Abstract
Tea tree oil (TTO) exhibits a potent antioxidant, antibacterial, and anti-inflammatory activity and is commonly used in skincare products. However, it is not clear whether TTO can protect gut barrier damage in inflammatory bowel disease (IBD) patients. Herein, we report the impact of terpinen-4-ol (TER, the primary constituent of TTO), on lipopolysaccharide (LPS)-induced intestinal epithelial cell barrier function impairment in intestinal porcine epithelial cell lines (IPEC-J2) and dextran sulfate sodium (DSS)-induced IBD in mice. TER protected against LPS-induced damage in IPEC-J2 cells in vitro and attenuated DSS-induced colitis in vivo. Added TER promoted the tight junction (TJ) proteins expressing in vitro and in vivo and attenuated the LPS-induced upregulation of ERK phosphorylation in IPEC-J2 cells. However, when an inhibitor of ERK phosphorylation was added, TER did not promote the expression of TJ protein, denoting that the ERK signaling pathway mediates the upregulation of TJ proteins. Our data may propose the potential application of TER in treating IBD.
Keywords: inflammatory bowel disease, terpinen 4-ol, ERK1/2-signaling pathway, tight junction (TJ) proteins, mouse model
Introduction
Inflammatory bowel disease (IBDs) is a chronic inflammatory disease of the gastrointestinal tract, which comprises Crohn's disease (CD), ulcerative colitis (UC), and indeterminate colitis (1). This disease affects all ages, and the clinical features primarily include fever, weight loss, diarrhea, and blood in stool (2). Although the exact causes of CD and UC are not well documented, previous studies have shown that genetic factors, immune system dysfunction, and environmental factors might play a crucial role in its occurrence (3). The lesions of IBD patients are mainly confined to the colorectal mucosa and submucosa. They are characterized by mucosal barrier damage and impaired tight junction (TJ) functions, resulting in a loss in gut barrier integrity (4).
Tight junctions between epithelial cells play a role in maintaining the permeability and integrity of the intestinal mucosal barrier. Occludin, claudins, and zonula occludens-1 (ZO-1) are the essential adhesion proteins responsible for the efficient functioning of TJs in the intestinal lining (5). The intestinal mucosal barrier is damaged in IBD patients, and the expression of the TJ proteins ZO-1, claudin, and occludin was decreased in the intestine (6). Otherwise, the upregulation of both ZO-1 and occludin expression significantly improves the integrity, reduces the intestinal mucosal barrier permeability, and prevents the infiltration of harmful substances in IBD patients (7, 8).
There are no drugs to cure IBD, and, at best, they serve to minimize the disease process (9). Drugs commonly used for IBD treatment, include corticosteroids, immunosuppressants, and immunomodulators, which may induce adverse effects on long-term usage (10). Therefore, it has been proposed that IBD patients be treated to restore the mucosal barrier function and thereby relieve the clinical symptoms and cure IBD (11). Given that TJ protein expression levels are closely related to the mucosal barrier function, we speculated that developing a “natural” drug that can normalize TJ function may be an approach for treating IBD.
Tea tree oil (TTO) is extracted from Melaleuca alternifolia. Because of its broad-spectrum antimicrobial (bacteria, fungi, and viruses), anti-cancer, anti-tussive, and antioxidant properties (12). TTO is widely used to treat acne, athlete's foot, and contact dermatitis. TTO contains over 100 components, including terpineol, zinc, and diheptoxy-sulfanylidene-sulfido-λ 5–phosphane (13), the most crucial being terpinen-4-ol (TER), which accounts for >30% of all TTO components. Currently, TER is widely used in the food industry. For instance, as an antistaling agent, TER can alter the microbial biofilm formation in foods, thereby preventing food deterioration and reducing the incidence of certain foodborne illnesses (14). TER has inhibitory activity against certain aerobic heterotrophic bacteria in food (15). It has also been used as a spice to reduce breast cancer incidence (16). Mechanistically, TER can downregulate the secretion of the inflammatory signaling molecule, NO, which is induced by lipopolysaccharide (LPS) and dioctyl sodium sulfosuccinate (DSS) (17, 18). In addition, TER plays a role in regulating the mitogen-activated protein kinase (MAPK)-signaling pathway (19), which is intimately involved in the expression of TJ proteins (20). Hence, we hypothesized that TER would be a promising candidate for IBD therapy. In this study, we examined the effects and mechanisms of TER in protection against LPS/DSS-induced inflammation, both in vitro and in vivo.
Materials and Methods
Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
TER was a gift from Professor Sidong Li's laboratory. His group isolated TER from TTO, the essential oil extracted from Melaleuca alternifolia. The protocol of FTIR analysis was conducted as described by Linshi and modifications (21). Briefly, samples were dissolved in heavy water (D2O) and detected with a Bruker Tensor 27 infrared spectrometer (Shanghai, China) with a resolution of 4.0 cm−1 and a scan range of 400–4,000 cm−1. An average of 64 scans was required to obtain information about the structure.
Cell Culture
IPEC-J2 cells were generously donated by Dr. Bruce Schultz of Kansas State University. The cells were seeded in a T25 flask (Corning Inc., Corning, NY, USA) and cultured in an incubator at 37 °C and 5% CO2. The cells were grown in DMEM/F12 (Sigma-Aldrich, St. Louis, MO, USA) added with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum. After the cells were grown to sub-confluence in 24-well plates, the culture medium was removed, and the cells were washed twice with phosphate-buffered saline (PBS). Subsequently, the cells were exposed to LPS with or without TER.
Cell Viability Assay
A Cell Counting Kit-8 (CCK8, MedChemExpress, Shanghai, China) was used to detect the viability of cells as described by Linshi and modifications (21). Briefly, the IPEC-J2 cells were scattered into 96-well plates and cultured for 24 h, then treated with TER (0, 0.001, 0.002, 0.004, 0.006, 0.008, 0.01, 0.016, and 0.02%) for another 24 h, then incubated with 10 μL of CCK8 solution. Next, the absorbance at 450 nm was detected by the plate reader.
