undergoing neuroimaging studies, (4) age 50 and above, and (5) fluent in English or Spanish. Exclusion criteria includes (1) type 1 diabetes, (2) presence of active infection, (3) current/recent (12 month) cancer (other than skin cancer), (4) current severe mental illness that could impact cognition (other than depression), (5) recent (12 months) traumatic brain injury with loss of consciousness, (6) current/recent alcohol/substance abuse, (7) active severe medical condition that could impact cognition (e.g., end stage renal failure, chronic heart failure, chronic obstructive pulmonary disease), and (8) current diagnosis of dementia other than AD. Participant recruitment for HABLE includes a community‐based participatory research (CBPR) approach. 17 The CBPR approach has been used successfully as a recruitment modality for reaching underserved and minority populations. It involves collaborating with local communities through outreach (holding community events, seminars), word of mouth, marketing modalities (newspaper, television, radio), and providing information (clinical lab work, MRI clinical reads, neuropsychological test results) to the participants and their health‐care providers. The HABLE protocol includes an interview, functional exam, blood draw for clinical labs and biobanking, neuropsychological testing, and 3T MRI of the brain. A subset of participants (n = 55) underwent amyloid positron emission tomography (PET) scans using florbetaben (18F) as part of a pilot study with all participants currently undergoing amyloid PET scans at follow‐up visits. All aspects of the study protocol can be conducted in Spanish or English. Participants are provided with the opportunity to select the language (Spanish or English) in which they would like to conduct the entirety of the interview and neuropsychological assessment. The full neuropsychological assessment is completed in the language selected (Spanish or English) and participants are not provided with an opportunity to switch between languages during the assessment. Participants provide a self‐report regarding race and ethnicity. For this study, only those who self‐identified as Hispanic Mexican American, and thus deriving from Mexico, were included in this study and analysis outlined below. The HABLE study is conducted under institutional review board approved protocols and each participant (or his/her legal representative) signs written informed consent.
2.2. Interview and neuropsychological assessment
An interview is conducted as part of the HABLE protocol, which includes a self‐report of race and ethnicity, education level, sex, annual household income, as well as questionnaires regarding acculturation (12‐item Short Acculturation Scale for Hispanics [SASH] 18 , 19 ), social support (12‐item Interpersonal Support Evaluation List [ISEL‐12] 20 ), and chronic stress (8‐item Chronic Burden Scale 21 ). The neuropsychological test battery includes the following: Mini Mental Status Exam (MMSE), 22 Wechsler Memory Scale‐Third Edition (WMS‐III) Digit Span and Logical Memory, 23 Digit Symbol Substitution, Trail Making Test Parts A and B, 24 Spanish‐English Verbal Learning Test (SEVLT), 25 Animal Naming (semantic fluency), 25 F‐A‐S Test (phonemic fluency) 26 as well as the American National Adult Reading Test (English‐speakers), 27 Word Accentuation Test (Spanish‐speakers), 28 and 30‐item Geriatric Depression Scale. An informant interview is also conducted for completion of the Clinical Dementia Rating (CDR) scale 1 by clinicians with expertise in dementia to evaluate functional declines. Normative references 29 for the neuropsychological assessments included an adjustment for age and education.
2.3. Diagnostic classification
Cognitive diagnoses are assigned algorithmically (decision tree) and verified at consensus review as follows: normal control (NC) = no cognitive complaints, CDR sum of boxes score of 0, and cognitive tests scores broadly within normal limits (i.e., performance greater than that defined as meeting diagnostic criteria for MCI [i.e., < = 1.5 standard deviations below the normative range]); MCI: cognitive complaint (self or other), CDR sum of boxes score between 0.5 and 2.0, and at least one cognitive test score falling < = 1.5 standard deviation below normative ranges; dementia: CDR sum of boxes score > = 2.5 and at least two cognitive test scores 2 standard deviations below normative ranges. Medical diagnoses are assigned by licensed professionals (NP, DO, and/or MD) during consensus review based on fasting clinical labs, objective measures, self‐report, and current medications. Clinical labs were conducted by Quest Diagnostics.
RESEARCH IN CONTEXT
Systematic Review: The authors used traditional (e.g., PubMed) sources. Hispanics are the largest ethnic minority group in the United States; however, this population remains underrepresented in Alzheimer's disease (AD) research. The Health & Aging Brain among Latino Elders (HABLE) study was created to study a broad range of factors contributing to health disparities in mild cognitive impairment (MCI) and AD among Mexican Americans, the largest segment of the US Hispanic population.
Interpretation: Our findings demonstrate that the neurodegeneration (N) biomarker from the AT(N) (amyloid/tau/neurodegeneration) framework is different among Mexican Americans compared to non‐Hispanic Whites. The underlying contributing factors to N among Mexican Americans were diabetes and sociodemographic in nature compared to the cardiovascular and cerebrovascular factors among non‐Hispanic Whites.
Future Directions: The life course trajectory of N will be examined to identify appropriate, timely, and ethnically appropriate intervention strategies.
2.4. Neuroimaging
2.4.1. MRI data
The HABLE MRI protocol is based on that of Alzheimer's Disease Neuroimaging Initiative 3 (ADNI3) using a 3T Siemens Magnetom SKYRA whole‐body scanner. We acquired the following scan sequences: T1‐weighted whole brain volumetric spoiled magnetization‐prepared rapid gradient (MPRAGE), whole brain volumetric fluid attenuated inversion recovery (FLAIR), susceptibility‐weighted imaging (SWI), diffusion tensor MRI (dMRI), 3D arterial spin labeling (3DPASL), resting‐state functional (rsfMRI), and high‐resolution (0.4 × 0.4 mm x 2 mm) T2‐weighted hippocampal high resolution (HHR) scans. For this study, the N component of the AT(N) framework 15 was examined as outlined by Jack et.al. 15 as the “meta‐ROI,” which comprises the surface‐area weighted average of the mean cortical thickness in individual ROIs of the entorhinal cortex, fusiform, inferior temporal gyri, and middle temporal gyri. N+ was determined based on a cut‐off of 2.68 mm for cortical thickness. 15 Participants who failed quality checks (quality assurance [QA]) for the FreeSurfer software version 5.3.0 segmentation for at least one of the individual ROI sections (referenced above) were excluded when calculating meta‐ROI. Meta‐ROI was calculated based on the sum of each region in each hemisphere times the surface area for that region divided by the sum of surface areas for all regions included.
