Abstract
Background
Multiple lumen umbilical venous catheters (ML‐UVCs) instead of single lumen UVCs (SL‐UVCs) may decrease the need for additional venous lines. Although it seems self‐evident that ML‐UVCs would reduce the need of additional venous lines, the rates of associated complications might be different.
Objectives
To compare the effectiveness and the safety of ML‐UVCs versus SL‐UVCs in terms of need of additional vascular access, rates of complications, morbidity and mortality in newborn infants.
Search methods
Randomized and quasi‐randomized trials were identified by searching the MEDLINE (1966 ‐ February 2005), EMBASE (1980‐ February 2005), CINAHL (1982 ‐ February 2005), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 4, 2004) and Science Direct (subject area: medicine, journal and abstract database; 1967 to February 2005). Literature search also included a manual search of the abstracts of scientific meetings published in Pediatric Research (1990‐2004). Additional citations were sought using references in articles retrieved from searches. Subject experts were contacted to identify the unpublished and ongoing studies.
Selection criteria
Randomized and quasi‐randomized controlled clinical trials comparing safety and efficacy of multiple versus single lumen umbilical venous catheter in neonates (both term and preterm) who were in need of umbilical venous catheter insertion for vascular access in first four weeks of life.
Data collection and analysis
Each review author performed data extraction independently and differences were resolved by discussion. The following outcomes were determined: total number of additional peripheral intravenous lines per baby in first week and first four weeks of life, total number of additional percutaneously and surgically placed central venous lines per baby in first four weeks of life, and other safety and efficacy measures. The treatment effect estimators used were RR, RD, and WMD when appropriate along with their 95% CI. If RD was statistically significant, then number needed to treat (NNT) or number needed to harm (NNH) was calculated.
Main results
Three studies qualified for inclusion in this review (Khilnani 1991; Loisel 1996; Soupre 1998). There was a decrease in the ML‐UVCs group in the number of additional PIVs used in the first week of life [WMD ‐1.42, (95% CI ‐1.74, ‐1.10), p<0.00001, number of infants (n) = 99]. There was no significant effect on the number of additional PIVs used in the first four weeks of life [MD ‐2.30, (95% CI ‐6.65, 2.05), n=36]. There was an increase in catheter malfunction in the ML‐UVCs group [typical RR 3.69 (95% CI 0.99, 13.81), p=0.05; RD 0.15 (95% CI 0.03, 0.27), p=0.01; NNH was 7, 95% CI 4, 33; n=99]. The following outcomes were not significantly different in the two groups: clinical sepsis, catheter related blood stream infection, catheter‐associated thrombosis, complications related to catheter malposition in heart and great vessels, NEC and early neonatal mortality.
Authors' conclusions
The use of ML‐UVCs in comparison to SL‐UVCs in neonates is associated with decrease in the usage of PIVs in first week of life, but an increase in catheter malfunctions. As the quality of included randomized studies is poor and the estimates of clinically important complications are imprecise, no firm recommendations can be made regarding the choice of UVC. Adequately powered, properly randomized and properly blinded controlled trials are needed that address the effectiveness and safety of ML‐UVCs (double and triple lumen) in comparison to SL‐UVCs. These studies should also address the impact of type of catheter material.
Plain language summary
Multiple versus single lumen umbilical venous catheters for newborn infants
Umbilical venous catheters (UVCs) are frequently used in newborn infants. These tubes into the body can have a single channel (lumen) or they can have two or three channels (multiple lumens). With more than one channel, multi‐lumen umbilical venous catheters (ML‐UVCs) can provide access for multiple purposes: for instance, administration of nutrition, blood products, or therapeutic drugs. It seems logical that use of ML‐UVCs would lower the need for additional venous lines, but this needs confirmation. Complications associated with UVCs are also a consideration. The channel diameter is generally narrower in ML‐UVCs. This could increase blockage and blood clot risks. Three clinical trials involving/completed by 113 babies were identified that compared double‐lumen catheters to single‐lumen catheters. None of the studies used triple‐lumen catheters. All three trials found that use of a double‐lumen catheter lowered the number of additional venous placements needed during the first week of life. The double‐lumen catheters, however, clogged, leaked, and broke more often. In these studies, no significant difference was found in catheter placement difficulty and misplacement, catheter‐related infections or blood clots, other serious complications, or rate of infant mortality. But the quality of studies was poor, and sample sizes were too small to draw valid conclusions about many complication rates. Available clinical trials at present do not provide a basis for recommending one catheter type over another in this setting.
Background
Reliable vascular access can be problematic in sick neonates (Ramachandran 1994). In newborn babies, peripheral intravenous lines (PIVs), umbilical venous catheters (UVCs) and percutaneously inserted central venous catheters (PICCs) are most often used to establish venous access (Moller 1995; Lesser 1996). The peripheral veins of a neonate are small and friable, can be difficult to cannulate, and are easily injured by irritating fluids and medications (Loisel 1996). The dwell time of PIVs can be shorter (Johnson 1988; Sheehan 1992). UVCs are one form of vascular access that can be used in neonates to deal with these difficulties (Ramachandran 1994; Moller 1995; Seguin 1994; Green 1998; Grupo 2000; MacDonald 1993; Pereira 1992). The umbilical vein was first used in 1947 for vascular access in newborn babies for exchange transfusion (Diamond 1947). The commonest complication associated with umbilical venous catheterization is nosocomial sepsis. The rates of UVC related nosocomial sepsis vary from 3 to 16% (Landers 1991; Seguin 1994; Raval 1995; Bhandari 1997; Hogan 1999). In one study, it was observed that the neonates with higher birth weight and receiving parenteral nutrition fluids were at increased risk of catheter related sepsis (Landers 1991). In same study, it was found that the duration of UVC in situ was not an independent risk factor for catheter related sepsis (Landers 1991). The other complications associated with umbilical venous catheterization include thrombo‐embolism (MacDonald 1986; Boo 1999; Roy 2002), catheter malpositioning in heart and great vessels (Savani 1990; Sigda 1992; Crie 1989; Johnson 1981) or portal venous system (Venkataraman 1984; Levkoff 1990; Narla 1991; Kim 2001), hepatic necrosis (Zipursky 1987), phlebitis (Zipursky 1987) and intrahepatic calcification (Richter 1984). Based on the evidence from two studies (Seguin 1994; Loisel 1996), the recent recommendations from The Hospital Infection Control Practices Advisory Committee, Centre for Disease Control and Prevention are: UVCs should be removed as soon as possible when no longer needed but can be used up to 14 days if managed aseptically (O'Grady 2002).
