Abstract
The gene encoding glycoprotein D (gD) of the monkey B virus (Cercopithecine herpesvirus 1) was cloned into a mammalian expression vector, pcDNA3.1(−), and the recombinant plasmid DNA was transfected into COS7 cells. The expression of gD in transfected COS7 cells was detected by indirect immunofluorescence assay or radioimmunoprecipitation analysis (RIPA). Although the expressed gD protein was revealed to react well with sera from monkeys naturally infected with B virus by RIPA, some sera showed reduced reactivity when analyzed by the Western blotting (WB) method. Some sera also showed relatively high background when the WB was performed using gD expressed from recombinant plasmid. The mutant gD protein lacking the transmembrane domain (TM) and cytoplasmic tail (CT) was next expressed in COS7 cells. The mutant protein was secreted into culture medium without apparent loss of the antigenicity. Using the secretory form of the gD protein as antigen in dot blot analysis, sera from B virus-infected monkeys were shown to react with the mutant protein without nonspecific reaction. Since the recombinant gD or its derivative lacking TM and CT could be expressed in mammalian cells with proper antigenicity, these antigens appeared to be useful for serological detection of B virus infection in monkeys.
B virus (Cercopithecine herpesvirus 1) is a member of subfamily Alphaherpesvirinae and is classified into the genus Simplexvirus together with human herpes simplex virus 1 (HSV-1) and HSV-2 (8). It is enzootic in rhesus monkeys (Macaca mulatta), cynomolgus monkeys (Macaca fascicularis), and other Asiatic species of the genus Macaca. Clinical manifestation of B virus infection is generally mild in these natural hosts. Human infections with this virus, although the incidence is quite low, result in severe and sometimes fatal encephalitis (14, 15). Since nonhuman primates are important experimental animals for biomedical research, it is very important to reduce the risk of B virus infection in animal handlers, laboratory workers, and researchers. For this purpose, efforts have been made to establish B virus-free monkey colonies (6, 12, 13).
Serological diagnosis of B virus infection in macaques is usually conducted by antibody detection using enzyme-linked immunosorbent assay (ELISA) or Western blotting (WB) analysis. The antigens used in these serological tests are prepared from B virus-infected cells; however, for propagation of this virus a special biosafety containment is required, since the virus is classified as a class 4 pathogen. Viruses antigenically closely related to B virus, such as human HSV-1, herpesvirus papio 2, or simian agent 8 (SA8), thus have been used as alternative antigens for detection of B virus infection (7, 10, 15). Another way to prepare the antigens for serological tests is expression of a viral protein by means of the recombinant DNA technique. A part of the C terminus of glycoprotein G (gG) of B virus expressed in Escherichia coli as a fusion protein with glutathione S-transferase has been shown to react with sera taken from B virus-infected monkeys (9). Since glycosylation of the glycoprotein does not take place in bacteria, it seems likely that the tertiary structure of the protein is altered and that the antigenic property of the protein is possibly affected. We therefore attempted to express one of the essential and major viral glycoproteins, gD, of B virus in mammalian cells and to assess the possibility of using the recombinant gD protein for serodiagnosis of B virus infection in macaques.
MATERIALS AND METHODS
Cells.
COS7 cells were maintained in Dulbecco's modified Eagle's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum.
Construction of gD expressing recombinant plasmid.
A recombinant plasmid carrying the B virus gD gene, pBlueSK+2.6 (1), was generously provided by Alice Bennett (Defense Evaluation and Research Agency, Chemical and Biological Defense, Salisbury, United Kingdom). The DNA fragment including the entire region of the open reading frame of the gD gene was excised by CspI digestion and blunted by T4 DNA polymerase, followed by digestion with XhoI. The resulting DNA fragment was subcloned into the XhoI-EcoRV site of pcDNA3.1(−) expression vector (Invitrogen, Carlsbad, Calif.). The recombinant plasmid, designated pBgD, was introduced into E. coli DH5α cells, and the amplified plasmid DNA was purified with a plasmid kit (Qiagen) according to the manufacturer's instructions.
Construction of mutant gD-expressing recombinant plasmid.