2.4.2. PET scans
In a pilot study, n = 61 participants underwent amyloid positron emission tomography (PET) scans using Siemens Biograph Vision 450 whole‐body PET/computed tomography (CT) scanner following the ADNI3 protocol for florbetaben scans. Scans were processed to derive standardized uptake value ratio (SUVR) levels at the University of Southern California Stevens Neuroimaging and Informatics Institute per ADNI protocols, using FreeSurfer‐derived ROIs and whole cerebellum as the reference region. Consistent with the ADNI3 protocols, a global ROI was composed of frontal, anterior/posterior cingulate, lateral parietal, and lateral temporal cortex. A global SUVR >1.08 was considered to be amyloid positive, which is consistent with the ADNI3 protocol cut‐off.
2.5. Blood collection and processing procedures
Fasting blood collection and processing were completed based on the international guidelines for AD biomarker studies and processed within 2 hours (stick‐to‐freezer). 30 Plasma neurofilament light chain (NfL), amyloid beta 40 (Aβ)40, Aβ42, and total tau (t‐tau) were assayed using the ultra‐sensitive Simoa (single molecule array) technology platform HD‐1 (Quanterix.com). The ITR Biomarker Core has conducted >5000 assays using this platform and all coefficients of variability (CVs) were <4%. Average CV for Aβ40 was 0.043 pg/mL (lowest limit of detection [LLOD] = 0.196 pg/mL; highest limit of detection [HLOD] = 560 pg/mL), Aβ42 was 0.043 pg/mL (LLOD = 0.045 pg/mL; HLOD = 240 pg/mL), t‐tau was 0.061 pg/mL (LLOD = 0.019 pg/mL; HLOD = 400 pg/mL), and NfL was 0.038 pg/mL (LLOD = 0.038 pg/mL; HLOD = 1800 pg/mL).
2.6. Statistical analyses
Statistical analyses were conducted in SPSS 25 (IBM) and R. 15 Group differences between ethnicities (Mexican American, Non‐Hispanic White) were examined using independent t‐tests and Mann Whitney U and Chi square tests, the latter for non‐normally distributed data for categorical (presence hypertension [dHTN] [yes/no], diabetes [yes/no], dyslipidemia [dDLP], sex) and continuous variables (age, education, N, N+). Pearson correlations were then conducted to examine correlations among demographic characteristics (age, sex, education), social and behavioral factors (household income, acculturation, social support, chronic stress, and depression), medical conditions and their duration (diabetes mellitus [dDm], dHTN, dDLP), cerebral A and AT(N) plasma biomarkers, and an MRI‐based measure of N (i.e., “meta‐ROI” defined previously). Univariate analyses were conducted to examine potential interaction effects of ethnicity and select independent variables on the primary outcome N. Linear regression models were further used (in separate analyses) to examine the impact of social and behavioral factors, medical conditions, duration of medical conditions, and plasma biomarkers on N. Logistic regressions were also conducted with covariates of age, sex, and education to examine the predictive ability of N in detecting cognitive impairment (MCI, dementia). Covariates of age, sex, and education were included across all models. Statistical significance was set at P < .05 unless otherwise specified to correct for multiple comparisons.
3. RESULTS
Of the total n = 1761 actively enrolled participants, a total of n = 1305 participants had all requisite data (i.e., passed all QA for all bilateral reference regions of interest for calculation of the weighted meta‐ROI) for inclusion in the current analyses (Mexican American n = 688, non‐Hispanic White n = 617). The Mexican American group was significantly younger (P < .001) and obtained fewer years of education (P < .001) than non‐Hispanic Whites. There was also a significant sex difference between groups with a higher number of females included among those who self‐reported as Mexican American (P < .001). Additionally, differences in medical comorbidities were found with Mexican Americans being more likely to have a diagnosis of diabetes (P < 0001) than non‐Hispanic Whites. A significant difference was also found between groups regarding social and behavioral factors with Mexican Americans reporting lower mean household annual incomes (P < .001), lower social support (P < .001), lower acculturation (P < .001), and a higher number of depression symptoms (P < .001) compared to non‐Hispanic Whites. Mexican Americans also reported lower levels of chronic stress (P = .043) than non‐Hispanic Whites. Regarding plasma biomarkers of Aβ, tau, and neurodegeneration (NfL), Mexican Americans were found to have lower mean levels of plasma NfL (P < .001) and Aβ40 (P < .001), along with higher levels of t‐tau (P = .001) compared to non‐Hispanic Whites. A mean difference was not found for Aβ42 (P = .096), N (P = .081), or N+ (P = .389) between ethnic groups. On neuropsychological test performance, mean differences were found between ethnic groups with Mexican Americans performing lower across all cognitive domains (P < .001; see Table 1).
TABLE 1.