Multiple venous lines are often required in sick newborn babies, either because of a need for continuous infusion of drugs (e.g. vasoactive agents) or because of drug‐drug incompatibilities. This problem can theoretically be overcome by using multiple lumen‐UVCs. Use of multiple lumen UVCs (ML‐UVCs) instead of using single lumen UVCs (SL‐UVCs) may decrease the need for additional venous lines (Khilnani 1991;Ginsberg 1997; Goldstein 1992; Pinheiro 1992; Ramachandran 1992; Ramachandran 1994;Loisel 1996; Soupre 1998; Storme 1999). The possible theoretical risks involved with usage of ML‐UVCs in comparison to SL‐UVCs are increase in risks of blockage and thrombosis because of the narrow diameter of internal lumen. There seems to be significant variation between NICUs in choice and usage of ML‐UVCs versus SL‐UVCs. There are also variations in choice of material ("PVC"‐polyvinyl chloride, polyurethane, and "Silastic"‐ a silicon based polymer) from which UVCs are constructed. Newer materials like polyurethane and silastic based polymers are said to be less thrombogenic than standard polyvinyl chloride. In a systematic review, looking at the effect of catheter material on adverse outcomes associated with umbilical artery catheterization, there were no significant differences in outcomes between the standard PVC and other materials (Barrington 2000).
The aim of this systematic review was to collate and analyze data on effectiveness and safety from randomized controlled trials, comparing ML‐UVCs with SL‐UVCs. Although it seems self‐evident that ML‐UVCs would reduce the need of additional venous lines, the rates of associated complications might be different.
Objectives
The overall objective of this systematic review was to compare the effectiveness and the safety of ML‐UVCs versus SL‐UVCs. The primary objective was to compare the effectiveness of ML‐UVCs versus SL‐UVCs inserted in first four weeks of life in preterm and term neonates needing vascular access. This was assessed by estimating the total number of additional peripheral intravenous lines and total number of additional percutaneously and/or surgically placed central lines in first 4 weeks of life.
Secondary objectives were to compare the effectiveness of ML‐UVCs versus SL‐UVCs on: failure of insertion of UVCs, failure to insert the UVC tip position in desired position, duration of catheter in situ in days (catheter life span), catheter malfunction (leak around catheter, catheter obstruction, catheter breakage), complications (incidence of clinical sepsis, incidence of catheter related blood stream infection, catheter associated thrombosis, complications related to catheter malposition in heart and great vessels, complications related to catheter malposition in portal venous system, necrotizing enterocolitis, intraventricular haemorrhage), success of enteral feeding, and mortality.
The following subgroup analyses would be conducted to determine whether the results differ for:
Preterm infants (gestational age < 37 weeks) in comparison to term (gestational age of 37 to 41 weeks) infants
Postnatal age at study entry (up to first 7 days of life versus day 8‐28 of life)
Double vs triple lumen catheters
Catheter material (polyvinyl chloride, polyurethane, and "silastic"‐ a silicon based polymer)
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled clinical trials.
Types of participants
Both term (gestational age of 37 to 41 weeks) and preterm (gestational age < 37 weeks) infants who are in need of umbilical venous catheter insertion for vascular access in first four weeks of life, as defined by the attending medical staff.
Types of interventions
ML‐UVCs compared to SL‐UVCs for first and any subsequent umbilical venous catheterization. Studies in which different types of UVCs are inserted only for the purpose exchange transfusion were not eligible.
Types of outcome measures
Studies that report one or more of following outcomes amongst randomized The primary outcome measures are to compare the effectiveness of ML‐UVCs versus SL‐UVCs on:
Total number of additional peripheral intravenous lines per baby in first week of life
Total number of additional peripheral intravenous lines per baby in first four weeks of life
Total number of additional percutaneously and surgically placed central venous lines per baby in first four weeks of life
Secondary outcome measures are to compare the effectiveness and safety of ML‐UVCs versus SL‐UVCs on following. All of the secondary outcome measures except 'duration of catheter in situ in days' and 'success of enteral feeding' are dichotomous outcome measures (failure‐yes/no).
Failure of insertion of UVCs (proportion of babies having one or more such episode): defined as inability to insert the UVC in umbilical vein
Failure to insert the UVC tip position in desired position (proportion of babies having one or more such episode): the desired position of catheter tip is in the supradiaphragmatic part of inferior vena cava (IVC), proximal to IVC‐right atrium (RA) junction.
Duration of catheter in situ in days (catheter life span)
Catheter malfunction (proportion of babies having one or more such episode): The following three malfunctions were combined into a single dichotomous measure.
(a) Leak around catheter: defined as any leakage of infusion fluid around catheter at umbilical base (b) Catheter obstruction defined as complete blockage of the one or more catheter lumens: obstruction leading to inability to infuse the infusate or inability to sample blood through that lumen, necessitating abandoning the use of that lumen or removal of UVC (c) Catheter breakage: any fracture or breakage of UVC
Catheter complications:
(a) Incidence of clinical sepsis (proportion of babies having one or more such episode): should meet at least one of the following criteria: at least one of the following clinical signs or symptoms with no other recognized cause: fever (> 100.4°F [> 38°C]), hypothermia (< 98.6°F [< 37°C]), apnea, or bradycardia, and blood culture not done or no organisms or antigen detected in blood and no apparent infection at another site, and physician institutes treatment for sepsis (O'Grady 2002) (b) Incidence of catheter related bloodstream infection (CRBSI), (proportion of babies having one or more such episode): Bacteremia/fungemia in an infant with an intravascular catheter with at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infections (i.e., fever, chills, and/or hypotension), and no apparent source for the BSI except the catheter. One of the following should be present: a positive semiquantitative (> 15 CFU/catheter segment) or quantitative (> 103 CFU/catheter segment catheter) culture whereby the same organism (species and antibiogram) is isolated from the catheter segment and peripheral blood; simultaneous quantitative blood cultures with a > 5:1 ratio CVC versus peripheral; differential period of CVC culture versus peripheral blood culture positivity of > 2 hours (O'Grady 2002) (c) Catheter‐associated thrombosis (proportion of babies having one or more such episode): diagnosed either by contrast venography or ultrasound color doppler examination (d) Complications related to catheter malposition in heart and great vessels (proportion of babies having one or more such episode): pericardial effusion/cardiac tamponade, cardiac arrhythmias, thrombotic endocarditis, and hydrothorax (e) Complications related to catheter malposition in portal venous system (proportion of babies having one or more such episode): hepatic necrosis, hepatic cyst, and portal hypertension (f) Necrotizing enterocolitis (NEC) (Bell's stage II and III) (Bell 1978) (g) Intraventricular haemorrhage (IVH) any grade [defined as per Papile et al (Papile 1978)
Success of enteral feeding measured by
(a) Age in days at initiation of enteral feedings (b) Age in days of accomplishing full enteral feedings
All cause mortality (expressed as early neonatal mortality: death up to first seven days of age, and late neonatal mortality: death from day 8 to 28)
Search methods for identification of studies
See: Collaborative Review Group Search Strategy.