Determination of Epithelial Cell Integrity
The protocol of epithelial cell integrity detection was according to the methods reported by Linshi et al. (21). Briefly, cells were scattered at a 1 × 105 cells/mL density into Trans-well-COL (Corning). The cells were cultured in a 37 °C, 5% CO2 incubator for 13–15 days until they reached a complete polarization, and the culture media was refreshed per 2 days. After the fused monolayer epithelial cells were formed, serial concentrations (as described above) of TER were added and treated for 24 h. Next, 10 μg/mL LPS was added to Trans-well plates' upper compartment (22). The Millicell ERS-2 Voltohmmeter (Millipore, Billerica, MA, USA) were used to measure Trans-epithelial electrical resistance (TEER) of monolayer epithelia at 0, 12, and 24 h. 4-kDa fluorescein isothiocyanate-labeled dextran (FITC-dextran, Sigma-Aldrich). FITC-dextran was dissolved in DMEM/F12 and added to the upper compartment with a 2.2 mg/mL concentration. Two hours later, the lower compartment fluorescence intensity was detected by fluorometry (Tecan Group, Switzerland; excitation, 490 nm; emission, 520 nm).
qRT-PCR
The total RNA of cells and tissue were extracted by the standard method using TRIzol® Reagent (Takara, Dalian, China). The quality of extracted RNA was analyzed by the ratio of A260/A280 using spectrometry. Then, the RNA was reverse transcribed to cDNA using the PrimeScript RT reagent kit (Takara, Dalian, China). Next, the SYBR® Premix Ex Taq™ II (Takara, Dalian, China) were used to perform qRT-PCR. The gene expression level was analyzed by the ΔΔCT method (23). The primers used in the reaction are shown in Table 1.
Table 1.
Primer sequence.
| Primer | Sequence (5′-3′) |
|---|---|
| claudin-1 forward | GTGGATGTCCTGCGTGTC |
| claudin-1 reverse | GTGTTGGGTAAGATGT TGTTTT |
| claudin-4 forward | TTCATCGGCAGCAACAT |
| claudin-4 reverse | AGGACACGGGCACCAT |
| Occludin forward | ATCAACAAAGGCAA |
| Occludin reverse | CTCCGTAATGACCAGA |
| ZO-1 forward | GCCTCCTGAGTTTGATAGTG |
| ZO-1 reverse | TCGGCAGACCTTGAAATAGA |
| β-actin forward | TGCGGGACATCAAGGAGAAG |
| β-actin reverse | AGTTGAAGGTAGT TTCGTGG |
Western Blotting
The western blotting was performed as Linshi reported methods and modification. RIPA Lysis Buffer (Beyotime Biotechnology, Shanghai, China) and extraction kits (ThermoFisher Scientific, Shanghai, China) were used to isolate the total proteins, a nuclear protein, and cytoplasmic proteins, respectively. The protein concentration was analyzed using a BCA reagent (CWBIO, Beijing, China) followed by SDS-PAGE and electrotransfer of separated protein lysates to nitrocellulose membranes (Millipore). After 1 h of blocking, the membranes were incubated with a primary antibody at 4°C for overnight, then incubated with the secondary antibody for 2 h at room temperature. Positive bands were measured by enhanced chemo-luminescence (Tanon, Shanghai, China). Gel-Pro Analyzer software version 4.0 (Media Cybernetics, Silver Spring, MD, USA) was used to analyse the densitometry. Antibodies against ZO-1 (ab96587), occludin (ab167161), claudin-1 (ab129119), and claudin-4 (ab15104) were obtained from Abcam (Cambridge, MA, USA). The antibody against ERK (4695S), P-ERK (4370S), and β-actin (4970S) were secured from CST (CST, MA, USA).
Enzyme-Linked Immunosorbent Assay
The cell culture supernatant was stored at −20 °C before analysis. According to the manufacturer's instructions, the IL-6 concentration and TNF-α concentration were detected by ELISA Kit (IL-6, P6000B; TNF-α, PTA00; R & D Systems, Inc. Minneapolis, MN, USA). The plates were read by a microplate reader (BioTek Instruments, INC, USA) at 405 nm wavelength.
Animal Experiments
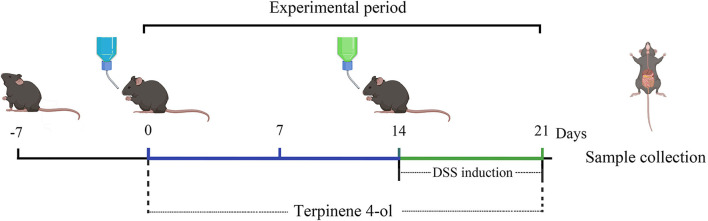
The six-week-old male mice (C57BL6/J) were reared in the Animal Housing Unit and controlled environmental temperature around 24 ± 1°C with a 12-h light/12-h dark cycle. The experimental protocols used were as follows (a brief protocol is shown in Figure 1). The mice were randomly allocated into one of six groups (five animals per group), namely the control, PBS (PBS, i.p.), DSS, DSS + TER-L (5 mg·kg−1·day−1, i.p.), DSS + TER-M (10 mg·kg−1·day−1, i.p.), and DSS + TER-H (20 mg·kg−1·day−1, i.p.). After TER pre-treatment, DSS (2%, v/w, MW 36,000–50,000, MP Biomedicals, Aurora, OH) was added to drinking water from days 15 to 21. All experimental protocols were approved by the Animal Ethics Committee of Guangdong Ocean University, China (Clearance No. 2018-0008) and performed according to the European Community Ethical Guidelines (Directive 2010/63/EU).
Figure 1.
Experimental design demonstrating the effect of terpinen-4-ol (TER) on ameliorating colitis.
Assessment of Colitis
The method for colitis assessment was followed by Linshi's reported and minimal modification (21). The animal's body weights (BWs) were recorded daily. All animals were euthanized on day 21 with CO2, and the blood, colon, and colon contents were collected. Two 5-mm sections of each colon were obtained and fixed in 4% paraformaldehyde and Carnoy's fixative (dry methanol: chloroform: glacial acetic acid at a volume ratio of 60:30:10). The rest of the colon was rinsed with saline and stored at −80°C pending analysis. After fixation, colon samples were dehydrated and embedded in paraffin wax. 5 μm slices were prepared using a microtome (Thermo Fisher) and stained with haematoxylin/eosin (H&E) and periodic acid–Schiff (PAS). The stained slices were covered with coverslips using neutral balsam as an adhesive. The thickness of the muscle layer, the height of the villus, and the number of goblet cells was calculated by Image-Pro Plus software, version 6.0 (Media Cybernetics, Silver Spring, MD, USA).
Statistical Analyses
GraphPad Prism® 5.0 (GraphPad Software, La Jolla, CA, USA) was used for all statistical analyses and graphs. Error bars refer to the standard error of the mean (SEM). The means ± SEM presented are from at least three experiments. The two means were compared using Student's t-test.
