Abstract
Desmoid tumors (fibromatoses) are rare but locally aggressive tumors that do not metastasize. They are non-encapsulated, well-differentiated lesions made of fibroblasts and collagen, which mainly appear in the mesentery and abdominal wall. Rarely, these tumors can also occur in breasts, making up approximately 0.2% of all breast neoplasms. Treatment typically includes surgical excision and/or medical management.
We describe a case of a 31-year-old female presenting with a mass in her left axilla that was biopsy proven to be a desmoid tumor. In this case report, we discuss the various imaging findings present on ultrasound, mammography, computed tomography, and magnetic resonance imaging.
Keywords: Desmoid tumor, Fibromatoses, Breast masses, MRI, CT, Ultrasound, Mammography
Background
Desmoid tumors (fibromatoses) are rare but locally aggressive tumors that do not metastasize. Desmoid fibromatoses typically occur between ages 15 and 60 years and they appear twice as often in women than in men. Desmoid tumors are non-encapsulated, well-differentiated lesions made of fibroblasts and collagen and, although can occur sporadically, are often associated with Gardner syndrome and mutations in the APC/beta-catenin pathway [1]. Therefore, it mainly appears in the mesentery, abdominal wall, and less often in the extremities. Rarely, these tumors can also occur in breasts, making up approximately 0.2% of all breast neoplasms [1,2]. It has been proposed that desmoid tumors can arise de novo or from the rectus abdominal muscle aponeurosis. Specifically, in breast cases it is hypothesized that they may arise from prior trauma or breast surgery [3]. This case is of a woman with no past medical history of malignancy who presented with a mass in her left axilla that was biopsy proven to be a desmoid tumor. Below is a discussion of the imaging findings on ultrasound, CT, mammography, and MRI.
Case report
A 31-year-old female presented to an outside facility with a 2-month history of a progressively enlarging and painful lump in her left axilla. The patient had no other breast findings. The patient had no significant medical comorbidities or family history of breast cancer. An ultrasound was ordered that showed a round, ill-defined, spiculated, heterogenous, hypoechoic mass measuring approximately 4.4 × 3.0 × 4.8 cm with minimal internal vascularity concerning for neoplasm. Additionally, there was an irregular, enlarged adjacent lymph node measuring 2.9 × 1.0 × 1.6 cm. A CT chest with intravenous contrast was recommended to further characterize this region.
Contrast-enhanced CT imaging was performed at the same outside facility that showed an asymmetric, enhancing mass measuring 5 × 4.3 × 5.5 cm intercalating between the teres major and teres minor. At this time, tissue sampling was recommended.
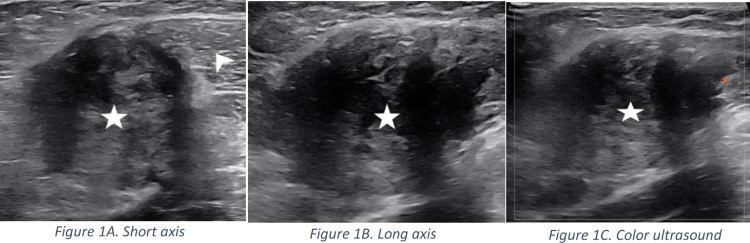
Subsequently, the patient presented to the comprehensive breast care center at our institution for evaluation and management. Unfortunately, the patient presented with a history of a palpable axillary finding and the outside imaging studies and reports were not available for review. A mammogram was obtained which showed no suspicious masses, distortion, or microcalcifications within the ipsilateral breast, however it did not include the area of concern in the axilla. A left axillary ultrasound was performed in the area of palpable concern showing an irregular, avascular, intramuscular mass with indistinct margins measuring 3.2 × 3.2 × 5.1 cm (Figs. 1A, B and C). Given its intramuscular location it was deemed inappropriate to biopsy the mass in the breast clinic and therefore the patient was referred to orthopedic oncology for management.
Fig. 1.
Short axis (Fig. 1A) and long axis (Fig. 1B) ultrasound images obtained in the Breast clinic. Mammography is not shown as the area of interest was not included in the images. A heterogeneous mass (star) with irregular margins and areas of through transmission measuring 3.2 × 3.2 × 5.1 cm is noted within a muscle (arrowhead) of the shoulder. Color ultrasound image (Fig. 1C) does not demonstrate internal vascularity.
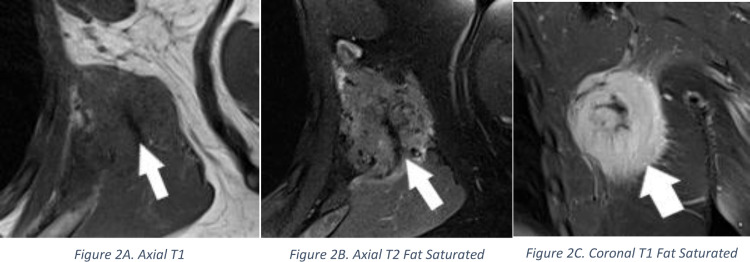
Orthopedic oncology ordered an MRI of the chest wall with and without contrast to further evaluate this mass. MRI showed a 3.9 × 4.4 × 4.9 cm enhancing left chest wall mass involving the latissimus dorsi muscle (Figs. 2A, B and C). Imaging features were nonspecific. Soft tissue sarcoma was considered the diagnosis of exclusion. A core biopsy was recommended, and the needle pathway was discussed with orthopedic oncology prior to biopsy.
