Highlights
-
•
AM enhances nutrient uptake, specially phosphorus and other less mobile elements mainly by increasing absorptive surface of hyphal exploration in rhizosprhere.
-
•
AM act as biocontrol and soil reclamation agent for polluted or contaminated soil.
-
•
Agrochemicals and fertilizers hamper AM function and diversity in agricultural field.
-
•
Crop rotation with nonmycorrhizal host, tillage hampers AM function and diversity.
-
•
in agricultural field.
-
•
Challenges in applying AMF in agriculture and how we can address these issues.
Keywords: Agro-ecosystem, Agrochemicals, Biocontrol, Mycorrhization, Mycorrhizae helper organism, Plant nutrition, Tillage, Symbiosis
Abstract
The rapid growth of human population on globe and reduction in agricultural land exerts huge pressure on crop productivity, food security and soil health; specially, in developing countries. Improper land management with excessive dependency on chemical fertilizers and agrochemicals to secure productivity tolls on human health, environment, biodiversity and sustainability. The utilization of arbuscular mycorrhizal fungi (AMF) as bio-fertilizer and in consortia with other beneficial microbes has become an increasing area of research in agriculture and life sciences. Former investigations revealed the positive influence of AM in nutrition, growth, yield of crops, soil quality increasing biological soil fertility and pathogen resistance. AMF symbionts are highly beneficial in plant abiotic stress tolerance. Along with other beneficial rhiozobacteria AM is almost substitute of chemical fertilizers in modern sustainable organic agricultural systems. But conventional agriculture in most countries is beyond to reach these benefits of AM. The issues which hinder the utilization also contradict to sustainability to some degrees. The present review highlights on the issues of hindrances in applicability of AM to the agricultural fields focusing on the mode of functions, maintaining soil and environmental sustainability; interactions with other biofertilizers and impact of various agrochemicals and agro-practices including tillage and crop rotation. The procedures to avail the full benefit of AM in agricultural field for sustainable system are discussed here.
Graphical abstract
The illustration of the benefit of AM symbiosis and diversity and the impact of agriculture related factors on the same.
1. Introduction
Agricultural practices in upcoming decades in the 21st century is going to face tremendous challenges to produce enough healthy food to cater the global population due to unstable economy, climate change, and biodiversity degradation (FAO, 2020; Zhao et al., 2017). The hiking of prices of inorganic fertilizers will continue to rise globally and the demand for chemical fertilizers in is conventional agro-productivity is rising continuously, which has a direct impact on production cost increment and high energy consumption. Moreover, it is quite disappointing that, the yield of maximum major crops has been declining in respect to the ever-increasing human population (FAO, 2020; Grassini et al., 2013; Zhao et al., 2017). About 30 years before, our country was accustomed to traditional organic based or integrated agricultural practices with organic manure, organic control or biocontrol with predator animals, integrated fishery etc. and a sustainable agriculture as well as ecosystem with sound biodiversity. Within these years the scenario changed to high input chemical dependent agriculture destroying soil sustainability with impact on water and environment too, inviting health hazards. Fertilizers compensate the need of nutrients in soil for optimum growth of plant. The dose of fertilizer application must be balanced to the nutrient deficiency in soil and according the requirement of the particular crop (Selim, 2020).
In conventional agricultural practices this logic is over ruled and fertilizers are used in doses higher than actually required to boost up the yield within short time. The high yielding varieties demands quick supply of nutrients and chemical control (Singh and Singh, 2017). Most portion of the applied fertilizer remain unavailable to plants, leading soil toxicity, deterioration of soil quality, loss of beneficial soil microflora, leaching of toxic elements to water table and run off to water resources; ultimately leading to human health issues and loss of sustainability in whole ecosystem (Meena et al., 2020; Prashar and Shah, 2016). All these result in huge loss of biodiversity is realized with time. This type of practice initiates short-term increment of yield but the long-term negative effects promoted on ecological issues, directly challenge healthy food security (GSDR, 2019; Godfray and Garnett, 2014;. All these affect the nutrient cycle governed by soil microflora to release inorganic elements in soluble form, available to plants; will ultimately hamper production in near future. In an ecologist's view, shifting from low input well balanced near natural system to high energy input near synthetic system is merely wastage of energy and leading to in verge of misbalance.
Most developed and developing countries are now opting for organic food production, consumption and import. Developing countries are also adopting organic farming for export and profit. In the recent past few years, public awareness has been developing related to the negative impacts on ecological diversity, safe food, environment, and economy because of on modern agriculture (Willer et al., 2020). Therefore, the concept of sustainable agricultural management is emerging in, where enough crop production is possible without any ecological and health y damage (Andres and Bhullar, 2016; Pretty and Bharucha, 2014). Organic agriculture is an alternative biological agro-systematic way that maintains cost-effective and environment-friendly secure food production. Shifting from high input conventional agriculture to organic farming should never balance in yield overnight. Organic manure is processed and utilized in presence and function of various soil microbes. Organic farming with suitable microbial consortia for particular environment, soil conditions and crop are vital for yield replacement in successive years (Alori and Babalola, 2018; Santos et al., 2019); and to maintain long-term soil fertility and safe, high-quality food productivity (Bender and van der Heijden, 2015; Philippot et al., 2013).
Application of arbuscular mycorrhizal fungi (AMF) as bio-inoculation could be an effective alternative, facilitate major benefit in long term soil fertility, plant nutrition, and protection, has a promising potency in sustainable agriculture (Cavagnaro et al., 2015; Thirkell et al., 2017). Mycorrhiza is the important mutualistic association between the two kingdoms, Plantae and Fungi. Arbuscular mycorrhizae (AM), the common endotrophicsymbiont, are taxonomically and functionally diverse (Lee et al., 2013) and members of the monophyletic phylum, Glomeromycota Spatafora et al., 2016), are present in more than 90% of land plants (Davison et al., 2015). They form two unique structures: a finely branched hyphal tip arbuscules for nutritional exchanges; and a balloon like vesicles for storage of nutrients within the plant root cortical cells of the host (Pepe et al., 2016). AMF may use as a biocontrol agent also to protect the host plant diseases against soil-borne pathogens (Veresoglou and Rillig, 2012).
1.1. How AM benefit plants? why we need to take the challenges in AMF application?
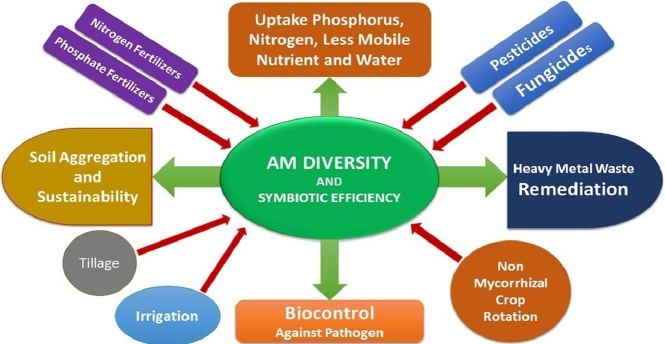
AM fungi are the most prevalent in soil that promotes essential agro-ecosystem benefits in organic farming systems (Berruti et al., 2016; Cavagnaro, 2014). AMF symbiosis is probably more favorable in conservative and sustainable agriculture to having the potentiality of major beneficial functions are like, (1) increase of plant growth and nutrition by gaining more nitrogen (N), phosphorus (P), and other less mobile nutrients, (2) increase water uptake and water holding capacity that initiate drought tolerance, (3) increase tolerance to other abiotic stresses such as, soil salinity, heavy metal toxicity, etc., (4) overcome biotic stresses and offering bioprotection against pathogen, (5) improve soil quality (6) Enhance plant vigor and yield. These multifunctional options may employ AM association towards agricultural sustainable intensification (Garnett et al., 2013).The graphical abstract (Graphical abstract) indicates the role of AM in the above mentioned criteria.
To comprehend the role of AM in sustainability, we need firstly to know the physiological functioning of this symbiosis. AM symbioses with its host in exchange of upto 30% host photosynthates; in turn of which it offers the above functions (Kiers et al., 2011).
1.1.1. Nutrient and water absorption mechanism
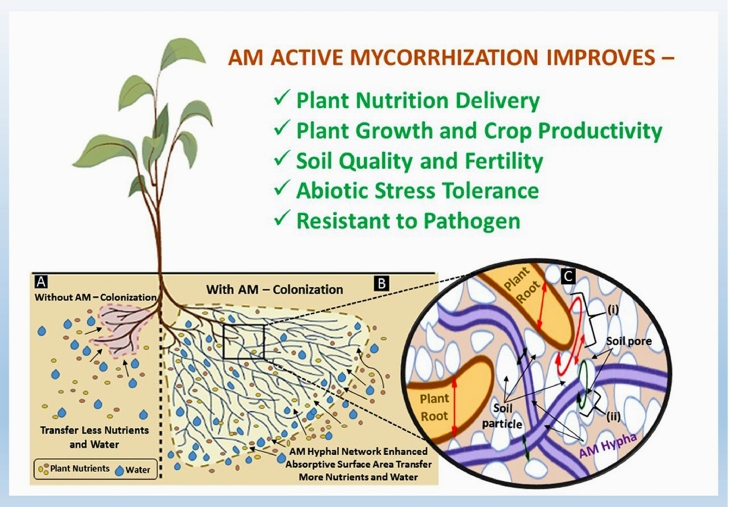
There are two modes of absorption for nutrients and water by AM fungi. After germination in asymbiotic phase, hyphal morphogenesis occurs in the availability of root exudates released by host plants (Coelho et al., 2019; The germling hyphae, while elongate and grow in branching pattern, come in physical contact with the host roots, grow inter-cellularly with the help of appressoria by penetrating in root cells starts and produces arbuscules within the root cortex (Berruti et al., 2016; Giovannini et al., 2020). Rapidly spreading extracellular hyphal network gain a high absorbing capacity and surface-volume, increase uptake and translocation of water and essential nutrients mainly phosphorus and nitrogen (Baum et al., 2015), with the activity of nutrient transporter genes present in the hyphae (Casieri et al., 2013). Hyphal diameter is less than 100 times than finest roots and 10–20 times lesser than root hairs. In drought condition when soil hydraulic potential is much low to be absorbed by root or root hairs, easily lifted by hyphae (Augé et al., 2015). Moreover these extraradical hyphae are capable to enter and procure nutrient and water from finer soil crevices beyond the entry of roots (Püschelet al., 2020); extensive mycelia network increase absorptive surface much more than root system and again this mycelia mat help to retain soil moisture also (Augé et al., 2015). Root system functions exhaust the nutrient from root depletion zone. Quick absorption from beyond root depletion zone by extensive mycelia network is the major mechanism of this symbiosis (Kobae, 2019; Johri et al., 2015). It has been observed that AM extraradical hyphae can extend upto 50 m in rhizosphere and translocation of phosphate as polyphpsphate granules through the hyphae by 32P labeled phosphate (Chiu and Paszkowski, 2019;Sato et al., 2019). As AM hyphae takes over the function of root hairs, in mycorrhizal plants they became obsolete. Physiologically too AM boost up and modify plant growth regulator functions to produce more tertiary as they roots and induce enlargement of root cortical cells as they colonize in cortex only (Gutjahr et al., 2013). The Fig. 1 depicts the nutrient and water absorption of AM.
Fig. 1.
Schematic representation of brief function of arbuscular mycorrhizal fungi (AMF): At the left portion (A),the zone around the plant root without AM colonization represents the limited absorptive surface area for nutrient and water for plant; In contrast, right side (B) the zone around the plant root with extensive AM network (mycorrhizosphere) represents the extended absorptive surface for nutrient and water for plant; In the rightmost part of the figure representing the comparative exploration pattern of plant root and the fungal hyphae into the soil (C). Plant root with larger diameter unable to access through fine pores into the soil particles, AMF hyphae being finer able to explore through finer soil pores (i) and absorb water from lower water potential (ii).