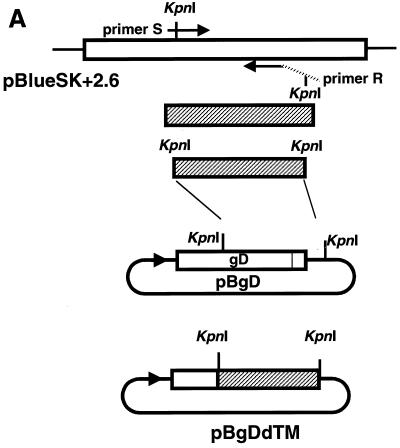
To delete the transmembrane domain (TM) and cytoplasmic tail (CT) of gD, PCR was performed using the forward oligonucleotide primer S (5′-CCTGGTACCGCACGAGCGAC-3′) and the reverse primer R (5′-CACGGTACCTCAATGATGATGATGATGATGGGGGCCCTGGATGGTGAC-3′) and recombinant plasmid pBlueSK+2.6 as a template. Both primers contained a KpnI recognition site (underlined). The reverse primer was designed to contain a stop codon (boldface type) and six extra histidine codons (italic letters). The PCR fragment was digested with KpnI and cloned into KpnI sites of pBgD. The nucleotide sequence of the resulting plasmid DNA was confirmed by sequencing analysis using an ABI PRISM 310 Genetic Analyzer. The recombinant plasmid, designated pBgDdTM, could encode 332 amino acids of gD. The construction strategy and protein structures are schematically shown in Fig. 1A and B, respectively.
FIG. 1.
(A) Strategy of construction of recombinant plasmid pBgDdTM. PCR was performed using pBlueSK+2.6 (1), which contains the gD gene of B virus, as the template together with primers S and R. The PCR product (shaded column) was replaced with a KpnI-KpnI fragment of pBgD. (B) Schematic representation of gD and gDdTM of B virus. The numbers indicate amino acid residues. The shaded columns represent the signal peptide (SP), TM, and CT. Positions of potential N-glycosylation sites (bar) and cysteine residues (inverted triangles) are illustrated on each of the columns.
Transient expression of wild-type and deletion mutant gD.
The recombinant plasmid DNA, pBgD or pBgDdTM, was introduced into COS7 cells by using Lipofectamine Plus Reagent (Gibco-BRL) according to the protocol recommended by the manufacturer.
Immunofluorescent staining.
COS7 cells grown on cover glasses were transfected with recombinant plasmid DNAs as described above. The cells were fixed with cold acetone and incubated with 1:100 diluted anti-gD monospecific antibody against SA8 [anti-gD(SA8)] (4) generously provided by R. Eberle (Oklahoma State University), followed by incubation with 1:100 diluted goat antibody against rabbit immunoglobulin G (IgG) conjugated with fluorescein isothiocyanate (Cappel, Aurora, Ohio). The stained cells were observed under a UV microscope.
Radioimmunoprecipitation analysis (RIPA).
COS7 cells transfected with recombinant plasmid DNAs were metabolically labeled with methionine- and cysteine-deficient Dulbecco's modified Eagle's minimal essential medium containing 100 μCi of the Redivue Pro-mix L-[35S] in vitro cell-labeling mix (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) per ml for 2 or 5 h at 40 h after transfection. The radiolabeled cells were lysed with cell dissociation buffer (10 mM Tris-HCl [pH 8.0], 250 mM NaCl, 0.5% Triton X-100, 0.5% sodium deoxycholate) and clarified by centrifugation (11). The cell extract was mixed with anti-gD(SA8), anti-B virus antibody (kindly provided by R. Eberle), or various sera, followed by precipitation with protein A-Sepharose (Amersham Pharmacia). The precipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10 or 7.5% polyacrylamide gels and were analyzed by the FLA 2000 system (Fuji Film, Tokyo, Japan).
WB analysis.
The cell extract or culture supernatant from recombinant plasmid DNA-transfected cells separated by SDS-PAGE were blotted onto polyvinylidene difluoride (PVDF) membrane (Immun-Blot PVDF; Bio-Rad Laboratories, Hercules, Calif.) with a semidry blotting system (Bio-Rad Laboratories). After blocking with BlockAce reagent (Daiiti-Kagaku, Tokyo, Japan), the membranes were incubated with rabbit anti-gD(SA8) antibody or monkey sera at room temperature for 1 h. After being washed, the membrane was incubated with peroxidase-conjugated anti-rabbit or anti-monkey IgG (Amersham Pharmacia Biotech or Cappel, respectively) at room temperature for 1 h. The specific signals were visualized using the ECL Plus Western blotting detection system (Amersham Pharmacia Biotech) according to the protocol supplied by the manufacturer.