Randomized controlled and quasi‐randomized trials comparing multiple lumen versus single lumen umbilical venous catheter for vascular access in preterm and term neonates were identified from MEDLINE: PubMed‐National Library of Medicine (1966 to February 2005) using following subject headings (MeSH) and text word terms: neonate(s), newborn(s), infant(s), umbilic*, vein* OR venous*, dual* OR double*, multiple*, triple*, catheter*, umbilical venous catheter, double lumen catheter. No language restrictions were applied. After identifying the relevant articles, "related articles" function from MEDLINE was used to identify additional studies.
Other electronic databases that were searched including: EMBASE (1980 to February 2005), CINAHL (1982 to February 2005), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 4, 2004) and Science Direct (subject area: medicine, journal and abstract database; 1967 to February 2005). Three review authors (NS, MK and SS) performed the electronic database searches independently. Literature search also included a manual search of the abstracts of scientific meetings published in Pediatric Research (1990‐2004). Additional citations were sought using references in articles retrieved from searches. Subject experts were contacted to identify the unpublished and ongoing studies. Three review authors (NS, MK, and SS) independently screened the candidate articles to check the eligibility for inclusion in the review.
Data collection and analysis
The standardized review methods of the Cochrane Neonatal Review Group (CNRG) were used to assess the methodological quality of the studies.
For each trial, information was sought regarding method of randomization, blinding of allocation, blinding of intervention, completeness of follow up and blinding of outcome measure assessment for all the infants enrolled in trial. For the published article providing inadequate information for the review, an attempt was made to obtain additional information by contacting the primary author. Retrieved articles were assessed for quality and the data was independently abstracted by three review authors (NS, MK and SS). The three review authors were not blinded to authors, institution or journal of publication. The treatment effect estimators used were RR, RD, and WMD when appropriate along with their 95% CI. If RD was statistically significant, then number needed to treat (NNT) or number needed to harm (NNH) was calculated. A fixed effect model was used for meta‐analyses. Review Manager 4.2 software was used for statistical analysis. To test the robustness of results, a sensitivity analysis (excluding the studies in which blinding of outcome assessment was not achieved) was planned.
Results
Description of studies
Seven studies describing usage of ML‐UVCs were identified. Four of these seven studies were excluded because of following reasons: non‐randomised prospective study (Storme 1999), case series with no comparison group (Pinheiro 1992), retrospective study (Ramachandran 1994), and cost analysis review (Ginsberg 1997), see: Table on "Characteristics of excluded studies".
Three studies qualified for inclusion in this review (Khilnani 1991; Loisel 1996; Soupre 1998). All three studies are published as full text articles. Details of each study are given in the table "Characteristics of included studies". All three studies compared double lumen UVCs (DL‐UVCs) with SL‐UVCs. The inclusion criteria, types of SL‐UVCs and DL‐UVCs, duration of intervention and duration of follow up varied between studies. There were no studies that compared triple lumen UVCs with either SL‐UVCs or DL‐UVCs.
Khilnani et al (Khilnani 1991) randomized 43 critically ill neonates to receive DL‐UVCs versus SL‐UVCs. Single and double lumen 5‐Fr radiopaque polyurethane umbilical venous catheters (#C‐DUCO 5.0‐30, Cook Critical Care, Bloomington, IN) were used. Catheters were 30 cm long, with an outer diameter of 0.16 ± 0.005 cm. The mean inner diameter of the lumen was 0.057± 0.005 cm in double lumen catheters. The outcomes assessed were efficacy (need of additional peripheral IV lines) and complications (sepsis, hepatic necrosis, phlebitis, or mechanical complications). Loisel et al (Loisel 1996) randomized 123 neonates to receive DL‐UVCs versus SL‐UVCs versus a control group (no UVC; peripheral IVs only). The DL‐UVCs and SL‐UVCs groups were eligible for inclusion in this review. In the DL‐UVCs group, Arrow brand 4F double‐lumen polyurethane UVC was used whereas in SL‐UVC group, Argyle brand 3.5F or 5F single‐lumen polyvinyl chloride UVC was used. The outcomes assessed were: number of venipunctures, number of PIVs, complications associated with access, and cost of line placement for staff and supplies.
Soupre et al (Soupre 1998) randomized 20 neonates to receive DL‐UVCs versus SL‐UVCs. In the DL‐UVCs group, Arrow brand percutaneous dual‐lumen polyurethane catheter (3.5 CH x 38 cm) was used. In SL‐UVCs group: Sherwood single lumen polyvinyl chloride UVC (4Fr x13 cm) was used. This study evaluated the ease of insertion and safety of DL‐ UVCs versus SL‐UVCs.
Across the studies the baseline characteristics including birth weight and gestational age were comparable. All studies involved preterm and/or term neonates needing vascular access in first week of life. In two studies (Khilnani 1991; Loisel 1996), an infusion of heparin (0.5 to 1 U/ml of IV fluid) was used as a co‐intervention. In two studies (Khilnani 1991; Soupre 1998) no UVC insertion failed. However, failure of insertion of UVC occurred frequently in the study by Loisel et al (Loisel 1996) (13 in the DL‐UVCs group and 14 in the SL‐UVCs group).
Risk of bias in included studies
Details of included studies are presented in the table "Characteristics of included studies".
Khilnani et al (Khilnani 1991): This was a single centre study. Infants were randomized to receive DL‐UVCs or SL‐UVCs. The intervention was not blinded. Outcome and data presented for all 43 babies enrolled in study. Outcome measures were not blinded.
Loisel et al (Loisel 1996): This was a single centre study. Infants were randomized to one of three treatment arms: (1) SL‐UVCs, (2) DL‐UVCs (3) no UVCs: peripheral intravenous lines only. Prior to start of study, sample size was estimated and a randomization scheme, based on a random digits table was completed and cards were sealed in separate envelopes. Group assignment was stratified by birth weight (< 1200, 1200 ‐ 2500, > 2500 gm). The intervention was not blinded. Outcome and data presented for 56 of 123 infants randomized in study: DL‐UVCs (46 randomized, 25 withdrawn, and 21 completed study), SL‐UVCs (41 randomized, 26 withdrawn, and 15 completed study), and Control group: no UVC (36 randomised, 16 withdrawn, and 20 completed study). Out of the 46 randomized to DL‐UVCs, 25 were withdrawn from study for various reasons (in 13 cases UVC insertion failed, in one case UVC was not placed by physician, in three cases SL‐UVCs was placed instead of DL‐UVCs, one patient died, one patient was transferred back at < 7 days, in one patient IV access was discontinued at < 7days, one patient went for ECMO, in two patients UVC fell out and was not replaced, in one case UVC was discontinued because of pericardial effusion, and in one case data was missing). Out of the 41 randomized to SL‐UVCs, 26 were withdrawn from study for various reasons (in 14 cases UVC insertion failed, in one case UVC was not placed by physician, one patient died, three patients were transferred back at < 7 days, in one patient study protocol was not followed, four patients went for ECMO, in one patient UVC fell out and was not replaced, and in one case data were missing). The validity of results from this study is limited because of the large number of infants withdrawn post randomization; outcomes are reported only on the remainder. Outcome measures were not blinded.