By chemical function to some degree AM solubilize phosphate by secretion of acid and alkaline phosphates and organic acids to mineralize nutrients and release (Sato et al., 2015). The uptake of nutrients especially P, also depends upon plant –fungal physiology. The translocation rate of nutrients depends on loading and unloading process through extracellular hyphae and cortical arbuscles. Plant requirement of nutrients together influences the whole process of uptake. AMF species, strains and environment too have a role (Cao et al., 2020).AMF has a key role in phosphate uptake particularly in P deficient soils by mobilizing P from rock phosphate (Etesami et al., 2021). Hyphae can decompose larger organic molecules (Bunn et al., 2019; Begum et al., 2019). Nitrogen transfer from organic matter to plant tissues through the AMF hyphae was evidenced to increase the plant biomass (Thirkell et al., 2016). The AM hyphal network is also able to uptake potassium (Zhao et al., 2015) and other important micronutrients like Mg, Zn, Cu, Ca, S, Na, Mn, B, Mo and Fe, essential for plant growth (Hajiboland et al., 2015). AMF involvement in nutrient cycling ensures adequate nutrient availability (Johnson et al., 2015) in infertile or less fertile soil.
1.1.2. AM in plant protection from biotic and abiotic stress
1.1.2.1. Abiotic stress
AM show a wide range of tolerance for abiotic factors, hence they are distributed and active worldwide in almost all ecosystems, soil conditions and environment; though the distribution, tolerance and efficacy vary with AM species and strains (Aguilera et al., 2015; Barbosa et al., 2017). It was well documented that, mycorrhizal inoculation with different AM species to plants under low or high temperature stress and different nitrogen level (Liu et al., 2013) able to reduce temperature stress and increase P content compared to non-AM plant (Liu et al., 2016; Hu et al., 2015; Mathur et al., 2018; Zhu et al., 2017). Zhu et al. (2015) showed that AM symbiosis increased glutamate oxaloacetate transaminase and glutamate pyruvate transaminase activities of maize plants under low temperature stress. AM symbiosis increases plant tolerance to alkalinity stresses and can withstand and alleviate stress in low pH acidic soil which restricts plant growth (Muthukumar et al., 2014). They promote osmotic adjustment under drought (Auge et al., 2015), and salinity stresses (Al-Karaki, 2013), salinity (Bothe et al., 2010) and heavy metals (Forgy, 2012). Application of AM in agriculture in water stressed or mined area and other waste places or convert these areas to agriculture field may be possible by reclamation with AM. As AM capture the hazardous elements within its mycelial mat though efficiency is species and strain dependent (Leal et al., 2016).
1.1.2.2. Biotic stress
AM increases the host plant resistance to pests and soil-borne diseases (Cameron et al., 2013; Poveda et al., 2020; Veresoglou and Rillig, 2012). They mainly increase host tolerance against root affecting nematodes and fungi present in the rhizosphere (Poveda et al., 2020; Schouteden et al., 2015). The AMF mediated bio control involves in direct competition with the pathogen for nutrients in rhizoplane and rhizisphere (Poveda et al., 2020; Santoyo et al., 2021). AM produce polysaccharides and phenolic compounds bound to cell walls which thicken the cell wall creating a mechanical barrier resistant to the entry of root pathogen to the host tissue of. Some AMF produces antifungal and antibacterial antibiotics resulting in pathogen resistance (Cameron et al., 2013). AM also indirectly induces host defense system by plant-mediated mechanisms to reduce the damage caused by soil-borne plant pathogen (Cameron et al., 2013); enhancing the tolerance of plant roots (Pieterse et al., 2014).
1.1.3. Role of AM in soil sustainability
Not merely in nutrient and water uptake, AMF has an important role in the improvement of soil structure and quality (Madhya, 2016), as external hyphal network promote soil aggregation by creating a skeletal structure in the mycorrhizosphere (Mardhiah et al., 2016). AMF improve soil structure by releasing various proteinaceous and non-proteinaceous organic compounds; the most effective protein glomalin to bind soil particles and these aggregates remain stable after six months of disappearance of the network. Arbuscular mycorrhization improves the soil organic matter content and water-holding capacity (Bitterlich et al., 2018; Zhang et al., 2019), which helps to maintain the conservation of the soil ecosystem. The extended hyphae play a crucial role to overcome water deficit in dry soil and reduce evaporation (Jayne and Quigley, 2014).
1.1.4. AM benefit in agriculture
The AM symbiosis is the potential component for sustainable agricultural systems as they have found positive effects on host plant nutrition, mineral cycling, and growth (Chahal et al., 2020; Thirkell et al., 2017).The symbiosis also increases chlorophyll, carotenoids, phenolics, etc. (Baslam et al., 2011; 2013). The early enhancement in chlorophyll and growth provides plant vigor and reproductive health boosting the yield. Improvement of growth and productivity of plants by the application of AM inocula has been established (Elbon and Whalen, 2015). Several recent works have been conducted with different crops like tomato, rice, wheat, maize, yam, potato etc. have shown the positive influence of plant growth and productivity (Hijri, 2016; Hu et al., 2014; Lu et al., 2015; Sabia et al., 2015). Moreover, the food quality of the crop in terms of antioxidants, flavonoids, vitamin C, etc. enhance by AMF colonization has been reported (Hart et al., 2015; Lu et al., 2015). AM may be used as a potential amendment to improve soil fertility, crop productivity, and yield quality as well as the revival of agro-ecosystem (Chen et al., 2018; The utilization of the beneficial effect of AM inoculated farming enhancing the plant growth and product quality of their hosts may be incorporated as sustainable agricultural systems (Bardgett and van der Putten, 2014). They also increase the formation of the nodule in leguminous plants and also free nitrogen fixation (Wang et al., 2018). The AMF has potential use as a biofertilizer and replaces the fertilizer requirements of crop production. Therefore, a reduction in the need for chemical fertilizer takes place. AM plants produce phytochemicals like, carotenoids, flavonoids, etc., that reduce oxidative damages, beneficial for human health (Sbrana et al., 2014).
2. Interactions with other biofertilizers
AM has binary treatments towards soil microflora; with pathogenic soil microflora they act antagonistically, but with plant growth promoting rhizomicrobes (PGPR) they act positively towards a synergistic action benefiting both plant and PGPR, these organisms mostly help mycorrhizae too, are also known as Mycorrhizae Helper organism (MHO)(Pérez-de-Luque et al., 2017;Jie et al., 2015; Raklami et al., 2019). These mycorrhizae invite theseassociated ‘mycorrhiza helper’ bacterial communities living in surrounding mycorrhizosphere, root and spores surface, and extraradical hyphae by cellular signaling mechanism, promote their growth (Xu et al., 2019). In vice versa MHO also help in plant growth by establishing mycorrhizal symbiosis, improving nitrogen fixation, and phosphate solubilisation. Mycorrhiza helpers also can increase spore germination of AM species. PGPR have also been shown to cause better colonization, increase in root mass, sporulation of AMF and in dual inoculation boost up the plant growth (Raklami et al., 2019) and greater viability of mycelia. Some mycorrhiza associate bacteria form siderophores, and antibiotics and release phytohormones improve plant growth and root formation (Rouphael et al., 2015; Vacheron et al., 2013). Mycorrhizal hyphae extract nitrogen and transport it from soil to plant through nitrogen reductase mechanism. But in considerable low N-status of soil this mechanism could not be effective. Dual inoculation with N fixers may be effective substitute for N and P fertilizers. Dual or triple inoculation of AM with N fixer and P solubilizer bacteria was found to increase plant alkaloid, growth, chlorophyll content, N, P, K significantly (Vardafar et al., 2014); while single inoculations of either AM or PGPR is not so effective (Nanjundappa et al., 2019). The combined inoculations in different combinations of PGPR and AM species has been found to increase yield, growth, biomass, growth promoting hormones, soil NPK content and soil sustainability (El Shawah et al., 2021; Nanjundappa et al., 2019; Raklami et al., 2019;) and pathogen resistance (Perez-de-Luque et al., 2020) of different crops in last decades.
3. Hindrances to utilize AMF in agriculture
In conventional agrochemical based agriculture all the above mentioned benefits of AM are beyond utilization as this practice hinders the symbiosis and efficacy of AM. High concentration of major fertilizers, specially phosphate and nitrogen; fungicides and pesticides, intensive tillage, crop rotation with nonmycorrhizal crops hampers AM association, diversity and activity. Hence in agricultural field the diversity and population of AM flora and root colonization is altered and poor compared to adjacent natural soil (Mbuthia et al., 2015). How different agriculture related management impact negatively is represented in graphical figure.
3.1. Impact of fertilizers and doses
The high P concentrations in plant induced by high P-fertilization in soil is found responsible for inhibition of mycorrhizal symbiosis (Balzergue et al., 2013). At high P fertilizer application, plant can up take enough phosphorous without sharing its carbohydrate (García-Caparrós et al., 2021;Kiers et al., 2011; M. Willmann et al., 2013). The P–fertilizer application decreases the supply of soluble carbohydrate in roots; absence of signal carbohydrates reduces the appresoria formation and fresh infection (García-Caparrós et al., 2021; López-Ráez et al., 2017).AM colonization, specially, arbuscle formation and active P transfer to plants is reduced in high P content in soil (Kobae et al., 2016). AM fungi demands carbon source from plant in exchange of phosphate. The existence and activity of AM depends on cooperation of both partners; and plant itself choose the most compatible and efficient strains by partitioning more resources with them (Kiers et al., 2011; Arguello et al., 2016). The plant Fungal Pi: H+ symporter (PT) gene produced in extraradical mycelia, responsible for P uptake (Sawers et al., 2017). PT4 gene trigger symbiotic Pi uptake in low soil P condition and is involved in root architecture responses to low Pi (Volpe et al., 2016). The functional PT genes in both plant and fungi are responsible for arbuscle formation, longevity and P transfer, in high soil P; these are inactivated leading to AM parasitic nature (Gutjahr and Parniske, 2017). In high P content in soil, plant exudates less strignolactone to modulate the symbiosis nature (López-Ráez et al., 2017) and allocate less resource to inefficient AM that are mostly parasitic burden, though plant species vary in their ability to cut off resources (Balzergue et al., 2013).The host plant's P requirement and level of soil available P will also influence the extent of plant response to mycorrhizae. The P use quotient of the plants decreased as the amount of P applied increased, and the P use efficiency index increased at low P levels and decreased at high P levels. The highest mycorrhizal efficiency was observed when the soil contained between 7.8 and 25 mg kg-1 of P. (Balota et al., 2012).
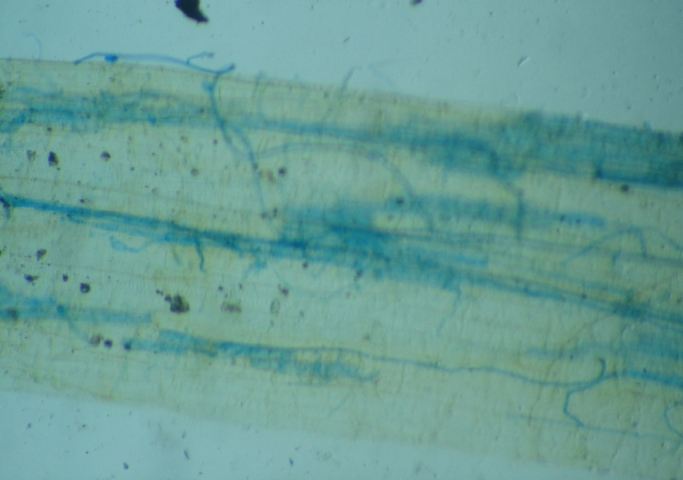
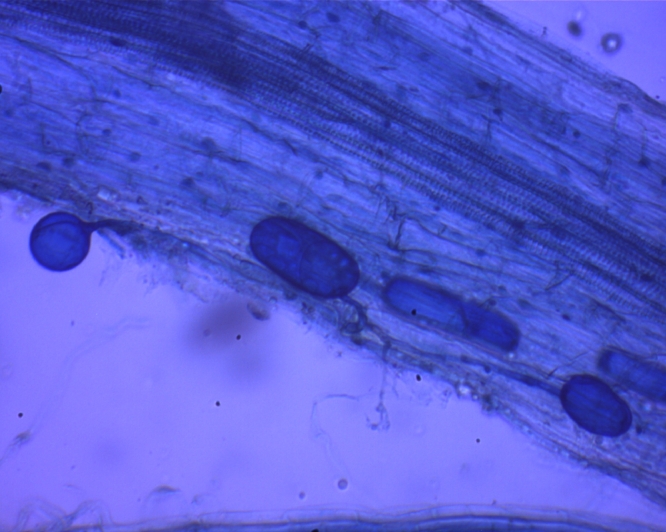
Many nitrogen fertilizers have been reported to decrease colonization in field and pot experiments (Getman-Pickering et al., 2021). Low to medium level increases the AM colonization and sporulation; plant growth and root formation. Higher-level nitrogen fertilizer application reduces AM colonization in plants (Lin et al., 2020). More than optimum potassium concentration, root exudation is decreased and soluble carbohydrates get accumulated in cortex, signaling for AM is hampered. The root of onion in conventional agriculture with mainly mycelial colonization only (Fig. 2).The root of Abelmoschus esculentus in compost based cultivation with agrochemicals is with vesicles and spore (left), though colonization intensity is visibly low(Fig. 3).