Dot blot analysis.
The secretory form of gD, gDdTM, was purified from the culture supernatant of COS7 cells transfected with pBgDdTM by chromatography on HisTrap and HiTrap desalting columns (Amersham Pharmacia Biotech) according to the manufacturer's protocol. Five microliters of purified protein, culture fluid of COS7 cells transfected with recombinant plasmid pBgDdTM, or vector DNA was directly spotted on the activated PVDF membrane. After blocking by the BlockAce reagent, the membranes were incubated with monkey sera (1:20) for 1.5 h, followed by incubation with peroxidase-conjugated anti-monkey IgG (1:1,000; Cappel) at room temperature for 1 h. The specific signals were visualized by HRP Conjugate Substrate Kit (Bio-Rad Laboratories) according to the protocol supplied by the manufacturer.
Monkey sera.
Sera were collected from cynomolgus monkeys raised at the Tsukuba Primate Center for Medical Science, the National Institute of Infectious Diseases, and stored at −80°C until use. Eight positive and five negative sera were selected and used throughout the study. An additional 61 positive and 36 negative sera from cynomolgus monkeys were also tested in the dot blot analysis. The presence of antibodies directed to inactivated B virus antigen was determined at the Corporation for Production and Research of Laboratory Primates with ELISA (10).
RESULTS
Expression of gD in mammalian cells.
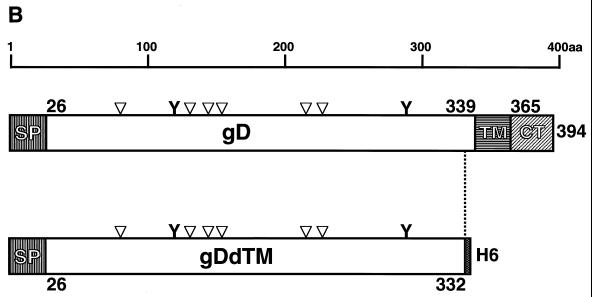
The recombinant plasmid DNA, pBgD, or vector DNA was transfected to COS7 cells, and the transfected cells were examined for the expression of the gD protein by immunofluorescence staining with anti-gD(SA8) antibody. Significant fluorescence was observed in cells transfected with pBgD (Fig. 2A, top), but not in cells transfected with vector DNA (Fig. 2A, bottom). Radioimmunoprecipitation analysis with anti-gD(SA8) or anti-B virus antibody of the extract prepared from COS7 cells transfected with pBgD revealed the presence of the protein with a molecular mass corresponding to that reported previously (2) (Fig. 2B).
FIG. 2.
Expression of B virus gD in COS7 cells was analyzed by indirect immunofluorescence staining (A) and RIPA (B). (A) COS7 cells transfected with pBgD (upper panel) or pcDNA3.1(−) vector (lower panel) were fixed with cold acetone and incubated with anti-gD(SA8) (dilution;1:100), followed by goat anti-rabbit IgG conjugated with fluorescein isothiocyanate. The cells were observed under a UV microscope. (B) The recombinant plasmid, pBgD (lanes 1 and 2), or vector DNA (lanes 3 and 4) was transfected to COS7 cells, and the cell extracts were precipitated with anti-gD(SA8) (lanes 1 and 3) or rabbit anti-B virus (lanes 2 and 4). The precipitates were analyzed on a 10% polyacrylamide gel containing SDS, followed by exposure to an imaging plate of the FLA 2000 system. The position of gD is indicated on the left, and molecular mass standards (in kilodaltons) are shown on the right.
Reactivity of gD expressed in transfected cells to monkey sera.