Soupre et al (Soupre 1998): This was a single centre study. Infants were randomized to receive SL‐UVCs or DL‐UVCs. The intervention was not blinded. Outcome data presented for all 20 babies enrolled in study. Outcome measures were not blinded.
Effects of interventions
Primary Outcome Measures: 1. Total number of additional peripheral intravenous lines per baby in first week of life (Table 01.01): This outcome was reported in all three trials (Khilnani 1991; Loisel 1996; Soupre 1998); there was a decrease in the usage of additional PIVs in ML‐UVCs group in first week of life [WMD ‐1.42, (95% CI ‐1.74, ‐1.10), p<0.00001; number of infants (n) = 99].
2. Total number of additional peripheral intravenous lines per baby in first four weeks of life (Table 01.02): One study (Loisel 1996) reported this outcome. There was no significant effect on the usage of additional PIVs in the first four weeks of life [MD ‐2.30, (95% CI ‐6.65, 2.05); n=36].
3. Total number of additional percutaneously and surgically placed central venous lines per baby in first four weeks of life: Although this outcome was reported in one study (Loisel 1996), the numbers per treatment group were not extractable.
Secondary Outcome Measures 4. Failure of insertion of UVCs (Table 01.03): This outcome was reported in all trials (Khilnani 1991; Loisel 1996; Soupre 1998), and no significant difference was found in any trial. In overall analysis, no statistically significant difference in failure of insertion of UVCs was found in between the two groups [typical RR 0.79 (95% CI 0.45, 1.40); typical RD ‐ 0.05 (95% CI ‐0.18, 0.08); n=126].
5. Failure to insert the UVC tip in desired position (Table 01.04): Two studies (Khilnani 1991; Soupre 1998) reported this outcome and neither study found a statistically significant difference. Meta‐analysis showed no significant effect: typical RR 2.12 (95% CI 0.76, 5.94); typical RD 0.15 (‐0.03, 0.33); n=63.
6. Duration of catheter in situ in days (Table 01.05): All three included studies (Khilnani 1991; Loisel 1996; Soupre 1998) reported this outcome, and none found a significant effect. In overall analysis there was no significant difference between the two groups in the duration of UVCs in situ [WMD 0.06 days (95% CI ‐0.70, 0.83); n = 99].
7. Catheter malfunction (Leak around catheter/ Catheter obstruction/ Catheter breakage) (Table 01.06): One or more components of this outcome were reported in all three trials (Khilnani 1991; Loisel 1996; Soupre 1998). Khilnani et al (Khilnani 1991) reported on leak around catheter and catheter obstruction, but did not report on catheter breakage. Loisel et al (Loisel 1996) reported on catheter obstruction (difficulty in drawing blood), but did not report on leak around catheter or catheter breakage. Soupre et al (Soupre 1998) reported on leak around catheter but not catheter obstruction or breakage. The typical estimates from meta‐analysis showed that catheter malfunction occurred more frequently in ML‐UVCs group [typical RR 3.69 (95% CI 0.99, 13.81), p=0.05; typical RD 0.15 (95% CI 0.03, 0.27), p=0.01; n=99]. The NNH was 7, 95% CI 4, 33. Catheter malfunction events occurred most frequently in the study by Loisel et al (Loisel 1996). In that study, episodes of catheter obstruction (difficulty in drawing blood) occurred in eight infants in DL‐UVCs group as compared to one infant in SL‐UVCs group.
8. Clinical sepsis (Table 01.07): This outcome was reported in one trial (Loisel 1996). There was no statistical difference in incidence of clinical sepsis in between the two groups [RR 0.89 (95% CI 0.29, 2.78); RD ‐0.02 (95% CI ‐0.21, 0.18); n=36].
9. Catheter related bloodstream infection (CRBSI) (Table 01.08): Two trials reported this outcome (Khilnani 1991; Soupre 1998) and there was no occurrence of CRBSI in either study.
10. Catheter‐associated thrombosis (Table 01.09): One study (Soupre 1998) reported this outcome, no event occurred in either group. Though this outcome was also reported on some of the patients in another study (Loisel 1996), data are not extractable for analysis.
11. Complications related to catheter malposition in heart and great vessels (Table 01.10): This outcome was reported in one trial (Loisel 1996). There was no statistical difference in incidence of this complication in between the two groups [RR 2.09 (95% CI 0.09, 48.04); RD 0.05 (95% CI ‐0.09, 0.18); n=37].
12. Necrotizing enterocolitis (NEC) (Bell's stage II and III) (Table 01.11): This outcome was reported in one study (Loisel 1996). There was no statistical difference in incidence of NEC in between the two groups [RR 0.24 (95% CI 0.01, 5.57); RD ‐0.07 (95% CI ‐0.22, 0.09); n=36].
13. All cause early neonatal mortality (Table 01.12): Two studies (Loisel 1996; Soupre 1998) reported mortality in first seven days of life. In Loisel 1996, one patient from each group died (cause: complications related to prematurity) on the first day in the study. There were no early neonatal deaths in Soupre 1998. There was no statistically significant effect on mortality in either trial, or in the meta‐analysis: typical RR 0.73 (95% CI 0.05, 10.78); typical RD ‐0.01 (95% CI ‐0.13, 0.11); n=58.
No relevant data for the following outcome were available for analysis: complications related to catheter malposition in portal venous system, IVH, late neonatal mortality, and success of enteral feeding.
The planned sensitivity analysis after excluding the studies in which blinding of outcome assessment was not achieved was not possible, because in all of the three studies included in this review, the blinding of outcome assessment was not achieved. Due to lack data, the proposed subgroup analyses for preterm infants versus term infants and postnatal age at study entry (up to first seven days of life versus day 8‐28 of life) were not possible. There were no studies that compared the effect of various numbers of lumens amongst the multiple lumen UVCs (double versus triple lumen). No studies were found that compared the impact of various types of catheter material types (polyvinyl chloride/ polyurethane/ silastic) used for UVC construction.