Results
Chemical Profile of TER
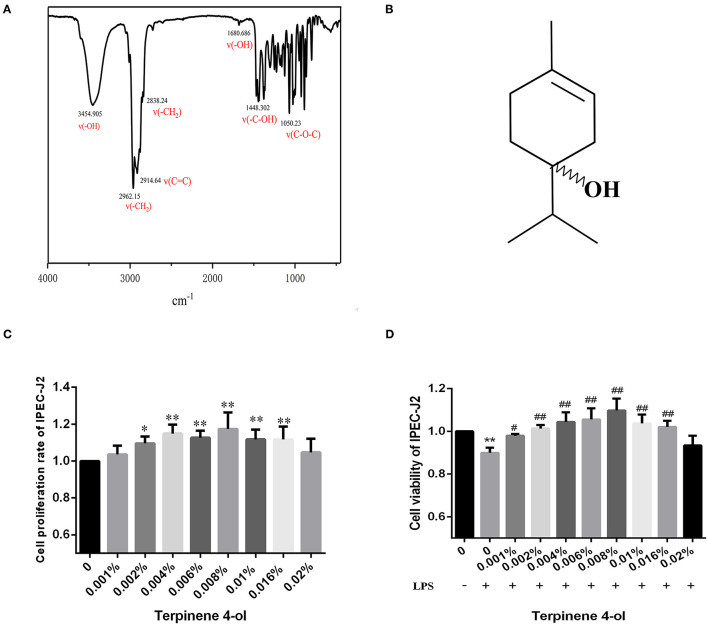
As assessed by FTIR spectroscopy, the structure characteristics and molecular mass of TER are shown in Figures 2A,B. The IR spectra revealed detection at 3,454 cm−1 (-OH stretching); 2,962 cm−1, 2,838 cm−1 (-CH2 stretching); 2,914 cm−1 (C=C stretching); 1,448 cm−1 (-C-OH); 3,400 cm−1 (-OH stretching vibration); 1,680 cm−1 (-OH bending); and 1,050 cm−1 (C-O-C stretching). The spectral results were similar to the TER structure reported in previous studies (24, 25).
Figure 2.
The characterization of TER. (A) The infrared spectrum of TER. (B) The chemical structure of TER intestinal epithelial cells pretreated with different TER concentrations for 24 h followed by treatment with LPS (10 μg/mL) for 24 h. The effects of TER on (B,C) cytotoxicity and (D) cell viability were observed in IPEC-J2 cells exposed to LPS. The data are expressed as the mean ± standard error of three independent experiments. * P < 0.05, ** P < 0.01, compared with the blank control group. # P < 0.05, ## P < 0.01, compared with the blank LPS group.
Cell Viability After Exposed to TER
Cells were treated with different TER concentrations for 24 h to determine the highest non-toxic TER concentration. Figure 2C shows that TER did not significantly inhibit IPEC-J2 cell proliferation. At concentrations between 0.002% and 0.016%, the viability rate of IPEC-J2 significantly increased (P < 0.05). The viability rate of IPEC-J2 cells significantly decreased following LPS exposure (Figure 2D). However, upon TER pretreatment, the cell viability rate increased significantly (P < 0.05). The most effective TER concentrations ranged between 0.001% and 0.016%.
Effect of TER on the Intestinal Epithelial Cell Monolayer Integrity Exposed to LPS
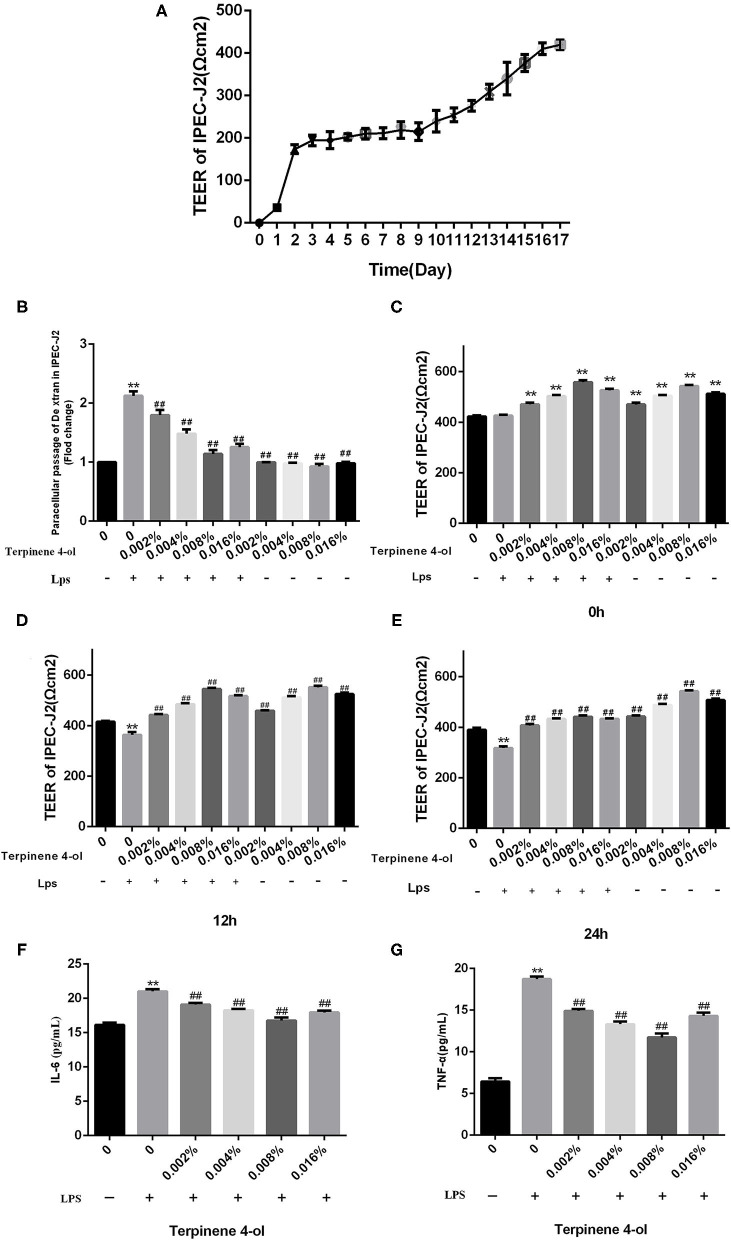
The effects of TER on intestinal epithelial cell permeability were determined by gauging TEER values and concentration of FITC-dextran after being exposed to LPS. The TEER values of IPEC-J2 epithelial cells fused monolayers were stable at 16 d after seeding, indicating that the single-layered epithelium fusion was successfully constructed (Figure 3A). The TEER values of the epithelial monolayer significantly increased after pre-treating with different TER concentrations for 24 h. When fused IPEC-J2 epithelial cell monolayers were exposed to LPS (10 μg/mL), the TEER values significantly decreased (P < 0.05) and the permeability to FITC-dextran was remarkably increased (P < 0.01). Adding different concentrations of TER significantly (P < 0.01) reversed the LPS-dependent decrease in TEER values and increased FITC-dextran permeability in IPEC-J2 monolayers (Figures 3B–E).
Figure 3.