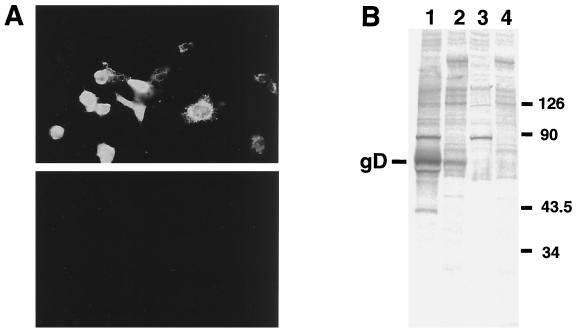
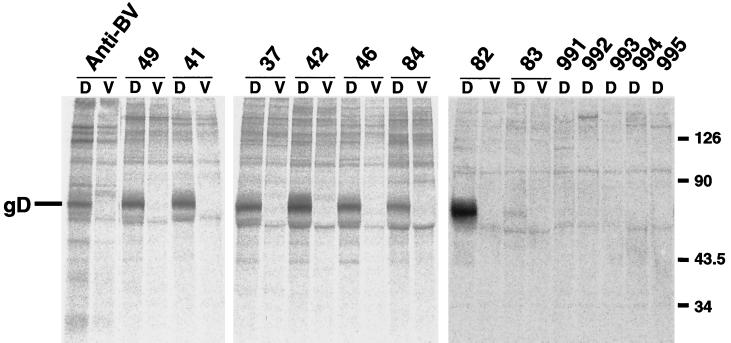
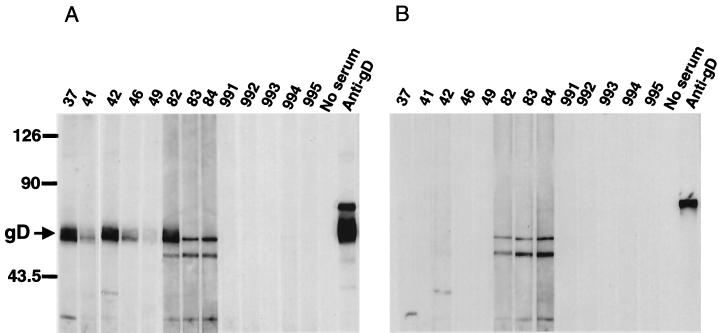
We next attempted to determine whether sera from monkeys naturally infected with B virus recognized gD expressed in COS7 cells from plasmid DNA. As shown in Fig. 3, the expressed gD was specifically precipitated with monkey sera which had been judged to contain antibodies against B virus by ELISA using inactivated B virus antigen (serum no. 49, 41, 37, 42, 46, 84, 82, and 83). No specific band was detected when sera from uninfected monkeys were examined (Fig. 3, no. 991 to 995). Since RIPA is time-consuming and labor-intensive and it also requires the use of radioactive materials, we have tried to develop other methods without any use of radioisotopes. To this end, cells transfected with pBgD were lysed and used as antigen for WB analysis (Fig. 4). In this method, three out of eight monkey sera reacted well, but the others (no. 41, 46, 49, 83, and 84) showed significantly reduced reactivity to gD. In addition nonspecific bands with mobility similar to that of gD were present when some monkey sera (no. 82 to 84) were reacted with the extracts from COS7 cells transfected with the pBgD as well as vector DNA.
FIG. 3.
Reactivity of recombinant gD to monkey sera in RIPA. Radiolabeled extracts from COS7 cells transfected with the pBgD (D) or pcDNA vector DNA (V) were immunoprecipitated with various sera (dilution, 1:50). Sera from monkeys regarded as B virus-infected (no. 37, 41, 42, 46, 49, 82, 83, and 84) and noninfected (no. 991,992, 993, 994, and 995) were used. Anti-BV serum was also included, and the position of the gD was indicated on the left of the panel. Molecular mass standards (in kilodaltons) are shown on the right.
FIG. 4.
Reactivity of expressed gD with monkey sera in WB analysis. The extracts from COS7 cells transfected with pBgD (A) or pcDNA3.1(−) (B) were examined for reactivity with sera obtained from monkeys. Sera from monkeys regarded as B virus infected (no. 37, 41, 42, 46, 49, 82, 83, and 84) and noninfected (no. 991,992, 993, 994, and 995) were used. Anti-gD(SA8) serum was also included. The position of gD (arrow) and molecular mass standards (in kilodaltons) are indicated on the left.
Expression of the deletion mutant gD in the culture supernatant.