Based on the protocol for this review, the study by Loisel et al (Loisel 1996) qualified for inclusion. However, in this study, 59% of the infants who were randomized to DL‐UVCs or SL‐UVCs were withdrawn after randomization. The reasons for these withdrawals are stated in the section on "Methodological qualities of included studies". Because of these post‐randomization withdrawals, the validity of the reported results from this study is limited. Therefore, although not specified in the protocol, a post hoc sensitivity analysis was performed after excluding the study by Loisel et al (Loisel 1996) in which completeness of follow‐up was not achieved on all randomized subjects. This sensitivity analysis could be performed on only three of the following outcomes.
Total number of additional peripheral intravenous lines per baby in first week of life: The results were similar to the original analysis; there was a decrease in the usage of PIVs in ML‐UVCs group in first week of life [WMD ‐1.50 (‐1.85, ‐1.15), p<0.00001; number of infants (n)=63]
Duration of catheter in situ in days: The results were similar to original analysis. There was no significant difference in the duration of UVCs in situ in between the two groups [WMD 0.02 (95% CI ‐0.78, 0.83); p=0.95, n = 63].
Catheter malfunction: The results for this outcome changed from borderline statistical significance to statistically insignificant. The typical estimates from meta‐analysis were [RR 2.19 (95% CI 0.35, 13.56), p=0.40; RD 0.06 (95% CI ‐0.07, 0.19), p=0.37; n=63].
The principal investigators of included trials are being contacted to get additional unreported information and, if further data become retrievable, they will be incorporated in a future update of this review.
Discussion
This review examines the evidence from randomized controlled trials on effectiveness and safety of the ML‐UVCs versus SL‐UVCs in neonates. Altogether in three studies (Khilnani 1991; Loisel 1996; Soupre 1998), a total of 150 neonates were randomized to DL‐UVCs versus SL‐UVCs, and outcomes were reported on 99 neonates. Only one (Loisel 1996) study described adequate randomization procedures and allocation concealment. None of the studies achieved blinding of intervention and blinding of outcome assessment. In two studies (Khilnani 1991; Soupre 1998), the follow‐up data were complete. In one study (Loisel 1996), outcomes were reported on 36 of 87 (41%) of infants who were randomized to either DL‐UVCs or SL‐UVCs.
The primary outcome measure of this review was the effectiveness of ML‐UVCs versus SL‐UVCs inserted in first four weeks of life in preterm and term neonates needing vascular access. This was assessed by estimating the total number of additional peripheral intravenous lines and total number of additional percutaneously and/or surgically placed central lines in first four weeks of life. Perhaps, arguably "additional line days" might have been a better primary outcome measure. "Additional line days" is a clinically more important than the "number of additional lines" as it takes into account the duration as well.
This review demonstrated that the use of ML‐UVCs is associated with decrease in the usage of PIVs in first week of life as compared to SL‐UVCs. The duration of UVCs in situ was less than a week in two studies (Khilnani 1991; Soupre 1998). There was no evidence of decrease in the usage of additional PIVs in ML‐UVCs group in first four weeks of life; this outcome was reported in only one study (Loisel 1996). This finding was not surprising as the duration of UVCs in situ was not up to first 4 weeks of life, but up to a maximum of 19 days in ML‐UVCs group and 17 days in SL‐UVCs group (Loisel 1996). It is expected that once the UVCs are removed the usage of PIVs will be likely to be similar in both groups.
This review also found that catheter malfunction occurred more frequently in ML‐UVCs group. However, in a post hoc sensitivity analysis that was performed after excluding the study by Loisel et al (Loisel 1996) in which completeness of follow‐up was not achieved on all randomized subjects, the results for this outcome changed from borderline statistically significant to insignificant. The results for this outcome were not robust.
There was no significant difference between the two groups in the incidence of failure of insertion of UVCs or failure to insert the UVC tip in desired position. There was no evidence of any difference between the two groups in the duration of UVCs in situ. The incidence of the following outcomes was not significantly different in the two groups: clinical sepsis, CRBSI, catheter‐associated thrombosis, complications related to catheter malposition in heart and great vessels, NEC and early neonatal mortality.
No relevant data for the following outcome were available for analysis: total number of additional percutaneously and surgically placed central venous lines per baby in first four weeks of life, complications related to catheter malposition in portal venous system, IVH, late neonatal mortality, and success of enteral feeding.
The strengths of this systematic review are a comprehensive literature search strategy and rigorous methodology. The validity of the results from this systematic review is restricted by the limitations in methodological quality of the primary studies. In addition, all three studies individually as well as the overall meta‐analysis have very small sample sizes and inadequate power to determine effects on some important clinical outcomes including: catheter malfunction, clinical sepsis, CRBSI, catheter obstruction, catheter associated thrombosis and mortality. These outcomes are of concern to clinicians, but there were too few events in the studies reviewed to draw any valid conclusions.
Authors' conclusions
Implications for practice.
The use of ML‐UVCs compared to SL‐UVCs is associated with decrease in the usage of PIVs in first week of life. The frequency of catheter malfunction is more frequent in ML‐UVCs group. As the quality of included randomized studies is poor and the estimates of clinically important complications are imprecise because of small sample sizes in studies, no firm recommendations can be made regarding the choice of UVC.
Implications for research.
Adequately powered, properly randomized and properly blinded controlled trials are needed that address the safety (clinically important complications) of ML‐UVCs (double and triple lumen) in comparison to SL‐UVCs in neonates. These studies should also address the impact of type of catheter material on these outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 15 October 2008 | Amended | Converted to new review format. |
Acknowledgements
Authors acknowledge Dr Samir Hussain for translation of two studies (Soupre 1998; Storme 1999) from French to English.
Data and analyses
Comparison 1. ML‐UVCs versus SL‐UVCs.
1.1. Analysis.
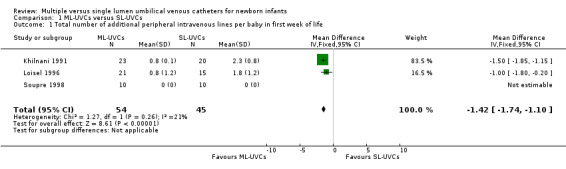
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 1 Total number of additional peripheral intravenous lines per baby in first week of life.
1.2. Analysis.
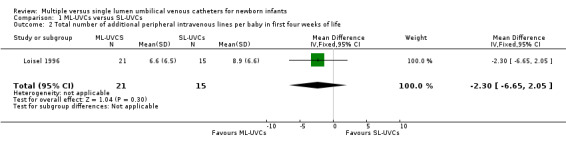
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 2 Total number of additional peripheral intravenous lines per baby in first four weeks of life.