Effects of TER on LPS-induced intestinal epithelial cell permeability and inflammatory cytokines. The protective effects of TER on fused monolayers of intestinal epithelial cells were examined by measuring the TEER values of fused intestinal epithelial monolayers at different time points and FITC-dextran permeability. (A) TEER values. Cells were treated with LPS on day 17 after differentiation, and TEER values were detected at different times (B–D). Cell permeability was measured by determining FITC-dextran permeability after 24 h of LPS exposure in TER-treated cells (E). The IL-6 (F) and TNF-α (G) levels in the culture supernatants of cells pretreated with TER for 24 h and then exposed to LPS for 3 h were measured by ELISA. ** and ## display similar means with above mentioned.
Effect of TER on IL-6 and TNF-α Expression in IPEC-J2 Cells Exposed to LPS
The cells were centrifuged, and the supernatant was collected to detect the expression of IL-6 and TNF-α. As shown in Figures 3F,G, the IL-6 and TNF-α levels, all of which were significantly increased (P < 0.01) in the supernatants of IPEC-J2 cells challenged by LPS, but were significantly decreased (P < 0.01) when pre-treated with TER.
Effect of TER on TJ Proteins Expressing in IPEC-J2 Cells Exposed to LPS
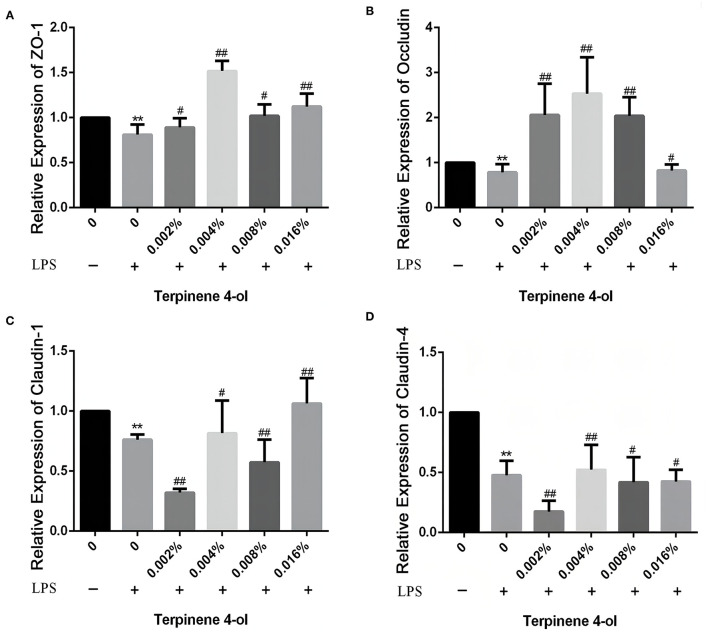
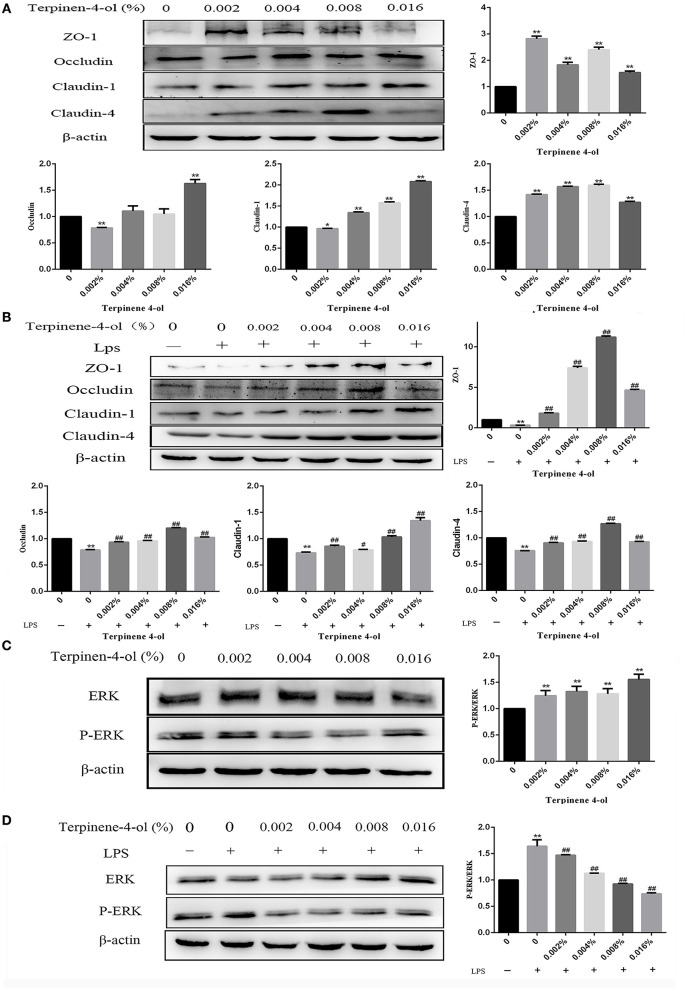
Following 24 h pretreatment with TER, the TJ protein expression in IPEC-J2 cells were measured by qPCR and western blotting. TER significantly upregulated the expression of tight junction proteins, such as ZO-1, occludin, claudin-1, and claudin-4. Interestingly, when cells were exposed to LPS for 3 h, the above-mentioned proteins level was significantly decreased (P < 0.05). However, TER pretreatment significantly inhibited LPS-induced decreased TJ proteins at both mRNA (Figures 4A–D) and protein (Figures 5A,B) levels.
Figure 4.
Effect of TER on tight junction proteins in IPEC-J2 cells. The expression of ZO-1 (A), occluding (B), claudin-1 (C), and claudin-4 (D) genes were analyzed by qRT-PCR. ** and ## display similar means with above mentioned.
Figure 5.
Effect of TER on tight junction (TJ) proteins and phosphorylated ERK expression in IPEC-J2 cells. (A) The TJ proteins expression were detected in cells exposed to a serial concentration of TER. (B) The TJ proteins' expression in cells incubated with TER for 24 h and then exposed to LPS. (C) ERK and phosphorylated ERK expression in cells incubated with TER for 24 h. (D) ERK and phosphorylated ERK expression in cells incubated with TER for 24 h and then exposed to LPS. ** and ## display similar means with above mentioned.
Effects of TER on ERK Protein and Its Phosphorylation in IPEC-J2 Cells
Following 24-h pretreatment with TER, ERK and phosphorylated ERK in cells were measured by immunoblotting. TER treatment was significantly downregulated (P < 0.05) the expression of phosphorylated ERK in IPEC-J2 cells (Figure 5C). Following exposure to LPS for 3 h, the phosphorylated ERK levels were significantly increased (P < 0.05). However, pre-treatment of the cells with TER for 24 h and subsequent exposure to LPS resulted in a phosphorylated ERK decrease (Figure 5D, P < 0.05).