Descriptive statistics of cohort (n = 1305)
| Total cohort | Mexican American N = 688 | Non‐Hispanic White N = 617 | P‐value | |
|---|---|---|---|---|
| Age, mean (SD) | 66.05 (8.64) | 63.60 (7.91) | 68.77 (8.62) | <.001* |
| Range | 50–92 | 50–91 | 50–92 | |
| Education, mean (SD) | 12.37 (4.73) | 9.62 (4.58) | 15.43 (2.46) | <.001* |
| Range | 0–20 | 0–20 | 7–20 | |
| Sex, N (% female) | 836 (64%) | 480 (70%) | 356 (58%) | <.001* |
| Hypertension (HTN), N (% yes) | 810 (62%) | 443 (64%) | 367 (60%) | .069 |
| Diabetes mellitus (DM), N (% yes) | 325 (25%) | 251 (36%) | 74 (12%) | <.001* |
| Dyslipidemia (DLP), N (% yes) | 849 (65%) | 460 (67%) | 389 (63%) | .150 |
| Household income, mean (SD) | $60,638.28 (73459.94) | $37,460.77 (52263.47) | $86,190.99 (84240.66) | <.001* |
| Range | 0–1,000,000.00 | 0–800,000.00 | 0–1,000,000.00 | |
| Chronic stress, mean (SD) | 7.20 (6.65) | 6.85 (6.50) | 7.60 (6.80) | .042* |
| Range | 0–37 | 0–32 | 0–37 | |
| Social support, mean (SD) | 40.70 (6.31) | 39.80 (6.34) | 41.71 (6.14) | <.001* |
| Range | 16–48 | 19–48 | 16–48 | |
| Acculturation—SASH total, mean (SD) | 3.54 (1.77) | 2.26 (1.56) | 4.97 (0.20) | <.001* |
| Range | 1‐5 | 1‐5 | 1‐5 | |
| Depression—GDS‐30, mean (SD) | 5.58 (5.79) | 6.41 (6.14) | 4.66 (5.24) | <.001* |
| Range | 0–29 | 0–29 | 0–29 | |
| Plasma NfL, mean (SD) | 19.34 (13.84) | 17.41 (12.87) | 20.78 (13.94) | <.001* |
| Range | 0.64–209.00 | 0.64–123.0 | 4.52–209.00 | |
| Plasma Aβ40, mean (SD) | 252.71 (68.40) | 241.98 (70.37) | 267.18 (64.73) | <.001* |
| Range | 51–627 | 51–627 | 63.2–560 | |
| Plasma Aβ42, mean (SD) | 12.06 (3.35) | 11.87 (3.51) | 12.18 (3.10) | .096 |
| Range | 3.56–30.90 | 3.82–30.90 | 3.92–29.20 | |
| Plasma total tau, mean (SD) | 2.48 (1.09) | 2.60 (1.08) | 2.39 (1.13) | .001* |
| Range | 0.17–17.60 | 0.58–16.00 | 0.29–17.60 | |
| N (meta‐ROI), mean (SD) | 2.72 (0.14) | 2.73 (0.14) | 2.71 (0.15) | .081 |
| Range | 2.01‐3.12 | 2.01‐3.07 | 2.04‐3.12 | |
| N+, N (%) | 435 (33%) | 222 (32%) | 213 (34%) | .389 |
| Cognitive diagnosis | .001* | |||
| Normal controls, N (%) | 1059 (81%) | 532 (77%) | 527 (85%) | |
| MCI, N (%) | 168 (13%) | 106 (15%) | 62 (10%) | |
| Dementia, N (%) | 78 (6%) | 50 (7%) | 28 (5%) | |
| MMSE, mean (SD) | 27.34 (3.31) | 26.11 (3.71) | 28.88 (1.70) | <.001* |
| Range | 3–30 | 3–30 | 14–30 | |
| WMS‐III digit span, mean (SD) | 13.66 (4.29) | 11.50 (3.60) | 16.19 (3.60) | <.001* |
| Range | 1–29 | 1–25 | 6–29 | |
| Trail Making Test Part A, mean (SD) | 44.17 (25.64) | 50.22 (29.01) | 35.34 (16.33) | <.001* |
| Range | 15–150 | 16–150 | 15–150 | |
| Trail Making Test Part B, mean (SD) | 129.05 (85.77) | 158.21 (93.10) | 90.08 (53.70) | <.001* |
| Range | 25–300 | 25–300 | 25–300 | |
| F‐A‐S, mean (SD) | 31.84 (12.25) | 27.57 (11.00) | 37.22 (11.02) | <.001* |
| Range | 1‐67 | 1‐65 | 9‐67 | |
| Animals, mean (SD) | 17.46 (5.16) | 16.42 (4.85) | 19.17 (5.04) | <.001* |
| Range | 0–37 | 0–33 | 2–37 | |
| WMS‐III logical memory I, mean (SD) | 35.16 (11.98) | 31.02 (10.71) | 40.78 (10.99) | <.001* |
| Range | 0–69 | 0–57 | 4–69 | |
| WMS‐III logical memory II, mean (SD) | 21.24 (8.95) | 18.77 (8.18) | 24.80 (8.70) | <.001* |
| Range | 0–44 | 0–40 | 0–44 | |
| SEVLT 1‐5 total, mean (SD) | 31.69 (9.08) | 29.34 (8.16) | 33.33 (9.02) | <.001* |
| Range | 0–53 | 0–53 | 3–53 | |
| SEVLT delayed recall, mean (SD) | 7.59 (3.45) | 7.13 (3.26) | 8.51 (3.32) | <.001* |
| Range | 0–15 | 0–15 | 0–15 |
Abbreviations: Aβ, amyloid beta; GDS, Geriatric Depression Scale; MMSE, Mini‐Mental State Examination; NfL, neurofilament light; SASH, Short Acculturation Scale for Hispanics; SD, standard deviation; SEVLT, Spanish English Verbal Learning Test; WMS, Weschler Memory Scale.
P < .05.
3.1. Demographic factor impact on N
Age was found to be significantly negatively correlated with the MRI‐based N marker (r2 = ‐0.40, P < .001) such that greater neurodegeneration was found with increasing age; however, no significant correlation was found for education (P > .05). Females were found to have significantly higher mean N values (2.74, standard deviation [SD] = 0.15) than males (2.70, SD = 0.15; F [1, 1303] = 11.93, P < .001). No interaction effect was not found for ethnicity for age (F [36, 1224] = 1.110, P = .303) or level of education (F [13, 1268] = 1.581, P = .0.084) when examined with the outcome measure of N.