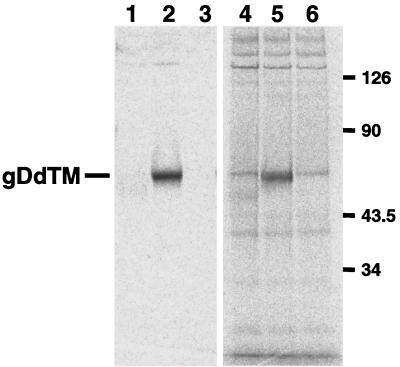
To reduce the nonspecific reaction we have next attempted to express the gD protein that would be secreted to the culture fluid. We therefore introduced a stop codon immediately adjacent to the first amino acid of the TM region of gD (Fig. 1). The plasmid DNA coding for the secretory form of gD was transfected to the COS7 cells, and the cells were subjected to immunofluorescence staining. Significant signals were observed in the cells (data not shown). In order to know whether the mutant protein was properly expressed and secreted into medium of the transfected-cell culture, the cells were labeled with the Redivue Pro-mix L-[35S] in vitro cell- labeling mix, and the clarified supernatant was analyzed by SDS-PAGE (Fig. 5). A band with a molecular mass corresponding to the truncated form of gD was detected when the medium of cells transfected with the deletion mutant gD was directly subjected to SDS-PAGE (Fig. 5, lane 5). Media obtained from cells transfected with neither pBgD nor pcDNA3.1(−) gave signals upon SDS-PAGE analysis (Fig. 5, lane 4 or 6, respectively). The culture media were then used as antigen in RIPA. Whereas a single discrete band was detected in the medium from cells transfected with pBgDdTM (Fig. 5, lane 2), no protein was precipitated when the media from cells transfected with pBgD or pcDNA3.1(−) (Fig. 5, lane 1 or 3, respectively) were examined. These results indicated that the gD protein lacking TM and CT was secreted into the culture medium without apparent loss of antigenicity.
FIG. 5.
Expression of the secretory form of gD. The culture fluids from COS7 cells transfected with pBgD (lanes 1 and 4), pBgDdTM (lanes 2 and 5), or pcDNA3.1(−) (lanes 3 and 6) and then labeled with [35S] were subjected to SDS-PAGE directly (lanes 4 to 6) or after immunoprecipitation with anti-gD(SA8) serum (lanes 1 to 3). The position of gDdTM is indicated on the left, and molecular mass standards (in kilodaltons) are shown on the right.
Reactivity of gDdTM to monkey sera by WB or dot blot method.
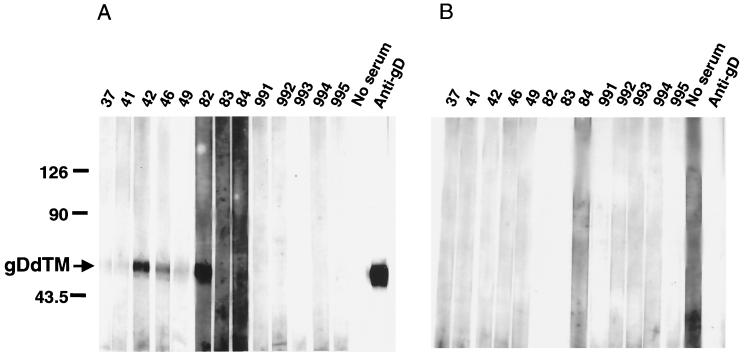
The culture supernatant of COS7 cells transfected with the pBgDdTM or vector plasmid was applied to the SDS-PAGE, and the separated proteins were transferred to a PVDF membrane. The membrane was separated into strips, and the strips were incubated with sera from monkeys. As expected, no nonspecific band was detected (Fig. 6B), indicating that the culture supernatant contained a minimal amount of cellular proteins. As shown in Fig. 6A, however, five out of eight positive sera (no. 37, 41, 49, 83, and 84) showed reduced reactivity in WB analysis using the lysate from cells transfected with pBgD. Since it seemed likely that stringent conditions used in the WB affected the antigenicity, we next employed the dot blot assay, which did not involve a transfer process of the proteins in the denaturing buffer.
FIG. 6.
Reactivity of the secretory form of gD, gDdTM, with monkey sera in WB analysis. Culture media from COS7 cells transfected with pBgDdTM (A) or pcDNA3.1(−) (B) were examined for their reactivities with monkey sera. The position of gDdTM (arrow) and molecular mass standards (in kilodaltons) are indicated on the left.