Fig. 2.
The root of onion in conventional agriculture with mainly mycelial colonization only.
Fig. 3.
The root of Abelmoschus esculentus in compost based cultivation with agrochemicals is with vesicles and spore (left), though colonization intensity is visibly low.
3.2. Other agrochemicals
The use of agrochemicals is now an essential part of technology dependent modern agriculture. The need is increasing as most high yielding crops are more susceptible to diseases than their wild varieties. Though these agrochemicals have more or less negative effects on soil, environment and human health none like to compromise with crop production. Both systemic and non-systemic fungicides are used to control, leaf, seed and soil borne pathogen; most have detrimental effects on AM spore germination, colonization, extraradical hyphal growth, sporulation (Buysens et al., 2015) and efficacy in P uptake by phosphatase activity (Channabasava et al., 2015; Zocco et al., 2011). Both fungicides effect the growth of AM dependent crops, by blocking the nutrient transfer mechanisms particularly in nutrient deficient dry soil. Supply of phosphate fertilizer at higher rate may help the host to overcome the adverse effect without depending on AMF 9Lanfranco et al., 2018). Organophosphate insecticides and nematicides like chloropeniphos, carbaryl, diazinon, ethoprop, malathione and parathione are generally neutral with little or no effect on mycorrhizal colonization (Amareshappa et al., 2015). Most fungicides are detrimental to AM in field recommend dose, some neutral, some inducing at low dose (Buysens et al., 2015; Rivera-Becerril et al., 2017; Rodriguez-Morelos et al., 2021). AM have the ability of phytoremediation of heavy metals, hence they are capable to withstand in low doses of Cu, Hg containing fungicides; but high doses are detrimental (Hage-Ahmed et al., 2019; Hildebrandt et al., 2007; Jakobsen et al., 2021). In different studies fungicides were found to affect root colonization (Calonne et al., 2012; Helander et al., 2018), spore germination (Buysens et al., 2015), transport of phosphorus from fungus to plant (Zocco et al., 2011), anastomosis formation (de Novais et al., 2019) and sterol biosynthesis pathway (Calonne et al., 2012); significantly correlated with doses.
Azoxystrobin is a systemic fungicide, belongs to the class of methoxyacrylates, worldwide used fungicide against several fungal diseases of many edible crops (Zhang et al., 2019b). Flutolanil is a systemic phenyl benzamide fungicide, used against diseases caused by Basidiomycota in crop plants (Zhao et al., 2019).
Fenpropimorph is a morpholine of broad-spectrum considered as a sterol biosynthesis inhibitor (SBI),active in low concentrations applied to control Blumeria (powdery mildew) and Puccinia (cereal rust) species in cereals (Stenzel and Vors, 2019). Pencycuron is a phenylurea fungicide of contact, which is highly specific to Rhizoctonia solani.
Systemic fungicides AllegianceTM FL, Apron Maxx® RTA®, Vitaflo® 280, Crown ® Trilex® AL, when applied as seed pretreatment in pea and chick pea, restricted mycorrhizal colonization, host growth and P uptake to different levels in absence of disease pressure. In contrast, fungicides Agrox® FL and Thiram 75WP had minimal effects on mycorrhizal colonization, host growth and P uptake. Though sporulation and glomalin production were not significantly affected by fungicides at an early host growth stage, the AMF community structure in host roots was significantly altered in response to Agrox® FL, AllegianceTM FL, Apron Maxx® RTA®, and Trilex® AL as revealed by pyrosequencing-based analysis of fungal 18S rRNA. These results indicate that the suppressive effects of seed applied fungicides on AMF development depend on specific fungicide-AMF interaction (Jin et al., 2013). It exhibits its fungicidal activity by Azoxystrobin was found detrimental for 2 AM fungi Gigaspora sp. MUCL 52,331 and Rhizophagus irregularis at 2 mg L − 1, while fenpropimorph stimulated R. irregularis at low and inhibited at high concentration. Flutolanil and pencycuron did not impact any of the 2 AM fungi (Rodriguez-Morelos et al., 2021). Pencycuron at 0.01 mg L − 1,0.02 and 2 mg L − 1did not impact the extraradical mycelial development or root colonization, but higher conc. affected both colonization and sporulation Buysens et al. (2015). Though soil application of azoxystrobin inhibited root colonization of Glomeraceae members (Vuyyuru et al., 2018), foliar application of azoxystrobin not affect AM root colonization (Campos et al., 2015). Benomyl, Bavistin, Captan and Mancozeb were tested on association of R. fasciculatus with Panicum miliaceum L.The results of this study showed significant (P ≤ 0.05) higher AM colonization, spore density, plant growth and grain yield treated with Captan compared to other fungicides and untreated controls. Benomyl showed most adverse effect in all parameters measured in inoculated plant (Channabasava et al., 2015).
3.3. Tillage
Tillage may physically crash the AM spores and soil disturbances destroy the hyphal network in soil, which in turn reduces the root colonization (Brito et al., 2012) Disruption of colonised root fragments and hyphal networks reduce the volume of extractable soil by AM. The disruption of extra – radical mycelia hampers the AM mediated P–transfer to plants especially in tillage in early season (Säle et al., 2015). The shifting of soil layer also changes the existing suitable conditions for AMF species. Tillage systems move the surface applied fertilizer and weed plant residue downward the top soil enhancing decomposition and release of nutrients. In contrast, in no tillage and reduced tillage, slow rate of decomposition rate (Brennan et al., 2013). Tillage may also add soil porosity that enhances microbial activity (Navarro-Noya et al., 2014). But reduced tillage was noticed to improve soil aggregation, increase the amount of soil organic carbon (SOC) in the surface layer, moisture content and reduce erosion (Wang et al., 2018). Percentage of macro-aggregates (0.25–2 mm) (Qin et al., 2017), SOCs in small macro-aggregates and micro-aggregates and SOC in bulk soil were positively related with the percentage and biomass of soil AM fungi (Lu et al., 2018). Conservation tillage stimulates AMF colonization to a greater extent (Higo et al., 2020). The increased soil microbial communities and increased AM diversity and richness (Lu et al., 2018; Ohl and Koch, 2018) can play important roles in soil aggregation, soil carbon sequestration, and soil nutrition; water use efficiencies; and influence crop yields (Palm et al., 2014).In early colonization stage, the direct effects of the conventional tillage systems are related to physical disruption of the extra radical mycelium network resulting AM activity in nutrient and water uptake and glomalin related soil aggregate formation (Brito et al., 2012) and bioprotection against soil pathogens (Patanita et al., 2020). However, long-term no-till treatment could decrease the soil AM fungal propagules because of the higher soil bulk density, and the lower C utilization efficiency of soil organisms (Schluter et al., 2018). The differences in soil properties, climatic conditions and the duration of no-till treatment also matters.
3.4. Other agriculture practices
Long fallow and crop rotation with non-mycorrhizal crops affect severely on AMF community, propagule density and activity. In crop rotations, colonised root and hyphae are important source of inoculum for the next crop (Brito et al., 2012; Muneeret al., 2020). Hence, fallow period, inclusion of non-host species affect the AMF propagule density and abundance (Higo et al., 2015), which adversely affect successive crop yield. In paddy cultivation, waterlogged soil hinders AM activity, as AM cannot grow in wet soil (Vallino et al., 2014), but after tillage AM may be applied; for upland paddy no such problem.
3.5. AM inocula preparation, host-strain, soil- strain compatibility
Preparation of AM inocula, specially, with effective species or strain and isolation of that is tiresome and time consuming. Unlike other microbial biofertilizer, they are not easily isolated in culture media or mass cultured for large-scale production within a short period. Being obligate symbiont, AM need host to grow in soil, hydroponics or aeroponics. All the process needs well equipped laboratory and glasshouse and skilled person and knowledge to avoid contamination. In developing countries there are surely limitations. But beyond this, differential efficacy of AM with crops (Higo et al., 2015), cultivars (Chu et al., 2013), soil type (Aguilera et al., 2014, 2017) with/and AM strains (Cruz-Paredes et al., 2017) persists. AM though has no such specificity for host, preference for host and soil condition matters (Kim et al., 2017). During application this matches are also needful to avail better production. Hence, after a century's extensive research, we are still to prepare a checklist of AM strain-crop/cultivar-soil utilize the datasets for benefit by motivating farmers and least organizations in some countries are interested in the hard-work to serve large scale production with application guidance.
4. Way out to utilize AM in agriculture
4.1. Fertilizer maintenance
Application of slow releasing fertilizer have an inducing effect on AM colonization and activity i.e. rock phosphate as easy but unconventional alternative source of phosphate to inorganic P fertilizer, can promote mycorrhizal growth and activity (Bender et al., 2015; Thirkell et al., 2017). Growth of AM colonized plants with rock phosphate was higher than non-AM plants with double dose of super phosphate. Low to medium level of nitrogen increases the AM colonization and sporulation; plant growth and root formation. Nitrogen supply at initial stage, sometimes offer a potential benefit in establishment of mycorrhizae (Getman-Pickering et al., 2021).
AM work better even reduced fertilizer doses uses (Jarosz et al., 2021). Zoe et al. (2021) found that low to moderate dose of fertilizer application, specially, organic fertilizer compared to inorganic, increased AM mediated plant growth and biocontrol ability. Even without mycorrhizal application biomass decreased under increasing P supply, while in low P application induced root branching to procure soil more P (García-Caparrós et al., 2021). Mycorrhizae mediated biomass increase is evident at low levels of fertilization and at high levels of fertilization biomass is decreased. Mycorrhizae also increase resistance to herbivores at medium levels of fertilization, but no effect to low and high levels of fertilization was noticed. Mycorrhizae improved resistance most strongly when plants were fertilized with a phosphorus rich organically derived fertilizer (Zoe et al., 2021). Besides increasing the absorptive surface and procure more nutrients and phosphates by hyphal network physically, mycorrhizae adopt some biochemical processes that involve to dissolve of insoluble phosphates and primary minerals by organic acids and mineralize P from organic sources directly by release of acid phosphatase (Sato et al., 2015). Some species can also hydrolyze organic P compounds (Li et al., 2020). Mycorrhizae stimulate bacteria that live in the mycorrhizosphere by sharing some of the photosynthate oozed by the plant (Azcón-Aguilar and Barea, 2015). Mycorrhizae may act in a consortium with other rhizospheric microorganisms (Schneider et al., 2019) and increase phosphate mineralization with help of those bacteria (Battini et al., 2017). These consortia may influence the mobilization of both inorganic and organic P into the soil solution P pool. Some modifications in conventional agricultural management practices towards integrated eco-friendly approach, such as avoiding over-fertilization, applying beneficial soil microorganisms and mycorrhizae helper bacteria which solubilise and enhance P uptake can move us toward more efficient P use, even lessen P toxicity in soil and P leaching to water table (Battini et al., 2017).
4.2. Conserve tillage
Conservation agriculture practices involving minimal soil disturbances and retention of crop residue (>30%) is being increasingly practiced worldwide, and recognized to enhance soil health by optimizing key soil attributes. Conservation tillage and application of organic manure can protect survivability and inoculation, improving soil aggregation and P uptake (Bottinelli et al., 2017; Wilkes et al., 2021). Tillage had no significant effect (P > 0.05) on crop yields after four crop cycles (Somasundaram et al., 2018).