3.2. Social and behavioral factor impact on N
A significant correlation was found between N and household income (r2 = 0.083, P = .003) and acculturation (r2 = 0.069, P = .014) with covariates of age, sex, and education. No other social or behavioral factor (social support, chronic stress, depression) was shown to be significantly correlated. A significant interaction effect was not found for ethnicity and any of the social and behavioral factors examined (i.e., household income [F (81, 955) = 1.083, P = .296], social support [F (28, 1241) = 1.336, P = .113], chronic stress [F (28 1241) = 0.794, P = .769], acculturation [F (7, 1279) = 1.159, P = .323], and depression [F (26, 1246) = 0.783, P = .773]). Individual linear regression models were conducted to examine the association among household income, social support, chronic stress, acculturation, and depression scores on the primary outcome measure of N with covariates of age, sex, and education. After adjusting for covariates, the N biomarker was found to be significantly associated with household income (standardized coefficients beta = 0.085, t = 2.995, P = .003) and acculturation (standardized coefficients beta = 0.109, t = 2.878, P = .004) reflecting a potential protective effect on N (see Table 2). Although an interaction effect was not shown among household income, acculturation, and ethnicity, supplementary stratified analyses revealed the protective effect of social factors applied only to those of Mexican American ethnicity (household income: standardized coefficients beta = 0.094, t‐value = 2.451, P = .015; acculturation: standardized coefficients beta = 0.118, t‐value = 2.773, P = .006; Table S1 in supporting information).
TABLE 2.
Association between neurodegeneration (N) and social and behavioral factors covarying for age, sex, and education in the combined sample
| Standardized coefficients beta | t‐value | P‐value | |
|---|---|---|---|
| Annual household income | 0.085 | 2.995 | .003* |
| Social support | 0.031 | 1.198 | .231 |
| Chronic stress | 0.020 | 0.798 | .425 |
| Acculturation | 0.109 | 2.878 | .004 |
| Depression | –0.017 | –0.629 | .529 |
Note: *Partial P < .01.
3.3. Medical condition impact on N
Examining the link between medical conditions (dHTN, dDLP, dDM) and N, those with a diagnosis of dDM were found to have significantly lower mean N values (2.70; SD = 0.14) compared to those without (2.73, SD = 0.14; F [1, 1303] = 0.404, t = 2.808, P = .005 [95% confidence interval (CI) = 0.007 to 0.044]). Additionally, those with a diagnosis of dHTN (2.71, SD = 0.14) were found to have lower mean N value than those without (2.73, SD = 0.15; F [1, 1303] = 0.433, t = 2.561, P = .011 [95% CI = 0.005 to 0.037]). Mean differences were otherwise not observed for dDLP (F [1, 1303] = 2.046, t = 0.959, P = .338 [95% CI = –0.008 to 0.024]). Ethnicity did not produce a significant interaction effect between the N biomarker and any of the medical conditions examined (dDM F [1, 1298] = 0.944, P = .332; dDLP F [1, 1298] = 2.284, P = .131; dHTN F [1, 1298] = 0.128, P = .720) with covariates of age, sex, and education.
After controlling for covariates (age, sex, and education), N was significantly correlated with fasting levels of total cholesterol (r2 = 0.07, P = .006), low‐density lipoprotein (LDL; r2 = 0.08, P = .005), glucose (r2 = –0.12, P < .001) and HbA1c (r2 = –0.13, P < .001). In the total sample, an interaction effect was not found for ethnicity (covarying for age, sex, education) with any of the bloodwork measures examined (fasting levels of total cholesterol F [142, 942] = 1.175, P = .093; LDL F [124, 981] = 0.999, P = .488; glucose F [79, 1046] = 0.881, P = .759; HbA1c F [39, 1165] = 0.604, P = .975); however, when split by ethnicity, N was found to be significantly correlated with total cholesterol (r2 = 0.08, P = .041) and LDL (r2 = 0.12, P = .003) among non‐Hispanic Whites and glucose (r2 = –0.16, P < .001) and HbA1c (r2 = 0.179 P < .001) among Mexican Americans reflecting differences across ethnic groups.
Given the differential impact of medical conditions, additional analyses were conducted to examine the impact of duration of disease on N for dDM, dHTN), and dDLP. In the total sample, dDM was significantly related to N after covarying for age, sex, and education (P = .001). Interestingly, neither dHTN nor dDLP were significantly associated with N (P > .05; see Table 3). Although an interaction effect was no found between ethnic group and duration of medication conditions (P > .05), when analyses were split by ethnic group, dDM (P < .001) was found to be significantly associated with N among Mexican Americans while no significant associations were found among Non‐Hispanic Whites (P > .05; see Table 3).
TABLE 3.
Association of duration of medical conditions and neurodegeneration (N) covarying for age, education, sex
| Standardized coefficients beta | t‐value | P‐value | |
|---|---|---|---|
| dDM | |||
| Total sample | –0.221 | –4.045 | <.001* |
| Non‐Hispanic White | –0.178 | –1.696 | .095 |
| Mexican American | –0.230 | –3.587 | <.001* |
| dHTN | |||
| Total sample | –0.057 | –1.530 | .126 |
| Non‐Hispanic White | –0.022 | –0.402 | .688 |
| Mexican American | –0.096 | –1.909 | .057 |
| dDLP | |||
| Total sample | –0.027 | –0.732 | .464 |
| Non‐Hispanic White | –0.067 | –1.308 | .192 |
| Mexican American | –0.003 | –0.071 | .943 |
Abbreviations: dDLP, duration of dyslipidemia in years; dDM, duration of diabetes mellitus in years; dHTN, duration of hypertension in years.