1.3. Analysis.
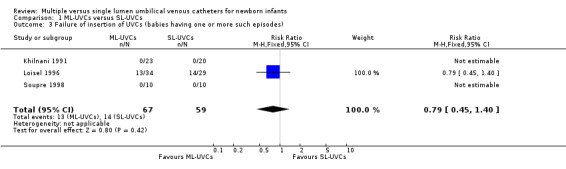
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 3 Failure of insertion of UVCs (babies having one or more such episodes).
1.4. Analysis.
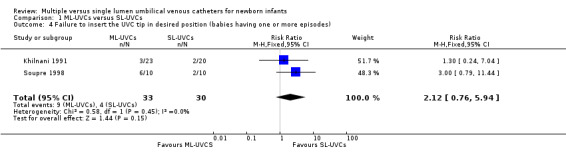
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 4 Failure to insert the UVC tip in desired position (babies having one or more episodes).
1.5. Analysis.
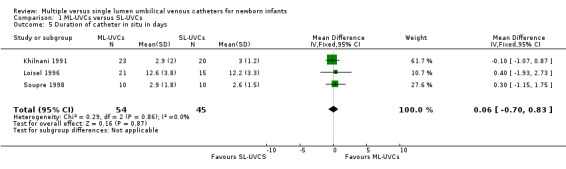
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 5 Duration of catheter in situ in days.
1.6. Analysis.
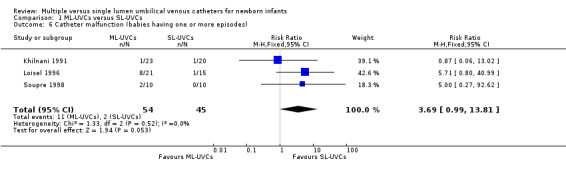
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 6 Catheter malfunction (babies having one or more episodes).
1.7. Analysis.
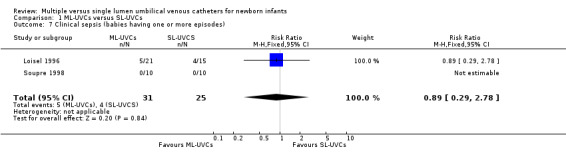
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 7 Clinical sepsis (babies having one or more episodes).
1.8. Analysis.
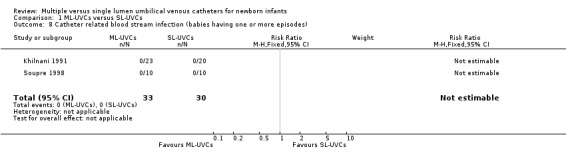
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 8 Catheter related blood stream infection (babies having one or more episodes).
1.9. Analysis.
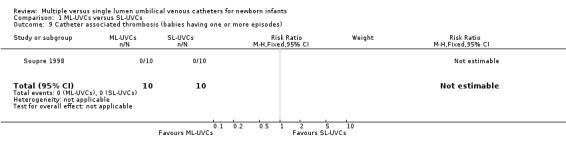
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 9 Catheter associated thrombosis (babies having one or more episodes).
1.10. Analysis.
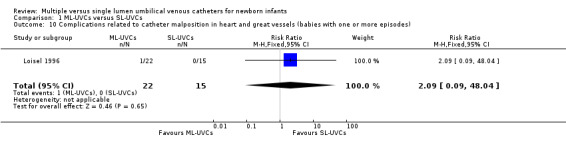
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 10 Complications related to catheter malposition in heart and great vessels (babies with one or more episodes).
1.11. Analysis.
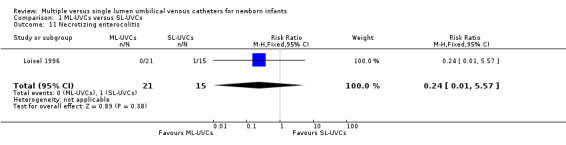
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 11 Necrotizing enterocolitis.
1.12. Analysis.
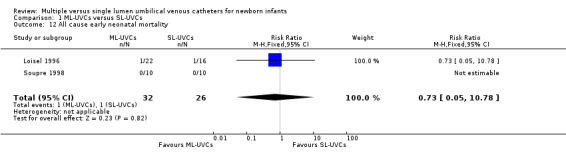
Comparison 1 ML‐UVCs versus SL‐UVCs, Outcome 12 All cause early neonatal mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Khilnani 1991.
| Methods | Single centre randomized study Blinding of randomization: Can't tell, methods not described Blinding of Intervention: No Blinding of outcome measurement: No Complete Follow‐up: Yes |
|
| Participants | 43 critically ill neonates. Demographic data (values presented as mean ± SD) 1. DL‐UVCs group: n=23, gestational age (weeks) 35.7 ± 4.3 2. SL‐UVCs group: n=20, gestational age (weeks) 35.3 ± 4.5 |
|
| Interventions | A standard UVC insertion technique was used. Single and double lumen 5‐Fr radiopaque polyurethane umbilical venous catheters (#C‐DUCO 5.0‐30, Cook Critical Care, Bloomington, IN) were used. Catheters were 30 cm long, with an outer diameter of 0.16 ± 0.005 cm. The mean inner diameter of the lumen was 0.057± 0.005 cm in double lumen catheters. Both lumens had distal openings at catheter. Both lumens of DL‐UVCs were used at all the times for the infusion of fluid and medications. Heparin (0.5U/ml) was used in all fluids infused through both SL‐UVCs and DL‐UVCs regardless of type of fluid infused. Types of fluid infused through both SL‐UVCs or DL‐UVCs included crystalloids, blood products, inotropic agents and medications. | |
| Outcomes | Efficacy and complications:
1. Need of additional peripheral IV lines
2. Complications: sepsis, hepatic necrosis, phlebitis, or mechanical complications. Catheter related sepsis was defined as two "positive" blood cultures for the same organism obtained at least 24 hours after umbilical catheter insertion. |
|
| Notes | This study centre had a policy of using soft silastic central venous catheters if central venous access was required for parenteral nutrition for prolonged periods, thereby limiting the use of UVCs to a maximum of 3 to 4 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Loisel 1996.
| Methods | Single centre randomized study Blinding of randomization: Yes, cards sealed in separate envelopes. Group assignment was stratified according to birth weight (<1200, 1200‐2500, >2500 gm) Blinding of intervention: No Blinding of outcome measurement: No Completeness of follow up: No |
|
| Participants | 123 neonates who met criteria were randomized. Infants became candidates for the study if they met the following criteria: age younger than 5 days, predicated need for intravenous therapy for more than 7 days, no complex congenital heart disease, not admitted to NICU because of the need for ECMO or operation, UVC not present, UVC not contraindicated, and informed parental consent. Randomized to 3 groups: 1. DL‐UVCs (46 randomized, 21 completed study). 2. SL‐UVCs (41 randomized, 15 completed study) 3. Control group: no UVC (36 randomised, 20 completed study) Demographic data (values presented as mean ± SD) of infants who completed the study: 1. DL‐UVCs group: n=21, gestational age (weeks) 30.0 ± 3.2, birth weight (grams) 1418 ± 813 2. SL‐UVC group: n=15, gestational age (weeks) 30.9 ± 3.4, birth weight (grams) 1605 ± 1031 3. Control group (no UVC): n=20, gestational age (weeks) 30.7 ±4.6, birth weight (grams) 1502 ± 816. |
|
| Interventions | 1. DL‐UVCs group: Arrow brand 4F double‐lumen polyurethane UVC.