4.3. Choice of crop, cultivar, cover crop, rotation
Choosing of highly mycotropic crops/ cultivars and crops with root architecture efficient in accessing sufficient P and forming active symbiosis with AMF is needed (Berruti et al., 2016). Avoiding crop rotation by nonmycorrhizal families as Brassicaceae; Amaranthace or Brassicaceae family releasing fungistatic compounds in soil, or growing mycorrhizal cover crops after these crops before next cropping may be beneficial (Karasawa et al., 2012; Njeru et al., 2014). It has been suggested that domestication may have decreased the ability of plants to respond positively to AMF in high soil P. Martinz-Robles et al. (2018) found both wild and domesticated species of 27 crops were benefited similarly by AMF at low Pi conditions. At high P conditions, response of 14 pairs of wild varieties to AMF was not differed much, whereas it strongly reduced growth in domesticated species. Hence, it is evident that, domesticated crop able to avail mycorrhizal benefit in low soil P concentration only.
Though AM fungi have no specificity for host, host preference till present (Bainard et al., 2014; Torrecillas et al., 2012; Higo et al., 2016), that also vary with, geographical distribution and land use. Though contrasting, the AM flora in different host may response similarly to soil Phosphorous gradient. Crop rotation may influence AM function positively with a highly mycotrophic crop of Fabaceae or Poaceae family, though AM species composition may vary with plant species and may take some time to be replaced (Higo et al., 2010, 2015), next mycotropic crop surely avails benefit (Isobe et al., 2014) with reduced requirement of phosphorus application; than fallow land without vegetation (Jemo et al., 2014).
4.4. AM inocula- host –soil compatibality
The native flora of AMF of upland ecosystem has been found to be very efficient and responsive to upland rice. Crop rotation with rice was found to uplift soil P content and native AM inocula (Maiti et al., 2012). Though AMF are not able to survive in wetland habitat generally, practice of application of AMF is negligible in lowland rice cultivation, but some species as Glomus etunicatum, G. mosseae and G. intraradices were found to perform very well, increasing P uptake and root colonization under flooded condition both in high- and low-fertility soil, promoting the nutrient acquisition in rice and increase yield (Watanarojanaporn et al., 2013). Glomus intraradices enhanced growth response, photosynthetic efficiency, and antioxidative responses of rice plant in drought stress also (Ruı´z-Sa´nchez et al., 2010).
Host-fungi compatibility is also a factor, the intraspecific strain diversity impact on efficiency of AM; differential responses of host cultivars to AM also exist. There are plenty variations of cultivars genotypes showing differential behavior with environments (Chu et al., 2013). The molecular mechanisms of plant determining fungal performance are almost unknown and may be related to the amount of carbohydrates and lipids shared. It was found the expression pattern of monosaccharide transporter genes from the AMF, Rhizophagus irregularis in intraradical vs. extraradical hyphae depended on the host plant (Ait Lahmidi et al., 2016); amount of Pi uptake. Biomass gain was also correlated to exraradical mycelial mass indicating a complex genotype–environment interaction (Sawers et al. (2017). Some studies on molecular diversity in roots have shown differences between AM community composition associated to wheat and N-fixing crops (Bainard et al., 2014; Higo et al., 2016). AM community composition associated to wheat also varied with the growing season, P fluxes and degree of fertilization (Bainard et al., 2014; Qin et al., 2015). Dominant AM species varied in conventional (Funneliformis spp.) and organic systems (Claroideoglomus spp.) also (Dai et al., 2014); indicating variation of AM efficiency with fertilizers, specially P (Cruz-Paredes et al., 2017).
Soil-AM strain compatibility is also a major criterion for effective AM application, variation of soil conditions also determine the effective AM species for same crop (Ricardo et al., 2011). Aguilera et al. (2014, 2017) found Acaulospora and Scutellospora are the dominant genera by analyzing spore morphology in an acidic soils under continuous wheat cropping, while, Castillo et al. (2016a) found a prevalence of Acaulospora and Claroideoglomus in acidic soils. Hence, application of just some AM inocula in any soil for any crop may not work from which we generally conclude about efficacy of AM inocula. The growth response of a single host crop species may differ with different AM fungal species, and similarly, the same AM fungal isolate can result in different growth responses with different plant species or cultivars or genotypes (Castillo et al., 2016b). Soil conditions were found to control the native AM dominance and affectivity in rhizosphere as noticed in rice field in Ghana by Rhizophagus and Glomus or Scutellospora and Acaulospora (Sarkodee-Addo et al., 2020).
4.5. Disease control measures
From previous chapter, it is noted that Captan, Flutolanil and Pencycuron have very low impact on AMF Buysens et al. (2015). Though soil application of Azoxystrobin inhibited root colonization of Glomeraceae members, foliar application is safe (Vuyyuru et al., 2018). Alternative use of similar less harmful fungicides or low doses may be practiced. Some fungicides have positive effect on infection and sporulation of AM fungi. Most pesticides or herbicides have neutral, near neutral or positive effect on AM at reduced doses which may be useful in inocula production too (Hage-Ahmed et al., 2019; which is surely a facility to utilize. Actually in AM species community if less difference is noticed in conventional and organic or low input agricultural practices, but efficacy differs, and G.claroideum largely adjusted species found by Vestberg et al. (2011).
Use of biological or integrated disease control, reducing use of fungicides and pesticides, specially, those negatively affect AM, adopt to use neutral or inducing pesticides, lower doses, foliar spray should be effective. In past years, AMF have been reported to decrease the incidence of several fungal pathogens in primary agriculture crops like potato (Jung et al., 2012); against Fusarium oxysporum (Jie et al., 2015) and many other pathogens. Inoculation with Rhizophagus irregularis in potato plants reduced symptoms of potato virus Y (PVY) (Maffei et al., 2014). Application of biocontrol microorganisms including plant growth-promoting bacteria (e.g., Pseudomonas and Bacillus spp.) with AMF can boost up the partial resistance to pathogens by several mechanisms such as induced systemic resistance (ISR) and mycorrhiza-induced resistance (MIR), respectively (Cameron et al., 2013).
From these discussion its evident though specific consortia preparation, maintenance and applications for different requirements in large scale demands some external assistance and administration facility; and farmers awareness to step out is also necessary, but logically AM benefit may be fully and easily availed in agriculture field by following several protocols of – (1) Limiting NPK, specially P fertilization, or use slowly releasing P substitute (2) Using organic manure and integrated control mechanism (3) Using reduced doses of fungicides and non-affecting neutral or inducing fungicides (4) Limiting tillage or application inocula after tillage (5) Avoid fallowing lands (6) Application of suitable consortia of AM along with MHO. The effects also vary with timing of application of fertilizer or fungicides. Most pesticides or herbicides have neutral, near neutral or positive effect on AM at reduced doses (Hage-Ahmed et al., 2019; Qi et al., 2020), which is surely a facility to utilize. AM strains from low phosphate zone are more sensitive to high P–fertilization or P-rich agricultural land and become pathogenic or parasite. Hence, in order to avoid the negative effect and avail the mycorrhizal benefit for any crop, the plant available P and P-fixing capacity of soil is to be studied. Data on mycorrhizal dependency of the crop at different P-level could certainly provide extra facility to decide level of phosphate suitable of availing maximum mycorrhizal benefit.
Then to avail full benefit of AM, we need to switch off from conventional high chemical input agriculture to organic or integrated farming based culture as Permaculture or sustainable dairy cropping systems (SDCS), that uses soil testing to recommend the appropriate fertilizer dose with attempt to synchronize nutrient availability and demand of crop. Dairy processing sludge is also a good replacement for nitrogen and phosphorus (Ashekuzzaman et al., 2020). To reduce the use of synthetic pesticides, the integrated pest management (IPM) is adopted in these means. SDCS include diverse crop rotations to increase natural biological control. Maintenance of cover crop is beneficial to add nutrients and enhance biofertilizer and biocontrol population for successive crops (Schipanski et al., 2013). Maintaining a highly mycotropic cover crop may actually reduce the allelopathic effects of mustards on next crop and AMF is important to maintain AMF populations (Barto et al., 2010). The system is almost entirely no‐till which is less disruptive to natural enemy populations. Organic agriculture with mycorrhizae-microbial consortia gradually suppresses pathogens by bio-control (Lu et al., 2018; Nepomuceno et al., 2019). Actually in a nutshell, AM is a complete package if we can use it properly and simply providing its above mentioned working environment. The production of AM is not as simple as other biofertilizers surely but can sustain prolonged in integrated cropping system or permaculture (Symanczik et al., 2017) or agro-silviculture (Dierks et al., 2021). Mixed native inocula from highly mycotropic graminaceous/ fabaceae plant rhizospere soil mass directly or permaculture soil or rhizosperic soil of same plant may be used as low cost non-labbased effective inocula (Symanczik et al., 2017). Biofertilizer production bodies may guide for inocula for crop, soil and application procedures. But it should be keep in mind introduced AMF species could alter existing AMF communities by decrease in diversity (Koch et al., 2011) and functionality (Symanczik et al., 2015) of native AMF. Details survey and knowledge regarding the soil condition, environment parameter, mycorrhizal dependency of crops to be cultivated, effective local strains for the soil conditions is required along with skilled personnel and well equipped laboratory. Deficiency in later in developing country may be mitigated if government or private organizations could supply crop/soil/environment friendly inocula or native mixed inocula. In India and abroad already enough researches are undertaken with the data available. Now in India and abroad, packaged AM inocula as biofertilizer are available, with some effective stains with wide ecological amplitude or tolerance. It is experimented in Kerala, India long ago and showed that the difference of production quantity with conventional practice gradually abolished in transitional period (van der Werf and de Jager, 1992). Ultimately for adopting AMF technology in agriculture, to change the way of thinking, awareness in grass root level is necessary and intention to move towards sustainable production and patience to compromise the reduced yield in transitional period. A comprehensive focus with challenge of overcoming the low productive switching period may reach the change to a sustainable agriculture.