3.4. Diagnosis and N positivity
A total n = 213 (35%) non‐Hispanic Whites and n = 222 (32%) Mexican Americans were classified as positive on the N biomarker based on previously published cut‐scores. 7 Split by diagnosis, N positivity (N+) was as follows: NC n = 313 (30%), MCI n = 70 (42%), dementia n = 52 (67%). Among Mexican Americans, N+ by diagnostic category was as follows: NC n = 153 (29%), MCI n = 36 (34%), dementia n = 33 (66%). Among non‐Hispanic Whites, the N+ was as follows: NC n = 160 (30%), MCI n = 34 (55%), dementia n = 19 (68%). Although an interaction effect was not found between ethnicity and cognitive diagnosis, the rate of N+ was significantly lower among the Mexican American MCI group compared to the non‐Hispanic White group (χ2 = 13.37, P < .001); no difference was shown between ethnic groups for those with dementia (χ2 = 2.79, P = .095). In separate logistic regression analyses with covariates of age, sex, and education, N+ was a significant predictor of MCI diagnosis among non‐Hispanic Whites (B [S.E.] = 0.794 [0.299], Wald = 7.02, P = .008, Exp [B] = 2.212 [95% CI = 1.230 to 3.978]) but not among Mexican Americans (B [S.E.] = 0.248 [0.238], Wald = 1.083, P = .298, Exp [B] = 1.281 [95% CI = 0.803 to 2.045]). N+ was a significant predictor of dementia diagnosis among both Mexican Americans (B [S.E.] = 1.247 [0.341], Wald = 13.373, P < .001, Exp [B] = 3.482 [95% CI = 1.784 to 6.794]) and non‐Hispanic Whites (B [S.E.] = 1.615 [0.447], Wald = 13.041, P < 0.001, Exp [B] = 5.026 [95% CI = 2.093 to 12.074]).
3.5. Age of N positivity
Controlling for sex and education, age was shown to be significantly related to N+ (standardized coefficients beta = –0.404, t‐value = –15.868, P < .001). An interaction between age and ethnicity was not found (F [36, 1221] = 0.681, P = .925) for N+ when entered into a model with covariates of sex and education; however, the average age of N+ among Mexican Americans was 67.33 (SD = 8.38) compared to non‐Hispanic White age of 72.88 (SD = 8.40), which did support a significant mean group difference (P < .001; see Figure 1A). When medical comorbidities were included as covariates along with age, sex, and education, the only medical comorbidity found to be a significant within the model was diabetes (standardized coefficients beta = –0.085, t = ‐3.168, P = .002). Sex was also a significant covariate within the model (standardized coefficients beta = 0.068, t = 2.667, P = .008). When the analyses were expanded to examine the relationship among cognitive diagnosis, age, and ethnicity, an interaction was not found (P > .05) with covariates previously noted; however, Figure 1B highlights the difference that can be seen in age of N+ across diagnostic groups (NC, MCI, dementia). In our prior work, we have shown that Mexican Americans develop MCI at significantly younger ages compared to non‐Hispanic Whites; therefore, we examined age of N+ among MCI cases. Mexican American participants categorized as MCI and N+ were significantly younger (66.19, SD = 8.24) than non‐Hispanic White participants (74.29, SD = 10.26) who were N+ and diagnosed as MCI (P = .001).
FIGURE 1.
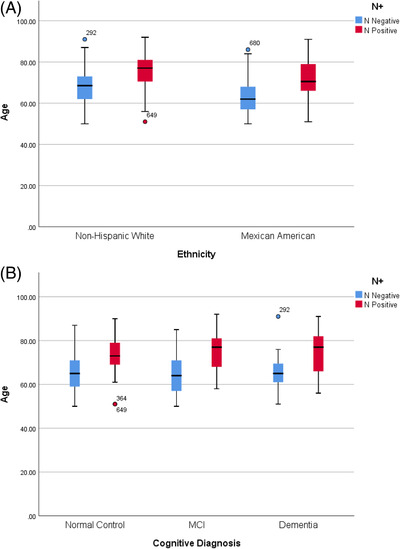
A, Age of neurodegeneration positivity (N+) separated by ethnic group. B, Age of N+ separated by cognitive diagnosis. MCI, mild cognitive impairment
3.6. Neuropsychological testing
The link between N and neuropsychological test performance was also examined. An interaction effect was shown between ethnicity and select cognitive measures including tests of global cognitive functioning (MMSE, F [11, 1266] = 6.072, P < .001), working memory (WMS‐III Digit Span, F [18, 1239] = 2.466, P = .001), and immediate episodic memory (WMS‐III Logical Memory II, F [47, 1186] = 1.912, P < .001) with covariates of age, sex, and education and after correcting for multiple comparisons (partial P‐value = .005). Interaction effects between cognitive diagnosis and select cognitive measures (covarying for age, sex, and education) were no longer significant after correcting for multiple comparisons (partial P‐value = .005).
In separate linear regression models, findings revealed that after covarying for age, education, and sex, less N (represented by higher meta‐ROI) was associated in the total sample with better performance across measures all cognitive measures (P < .001; see Table 4). When split by ethnic group, less N was found to be associated with better performance across measures of global cognition (MMSE, P = .001), attention and processing speed (Trail Making Test Part A, P < .001), executive functioning (Trail Making Test Part B, P < .001), immediate and delayed verbal episodic memory (WMS‐III Logical Memory I, P < .001; WMS‐III Logical Memory II, P = .002), as well as immediate route verbal learning (SEVLT Trial 1‐5 recall, P < .001) for Non‐Hispanic Whites. Fewer associations were found among Mexican Americans between a measure of N and cognitive test performance. Of those, less N among this group was found to be associated with better neuropsychological test performance on measures of global cognition (MMSE, P < .001), working memory (WMS‐II Digit Span, P = .004), phonemic fluency (FAS, P = .002), as well as route verbal learning and memory (SEVLT Trial 1 to 5 recall, P = .001; SEVLT delayed recall, P < .001; see Table 4).
TABLE 4.