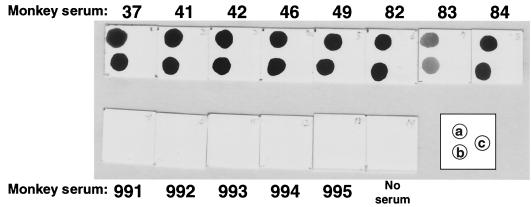
We first tested the same set of sera used in the experiment mentioned above using purified gDdTM as the antigen. The results were shown in Fig. 7. All eight sera (no. 37 to 84) from monkeys that had been regarded as being infected with B virus reacted well with both the purified protein (Fig. 7, position a) and culture supernatant (Fig. 7, position b) blotted directly onto the membrane. No signal was observed when the membrane was spotted with a culture fluid from cells transfected with pcDNA3.1(−) (Fig. 7, position c). Five sera (no. 991 to 995) from uninfected monkeys did not show significant reactivity against any antigen, including unpurified gDdTM. Therefore, we used the culture fluid from cells transfected with pBgDdTM as the antigen without purification in the dot blot method to test additional monkey sera. Sixty-one sera from monkeys that had been regarded as positive by ELISA using inactivated B virus-infected cell lysate showed significant positive signals to the culture fluid containing gDdTM but not to the vector control in dot blot analysis. Thirty-six negative sera showed significant signals neither to gDdTM nor to the vector control.
FIG. 7.
Detection of antibody to the secretory form of gD, gDdTM, in monkey sera by the dot blot method. The position of the purified gDdTM (a), culture supernatant from COS7 cells transfected with pBgDdTM (b), or vector DNA (c) on each membrane is schematically indicated in the inset box. After blocking, each membrane was incubated with individual monkey serum.
DISCUSSION
Since B virus infection often results in devastating and fatal disease, establishment of macaque colonies free from B virus is urgently required. Although serological surveillance of a herd of macaques seems to be a powerful tool to detect B virus infection, it has been hampered to obtain B virus-specific antigens because a biosafety level 4 facility is necessary for propagation of live virus. To circumvent this problem we attempted to express one of the major glycoproteins of B virus, gD, in mammalian cells. The B virus gD gene was therefore subcloned into the expression vector pcDNA3.1(−) downstream of the cytomegalovirus promoter. The nucleotide sequencing of the insert revealed that three nucleotides that were reported before at positions 891, 898, and 907 (the number 1 is A of the initiation codon of gD) were missing (1). The nucleotide deletions resulted in one amino acid deletion at position 303 as well as amino acid substitutions at positions 297 to 302 (data not shown).
Upon transfection of the recombinant plasmid DNA into COS7 cells, the protein that reacted with the antibody against gD raised against gel-purified gD of SA8 or inactivated B virus was detected in transfected cells by RIPA. RIPA revealed that the protein also reacted with sera from monkeys which had been shown to be infected with B virus by an ELISA using inactivated B virus-infected cell lysate. These observations indicated that the gD protein expressed from plasmid DNA was antigenically indistinguishable from that synthesized in virus-infected cells.
We next tried to use the expressed gD in WB analysis, since the method did not require any radioactive materials. Reduced reactivities, however, were noticed for some sera. Nonspecific binding to possible cellular components was also noticed. The reduced reactivities of some sera observed in WB analysis was probably due to the loss of the antigenic epitopes on gD under the condition utilized in PAGE and membrane transfer. Monoclonal antibodies to the discontinuous epitopes of gD of HSV-1 were previously reported to lose their reactivities to gD in the WB analysis (3, 5).
We attempted to express a secretory form of gD lacking TM and CT to minimize contaminating cellular component. Upon transfection of COS7 cells with the plasmid pBgDdTM, mutant gD was secreted into the culture fluid. The secreted proteins were shown to react not only with anti-gD(SA8) and anti-B virus rabbit serum but also with sera from a monkey that had been shown by RIPA to be seropositive for B virus (data not shown). As expected, nonspecific reactions to the cellular components were hardly observed in WB analysis. Since some sera still showed reduced reactivities to the secretory form of gD in WB and since the culture fluid exclusively containing truncated gD showed comparable reactivity in terms of specificity and sensitivity, dot blot analysis was employed. The results from the experiments using a large number of monkey sera indicated that this dot blot assay could be applied to the detection of B virus antibody in the monkey sera. Although we have analyzed only one viral component, our data indicated that gD was one of the most useful antigens for serodiagnosis of B virus infection in monkeys.