5. Conclusion
From this discussion and evidences it is prominent that AM fungi function not merely as biofertilizer, – substitute of N, P and trace elements; efficacy as bioprotector from biotic and abiotic stresses and maintaining sustainability of soil and ecosystem are parts of its extended activity. In dry, drought affected nutrient poor soils they are most active, functioning synergistically with ‘plant growth promoting rhizospheric microorganisms’ ‘which act also as ‘mycorrhizae helper organism’. Along with these PGPRs suitable AM strain for the particular soil and crop may be successful to cater plant needs for nutrient, water and substitute of agrochemicals. In organic and integrated farming they may be utilized as essential part in boost of yield and maintaining soil and consumer's health, if properly used. To avail the benefit most, we just need the knowledge of detrimental and inducing effects and doses of various agrochemicals e.g. fertilizers, fungicides, pesticides, the application time and other agricultural process, as crop rotation, tillage. Information regarding active AM species/strain for different crop/cultivar/soil/environment and synergistic behavior with other plant growth promoting microbes in soil sustainability and disease prevention also vital to avail maximum benefit of AM as a tool for sustainable agriculture. But all data are available since half century before regarding AM species compatibility with different crops and soil, AM inducing and reducing agrochemicals etc. Just shifting to a careful use and handling of these, we can shift to a low cost profitable and sustainable agriculture system with less health hazards without compromising the production in future. Moreover, for marginal farmer a little help with the inocula or consortia and guidance may be great help to reduce cost benefit ratio. In shifting cultivation, specially, in slow decomposed soil; wasteland or fallow land utilized for agriculture the technology would be more effective. In conservation of indigenous seeds the technology would be beneficial than conventional agriculture. The ultimate need is to assimilate the above discussed interacting data regarding AM, provide suitable inocula /consortia for specific soil, crop and guidance regarding related agro practices. Some effective AM fungi are being utilized for broad range application, some are soil type favoring, yet the local strains may be more effective and promising, specially, in developing countries, where skilled personnel and sophisticated laboratory is a limiting factor, soil based mixed native inocula may be utilized. Moreover patience for a transition period to switch over AM mediated sustainable production is necessary.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:No
References
- Aguilera P., Conejos P., Bore F., Barea J.M., von Baer E., Oehl F. Diversity of arbuscular mycorrhizal fungi associated with Triticumaestivum L. plants growing in an Andosol with high aluminum level. Agric. Ecosyst. Environ. 2014;186:178–184. doi: 10.1016/j.agee.2014.01.029. [DOI] [Google Scholar]
- Aguilera P., Cumming J., Oehl F., Cornejo P., Borie F. In: Aluminum Stress Adaptation in Plants. Panda S.K., Balusˇka F., editors. Springer International Publishing; Switzerland: 2015. Diversity of arbuscular mycorrhizal fungi in acidic soils and their contribution to aluminum phytotoxicity alleviation; pp. 203–228. [DOI] [Google Scholar]
- Aguilera P., Marín C., Oehl F., Godoy R., Borie F., Cornejo P. Selection of aluminum tolerant cereal genotypes strongly influences the arbuscular mycorrhizal fungal communities in an acidic Andosol. Agric. Ecosyst. Environ. 2017;246:86–93. 10.1016/j.agee.2017.05.031. [Google Scholar]
- Ait Lahmidi N., Courty P.E., Brulé D., Chatagnier O., Arnould C., Doidy J., Berta G., Lingua G., Wipf D., Bonneau L. Sugar exchanges in arbuscular mycorrhiza: riMST5 and RiMST6, two novel Rhizophagusirregularis monosaccharide transporters, are involved in both sugar uptake from the soil and from the plant partner. Plant PhysiolBiochem. 2016;107:354–363. doi: 10.1016/j.plaphy.2016.06.023. 10.1016/j.plaphy.2016.06.023. [DOI] [PubMed] [Google Scholar]
- Al-Karaki G.N. In: Developments in Soil Classification, Land Use Planning and Policy Implications: Innovative Thinking of Soil Inventory For Land Use Planning and Management of Land Resources.Springer Science Business Media. Shahid S.A., Taha F.K., Abdelfattah M.A., editors. pp.; Dordrecht: 2013. The role of mycorrhiza in the reclamation of degraded lands in arid environments; pp. 823–836. [Google Scholar]
- Alori E.T., Babalola O.O. Microbial inoculants for improving crop quality and human health in. Africa. Front Microbiol. 2018;9:2213. doi: 10.3389/fmicb.2018.02213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amareshappa D., Channabasava R., Lakshman H.C., Jorquera M. Effect of fungicides on association of arbuscular mycorrhiza fungus Rhizophagusfasciculatus and growth of Proso millet (Panicummiliaceum L.) J. Soil Sci. Plant Nutr. 2015;15:35–45. doi: 10.4067/S0718-95162015005000004. [DOI] [Google Scholar]
- Andres C., Bhullar G.S. Sustainable intensification of tropical agro-ecosystems: need and potentials. Front. Environ. Sci. 2016;4:5. doi: 10.3389/fenvs.2016.00005. [DOI] [Google Scholar]
- Argüello A., O'Brien M.J., van der Heijden M.G., Wiemken A., Schmid B., Niklaus P.A. Options of partners improve carbon for phosphorus trade in the arbuscular mycorrhizal mutualism. Ecol. Lett. 2016;19(6):648–656. doi: 10.1111/ele.12601. [DOI] [PubMed] [Google Scholar]
- Augé R.M., Toler H.D., Saxton A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza. 2015;25:13–24. doi: 10.1007/s00572-014-0585-4. 10.1007/s00572-014-0585-4. [DOI] [PubMed] [Google Scholar]
- Azcón-Aguilar C., Barea J.M. Nutrient cycling in the mycorrhizosphere. J. Soil Sci. Plant Nutr. 2015;15(2):372–396. doi: 10.4067/S0718-95162015005000035. [DOI] [Google Scholar]
- Bainard L.D., Bainard J.D., Hamel C., Gan Y. Spatial and temporal structuring of arbuscular mycorrhizal communities is differentially influenced by abiotic factors and host crop in a semi-arid prairie agroecosystem. FEMS Microbiol. Ecol. 2014;88:333–344. doi: 10.1111/1574-6941.12300. [DOI] [PubMed] [Google Scholar]
- Balota E.L., Machineski O., Scherer A. Mycorrhizal effectiveness on physic nut as influenced by phosphate fertilization levels. Rev. Bras. Ciênc. Solo. 2012;36(1):23–32. 10.1590/S0100-06832012000100003. [Google Scholar]
- Balzergue C., Chabaud M., Barker D.G., Bécard G., Rochange S.F. High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spiking responses to the fungus. Front. Plant Sc. 2013;4:426. doi: 10.3389/fpls.2013.00426. 10.3389/fpls.2013.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M.V., Pereira E.A., Cury J.C., Carneiro M.A.C. Occurrence of arbuscular mycorrhizal fungi on King George Island, South Shetland Islands, Antarctica. An. Acad. Bras. Cienc. 2017;89(3):1737–1743. doi: 10.1590/0001-3765201720170119. 10.1590/0001-3765201720170119. [DOI] [PubMed] [Google Scholar]
- Bardgett R.D., van der Putten W.H. Belowground biodiversity and ecosystem functioning. Nature. 2014;515:505–511. doi: 10.1038/nature13855. [DOI] [PubMed] [Google Scholar]
- Barto K., Friese C., Cipollini D. Arbuscular mycorrhizal fungi protect a native plant from allelopathic effects of an invader. J. Chem. Ecol. 2010;36:351–360. doi: 10.1007/s10886-010-9768-4. [DOI] [PubMed] [Google Scholar]
- Baslam M., Esteban R., García-Plazaola J.I., Goicoechea N. Effectiveness of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of major carotenoids, chlorophylls and tocopherol in green and red leaf lettuces. Appl. Microbiol. Biotechnol. 2013;97(7):3119–3128. doi: 10.1007/s00253-012-4526-x. [DOI] [PubMed] [Google Scholar]
- Baslam M., Garmendia I., Goicoechea N. Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse grown lettuce. J. Agric. Food Chem. 2011;59:5504–5515. doi: 10.1021/jf200501c. [DOI] [PubMed] [Google Scholar]
- Battini F., Grønlund M., Agnolucci M., Jakobsen I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017;7:4686. doi: 10.1038/s41598-017-04959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum C., El-Tohamy W., Gruda N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: a review. Sci. Hortic. Amsterdam. 2015;187:131–141. [Google Scholar]
- Begum N., Qin C., Ahanger M.A., Raza S., Khan M.I., Ashraf M., Ahmed N., Zhang L. Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front. Plant Sci. 2019;10:1068. doi: 10.3389/fpls.2019.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender S.F., van der Heijden M.G.A. Soil biota enhance agricultural sustainability by improving crop yield, nutrient uptake and reducing nitrogen leaching losses. J. Appl. Ecol. 2015;52:228–239. doi: 10.1111/1365-2664.12351. [DOI] [Google Scholar]
- Berruti A., Lumini E., Balestrini R., Bianciotto V. Arbuscular mycorrhizal fungi as natural biofertilizers: let's benefit from past successes. Front. Microbiol. 2016;6:1559. doi: 10.3389/fmicb.2015.01559. 10.3389/fmicb.2015.01559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterlich M., Franken P., Graefe J. Arbuscular mycorrhiza improves substrate hydraulic conductivity in the plant available moisture range under root growth exclusion. Front. Plant Sci. 2018;9:301. doi: 10.3389/fpls.2018.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe H., Regvar M., Turnau K. Springer; Heidelberg, Berlin: 2010. Arbuscular mycorrhiza, Heavy metal, and Salt tolerance.In: Soil Heavy Metals; pp. 87–111. [Google Scholar]
- Bottinelli N., Angers D.A., Hallaire V., Michot D., Le Guillou C., Cluzeau D., Heddadj D., Menasseri-Aubry S. Tillage and fertilization practices affect soil aggregate stabilityin a HumicCambisol of Northwest France. Soil Tillage Res. 2017;170:14–17. doi: 10.1016/j.still.2017.02.008. [DOI] [Google Scholar]
- Brito I., Goss M.J., de Carvalho M. Effect of tillage and crop on arbuscular mycorrhiza colonization of winter wheat and triticale under Mediterranean conditions. Soil Use Manag. 2012;28:202–208. doi: 10.1111/j.1475-2743.2012.00404.x. [DOI] [Google Scholar]
- Bunn R.A., Simpson D.T., Bullington L.S., Lekberg Y., Janos D.P. Revisiting the ‘direct mineral cycling’ hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? ISME J. 2019;13:1891–1898. doi: 10.1038/s41396-019-0403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysens C., Dupré de Boulois H., Declerck S. Do fungicides used to controlRhizoctoniasolaniimpact the non-target arbuscular mycorrhizal fungus Rhizophagusirregularis? Mycorrhiza. 2015;25:277–288. doi: 10.1007/s00572-014-0610-7. 10.1007/s00572-014-0610-7. [DOI] [PubMed] [Google Scholar]
- Cameron D.D., Neal A.L., van Wees S.C., Ton J. Mycorrhizainducedresistance: more than the sum of its parts? Trends Plant Sci. 2013;18:539–545. doi: 10.1016/j.tplants.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos A.A.B., Scotton J.C., Costa W.L.F., Giassi V., Pinto D.F.P., Homma S.K. Seleção de fungicidasvisando à preservação de fungosmicorrízicosarbuscularesnativos no cultivo do feijoeiro. Rev. Bras. Eng.Agríc. Ambient. 2015;19:898–902. 10.1590/1807-1929/agriambi.v19n9p898-902. [Google Scholar]
- Cao Y., Wu X., Zhukova A., Tang Z., Weng Y., Li Z., Yang Y. Arbuscular mycorrhizal fungi (AMF) species and abundance exhibit different effects on saline-alkaline tolerance in Leymus chinensis. J. Plant Inter. 2020;15(1):266–279. doi: 10.1080/17429145.2020.1802524. [DOI] [Google Scholar]
- Casieri L., AitLahmidi N., Doidy J., Veneault-Fourrey C., Migeon A., Bonneau L., Courty P.E. Biotrophictransportome in mutualistic plant-fungal interactions. Mycorrhiza. 2013;23:597–625. doi: 10.1007/s00572-013-0496-9. [DOI] [PubMed] [Google Scholar]