Association of neuropsychological test performance and neurodegeneration (N) covarying for age, education, and sex
| Standardized coefficients beta | t‐score | P‐value | |
|---|---|---|---|
| MMSE | |||
| Total sample | –0.160 | –4.829 | <.001* |
| Non‐Hispanic White | –0.131 | –3.403 | .001* |
| Mexican American | –0.175 | –3.951 | <.001* |
| WMS‐III digit span | |||
| Total sample | –0.145 | –4.404 | <.001* |
| Non‐Hispanic White | –0.106 | –2.716 | .007 |
| Mexican American | –0.126 | –2.905 | .004* |
| Trail Making Test Part A | |||
| Total sample | 0.127 | 3.975 | <.001* |
| Non‐Hispanic White | 0.166 | 4.105 | <.001* |
| Mexican American | 0.109 | 2.453 | .014 |
| Trail Making Test Part B | |||
| Total sample | 0.162 | 4.560 | <.001* |
| Non‐Hispanic White | 0.173 | 4.186 | <.001* |
| Mexican American | 0.138 | 2.828 | .005 |
| F‐A‐S | |||
| Total sample | –0.136 | –4.291 | <.001* |
| Non‐Hispanic White | –0.106 | –2.660 | .008 |
| Mexican American | –0.132 | –3.065 | .002* |
| Animals | |||
| Total sample | –0.106 | –3.548 | <.001* |
| Non‐Hispanic White | –0.091 | –2.239 | .025 |
| Mexican American | –0.100 | –2.411 | .016 |
| WMS‐III logical memory I | |||
| Total sample | –0.146 | –4.822 | <.001* |
| Non‐Hispanic White | –0.159 | –3.960 | <.001* |
| Mexican American | –0.089 | –2.212 | .027 |
| WMS‐III logical memory II | |||
| Total sample | –0.126 | –4.252 | <.001* |
| Non‐Hispanic White | –0.127 | –3.151 | .002* |
| Mexican American | –0.092 | –2.289 | .022 |
| SEVLT 1‐5 total | |||
| Total sample | –0.159 | –5.210 | <.001* |
| Non‐Hispanic White | ‐0.156 | ‐3.594 | <.001* |
| Mexican American | –0.141 | –3.369 | .001* |
| SEVLT delayed recall | |||
| Total sample | –0.140 | –4.604 | <.001* |
| Non‐Hispanic White | –0.089 | –2.084 | .038 |
| Mexican American | –0.174 | –4.158 | <.001* |
Abbreviations: MMSE, Mini‐Mental State Examination; WMS, Weschler Memory Scale; SEVLT, Spanish English Verbal Learning Test.
Partial P < .005.
3.7. Cerebral amyloid and AT(N) plasma biomarkers
A subset of the HABLE study included available amyloid PET scans (n = 61 [n = 39 Mexican Americans; n = 22 non‐Hispanic Whites]). Of those, n = 13 were A+ while n = 48 were A–. The mean age of this subset was 66.25 (SD = 10.14) with a mean education level of 11.51 years (SD = 5.34). There was no significant difference between demographic variables between the subset of HABLE participants who underwent an amyloid PET scan and those who did not (P > .05). Neither the MRI‐derived N biomarker or N+ positivity was found to be correlated with global cerebral amyloid SUVR levels (P > .05) after controlling for age, sex, and education. A similar finding was shown when separated by ethnic group (P > .05).
In regard to AT(N) plasma biomarkers in the total sample, partial correlations with covariates of age, sex, and education revealed a significant negative correlation between N and the plasma biomarker NfL (r2 = –0.12, P < .001); significant correlations were not found among the other plasma biomarkers of Aβ40 (r2 = –0.05, P = .060), Aβ42 (r2 = 0.01, P = .628), or total T (r2 = –0.03, P = .231). Although ethnicity did not produce a significant interaction effect with the plasma AD biomarkers on N, analyses were further conducted separated by ethnic group, which revealed only one significant association found among Mexicans reflecting higher NfL levels associated with greater N (P < .001; see Table 5).
TABLE 5.
Association between AT(N) plasma biomarkers and neurodegeneration (N) covarying for age, sex, and education
| Standardized coefficients beta | t‐score | P‐value | |
|---|---|---|---|
| Aβ40 | |||
| Non‐Hispanic White | –0.018 | –0.451 | .652 |
| Mexican American | –0.081 | –2.205 | .028 |
| Aβ42 | |||
| Non‐Hispanic White | 0.065 | 1.718 | .086 |
| Mexican American | –0.027 | –0.737 | .462 |
| Total tau (t‐tau) | |||
| Non‐Hispanic White | –0.047 | –1.230 | .219 |
| Mexican American | –0.007 | –0.195 | .845 |
| NfL | |||
| Non‐Hispanic White | –0.066 | –1.675 | .095 |
| Mexican American | –0.170 | –4.610 | <.001* |
Note: *Partial P < .0125.
Abbreviations: Aβ, amyloid beta; NfL, neurofilament light chain.
4. DISCUSSION
This is, to our knowledge, the first large‐scale characterization of the N (derived from meta‐ROI) among Mexican Americans compared to non‐Hispanic Whites. Our data demonstrates that N is significantly related to the clinical outcomes that would be expected among both groups. However, our findings suggest that the underlying mechanisms driving N may vary between ethnic groups based on findings such as duration of diabetes being associated with N among Mexican Americans but not among non‐Hispanic Whites. It also suggests that N may begin earlier in the sequence of the biological cascade associated with cognitive loss among Mexican Americans compared to non‐Hispanic Whites, which will be important to further examine with longitudinal data. Given the younger age of onset of cognitive loss, these findings indicate a possibility that the sequence and timing of the AT(N) biomarkers and subsequent development of AD pathology may vary between ethnic groups.