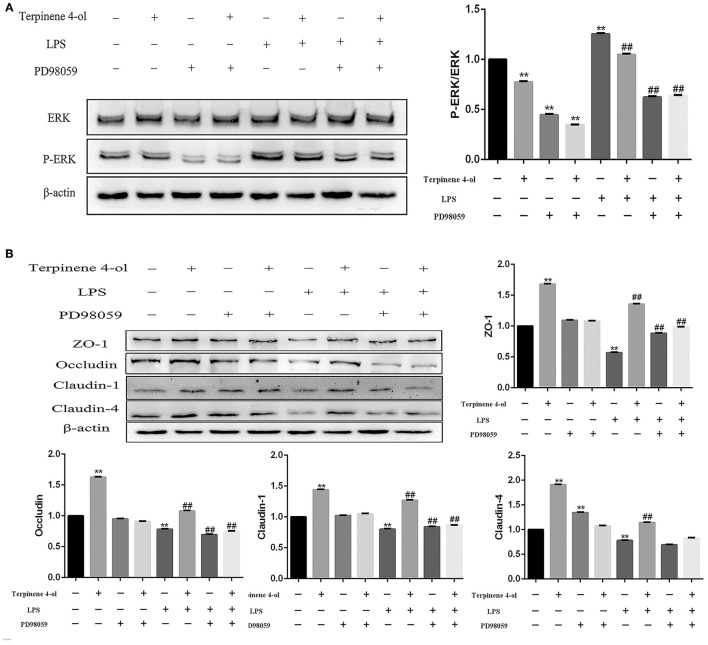
Effects of Blocking ERK Phosphorylation on TJ Protein Expression
To examine the role of ERK in regulating TJ proteins expression, cells were incubated with an ERK inhibitor (PD98059, 50 nM) for 1 h before treatment with TER (0.008%) or LPS (10 μg/mL). The level of phosphorylated ERK was significantly decreased (P < 0.01) after adding the inhibitor (Figure 6A), suggesting that the ERK pathway was successfully blocked. Although the expression of TJ proteins was upregulated significantly (P < 0.01) in cells pre-treated with TER alone, but the ERK inhibitor significantly attenuated (P < 0.01) the upregulation of TJ proteins induced by TER. In TER-pretreated cells that were later exposed to LPS, the inhibitor significantly decreased (P < 0.01) the expression of TJ proteins (Figure 6B).
Figure 6.
Effect of ERK phosphorylation inhibition on tight junction (TJ) protein expression. (A) The expression of phosphorylated ERK and (B) TJ proteins were detected in cells pretreated with the ERK inhibitor PD98059 (50 μM) for 1 h before treatment with TER or LPS. ** and ## display similar means with above mentioned.
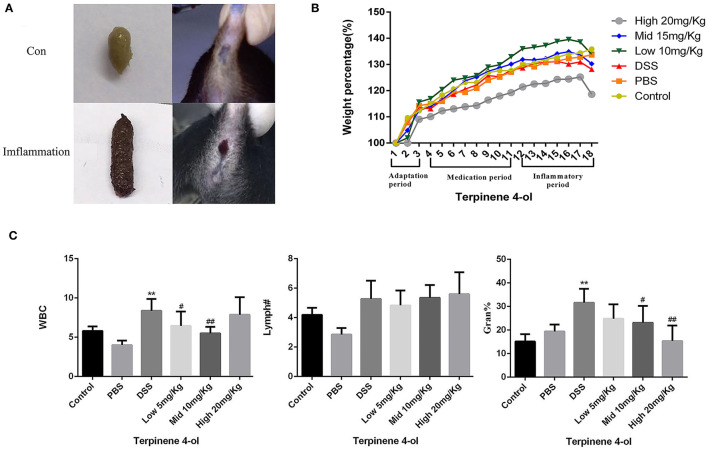
Effects of TER on DSS-Induced Colonic Inflammation in Mice
The mice given DSS-containing water showed reduced food intake, lethargy, dry hair, slow responses to stimulation and impotence. Severe bloody stools and/or anal bleeding was also noticed (Figure 7A). The BWs of mice in the control and the PBS-treated groups increased steadily, whereas it decreased in those administered DSS alone on day 3 post-treatment. Lower BW losses were observed in mice exposed to both low- and moderate- levels of TER than in the DSS group in contrast to higher BW losses in mice exposed to a high TER level (Figure 7B). Routine blood test results showed that the proportions of leukocytes, lymphocytes, and neutrophils increased in the DSS-treated mouse. However, TER (low and moderate doses) administration attenuated the increase of inflammatory response induced by DSS in mice (Figure 7C).
Figure 7.
Effect of TER on colonic inflammation in mice with DSS-induced colitis. Colonic inflammatory responses after drinking DSS solution. (A) Severe bloody stools, (B) relative BW changes, (C) and the percentages of white blood cells, lymphocytes, and neutrophils in mice treated with TER. ** and ## display similar means with above mentioned.
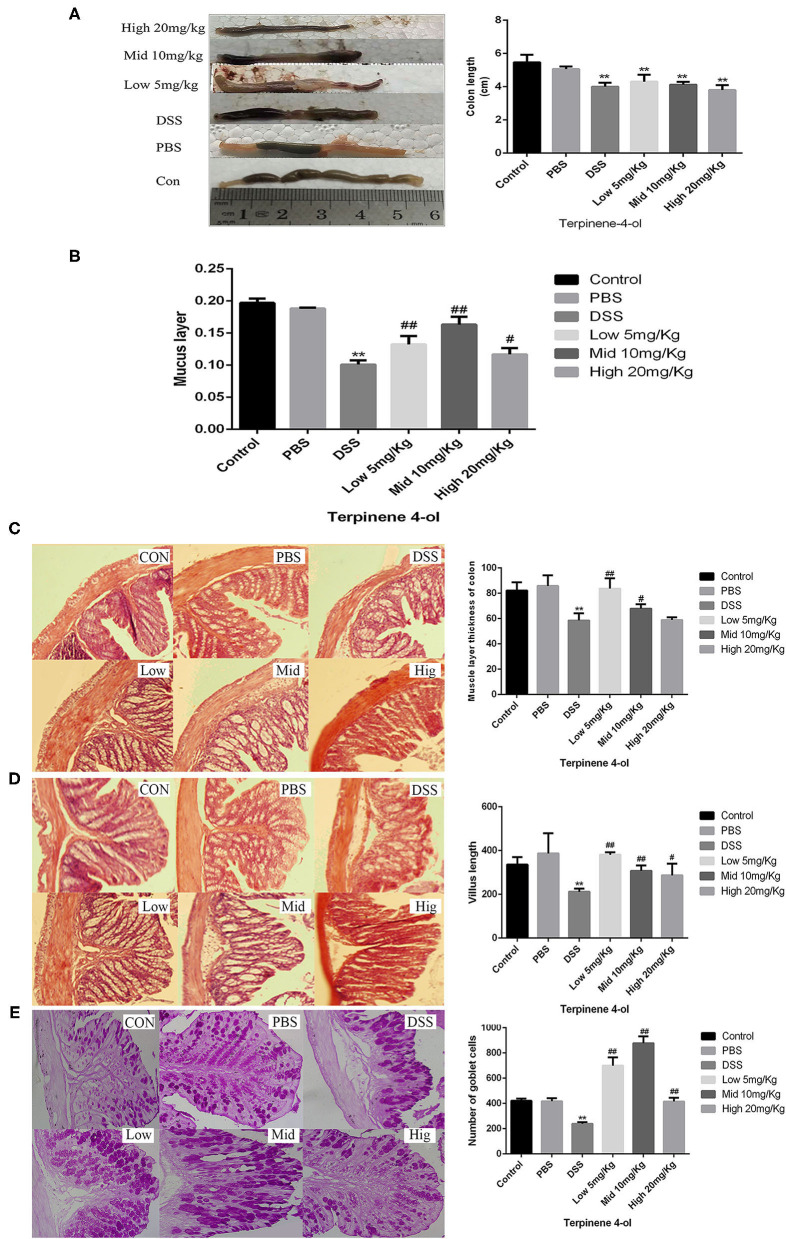
TER Attenuated Mice Colitis Induced by DSS
The colon structure in control mice was normal under the microscope. However, in DSS-treated mice, significant inflammatory responses, such as the short of colon length (Figure 8A), the disappear of intestinal mucous layer (Figure 8B), the thickness of the muscle layer of the colon (Figure 8C), the short of intestinal villus (Figure 8D), and marked decrease of the number of goblet cell (Figure 8E) have appeared. Interestedly, both lower concentration and middle concentration of TER added protected the goblet cells and the mucosal integrity in DSS-induced colitis mice.