2. SL‐UVC group: Argyle brand 3.5F or 5F single‐lumen polyvinyl chloride UVC
3. Control group (no UVCs): peripheral IVs only. Placement of UV catheter tip was confirmed by X‐ray and adjusted if necessary so that it would lie near the junction of right atrium and IVC. If the catheter failed to pass through ductus venosus, it was removed and patient was withdrawn from the study. Successfully placed UVCs were used up to 14 days to infuse blood products, hyperalimentation fluid, maintenance fluids, and medications and to obtain blood samples. Extra fluid not given to patients with DL‐UVCs. Maintenance fluid contained 1U of heparin/ ml. CVP monitoring was not done. Ultrasound examination of inferior vena cava was done 24‐48 hours before catheter removal. |
|
| Outcomes | 1. Number of venipunctures 2. Number of PIVs 3. Complications associated with access 4. Cost of line placement for staff and supplies | |
| Notes | The internal validity of the study was limited by the high rate of withdrawal, the randomness of which was not properly examined. Study results were based on treatment completers only, and the number of subjects enrolled was lower than that were determined adequate in the power calculations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Soupre 1998.
| Methods | Single centre randomized study Blinding of randomization: Can't tell, methods not described Blinding of intervention: No Blinding of outcome measurement: No Completeness of follow‐up: Yes |
|
| Participants | 20 neonates whose clinical condition required a central venous line were randomized to either DL‐UVCs or SL‐ UVCs Demographic data (values presented as mean ± SD): 1. DL‐UVCs group, n=10, gestational age (weeks) 32 ± 3, birth wight (grams) 1665 ± 643. 2. SL‐UVC group: n=10, gestational age (weeks) 32 ± 3, birth weight (grams) 1632 ± 656. |
|
| Interventions | 1. DL‐UVCs group: Arrow brand percutaneous dual‐lumen polyurethane catheter (3.5 CH x 38 cm) 2. SL‐UVCs group: Sherwood single lumen polyvinyl chloride UVC (4Fr x13 cm). Heparin infusion was not used with infusion fluids. |
|
| Outcomes | Evaluated the ease of insertion and safety of DL‐ UVCs versus SL‐UVCs. | |
| Notes | The duration of UVCs in situ was relatively short (<1 week). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ginsberg 1997 | This study was excluded because it was a cost analysis of DL‐UVCs versus SL‐UVCs. |
| Pinheiro 1992 | This study was excluded because it was a case series with no control group. Triple lumen catheters were inserted through umbilical vein in 13 neonates. |
| Ramachandran 1994 | This study was excluded because it was a retrospective study. 128 neonates: 70 in SL‐UVCs group, 58 in DL‐UVCs group. |
| Storme 1999 | This study was excluded because the treatment allocation was not random or quasi‐random. The population was divided in to two groups according to severity of respiratory failure. Group I (n = 52): normal hemodynamic parameters and moderate respiratory failure (FIO2 < 0.6), only SL‐UVC was used. Group II (n = 56): low systemic pressure requiring vascular filling and/or inotropic drug infusion, and/or severe respiratory failure (FIO2 > 0.6), in this group either SL‐UVC or DL‐UVC was used. |
Contributions of authors
Nandkishor Kabra: writing of protocol, literature search, abstraction and analysis of data, writing of review. Manoj Kumar: writing of protocol, literature search, abstraction and analysis of data and editing of review. Sachin Shah: writing of protocol, literature search, abstraction and analysis of data and editing of review.
Sources of support
Internal sources
McMaster University Medical Centre, Hamilton, Ontario, Canada.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Khilnani 1991 {published data only}
- Khilnani P, Goldstein B, Todres ID. Double lumen umbilical venous catheters in critically ill neonates: a randomized prospective study. Critical Care Medicine 1991;19:1348‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Loisel 1996 {published data only}
- Loisel DB, Smith MM, MacDonald MG, Martin GR. Intravenous access in newborn infants: impact of extended umbilical venous catheter use on requirement for peripheral venous lines. Journal of Perinatology 1996;16:461‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Soupre 1998 {published data only}
- Soupre D, Sizun J, De PL, Alix D. Use of dual‐lumen umbilical venous catheters in neonates [Utilisation de catheters a double lumiere par voie ombilicale chez le nouveau‐ne]. Annales De Pediatrie 1998;45:394‐8. [1998201865] [Google Scholar]
References to studies excluded from this review
Ginsberg 1997 {published data only}
- Ginsberg HG. Advantages of dual‐lumen umbilical vessel catheters versus single‐lumen umbilical vessel catheters and additional peripheral intravenous catheters. Journal of Perinatology 1997;17:218‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Pinheiro 1992 {published data only}
- Pinheiro JM, Fisher MA. Use of a triple‐lumen catheter for umbilical venous access in the neonate [comment in: J Pediatr 1992;121:499‐500]. Journal of Pediatrics 1992;120:624‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramachandran 1994 {published data only}
- Ramachandran P, Cohen RS, Kim EH, Glasscock GF. Experience with double‐lumen umbilical venous catheters in the low‐birth‐weight neonate. Journal of Perinatology 1994;14:280‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Storme 1999 {published data only}
- Storme L, Ouali M, Ganga‐Zandzou PS, Klosowski S, Rakza T, Fassler C, et al. Use of double‐lumen umbilical vein catheters in a neonatal intensive care unit [Utilisation de catheters veineux ombilicaux double voie en unite de soins intensifs neonatals]. Archives of Pediatrics 1999;6:386‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Barrington 2000
- Barrington KJ. Umbilical artery cathters in the newborn: effects of catheter materials. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000949] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhandari 1997
- Bhandari V, Eisenfeld L, Lerer T, Holman M, Rowe J. Nosocomial sepsis in neonates with single lumen vascular catheters. Indian Journal of Pediatrics 1997;64:529‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boo 1999
- Boo NY, Wong NC, Zulkifli SS, Lye MS. Risk factors associated with umbilical vascular catheter‐associated thrombosis in newborn infants. Journal of Paediatrics and Child Health 1999;35:460‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crie 1989