- Castillo C.G., Borie F., Oehl F., Sieverding E. Arbuscular mycorrhizal fungi biodiversity: prospecting in Southern-Central zone of Chile. a review. J. Soil Sci. Plant Nutr. 2016;16:400–422. 10.4067/S0718-95162016005000036. [Google Scholar]
- Castillo C.G., Oehl F., Sieverding E. Arbuscular mycorrhizal fungal diversity in wheat agro-ecosystems in Southern Chile and effects of seed treatment with natural products. J. Soil Sci. Plant Nutr. 2016;16:967–978. 10.4067/S0718-95162016005000069. [Google Scholar]
- Cavagnaro T.R. Impacts of compost application on the formation and functioning of arbuscular mycorrhizas. Soil Biol. Biochem. 2014;78:38–44. [Google Scholar]
- Cavagnaro T.R., Bender S.F., Asghari H.R., van der Heijden M.G.A. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015;20(5):283–290. doi: 10.1016/j.tplants.2015.03.004. 10.1016/j.tplants.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Channabasava A., Lakshman H.C., Jorquera M.A. Effect of fungicides on association of arbuscular mycorrhiza fungus Rhizophagusfasciculatus and growth of Proso millet (Panicummiliaceum L.) J. Soil Sci. Plant Nutr. 2015;15(1):35–45. 10.4067/S0718-95162015005000004. [Google Scholar]
- Chen M., Arato M., Borghi L., Nouri E., Reinhardt D. Beneficial services of arbuscular mycorrhizal fungi – from ecology to application. Front. Plant Sci. 2018;9:1270. doi: 10.3389/fpls.2018.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C.H., Paszkowski U. Mechanisms and impact of symbiotic phosphate acquisition. Cold Spring Harb. Perspect. Biol. 2019;11 doi: 10.1101/cshperspect.a034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Q., Wang X., Yang Y., Chen F., Zhang F., Feng G. Mycorrhizal responsiveness of maize (Zea mays L.) genotypes as related to releasing date and available P content in soil. Mycorrhiza. 2013;23:497–505. doi: 10.1007/s00572-013-0492-0. 10.1007/s00572-013-0492-0. [DOI] [PubMed] [Google Scholar]
- Coelho L.C.S., Mignoni D.S.B., Silva F.S.B., Braga M.R. Seed exudates of Sesbania virgata (Cav.) Pers. stimulate the asymbiotic phase of the arbuscular mycorrhizal fungus Gigaspora albida Becker & Hall. Hoehnea. 2019;46 doi: 10.1590/2236-8906-27/2018. [DOI] [Google Scholar]
- Cruz-Paredes C., López-García Á., Rubæk G.H., Hovmand M.F., Sørensen P., Kjøller R. Risk assessment of replacing conventional P fertilizers with biomass ash: residual effects on plant yield, nutrition, cadmium accumulation and mycorrhizal status. Sci. Total Environ. 2017;575:1168–1176. doi: 10.1016/j.scitotenv.2016.09.194. 10.1016/j.scitotenv.2016.09.194. [DOI] [PubMed] [Google Scholar]
- Dai M., Hamel C., Bainard L.D., Arnaud M.S., Grant C.A., Lupwayi N.Z., et al. Negative and positive contributions of arbuscular mycorrhizal fungal taxa to wheat production and nutrient uptake efficiency in organic and conventional systems in the Canadian prairie. Soil Biol. Biochem. 2014;74:156–166. 10.1016/j.soilbio.2014.03.016. [Google Scholar]
- Davison J., Moora M., Öpik M., Adholeya A., Ainsaar L., Bâ A., Burla S., et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science. 2015;349(6251):970–973. doi: 10.1126/science.aab1161. [DOI] [PubMed] [Google Scholar]
- de Novais C.B., Giovannetti M., De Faria S.M., Sbrana C. Two herbicides, two fungicides and spore-associated bacteria affect Funneliformismosseaeextraradical mycelium structural traits and viability. Mycorrhiza. 2019;29:341–349. doi: 10.1007/s00572-019-00901-6. [DOI] [PubMed] [Google Scholar]
- Dierksa J., Blaser-Harta W.J., Gamperb H.A., Nyokac I.B., Barriosd E., Sixadoi J. Trees enhance abundance of arbuscular mycorrhizal fungi, soil structure, and nutrient retention in low-input maize cropping systems Agriculture. Ecosyst. Environ. 2021;318 doi: 10.1016/j.agee.2021.107487. [DOI] [Google Scholar]
- Elbon A., Whalen J.K. Phosphorus supply to vegetable crops from arbuscular mycorrhizal fungi: a review. Biol. Agric. Hortic. 2015;31:73–90. [Google Scholar]
- El-Sawah A.M., El-Keblawy A., Ali D.F.I., Ibrahim H.M., El-Sheikh M.A., Sharma A., AlhajHamoud Y., Shaghaleh H., Brestic M., Skalicky M., et al. Arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria enhance soil key enzymes, plant growth, seed yield, and qualitative attributes of guar. Agriculture. 2021;11(3):194. 10.3390/agriculture11030194. [Google Scholar]
- Etesami H., Jeong B.R., Glick B.R. Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to P Uptake by plant. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.699618. 699618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) Crop prospects and food situation – quarterly global report No. 1. Rome. 2020 doi: 10.4060/ca8032en. [DOI] [Google Scholar]
- Forgy D. Arbuscular mycorrhizal fungi can benefit heavy metal tolerance and phytoremediation. J. Nat.Resour. Life Sci. Educ. 2012;41:23–26. [Google Scholar]
- García-Caparrós P., Lao M.T., Preciado-Rangel P., Sanchez E. Phosphorus and carbohydrate metabolism in green bean plants subjected to increasing phosphorus concentration in the nutrient solution. Agronomy. 2021;11(2):245. doi: 10.3390/agronomy11020245. [DOI] [Google Scholar]
- Garnett T., Appleby M.C., Balmford A., Bateman I.J., Benton T.G., Bloomer P. Sustainable intensification in agriculture: premises and policies. Science. 2013;341:33–34. doi: 10.1126/science.1234485. [DOI] [PubMed] [Google Scholar]
- Getman-Pickering Z.L., Stack G.M., Thaler J.S. Fertilizer quantity and type alter mycorrhizae-conferred growth and resistance to herbivores. J. Appl. Ecol. 2021;58:931–940. doi: 10.1111/1365-2664.13833. [DOI] [Google Scholar]
- Giovannini L., Palla M., Agnolucci M., Avio L., Sbrana C., Turrini A., Giovannetti M. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: research strategies for the selection of the best performing Inocula. Agronomy. 2020;10(1):106. doi: 10.3390/agronomy10010106. [DOI] [Google Scholar]
- Godfray H.C.J., Garnett T. Food security and sustainable intensification. Philos. Trans. R. Soc. Lond. (B) 2014;369:101–120. doi: 10.1098/rstb.2012.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassini P., Eskridge K.M., Cassman K.G. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat. Commun. 2013;4:2918. doi: 10.1038/ncomms3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GSDR (Global Sustainable Development Report) 2019. The future is now – science for achieving sustainable development, independent group of scientists appointed by the secretary-general, United Nations, New York. https://sustainabledevelopment.un.org/content/documents/24797GSDR_report_2019.pdf.
- Gutjahr C., Parniske M. Cell biology: control of partner lifetime in a plant–fungus relationship. Curr. Biol. 2017;27:420–423. doi: 10.1016/j.cub.2017.04.020. 10.1016/j.cub.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Gutjahr C., Paszkowski U. Multiple control levels of root system remodeling in arbuscular mycorrhizal symbiosis. Plant Sci. 2013;4:204. doi: 10.3389/fpls.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage-Ahmed K., Rosner K., Steinkellner S. Arbuscular mycorrhizal fungi and their response to pesticides. Pest Manag. Sci. 2019;75(3):583–590. doi: 10.1002/ps.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajiboland R., Dashtebani F., Aliasgharzad N. Physiological responses of halophytic C4 grass, Aeluropuslittoralis to salinity and arbuscular mycorrhizal fungi colonization. Photosynthetica. 2015;53(4):572–584. doi: 10.1007/s11099-015-0131-4. [DOI] [Google Scholar]
- Hart M., Ehret D.L., Krumbein A., Leung C., Murch S., Turi C. Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza. 2015;25:359–376. doi: 10.1007/s00572-014-0617-0. [DOI] [PubMed] [Google Scholar]
- Helander M., Saloniemi I., Omacini M., Druille M., Salminen J.P., Saikkonen K. Glyphosate decreases mycorrhizal colonization and affects plant-soil feedback. Sci. Total Environ. 2018;642:285–291. doi: 10.1016/j.scitotenv.2018.05.377. [DOI] [PubMed] [Google Scholar]
- Higo M., Isobe K., Kang D.J., Ujiie K., Drijber R.A., Ishii R. Inoculation with arbuscular mycorrhizal fungior crop rotation with mycorrhizal plants improves the growth of maize in limed acid sulfate soil. Plant Prod. Sci. 2010;13:74–79. doi: 10.1626/pps.13.74. [DOI] [Google Scholar]
- Higo M., Isobe K., Kondo T., Yamaguchi M., Takeyama S., Drijber R.A., Torigoe Y. Temporal variation of the molecular diversity ofarbuscular mycorrhizal communities in three different winter cover crop rotational systems. Biol. Fertil. Soils. 2015;51:21–32. doi: 10.1007/s00374-014-0945-4. [DOI] [Google Scholar]
- Higo M., Isobe K., Miyazawa Y., Matsuda Y., Drijber R.A., Torigoe Y. Molecular diversity and distribution of indigenous arbuscular mycorrhizal communities colonizing roots of two different winter cover crops in response to their root proliferation. J. Microbiol. Ecol. 2016;54:86–97. doi: 10.1007/s12275-016-5379-2. [DOI] [PubMed] [Google Scholar]
- Higo M., Tatewaki Y., ida K., Yokota K., Isobe K. Amplicon sequencing analysis of arbuscular mycorrhizal fungalcommunities colonizing maize roots in different covercropping and tillage systems. Sci. Rep. 2020;10(1):6039. doi: 10.1038/s41598-020-58942-3. 10.1038/s41598-020-58942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijri M. Analysis of a large dataset form field mycorrhizal inoculation trials on potato showed highly significant increase in yield. Mycorrhiza. 2016;2:209–214. doi: 10.1007/s00572-015-0661-4. [DOI] [PubMed] [Google Scholar]
- Hildebrandt U., Regvar M., Bothe H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry. 2007;68:139–146. doi: 10.1016/j.phytochem.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Hu J., Cui X.C., Dai J., Wang J.H., Chen R.R., Yin R., Lin X.G. Interactive effects of arbuscular mycorrhizae and maize (Zea mays L.) straws on wheat (Triticumaestivum L.) growth and organic carbon storage in a sandy loam soil. Soil Water Res. 2014;9:119–126. [Google Scholar]
- Hu Y., Wu S., Sun Y., Li T., Zhang X., Chen C., Lin G., Chen B. Arbuscular mycorrhizal symbiosis can mitigate the negative effects of night warming on physiological traits of Medicagotruncatula L. Mycorrhiza. 2015;25(2):131–142. doi: 10.1007/s00572-014-0595-2. 10.1007/s00572-014-0595-2. [DOI] [PubMed] [Google Scholar]
- Isobe K., Higo M., Kondo T., Sato N., Takeyama S., Torigoe Y. Effect of winter crop species on arbuscular mycorrhizal fungal colonization and subsequent soybean yields. Plant Prod. Sci. 2014;17(3):260–267. doi: 10.1626/pps.17.260. [DOI] [Google Scholar]
- Jakobsen I., Murmann L.M., Rosendahl S. Hormetic responses in arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2021;159 doi: 10.1016/j.soilbio.2021.108299. [DOI] [Google Scholar]
- Jarosz Z., Michałoj´c Z., Pitura K., Dzida K., Koter M. Influence of fertilization and mycorrhizae on the nutritional status of ahododendron (Rhododendron hybridum) in a nursery. Agriculture. 2021;11:538. 10.3390/agriculture11060538. [Google Scholar]
- Jayne B., Quigley M. Influence of arbuscular mycorrhiza on growth and reproductive response of plants under water deficit: a meta-analysis. Mycorrhiza. 2014;24:109–119. doi: 10.1007/s00572-013-0515-x. [DOI] [PubMed] [Google Scholar]
- Jemo M., Souleymanou A., Frossard E., Jansa J. Cropping enhances mycorrhizal benefits to maize in a tropical soil. Soil Biol. Biochem. 2014;79:117–124. doi: 10.1016/j.soilbio.2014.09.014. [DOI] [Google Scholar]
- Jie W., Bai L., Yu W., Cai B. Analysis of interspecific relationships between Funneliformismosseae and Fusarium oxysporum in the continuous cropping of soybean rhizosphere soil during the branching period. Biocontrol Sc. Tech. 2015;25(9):1036–1051. doi: 10.1080/09583157.2015.1028891. [DOI] [Google Scholar]
- Jin, H., Fran, L.,Walley, J.,Germida, J., 2013. Suppressive effects of seed-applied fungicides on arbuscular mycorrhizal fungi (AMF) differ with fungicide mode of act and AMF species.72, 22–30. 10.1016/j.apsoil.2013.05.013.