Figure 8.
Effect of terpinen-4-ol (TER) treatment on colonic histopathology and the intestinal mucosal layer thickness in dextran sulfate sodium (DSS)-induced colitis. Effect of TER treatment on the colon length (A), mucus layer thickness (B), muscle layer thickness (C), villus length (D), and the number of goblet cells (E) in mice with DSS-induced colitis. ** and ## display similar means with above mentioned.
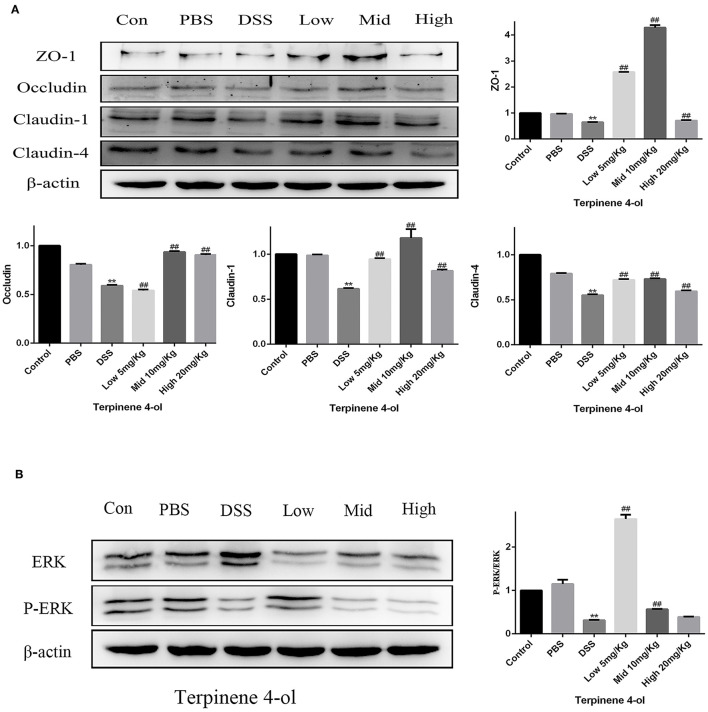
Effect of TER on TJ Proteins and ERK-Pathway Protein Expression in vivo
Compared with the control and PBS groups, TJ protein expression levels were markedly reduced (P < 0.05) in the DSS treated group but increased in TER pre-treated groups (Figure 9A, P < 0.05). Meanwhile, the expression of phosphorylated ERK was remarkably decreased in the DSS treated group compared with control but increased in mice with a lower dose of TER administration (Figure 9B, P < 0.05), suggesting that TER upregulated the expression of tight junction proteins was related to phosphorylated ERK.
Figure 9.
Effect of terpinen-4-ol (TER) on the tight junction (TJ) and ERK-pathway protein expression in mice. (A) Expression of colon TJ proteins and (B) ERK-signaling proteins in mice after drinking DSS solution. ** and ## display similar means with above mentioned.
Discussion
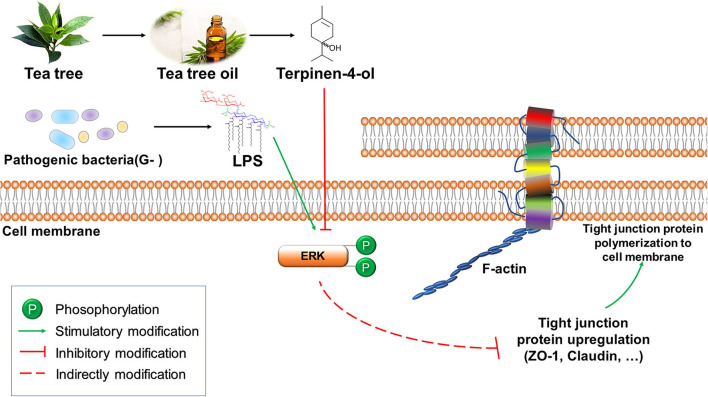
TER has attracted considerable interest as a bactericidal product in potential applications due to its unique biological activity, including antibacterial and antioxidant activities. Our study showed that TER protected IPEC-J2 cells against LPS-induced inflammation and DSS-induced colitis. Furthermore, we also showed the role of TJ proteins in LPS-or DSS-induced gut barrier damage via the ERK1/2-signaling pathway (Figure 10).
Figure 10.
The putative signal pathway of terpinen-4-ol (TER) protects gut integrity in IPEC-J2 cells. TER attenuated LPS induces downregulation of tight junction proteins via the ERK1/2 signal pathway.
During growth and metabolic processes, cells secrete highly active biological oxidants, such as NO and hydroxyl radicals. Physiological doses of biological oxidants are essential for normal cellular metabolic processes and regulating various physiological functions. However, excessive oxidants will induce oxidative stress and subsequent cellular damage in the animal. A variety of pathogens and their secreted molecules, such as LPS, induce cells to produce high levels of oxidants (26). Essential oils containing TER can downregulate LPS-induced NO secretion (17), allowing normal cell growth and metabolism (27). In this study, we found a significant decrease in the viability of IPEC-J2 cells when exposed to LPS. On the contrary, the viability rate was significantly increased when pretreated with TER before LPS exposure. This phenomenon could be due to (i) pretreatment of TER significantly increased the IPEC-J2 cell proliferation rate, and (ii) TER protecting the intestinal barrier integrity, which attenuated the toxicity of LPS.