- Crie JS, Hajar R, Folger G. Umbilical catheter masquerading at echocardiography as a left atrial mass. Clinical Cardiology 1989;12:728‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diamond 1947
- Diamond LK. Erythroblastosis foetalis or haemolytic disease of the newborn. Proceedings of the Royal Society of Medicine 1947;40:546‐50. [PMC free article] [PubMed] [Google Scholar]
Goldstein 1992
- Goldstein B. Multiple‐lumen umbilical venous catheters [comment on: J Pediatr 1992;120:624‐6]. Journal of Pediatrics 1992;121:499‐500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Green 1998
- Green C, Yohannan MD. Umbilical arterial and venous catheters: placement, use, and complications. Neonatal Network 1998;17:23‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Grupo 2000
- Grupo de Hospitales Castrillo. Prospective evaluation of umbilical catheters in newborn infants. The Castrillo Hospital Group [Estudio prospectvo sobre el empleo de cateteres umbilcales en el recien nacido]. Anales Espanoles de Pediatria 2000;53:470‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Hogan 1999
- Hogan MJ. Neonatal vascular catheters and their complications. Radiologic Clinics of North America 1999;37:1109‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 1981
- Johnson DE, Base JL, Thompson TR, Foker JE, Speert DP, Kaplan EL. Candida septicemia and right atrial mass secondary to umbilical vein catheterization. American Journal of Diseases of Children 1981;135:275‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 1988
- Johnson RV, Donn SM. Life span of intravenous canulas in neonatal intensive care unit. American Journal of Diseases of Children 1988;142:968‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2001
- Kim JH, Lee YS, Kim SH, Lee SK, Lim MK, Kim HS. Does umbilical vein catheterization lead to portal vein thrombosis? Prospective US evaluation of 100 neonates. Radiology 2001;219:645‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Landers 1991
- Landers S, Moise AA, Fraley JK, Smith EO, Baker CJ. Factors associated with umbilical catheter‐related sepsis in neonates. American Journal of Diseases of Children 1991;145:675‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lesser 1996
- Lesser E, Chabra R, Brion LP, Suresh BR. Use of midline catheters in low birth weight infants. Journal of Perinatology 1996;16:205‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Levkoff 1990
- Levkoff AH, Macpherson RI. Intrahepatic encystment of umbilical vein catheter infusate. Pediatric Radiology 1990;20:360‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MacDonald 1986
- MacDonald MG, Chou MM. Preventing complications from lines and tubes. Seminars in Perinatology 1986;10:224‐33. [MEDLINE: ] [PubMed] [Google Scholar]
MacDonald 1993
- MacDonald MG. Umbilical vein catheterisation. In: Fletcher MA, MacDonald MG editor(s). Atlas of Procedures in Neonatology. 2nd Edition. Philadelphia: JB Lippincott, 1993:178‐87. [Google Scholar]
Moller 1995
- Moller JC, Reiss I, Schaible T. Vascular access in neonates and infants‐‐indications, routes, techniques and devices, complications. Intensive Care World 1995;12:48‐53. [MEDLINE: ] [PubMed] [Google Scholar]
Narla 1991
- Narla LD, Hom M, Lofland GK, Moskowitz WB. Evaluation of umbilical catheter and tube placement in premature infants. Radiographics 1991;11:849‐63. [DOI] [PubMed] [Google Scholar]
O'Grady 2002
- O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter‐related infections. The Hospital Infection Control Practices Advisory Committee, Center for Disease Control and Prevention. Pediatrics 2002;110:e51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92:529‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pereira 1992
- Pereira GR, Lim BK, Ing C, Medeiros HF. Umbilical vs peripheral vein catheterization for parenteral nutrition in sick premature neonates. Yonsei Medical Journal 1992;33:224‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramachandran 1992
- Ramachandran P, Cohen RS, Kim EH. Multiple‐lumen umbilical venous catheters [comment on: J Pediatr 1992;120:624‐6]. Journal of Pediatrics 1992;121:499. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raval 1995
- Raval NC, Gonzalez E, Bhat AM, Pearlman SA, Stefano JL. Umbilical venous catheters: evaluation of radiographics to determine position and associated complications of malpositioned umbilical venous catheters. American Journal of Perinatology 1995;12:201‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Richter 1984
- Richter E, Globl H, Holthusen W, Lassrich MA. Intrahepatic calcifications in infants and children following umbilical vein catheterization [Calcifications intra‐hepatiques du nourrisson et de l'enfant ares catheterisme de la veine ombilicale]. Annals of Radiology (Paris) 1984;27:117‐24. [MEDLINE: ] [PubMed] [Google Scholar]
Roy 2002
- Roy M, Turner‐Gomes S, Gill G, Way C, Mernagh J, Schmidt B. Accuracy of Doppler echocardiography for the diagnosis of thrombosis associated with umbilical venous catheters. Journal of Pediatrics 2002;140:131‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Savani 1990
- Savani RC, Valentini RP, Mimouni F. Neonatal radiology casebook. Pericardial effusion as a complication of umbilical catheterization. Journal of Perinatology 1990;10:443‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Seguin 1994
- Seguin J, Fletcher MA, Landers S, Brown D, Macpherson T. Umbilical venous catheterizations: audit by the Study Group for Complications of Perinatal Care. American Journal of Perinatology 1994;11:67‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sheehan 1992
- Sheehan AM, Palange K, Rasor JS, Moran MA. Significantly improved peripheral intravenous catheter performance in neonates: insertion of ease, dwell time, complication rate, and costs. Journal of Perinatology 1992;12:369‐76. [MEDLINE: ] [PubMed] [Google Scholar]
Sigda 1992
- Sigda M, Speights C, Thigpen J. Pericardial tamponade due to umbilical venous catheterization. Neonatal Network 1992;11:7‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Venkataraman 1984
- Venkataraman PS, Babcock DS, Tsang RC, Ballard JL. Hepatic injury: a possible complication of dopamine infusion through an inappropriately placed umbilical vein catheter. American Journal of Perinatology 1984;1:351‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zipursky 1987
- Zipursky A, Schmidt B. Neonatal thrombosis and embolism. In: Avery GB editor(s). Neonatology: Pathophysiology and management of the newborn. 3rd Edition. Philadelphia: JB Lippincott, 1987:687‐8. [Google Scholar]