ACKNOWLEDGMENTS
We are indebted to Alice Bennett (Defense Evaluation and Research Agency, Chemical and Biological Defense) for kindly providing a plasmid containing the gD gene of B virus and to Richard Eberle (Oklahoma State University) for providing anti-gD and anti-B virus sera. We also thank Koji Fujimoto and Toyoko Narita (The Corporation for Production and Research of Laboratory Primates) for measuring the serum antibody by ELISA using inactivated B virus antigen.
This work was supported in part by a grant from the Ministry of Health and Welfare.
REFERENCES
- 1.Bennett A M, Harrington L, Kelly D C. Nucleotide sequence analysis of genes encoding glycoproteins D and J in simian herpes B virus. J Gen Virol. 1992;73:2963–2967. doi: 10.1099/0022-1317-73-11-2963. [DOI] [PubMed] [Google Scholar]
- 2.Bennett A M, Slomka M J, Brown D W G, Lloyd G, Mackett M. Protection against herpes B virus infection in rabbits with a recombinant vaccinia virus expressing glycoprotein D. J Med Virol. 1999;57:47–56. doi: 10.1002/(sici)1096-9071(199901)57:1<47::aid-jmv7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Cohen G H, Isola V J, Kuhns J, Berman P W, Eisenberg R J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986;60:157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberle R, Black D, Hilliard J K. Relatedness of glycoproteins expressed on the surface of simian herpesvirus virions and infected cells to specific HSV glycoproteins. Arch Virol. 1989;109:233–252. doi: 10.1007/BF01311084. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg R J, Long D, Ponce de Leon M, Mattews J T, Spear P G, Gibson M G, Lasky L A, Perman P, Golub E, Cohen G H. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J Virol. 1985;53:634–644. doi: 10.1128/jvi.53.2.634-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilliard J K, Ward J A. B-virus specific-pathogen-free breeding colonies of macaques (Macaca mulatta): retrospective study of seven years of testing. Lab Anim Sci. 1999;49:144–148. [PubMed] [Google Scholar]
- 7.Ohsawa K, Lehenbauer T W, Eberle R. Herpesvirus papio 2: alternative antigen for use in monkey B virus diagnostic assays. Lab Anim Sci. 1999;49:605–616. [PubMed] [Google Scholar]
- 8.Roizman B. Herpesviridae. In: Fields B N, Knipe D K, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2221–2230. [Google Scholar]
- 9.Slomka M J, Harrington L, Arnold C, Norcott J P N, Brown D W G. Complete nucleotide sequence of the herpesvirus simiae glycoprotein G gene and its expression as an immunogenic fusion protein in bacteria. J Gen Virol. 1995;76:2161–2168. doi: 10.1099/0022-1317-76-9-2161. [DOI] [PubMed] [Google Scholar]
- 10.Takano, J., T. Narita, K. Fujimoto, R. Mukai, and A. Yamada. Detection of B virus infection in cynomolgus monkeys by ELISA using simian agent 8 as alternative antigen. Exp. Animals, in press. [DOI] [PubMed]
- 11.Tanabayashi K, Compans R W. Functional interaction of paramyxovirus glycoproteins: identification of a domain in Sendai virus HN which promotes cell fusion. J Virol. 1996;70:6112–6118. doi: 10.1128/jvi.70.9.6112-6118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward J A, Hilliard J K. B virus-specific pathogen-free (SPF) breeding colonies of macaques: issues, surveillance, and results in 1992. Lab Anim Sci. 1994;44:222–228. [PubMed] [Google Scholar]
- 13.Ward J A, Hilliard J K, Pearson S. Herpes B-virus specific-pathogen-free breeding colonies of macaques (Macaca mulatta): diagnostic testing before and after elimination of the infection. Comp Med. 2000;50:317–322. [PubMed] [Google Scholar]
- 14.Weigler B J. Biology of B virus in macaque and human hosts: a review. Clin Infect Dis. 1992;14:555–567. doi: 10.1093/clinids/14.2.555. [DOI] [PubMed] [Google Scholar]
- 15.Whitley R J. Cercopithecine herpes virus 1 (B virus) In: Fields B N, Knipe D K, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2623–2635. [Google Scholar]