- Johnson N.C., Wilson G.W.T., Wilson J.A., Miller R.M., Bowker M.A. Mycorrhizal phenotypes and the Law of the Minimum. New Phytol. 2015;205:1473–1484. doi: 10.1111/nph.13172. [DOI] [PubMed] [Google Scholar]
- Johri A.K., Oelmüller R., Dua M., Yadav V., Kumar M., Tuteja N., Varma A., Bonfante P., Persson B.L., Stroud R.M. Fungal association and utilization of phosphate by plants: success, limitations, and future prospects. Front. Microbiol. 2015;6:984. doi: 10.3389/fmicb.2015.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.C., Martinez-Medina A., Lopez-Raez J.A., Pozo M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012;38:651–664. doi: 10.1007/s10886-012-0134-6. [DOI] [PubMed] [Google Scholar]
- Karasawa T., Takebe M. Temporal or spatial arrangements of covercrops to promote arbuscular mycorrhizalcolonization and P uptake of upland cropsgrown after non-mycorrhizal crops. Plant Soil. 2012;353:355–366. doi: 10.1007/s11104-011-1036-z. [DOI] [Google Scholar]
- Kiers, E.T., Duhamel, M., Beesetty, Y., Mensah, J.A., Franken, O., Verbruggen, E., Fellbaum, C.R., Kowalchuk, G.A., Hart, M.M., Bago, A., Palmer, T.M., West, S.A., Vandenkoornhuyse, P., Jansa, J., & Bücking, H., 2011. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. 333(6044), 880–882. 10.1126/science.1208473. [DOI] [PubMed]
- Kim S.J., Eo J.K., Lee E.H., Park H., Eom A.H. Effects of arbuscular mycorrhizal fungi and soil conditions on crop plant growth. Mycobiology. 2017;45(1):20–24. doi: 10.5941/MYCO.2017.45.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobae Y. Dynamic phosphate uptake in arbuscular mycorrhizal roots under field conditions. Front. Environ. Sci. 2019;6:159. doi: 10.3389/fenvs.2018.00159. [DOI] [Google Scholar]
- Kobae Y., Ohmori Y., Saito C., Yano K., Ohtomo R., Fujiwara T. Phosphate treatment strongly inhibits new Arbuscule development but not the maintenance of Arbuscule in mycorrhizal rice roots. Plant Physiol. 2016;171(1):566–579. doi: 10.1104/pp.16.00127. 10.1104/pp.16.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A.M., Antunes P.M., Barto E.K., Cipollini D., Mummey D.L., Klironomos J.N. The effects of arbuscular mycorrhizal (AM) fungal and garlic mustard introductions on native AM fungal diversity. Biol. Invasions. 2011;13:1627–1639. 10.1007/s10530-010-9920-7. [Google Scholar]
- Lanfranco L., Fiorilli V., Gutjahr C. Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytol. 2018;220:1031–1046. doi: 10.1111/nph.15230. [DOI] [PubMed] [Google Scholar]
- Leal P., Varon-Lopez M., Prado I., Santos J., Soares C., Siqueira O., Moreira F. Enrichment of arbuscular mycorrhizal fungi in a contaminated soil after rehabilitation. Brazilian J. Microbiol. 2016:47. doi: 10.1016/j.bjm.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.H., Eo J.K., Ka K.H., Eom A.H. Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems. Mycobiology. 2013;41(3):121–125. doi: 10.5941/MYCO.2013.41.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.F., Lü P.P., Wang Y.L., Yao H., Maitra P., Sun X., Zheng Y., Guo L.D. Response of arbuscular mycorrhizal fungal community in soil and roots to grazing differs in a wetland on the Qinghai-Tibet plateau. Peer J. 2020;8:e9375. doi: 10.7717/peerj.9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Wang Y., Liu M., et al. Effects of nitrogen deposition and phosphorus addition on arbuscular mycorrhizal fungi of Chinese fir (Cunninghamialanceolata) Sci. Rep. 2020;10:12260. doi: 10.1038/s41598-020-69213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Chen X., Song F., Liu F., Liu S., Zhu X. Effects of arbuscular mycorrhiza on growth and nutrition of maize plants under low temperature stress. Philipp. Agric. Sci. 2016;99(3):246–252. [Google Scholar]
- Liu Z.L., Li Y.J., Hou H.Y., Zhu X.C., Rai V., He X.Y., Tian C.J. Differences in the arbuscular mycorrhizal fungi-improved rice resistance to low temperature at two N levels: aspects of N and C metabolism on the plant side. Plant PhysiolBiochem. 2013;71:87–95. doi: 10.1016/j.plaphy.2013.07.002. 10.1016/j.plaphy.2013.07.002. [DOI] [PubMed] [Google Scholar]
- López-Ráez J.A., Shirasu K., Foo E. Strigolactones in plant interactions with beneficial and detrimental organisms: the Yin and Yang. Trends Plant Sci. 2017;22:527–537. doi: 10.1016/j.tplants.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Lu F., Lee C., Wang C. The influence of arbuscular mycorrhizal fungi inoculation on yam (Dioscorea spp.) tuber weights and secondary metabolite content. Peer J. 2015;3:12–66. doi: 10.7717/peerj.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Lu X., Liao Y. Effect of tillage treatment onthe diversity of soil arbuscularmycorrhizal fungal and soil aggregate-associated carbon content. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02986. 2986.10.3389/fmicb.2018.02986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei G., Miozzi L., Fiorilli V., Novero M., Lanfranco L., Accotto G. The arbuscular mycorrhizal symbiosis attenuates symptom severity and reduces virus concentration in tomato infected by Tomato yellow leaf curl Sardinia virus (TYLCSV) Mycorrhiza. 2014;24(3):179–186. doi: 10.1007/s00572-013-0527-6. [DOI] [PubMed] [Google Scholar]
- Maiti D., Singh R.K., Variar M. Rice-based crop rotation for enhancing native arbuscular mycorrhizal (AM) activity to improve phosphorus nutrition of upland rice (Oryza sativa L.) Biol.Fertil. Soil. 2012;48(1):67–73. doi: 10.1007/s00374-011-0609-6. [DOI] [Google Scholar]
- Mathur S., Sharma M.P., Jajoo A. Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photoch.Photobio. 2018;180:149–154. doi: 10.1016/j.jphotobiol.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Mbuthia L.W., Acosta-Martínez V., DeBruyn J., Schaeffer S., Tyler D., Odoi E., et al. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: implications for soil quality. Soil Biol. Biochem. 2015;89:24–34. 10.1016/j.soilbio.2015.06.016. [Google Scholar]
- Meena R.S., Kumar S., Datta R., Lal R., Vijayakumar V., Brtnicky M. Impact of agrochemicals on soil microbiota and management: a review. Land (Basel) 2020;9(2):34. doi.org/10.3390/land9020034. [Google Scholar]
- Muneer M.A., Wang P., Zhang J., Li Y., Munir M.Z., Ji B. Formation of common mycorrhizal networks significantly affects plant biomass and soil properties of the neighboring plants under various nitrogen levels. Microorganisms. 2020;8(2):230. doi: 10.3390/microorganisms8020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumar T., Priyadharsini P., Uma E., Jaison S., Pandey R.R. In: Use of Microbes for the Alleviation of Soil Stresses. Miransari Md., editor. Springer; New York: 2014. Role of arbuscular mycorrhizal fungi in alleviation of acidity stress on plant growth; pp. 43–72. [Google Scholar]
- Nanjundappa A., Bagyaraj D.J., Saxena A.K., Kumar M., Chakdar H. Interaction between arbuscular mycorrhizal fungi and Bacillus spp. in soil enhancing growth of crop plants. Fungal Biol. Biotechnol. 2019;6:23. doi: 10.1186/s40694-019-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomuceno R.A., Brown C.M.B., Mojica P.N., Brown M.B. Biological control potential of vesicular arbuscular mycorrhizal root inoculant (VAMRI) and associated phosphate solubilizing bacteria, Pseudochrobactrumasaccharolyticum against soilbornephytopathogens of Onion (Allium cepa L. var. Red Creole) Arch. Phytopathol. Plant Prot. 2019;52:714–732. 10.1080/03235408.2019.1644058. [Google Scholar]
- Njeru E.M., Avio L., Sbrana C., Turrini A., Bocci G., Bàrberi P., Giovannetti M. First evidence for amajor cover crop effect on arbuscularmycorrhizal fungi and organic maize growth. Agron. Sustain. Dev. 2014;34:841–848. doi: 10.1007/s13593-013-0197-y. [DOI] [Google Scholar]
- Oehl F., Koch B. Diversity of arbuscular mycorrhizal fungi in no-till and conventionally tilled vineyards. J. Appl. Bot. Food Qual. 2018;91:56–60. doi: 10.5073/JABFQ.2018.091.008. [DOI] [Google Scholar]
- Palm C., Blanco-Canqui H., DeClerck F., Gatere L., Grace P. Conservation agriculture and ecosystem services: an overview. Agric. Ecosyst. Environ. 2014;187:87–105. doi: 10.1016/j.agee.2013.10.010. [DOI] [Google Scholar]
- Patanita M., Campos M.D., Félix M.D., Carvalho M., Brito I. Effect of tillage system and cover crops on maize mycorrhization and presence of magnaporthe maydis. Biology (Basel) 2020;9(3):46. doi: 10.3390/biology9030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe A., Giovannetti M., Sbrana C. Different levels of hyphal self-incompatibility modulate interconnectedness of mycorrhizal networks in three arbuscular mycorrhizal fungi within the Glomeraceae. Mycorrhiza. 2016;26:325–332. doi: 10.1007/s00572-015-0671-2. [DOI] [PubMed] [Google Scholar]
- Pérez-de-Luque A., Tille S., Johnson I., Pascual-Pardo D., Ton J., Cameron D.D. Arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Sci. Rep. 2017;7:16409. doi: 10.1038/s41598-017-16697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot L., Raaijmakers J.M., Lemanceau P., Van Der Putten W.H. Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013;11(11):789–799. doi: 10.1038/nrmicro3109. 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- Pieterse C.M.J., Zamioudis C., Berendsen R.L., Weller D.M., van Wees S.C.M., Bakker P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014;52:347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- Poveda J., Abril-Urias P., Escobar C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020;11:992. doi: 10.3389/fmicb.2020.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashar, P., Shah, S., 2016. Impact of fertilizers and pesticides on soil microflora in agriculture. doi.org/10.1007/978-3-319-26777-7_8.