Inflammatory responses represent the initial pathological process during tissue damage in multiple diseases (28). Nuclear factor-kappa B (NF-κB) is activated when cells are activated by LPS, leading to an increase of intracellular inflammatory factors (29, 30). TER can significantly inhibit the activation of NF-κB, thereby reducing the inflammatory response (31). IL-6 and TNF-α were upregulated in IPEC-J2 cells after LPS stimulation, but pretreatment of the cells with TER, the above-mentioned phenomenon, disappeared. This indicates that TER exhibited significant anti-inflammatory activity.
When a pathogen invades the intestinal mucosal barrier, it can cause epithelial cell damage, activate inflammatory factors and mucosal immune activity, impair intestinal TJs (32), all of which can result in intestinal inflammation (33). This study demonstrated that TER improved intestinal barrier integrity. The mechanism may be related to the expression of TJ proteins, as TER pretreatment significantly ameliorated LPS- or DSS-induced damage to the epithelial integrity and decrease of tight junction proteins. The activating of p38 MAPK- and ERK1/2-pathways is related to inflammatory cytokine secretion in intestinal cells (34). The amelioration of Lactobacillus pentosus-induced colitis was based on inhibiting MAPKs and TJ protein expression (20, 35). In human corneal epithelial cells, TJ disruption occurred when the ERK1/2 pathway activated. In this study, TER attenuated LPS induced tight junction proteins decrease and ERK1/2 activation.
Similarly, pretreatment of IPEC-J2 cells with ERK1/2 inhibitor effectively inhibited the LPS-induced ERK1/2 activation and the TJ protein decrease. Moreover, TER attenuated colitis in DSS-treated mice was accompanied by TJ protein upregulation and inhibition of ERK1/2 phosphorylation in mice exposed to moderate and high TER doses, suggesting that ERK1/2 signaling is involved in TJ protein regulation by TER. In the LPS-induced inflammatory response of RAW264.7 macrophages, pedunculoside remarkably inhibited the phosphorylation of ERK1/2 to reduce the inflammatory cytokine production (32). However, as the phosphorylated ERK1/2 was significantly decreased in DSS alone and low dose TER treated mice, we speculate that cyclic changes in ERK1/2 expression may occur during the treatment process in the mice. For example, heat stress (HS) increased intestinal permeability and associated cyclic changes in TJ gene expression within 7 days (36), and ERK1/2 expression is quickly upregulated in acute HS but decreased sharply in pigs subjected to chronic HS (37).
In our study, TER remarkably improved DSS-induced colitis in mice and enhanced immune function. In addition, TER inhibited potential oxidative stress factors caused by DSS, thereby potentially protecting the intestinal barrier integrity by inhibiting intestinal cell apoptosis. Goblet cells are important mucus-secreting cells in the body (38). When goblet cells are reduced, mucus is secreted in insufficient quantities. The mucosal barrier function is impaired, resulting in inflammation (39). Our research also showed that TER ameliorated the DSS-induced decrease in the number of colonic goblet cells and the mucus layer thickness in mice. However, further study is required to elucidate the mechanism(s) by which goblet cells respond to TER.
In conclusion, the data demonstrate that TER attenuates LPS- or DSS-induced downregulation of TJ proteins via the ERK1/2-signaling pathway, which could be used as a novel therapeutic approach for treating IBD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Animal Ethics Committee of Guangdong Ocean University.
Author Contributions
XJ, YY, and BF designed the study. YY, BF, YH, JL, TY, LW, CH, XL, ZY, and XM participated in the experiments. YY, BF, XJ, SL, RG, and AA wrote and revised the manuscript. All authors approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (Nos. 31472243 and 31902314), Natural Science Foundation of Guangdong Province, China (No. 019A1515011142), and Project of Enhancing School with Innovation of Guangdong Ocean University (No. GDOU230419057).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.805612/full#supplementary-material
References
- 1.Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol. (2014) 20:16389. 10.3748/wjg.v20.i44.16389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laugesen K.B, Tøttrup A. Colorectal cancer in ulcerative colitis. Ugeskr Laeger. (2014). 176. [PubMed] [Google Scholar]
- 3.Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intestinal Research. (2018) 16:26–42. 10.5217/ir.2018.16.1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, Hold G. N, et al. The gut microbiota and host health: A new clinical frontier. Gut. (2016) 65:330. 10.1136/gutjnl-2015-309990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: From simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. (2016) 17:564. 10.1038/nrm.2016.80 [DOI] [PubMed] [Google Scholar]
- 6.Hu CAA, Hou Y, Yi D, Qiu Y, Wu G, Kong X, et al. Autophagy and tight junction proteins in the intestine and intestinal diseases. Anim Nutr. (2015) 1:43–7. 10.1016/j.aninu.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y JZ, Zheng C, et al. Effects of protein sources and levels in antibiotic-free diets on diarrhea, intestinal morphology, and expression of tight junctions in weaned piglets. Anim Nutr. (2015) 1:90–6. 10.1016/j.aninu.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mccarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Roger RA, et al. Occludin is a functional component of the tight junction. J Cell Sci. (1996) 109:2287. 10.1242/jcs.109.9.2287 [DOI] [PubMed] [Google Scholar]
- 9.Baig MM., Fatima S, Fatima M, Siddiqui S, Ali S. A, Ilyas, Prescription pattern MN, and cost of illness (coi) of inflammatory bowel disease (ibd) in a tertiary care hospital. Int J Pharm Pharm Sci. (2016) 9:44. 10.22159/ijpps.2017v9i1.14248 [DOI] [Google Scholar]
- 10.Murthy SK, Nguyen GC. Increased risk of venous thromboembolic events with corticosteroid versus biologic therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. (2016) 14:166. 10.1016/j.cgh.2015.04.022 [DOI] [PubMed] [Google Scholar]
- 11.Groschwitz KR, Hogan SP. Intestinal barrier function: Molecular regulation and disease pathogenesis. J Allergy Clin Immunol. (2009) 124:3–20. 10.1016/j.jaci.2009.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammer; Carson; Riley. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. (2004) 53:1081–5. 10.1093/jac/dkh243 [DOI] [PubMed] [Google Scholar]