Ours is not the first study to point to potential impacts of race or ethnicity on AT(N) biomarkers. Howell et al. 6 , 7 recruited n = 135 older participants (Black n = 65, non‐Hispanic White n = 70) and found that Blacks had significantly different cerebrospinal fluid (CSF) p‐tau181, t‐tau, and Aβ40 levels despite similar Aβ42, NfL, white matter hyperintensity, and hippocampal volume. Cognitively impaired Blacks also had significantly different CSF t‐tau/Aβ42 and p‐tau181/Aβ42 levels. Morris et al. 6 , 9 found that CSF t‐tau and p‐tau181 were significantly lower among African Americans and the racial impact was associated with APOE ε4 genotype. This work suggests that ethnic‐specific CSF cut‐scores need to be examined. Graff‐Radford et al. 8 , 9 examined autopsy data from n = 110 Blacks and n = 2500 non‐Hispanic Whites in the National Alzheimer's Coordinating Center (NACC) database and found that Blacks had lower rates of Braak Stages 0, I–II, and III–IV than non‐Hispanic Whites but greater levels of Stages V–VI. Overall AD and vascular neuropathology were more common among Blacks. Barnes et al. 8 , 31 examined data from the Rush Alzheimer's Disease Clinical Core (n = 51 Black, n = 81 non‐Hispanic White) and found that Blacks were more likely to have mixed neuropathology. In the GERAS‐US study, cerebral amyloid positivity was found to be significantly lower among Hispanic patients clinically diagnosed as AD compared to non‐Hispanic Whites. 32 The IDEAS study also found that Blacks and Hispanics who were clinically diagnosed with AD and MCI had significantly lower amyloid PET positivity rates compared to non‐Hispanic Whites (Rabinovici personal communication; 2019 AAIC poster). In the A4 study, both Hispanics and Blacks had lower levels of amyloid positivity (unpublished data). Additionally, in the HABLE amyloid pilot study, Mexican Americans had lower levels of amyloid positivity compared to non‐Hispanic Whites. 16 The sample size of Hispanics included in these studies was small and the HABLE study is collecting A (and T) PET scans on all participants at Visit 2 and Visit 3, so a better estimation of the prevalence rates of A positivity among community‐dwelling Mexican Americans will be generated soon. Regarding MRI‐defined N, Burke et al., studied a sample of n = 75 Hispanics and n = 90 non‐Hispanic Whites and found that MRI measures of N changed among control, MCI, and dementia groups but that Hispanics had less N than non‐Hispanic Whites. 33 Therefore, there is significant reason to examine the presence, timing, sequence, progression, and clinical impact of these biomarkers.
Our findings highlight the different underlying mechanisms linked with N among Mexican Americans compared to non‐Hispanic Whites. Among Mexican Americans, diabetes‐related factors (e.g., glucose, HbA1c levels) were strongly related to N with duration of diabetes being the strongest factor. However, among non‐Hispanic Whites, cardiovascular (LDL) factors were significantly related to N. This differential pathway to neuronal loss between ethnic groups has powerful implications. First, if diabetes (and duration of diabetes) is a significant driver of N among Mexican Americans, this would suggest that targeted and timely interventions to prevent or control diabetes could have neuroprotective effects. Second, if cardiovascular and cerebrovascular pathways are powerful drivers of N among non‐Hispanic Whites, different sets of targeted and timely interventions to prevent and control these factors could be neuroprotective among non‐Hispanic Whites. Our work, combined with the work of others presented above, suggest that an expanded AT(N) framework that includes vascular, metabolic, and inflammatory (VMI) pathways should be considered, to allow for a more comprehensive picture of the biological factors contributing to cognitive loss among diverse communities.
A limitation to the current study is the focus on N in the context of the current AT(N) framework, which captures N as an MRI measure of cortical thickness as opposed to taking into consideration other factors such as vascular risks (e.g., white matter hyperintensities, infarcts). Current work is ongoing within the HABLE study to further examine such vascular risks on N through diffusion tensor imaging to understand how VMI factors can impact the AT(N) biomarkers. Future work should also expand to examine AT(N) biomarkers among the broader Hispanic population as our findings are focused on those who self‐identify as Mexican Americans. It will be important to understand how the framework may be similar or distinct among different populations. Additionally, longitudinal PET and MRI scans are being conducted within this cohort to better understand the timing, sequence, and trajectories of the AT(N) framework among diverse populations.
Meeker et al. 34 recently found that socioeconomic status (SES) mediated the race effects on N (as measured by cortical volumes) among older Blacks. Dougherty et al. 35 recently found that higher SES was associated with increased brain volume and that smoking status mediated this relationship in the CARDIA study. Shaked et al. 36 recently found that SES impacted diffusion tensor imaging fractional anisotropy (FA) measures across regions. Here we found that markers of SES (household income and education) were only significantly associated with N among Mexican Americans. When combined with the recent findings by Meeker et al., these findings strongly support the need to fully examine the AT(N) biomarkers within the National Institute on Aging Health Disparities Research Framework, 37 which is being implemented within the HABLE cohort. Additionally, to understand the AT(N) biomarkers within the Health Disparities Research Framework more comprehensively, the HABLE study is currently expanding to add n = 1000 Blacks to study these important factors across the three largest ethnic/racial groups in the United States. Overall, these findings suggest that neurodegenration as measured by a N‐marker of the AT(N) framework is significantly different among Mexican Americans compared to non‐Hispanic Whites including the age of positivity and the underlying contributing factors, despite comparable impacts on clinical outcomes. As such, ethnically appropriate interventions to prevent or treat N will be required.
CONFLICTS OF INTEREST
No authors report conflicts of interest with the work presented in this manuscript.
Supporting information
Supporting information
ACKNOWLEDGMENTS
Research reported here was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers R01AG054073 and R01AG058533. This work was also supported in part by NIH/NIBIB award P41‐EB015992. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research team also thanks the local Fort Worth community and participants of the HABLE study.
O'Bryant SE, Zhang F, Petersen M, et al. Neurodegeneration from the AT(N) framework is different among Mexican Americans compared to non‐Hispanic Whites: A Health & Aging Brain among Latino Elders (HABLE) Study. Alzheimer's Dement. 2022;14:e12267. 10.1002/dad2.12267
HABLE Study Team (now HABS‐HD Team): MPIs: Sid E. O'Bryant, Kristine Yaffe, Arthur Toga, Robert Rissman, & Leigh Johnson; and the HABLE/HABS‐HD Investigators: Meredith Braskie, Kevin King, James R. Hall, Melissa Petersen, Raymond Palmer, Robert Barber, Yonggang Shi, Fan Zhang, Rajesh Nandy, Roderick McColl, David Mason, Bradley Christian, Nicole Philips, Rocky Vig, Raul Vintimilla, Stephanie Large, Mark Mapstone, Michelle Mielke, Joseph Lee, Ozioma Okonkwo, Amy Kind, Monica Rivera‐Mindt, Rema Raman, Michael Donohue, and Carl Hill.