- Pretty J., Bharucha Z.P. Sustainable intensification in agricultural systems. Ann. Bot. 2014;114:1571–1596. doi: 10.1093/aob/mcu205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Püschel D., Bitterlich M., Rydlová J. Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: a Gordian knot of roots and hyphae. Mycorrhiza. 2020;30:299–313. doi: 10.1007/s00572020-00949-9. [DOI] [PubMed] [Google Scholar]
- Qi Y., Li J., Guan X. Effects of herbicides on non-target plant species diversity and the community composition of fallow fields in northern China. Sci. Rep. 2020;10:9967. doi: 10.1038/s41598-020-67025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H., Lu K., Strong P.J., Xu Q., Wu Q., Xu Z., et al. Long-termfertilizer application effects on the soil, root arbuscular mycorrhizal fungi andcommunity composition in rotation agriculture. Agric. Ecosyst. Environ. Appl.Soil Ecol. 2015;89:35–43. 10.1016/j.apsoil.2015.01.008. [Google Scholar]
- Qin H., Niu L.M., Wu Q.F., Chen J.H., Li Y.C., Liang C.F., et al. Bamboo forest expansion increases soil organic carbon through its effect on soil arbuscular mycorrhizal fungal community and abundance. Plant Soil. 2017;420:407–421. 10.1007/s11104-017-3415-6. [Google Scholar]
- Raklami A., Bechtaoui N., Tahiri A., Anli M., Meddich A., Oufdou K. Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition. Productivity and Soil Fertility. Front. Microbiol. 2019;10:1106. doi: 10.3389/fmicb.2019.01106. 10.3389/fmicb.2019.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Becerril F., Tuinen D.V., Chatagnier O., Rouard N., Béguet J., Kuszala C., et al. Impact of a pesticide cocktail (fenhexamid, folpel, deltamethrin) on the abundance of Glomeromycota in two agricultural soils. Sci. Total Environ. 2017;577:84–93. doi: 10.1016/j.scitotenv.2016.10.098. 10.1016/j.scitotenv.2016.10.098. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Morelos V.H., Calonne-Salmon M., Bremhorst V., Garcés-Ruiz M., Declerck S. Fungicides with contrasting mode of action differentially affect hyphal healing mechanism in Gigasporasp. and Rhizophagusirregularis. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.642094. 642094.10.3389/fpls.2021.642094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouphael Y., Franken P., Schneider C., Schwarz D., Giovannetti M., Agnolucci M., De Pascale S., Bonini P., Colla G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015;196:91–108. doi: 10.1016/j.scienta.2015.09.002. [DOI] [Google Scholar]
- Ruiz-Sánchez M., Aroca R., Muñoz Y., Polón R., Ruiz-Lozano J.M. The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J. Plant Physiol. 2010;167(11):862–869. doi: 10.1016/j.jplph.2010.01.018. 10.1016/j.jplph.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Sabia E., Claps S., Morone G., Bruno A., Sepe L., Aleandri R. Field inoculation of arbuscular mycorrhiza on maize (Zea mays L.) under low inputs: preliminary study on quantitative and qualitative aspects. Italian J. Agron. 2015;10:30–33. doi: 10.4081/ija.2015.607. [DOI] [Google Scholar]
- Säle V., Aguilera P., Laczko E., Mäder P., Berner A., Zihlmann U. Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2015;84:38–52. [Google Scholar]
- Santos M.S., Nogueira M.A., Hungria M. Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Expr. 2019;9:205. doi: 10.1186/s13568-019-0932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoyo G., Guzmán-Guzmán P., Parra-Cota F.I., Santos-Villalobos S., Orozco-Mosqueda M., Glick B.R. Plant growth stimulation by microbial consortia. Agronomy. 2021;11(2):219. doi: 10.3390/agronomy11020219. [DOI] [Google Scholar]
- Sarkodee-Addo E., Yasuda M., Lee C.G., Kanasugi M., Fujii Y., Omari R.A., Abebrese S.O., Bam R., Asuming-Brempong S., Khondoker M.G.D., Okazaki S. Arbuscular mycorrhizal fungi associated with rice (Oryza sativa L.) in Ghana: effect of regional locations and soil factors on diversity and community assembly. Agronomy. 2020;10:559. doi: 10.3390/agronomy10040559. [DOI] [Google Scholar]
- Sato T., Ezawa T., Cheng W.G., Tawaraya K. Release of acid phosphatase from extraradical hyphae of arbuscular mycorrhizal fungus Rhizophagusclarus. Soil Sci. Plant Nutr. 2015;61(2):269–274. doi: 10.1080/00380768.2014.993298. [DOI] [Google Scholar]
- Sato T., Hachiya S., Inamura N., Ezawa T., Cheng W., Tawaraya K. Secretion of acid phosphatase from extraradical hyphae of the arbuscular mycorrhizal fungus Rhizophagus clarus is regulated in response to phosphate availability. Mycorrhiza. 2019;29(6):599–605. doi: 10.1007/s00572-019-00923-0. [DOI] [PubMed] [Google Scholar]
- Sawers R.J.H., Svane S.F., Quan C., Grønlund M., Wozniak B., Gebreselassie M.N., Gonzalez-Munoz E.Chavez, Montes R.A., Baxter I., Goudet J., et al. Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root-external hyphae and the accumulation of transcripts encoding PHT1 phosphate transporters. New Phytol. 2017;214(2):632–643. doi: 10.1111/nph.14403. [DOI] [PubMed] [Google Scholar]
- Sbrana C., Avio L., Giovannetti M. Beneficial mycorrhizal symbionts afecting the production of health promoting phytochemicals. Electrophoresis. 2014;35:1535–1546. doi: 10.1002/elps.201300568. [DOI] [PubMed] [Google Scholar]
- Schipanski M.E., Barbercheck M., Douglas M.R., Finney D.M., Haider K., Kaye J.P., Kemanian A.R., Mortensen D.A., Ryan M.R., Tooker J., White C. A framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agric. Syst. 2013;125:12–22. doi: 10.1016/j.agsy.2013.11.004. [DOI] [Google Scholar]
- Schluter S., Grossmann C., Diel J., Wu G.M., Tischer S., Deubel A., et al. Long-term effects of conventional and reduced tillage on soil structure, soil ecological and soil hydraulic properties. Geoderma. 2018;332:10–19. 10.1016/j.geoderma.2018.07.001. [Google Scholar]
- Schneider K.D., Martens J.R.T., Zvomuya F., Reid D.K., Fraser T.D., Lynch D.H., O'Halloran I.P., Wilson H.F. Options for improved phosphorus cycling and use in agriculture at the field and regional scales. J. Environ. Qual. 2019;48(5):1247–1264. doi: 10.2134/jeq2019.02.0070. 10.2134/jeq2019.02.0070. [DOI] [PubMed] [Google Scholar]
- Schouteden N., De Waele D., Panis B., Vos C.M. Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: a review of the mechanisms involved. Front. Microbiol. 2015;6:1280. doi: 10.3389/fmicb.2015.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim M.M. Introduction to the integrated nutrient management strategies and their contribution to yield and soil properties. International Journal of Agronomy. 2020;2821678:1–14. doi: 10.1155/2020/2821678. [DOI] [Google Scholar]
- Singh B., Singh V.K. In: In: Rice Production Worldwide. Chauhan B.S., Jabran K., Mahajan G., editors. Springer International Publishing AG; Dordrecht, The Netherlands: 2017. Fertilizer management in rice; pp. 217–253. Eds.; [Google Scholar]
- Somasundaram J., Chaudhary R.S., Awanish Kumar D., Biswas A.K., Sinha N.K., Mohanty M., et al. Effect of contrasting tillage and cropping systems on soil aggregation, carbon pools and aggregate-associated carbon in rainfedVertisols. Eur. J. Soil Sci. 2018;69:879–891. doi: 10.1111/ejss.12692. [DOI] [Google Scholar]
- Spatafora J.W., Chang Y., Benny G.L., Lazarus K., Smith M.E., Berbee M.L., Bonito G. A phylum-level phylogenetic classification of zygomycetes fungi based on genome-scale data. Mycologia. 2016;108(5):1028–1046. doi: 10.3852/16-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel K., Vors J.P. In: Modern Crop Protection Compounds. Jeschke P., Witschel M., Krämer W., Schirmer U., editors. Wiley-VCH; Weinheim: 2019. Sterol biosynthesis inhibitors; pp. 797–844. [DOI] [Google Scholar]
- Symanczik S., Courty P.E., Boller T., Wiemken A., Al-Yahya'ei M.N. Impact of water regimes on an experimental community of four desert arbuscular mycorrhizal fungal (AMF) species, as affected by the introduction of a non-native AMF species. Mycorrhiza. 2015;25:639–647. doi: 10.1007/s00572-015-0638-3. [DOI] [PubMed] [Google Scholar]
- Symanczik S., Gisler M., Thonar C., Schlaeppi K., Van der Heijden M., Kahmen A., Boller T., Mäder P. Application of mycorrhiza and soil from a permaculture system improved phosphorus acquisition in Naranjilla. Front. Plant Sci. 2017;8:1263. doi: 10.3389/fpls.2017.01263. 10.3389/fpls.2017.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirkell T.J., Cameron D.D., Hodge A. Resolving the ‘nitrogen paradox’ of arbuscular mycorrhizas: fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant. Cell Environ. 2016;39:1683–1690. doi: 10.1111/pce.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirkell T.J., Charters M.D., Elliott A.J., Sait S.M., Field K.J. Ecological solutions to global food security: are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J. Ecol. 2017;105:921–929. doi: 10.1111/1365-2745.12788. [DOI] [Google Scholar]
- Torrecillas E., Alguacil M.M., Roldán A. Host preferences of arbuscular mycorrhizal fungi colonizing annual herbaceous plant species in semiarid mediterranean prairies. Appl. Environ. Microbiol. 2012;78(17):6180–6186. doi: 10.1128/AEM.01287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacheron J., Desbrosses G., Bouffaud M.L., Touraine B., Moënne-Loccoz Y., Muller D., et al. Plant growth-promoting rhizobacteriaandroot system functioning. Front. Plant Sci. 2013;4:356. doi: 10.3389/fpls.2013.00356. 10.3389/fpls.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallino M., Fiorilli V., Bonfante P. Rice flooding, root branching and arbuscular fungi. Plant Cell Environ. 2014;37:557–572. doi: 10.1111/pce.12177. [DOI] [PubMed] [Google Scholar]
- van der Werf E., de Jager A. Ecological agriculture in South India: an agro-economic comparison and study of transition. Agric. Econ. Res. Institute, The Hague, ETC-Foundation, Leusden. 1992 https://edepot.wur.nl/266019 [Google Scholar]
- Veresoglou S.D., Rillig M.C. Suppression of fungal and nematode plant pathogens through arbuscular mycorrhizal fungi. Biol. Letters. 2012;8:214–217. doi: 10.1098/rsbl.2011.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestberg M., Kahiluoto H., Wallius E. Arbuscular mycorrhizal fungal diversity and species dominance in a temperate soil with long-term conventional and low-input cropping systems. Mycorrhiza. 2011;21:351–361. doi: 10.1007/s00572-010-0346-y. DOI 10.1007/s00572-010-0346-y. [DOI] [PubMed] [Google Scholar]
- Volpe V., Giovannetti M., Sun X.G., Fiorilli V., Bonfante P. The phosphate transporters LjPT4 and MtPT4 mediate early root responses to phosphate status in non-mycorrhizal roots. Plant Cell Environ. 2016;39(3):660–671. doi: 10.1111/pce.12659. 10.1111/pce.12659. [DOI] [PubMed] [Google Scholar]
- Vuyyuru M., Sandhu H.S., McCray J.M., Raid R.N. Effects of soil-applied fungicides on sugarcane root and shoot growth, rhizosphere microbial communities, and nutrient uptake. Agronomy. 2018;8:223. 10.3390/agronomy8100223. [Google Scholar]
- Wang Y., Wang M., Li Y. Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0196408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanarojanaporn N., Boonkerd N., Tittabutr P., Longtonglang A., Young J.P., Teaumroong N. Effect of rice cultivation systems on indigenous arbuscular mycorrhizal fungal communitystructure. Microbes Environ. 2013;28(3):316–324. doi: 10.1264/jsme2.ME13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes T.I., Warner D.J., Edmonds-Brown V., Davies K.G., Denholm I. Zero tillage systems conserve arbuscular mycorrhizal fungi, enhancing soil glomalin and water stable aggregates with implications for soil stability. Soil Sys. 2021;5(1):4. doi: 10.3390/soilsystems5010004. [DOI] [Google Scholar]
- Willer H., Schlater B., Travnicek J., Kempe R.L., Lernaoud J. Research institute of Organic Agriculture. FiBL, Frick, IFOAM, Organic International; Bonn: 2020. The World of Organic agriculture: Summary. In: The world of Organic Agriculture-Statistic and Emerging Trends 2020; p. 20.https://orgprints.org/id/eprint/37222/9/willer-et-al-2020-full-document-2020-02-28-4th-corrigenda.pdf [Google Scholar]
- Willmann M., Gerlach N., Buer B., Polatajko A., Nagy R., Koebke E., Jansa J., Flisch R., Bucher M. Mycorrhizal phosphate uptake pathway in maize: vital for growth and cob development on nutrient poor agricultural and greenhouse soils. Front. Plant Sci. 2013;4:533. doi: 10.3389/fpls.2013.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Hongbo S., Lu Y. Arbuscular mycorrhiza fungi and related soil microbial activity drive carbon mineralization in the maize rhizosphere. Ecotoxicol. Environ. Saf. 2019;182 doi: 10.1016/j.ecoenv.2019.109476. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhu D., Ding J., Zheng F., Zhou S., Lu T., et al. The fungicide azoxystrobin perturbs the gut microbiota community and enriches antibiotic resistance genes in Enchytraeuscrypticus. Environ. Int. 2019;131 doi: 10.1016/j.envint.2019.104965. 10.1016/j.envint.2019.104965. [DOI] [PubMed] [Google Scholar]
- Zhao C., Liu B., Piao S., Wang X. In: Proceedings of the National Academy of Sciences. Turner B.L., editor. (PNAS); USA: 2017. Temperature increase reduces global yields; pp. 9326–9331. 114 (35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Zhang X., Hua H., Han C., Wu X. Sensitivity of Rhizoctonia spp. to flutolanil and characterization of the point mutation in succinate dehyrogenase conferring fungicide resistance. Eur. J. Plant Pathol. 2019;155:13–23. 10.1007/s10658-019-01739-6. [Google Scholar]
- Zhao R., Guo W., Bi N., Guo J., Wang L., Zhao J., et al. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 2015;88:41–49. doi: 10.1016/j.apsoil.2014.11.016. [DOI] [Google Scholar]
- Zhu X., Song F., Liu F. In: Arbuscular Mycorrhizas and Stress Tolerance of Plants. Wu Q.S., editor. Springer; Singapore: 2017. Arbuscular mycorrhizal fungi and tolerance of temperature stress in plants; pp. 163–194. [Google Scholar]
- Zhu X.C., Song F.B., Liu F.L., Liu S., Tian C. Carbon and nitrogen metabolism in arbuscular mycorrhizal maize plants under low-temperature stress. Crop Pasture Sci. 2015;66(1):62–70. doi: 10.1071/CP14159. [DOI] [Google Scholar]
- Zocco D., Van Aarle I.M., Oger E., Lanfranco L, Declerck S. Fenpropimorph and fenhexamid impact phosphorus translocation by arbuscular mycorrhizal fungi. Mycorrhiza. 2011;21:363–374. doi: 10.1007/s00572-010-0344-0. 10.1007/s00572- 010-0344-0. [DOI] [PubMed] [Google Scholar]