- 13.Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin Microbiol Rev. (2006) 19:50. 10.1128/CMR.19.1.50-62.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerekes E-B, Deak E, Takó M, Tserennadmid R, Petkovits T, VágvLgyi C, et al. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J Appl Microbiol. (2013) 115:933–42. 10.1111/jam.12289 [DOI] [PubMed] [Google Scholar]
- 15.Busatta C, Vidal RS, Popiolski AS, Mossi AJ, Dariva C, Rodrigues MRA, et al. Application of Origanum majorana l. Essential oil as an antimicrobial agent in sausage. Food Microbiology. (2008) 25:207–11. 10.1016/j.fm.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 16.Al-Kalaldeh JZ, Abu-Dahab R, Afifi FU. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr Res. (2010) 30:271–8. 10.1016/j.nutres.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 17.Gostner J M., Becker K, Ueberall F, Fuchs The good D, and bad of antioxidant foods: An immunological perspective. Food Chem Toxicol. (2015) 80:72–9. 10.1016/j.fct.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 18.Souza CF, Baldissera MD, Silva LdL, Geihs MA, Baldisserotto B, et al. Is monoterpene terpinen-4-ol the compound responsible for the anesthetic and antioxidant activity of, Melaleuca alternifolia, essential oil (tea tree oil) in silver catfish?[j]. Aquaculture. (2018) 486:217–23. 10.1016/j.aquaculture.2017.12.025 [DOI] [Google Scholar]
- 19.Nogueira MNM, Aquino SG, Junior CR, Spolidorio DMP. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of il-1β, il-6 and il-10 on human macrophages. Inflamm Res. (2014) 63:769–78. 10.1007/s00011-014-0749-x [DOI] [PubMed] [Google Scholar]
- 20.Jin-Ju J, Kyung-Ah K, Se-Eun J, Jae-Yeon W, Myung Joo H, Dong-Hyun K. Orally administrated Lactobacillus pentosus var. Plantarum c29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa b signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS ONE. (2015) 10:e0116533. 10.1371/journal.pone.0116533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi L, Fang B, Yong Y, Li X, Gong D, Li J, et al. Chitosan oligosaccharide-mediated attenuation of lps-induced inflammation in ipec-j2 cells is related to the tlr4/nf-kappab signaling pathway. Carbohydr Polym. (2019) 219:269–79. 10.1016/j.carbpol.2019.05.036 [DOI] [PubMed] [Google Scholar]
- 22.Lu YC, Yeh WC, Ohashi PS. Lps/tlr4 signal transduction pathway. Cytokine. (2008) 42:145–51. 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 23.Yoshida LS, Kakegawa T, Yuda Y, Takanoohmuro H. Shikonin changes the lipopolysaccharide-induced expression of inflammation-related genes in macrophages. J Nat Med. (2017) 71:1–12. 10.1007/s11418-017-1106-5 [DOI] [PubMed] [Google Scholar]
- 24.Xia JQ, De Yu Z, Rui L, Qing ZW, Jing D, Guang CX. In vitro evaluation of the biomedical properties of chitosan and quaternized chitosan for dental applications. Carbohydr Res. (2009) 344:1297–302. 10.1016/j.carres.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Xiao Z, Ji H. Solid inclusion complex of terpinen-4-ol/β-cyclodextrin: Kinetic release, mechanism and its antibacterial activity. Flavour Fragr J. (2015) 30:179–87. 10.1002/ffj.322925855820 [DOI] [Google Scholar]
- 26.Ray PD, Huang B-W, Tsuji Y. Reactive oxygen species (ros) homeostasis and redox regulation in cellular signaling. Cell Signal. (2012) 24:981–90. 10.1016/j.cellsig.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajimehdipoor H, Samadi N, Mozaffarian V, Rahimifard N, Shoeibi S, Hamedani MP. Chemical composition and antimicrobial activity of Oliveria decumbens volatile oil from west of Iran. J Medicinal Plants. (2010) 4:1411–8. [Google Scholar]
- 28.Kim N, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. (2012) 4:829–41. 10.1101/cshperspect.a006049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hart P H., Brand C, Carson C.F, Riley T.V, Prager R.H, Finlay-Jones, Terpinen-4-ol JJ, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflammat Res. (2000) 49:619–26. 10.1007/s000110050639 [DOI] [PubMed] [Google Scholar]
- 30.Homeyer DC, Sanchez CJ, Katrin M, Beckius ML, Murray CK, Wenke JC, et al. In vitro activity of Melaleuca alternifolia (tea tree) oil on filamentous fungi and toxicity to human cells. Medical Mycology. (2015) 53:285–94. 10.1093/mmy/myu072 [DOI] [PubMed] [Google Scholar]
- 31.Hua KF, Yang TJ, Chiu HW, Ho CL. Essential oil from leaves of Liquidambar formosana ameliorates inflammatory response in lipopolysaccharide-activated mouse macrophages. Nat Prod Commun. (2014) 9:869. 10.1177/1934578X1400900638 [DOI] [PubMed] [Google Scholar]
- 32.Liu K, Li G, Guo W, Zhang J. The protective effect and mechanism of pedunculoside on dss (dextran sulfate sodium) induced ulcerative colitis in mice. Int Immunopharmacol. 88: 107017. 10.1016/j.intimp.2020.107017 [DOI] [PubMed] [Google Scholar]
- 33.Fermín SDM, Isabel R C., Cristina M. Olga, Intestinal inflammation MA, and mucosal barrier function. Inflamm Bowel Dis. (2014) 20:2394–404. 10.1097/MIB.0000000000000204 [DOI] [PubMed] [Google Scholar]
- 34.O'Brien D OCT, Shanahan F., O'brien d, o'connor t, shanahan f, et al. Activation of the p38 mapk and erk1/2 pathways is required for fas-induced il-8 production in colonic epithelial cells. Ann N. Y. Acad Sci. (2002) 973:161–5. 10.1111/j.1749-6632.2002.tb04627.x [DOI] [PubMed] [Google Scholar]
- 35.Wu HL, Gao X, Jiang ZD, Duan ZT, Wang SK, He B., et al. Attenuated expression of the tight junction proteins is involved in clopidogrel-induced gastric injury through p38 mapk activation. Toxicology. (2013) 304:41–8. 10.1016/j.tox.2012.11.020 [DOI] [PubMed] [Google Scholar]
- 36.Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross J, et al. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PloS ONE. (2013) 8:e70215. 10.1371/journal.pone.0070215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross J., et al. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J Animal Sci. (2012) 90:257–9. 10.2527/jas.52339 [DOI] [PubMed] [Google Scholar]
- 38.Moors E, Singh T, Siderius C, Balakrishnan S, Mishra A. Climate change and waterborne diarrhoea in northern India: Impact and adaptation strategies. Sci Total Environ. (2013) 468–9. 10.1016/j.scitotenv.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 39.Deckers J, Madeira FB, Hammad H. Innate immune cells in asthma. Trends Immunol. (2013) 34:540–7. 10.1016/j.it.2013.08.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.