REFERENCES
- 1. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: update and areas of immediate need. Alzheimer's Dement 2019;15:292‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhaskaran SP, Chandratre K, Gupta H, et al. Germline variation in BRCA1/2 is highly ethnic‐specific: evidence from over 30,000 Chinese hereditary breast and ovarian cancer patients. Int J Cancer. 2019; 145: 962‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friebel TM, Andrulis IL, Balmaña J, et al. BRCA1 and BRCA2 pathogenic sequence variants in women of African origin or ancestry. Hum Mutat. 2019;40:1781‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim YC, Zhao L, Zhang H, et al. Prevalence and spectrum of BRCA germline variants in mainland Chinese familial breast and ovarian cancer patients. Oncotarget. 2016;7:9600‐9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris JC, Schindler SE, McCue LM, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76(3):264‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howell JC, Watts KD, Parker MW, et al. Race modifies the relationship between cognition and Alzheimer's disease cerebrospinal fluid biomarkers. Alzheimers Res Ther. 2017;9(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graff‐Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW. Neuropathologic differences by race from the National Alzheimer's Coordinating Center. Alzheimers Dement. 2016;12:669‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Census Bureau QuickFacts: United States n.d.. https://www.census.gov/quickfacts/fact/table/US/PST045219 (accessed December 8, 2020).
- 11. US Hispanic Population Reached New High in 2019, but Growth Slowed. Pew Research Center n.d; 2020. [Google Scholar]
- 12. Jacobsen LA, Kent M, Lee M, et al. America's aging population. Popul Bull. 2011;66:1‐16. [Google Scholar]
- 13. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015‐2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15:17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. CensusBureau US . American Fact Finder 2004. CensusBureau US; 2006. [Google Scholar]
- 15. Jack CR, Wiste HJ, Therneau TM, et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA. 2019;321:2316‐2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Bryant SE, Johnson LA, Barber R, et al, and Ya for the HSTeam . The Health and Aging Brain among Latino Elders (HABLE) study methods and participant characteristics. Alzheimer's Dement. 2021;13(1):e12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marin G, Sabogal F, Marin BV, Otero‐Sabogal R, Perez‐Stable EJ. Development of a short acculturation scale for Hispanics. Hisp J Behav Sci. 1987;9:183‐205. [Google Scholar]
- 18. Merz EL, Roesch SC, Malcarne VL, et al. Validation of interpersonal support evaluation list‐12 (ISEL‐12) scores among English‐ and Spanish‐speaking Hispanics/Latinos from the HCHS/SOL Sociocultural Ancillary Study. Psychol Assess. 2014;26:384‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen S, Mermelstein R, Kamarck T, Hoberman HM, Sarason IG, Sarason BR. Social Support: Theory, Research and Applications. Measuring the Functional Components of Social Support. 1985:73‐94. [Google Scholar]
- 20. Bromberger JT, Matthews KA. A longitudinal study of the effects of pessimism, trait anxiety, and life stress on depressive symptoms in middle‐aged women. Psychol Aging. 1996;11:207‐213. [DOI] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 22. Weschler D. Weschler Memory Scale—Revised. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- 23. Reitan RM, Wolfson D. The Halstead‐Reitan Neuropsychological Test Battery: Theory and Interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 24. González HM, Mungas D, Haan MN. A verbal learning and memory test for English‐ and Spanish‐speaking older Mexican‐American adults. Clin Neuropsychol. 2002;16:439‐451. [DOI] [PubMed] [Google Scholar]
- 25. Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed.. Oxford: Oxford University Press; 2004. [Google Scholar]
- 26. O'Bryant SE, Edwards M, Johnson L, Hall J, Gamboa A, O'jile J. Texas Mexican American adult normative studies: normative data for commonly used clinical neuropsychological measures for English‐ and Spanish‐speakers. Dev Neuropsychol. 2018;43(1):1‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sierra Sanjurjo N, Montañes P, Sierra Matamoros FA, Burin D. Estimating intelligence in Spanish: regression equations with the word accentuation test and demographic variables in Latin America. Appl Neuropsychol Adult. 2015;22:252‐261. [DOI] [PubMed] [Google Scholar]
- 28. Berg L. Clinical Dementia Rating (CDR). Psychopharmacol Bull. 1988;24(4):637‐639. [PubMed] [Google Scholar]
- 29. O'Bryant SE, Edwards M, Johnson L, Hall J, Gamboa A, O'jile J. Texas Mexican American adult normative studies: normative data for commonly used clinical neuropsychological measures for English‐ and Spanish‐speakers. Dev Neuropsychol. 2018;43:1‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. R_Development_Core_Team . R: A Language and Environment for Statistical Computing. R_Development_Core_Team; 2009. [Google Scholar]
- 31. Robinson RL, Rentz DM, Bruemmer V, et al. Observation of patient and caregiver burden associated with early Alzheimer's disease in the United States: design and baseline findings of the GERAS‐US Cohort Study. J Alzheimers Dis. 2019;72:279‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burke SL, Rodriguez MJ, Barker W, et al. Relationship between cognitive performance and measures of neurodegeneration among Hispanic and white non‐Hispanic individuals with normal cognition, mild cognitive impairment, and dementia. J Int Neuropsychol Soc. 2018;24:176‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adapt_Research_Group , Lyketsos CG, Breitner JC, Green RC, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800‐1808. [DOI] [PubMed] [Google Scholar]
- 34. Meeker K, Wisch J, Hudson D, et al. Socioeconomic status mediates racial differences seen using the AT(N) framework. Annals of Neurology. 2021;89(2):254‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dougherty RJ, Moonen J, Yaffe K, et al. Smoking mediates the relationship between SES and brain volume: the CARDIA study. PLoS One. 2020;15:e0239548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shaked D, Leibel DK, Katzel LI, et al. Disparities in diffuse cortical white matter integrity between socioeconomic groups. Front Hum Neurosci. 2019;13:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hill CV, Pérez‐Stable EJ, Anderson NA, Bernard MA. The national institute on aging health disparities research framework. Ethn Dis. 2015;25:245‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