Fig. 2.
Axial T1 (Fig. 2A), Axial T2 Fat Saturated (Fig. 2B), and Coronal T1 Fat Saturated Post Contrast (Fig. 2C) images demonstrate a low T1 and T2 signal mass (arrow) with avid post contrast enhancement measuring 3.9 × 4.4 × 4.9 cm within the latissimus dorsi muscle.
A core biopsy of this mass revealed bland spindle cells arranged in fascicles. No necrosis, mitotic activity or cytologic atypia was present. Immunohistochemical stains were performed, but the findings were not specific (positive for beta-catenin and SMA; negative for S100). Overall, the features were most suggestive of fibromatosis (desmoid tumor), with no evidence of malignancy.
Following discussion with the patient, Sorafenib therapy was initiated with plans to excise the mass at a future date.
Discussion
Desmoid tumors (fibromatoses) are locally aggressive benign tumors that do not metastasize. Due to a possible hormonal dependency of desmoid growth, these tumors occur slightly more frequently in women compared to men. Desmoid tumors may arise at anybody site, however they are more frequently encountered in the abdomen, extremities, neck, and chest wall. They are very rare with an annual incidence of approximately 900 cases in the United States and make up 0.03% of all tumors and 3% of soft tissue tumors [4]. Specifically, they represent approximately 0.2% of all breast neoplasms [1,2]. Additionally, there is a known association between hereditary cancer syndromes (eg, familial adenomatous polyposis and Gardener syndrome) and abdominal desmoid tumors.
Ultrasound is an important screening tool with many benefits including low-cost, accessibility, and lack of ionizing radiation. On ultrasound, desmoid tumors often have a variable appearance. They may appear as round, well-circumscribed, heterogenous masses, though they also commonly appear as irregular, spiculated, hypoechoic masses with color Doppler flow mimicking malignancy [1,5].
On mammography, desmoid tumors typically appear as an irregular, spiculated mass. Therefore, mammographic findings typically mimic malignancy [5,6].
CT imaging may be useful when evaluating abdominal desmoid tumors, however, it is rarely used when evaluating breast masses. On CT, desmoid tumors will have a highly variable appearance. They commonly appear as soft tissue attenuating masses that may be well-defined with spiculated margins (abdominal wall tumors), or as ill-defined masses (extra-abdominal wall tumors). Due to their collagen/myxoid mixture, most are high attenuating [7].
MR imaging of desmoid tumors is useful in evaluating extent of disease and in pre-surgical planning. On T1-weighted images, they will appear hypointense; on T2-weighted images, they will appear heterogenous and hyperintense; finally, on T1-weighted post contrast images, they will show enhancement [5].
Definitive diagnosis of desmoid tumors requires biopsy with histopathologic confirmation. On histology, the tumor will show an imprecise pattern with varying cellularity, including a mass of bland spindle cells arranged in fascicles. Due to its less aggressive nature, it will not typically show nuclear atypia or mitotic activity [6,8]. Many desmoid tumors are highly associated with APC/beta-catenin pathway, and so on immunohistochemistry, they are positive for beta-catenin [8]. Additionally, desmoid tumors are strongly positive with vimentin [4], variably positive with SMA, and negative with S100.
There are several treatment approaches that may be taken with regards to desmoid tumors. Often, patients with asymptomatic disease will opt for active surveillance. However, in symptomatic patients, or patient with cosmetic concerns, treatment is favorable. Typically, surgical resection is the standard first line treatment. This involves completely excising the mass with negative margins. Large tumor size and positive margins can result in high recurrence rates [8].
Patient with extra-abdominal desmoid tumors may undergo systematic therapy with chemotherapeutic agents such as tyrosine kinase inhibitors. While a reasonable alternative to surgery in shrinking tumor volume, it may only provide a partial response [9]. Other systemic therapies such as tamoxifen or NSAIDs have also been shown to provide varying treatment response.
Finally, radiation therapy is typically reserved for non-surgical candidates or patients with recurrences, but with longer regression times.
Conclusion
Desmoid tumors are locally aggressive, benign tumors that do not metastasize. They can be difficult to diagnose due to their variable appearance and malignant-mimicking features. Tissue sampling is necessary for definitive diagnosis. Treatment approach favors wide local excision if present in the breast, however large tumor size and positive margins can result in high recurrence rates [8]. Desmoid tumors outside of the breast are typically treated with chemotherapy, which includes, for example, tyrosine-kinase inhibitors (eg, Sorafenib) with possible surgical excision [9].
Patient consent
The author (s) should confirm that written informed consent has been obtained from the involved patient (s) or if appropriate from the parent, guardian, power of attorney of the involved patient (s); and, they have given approval for this information to be published in this case report (series).
Footnotes
Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Rammohan A, Wood JJ. Desmoid tumour of the breast as a manifestation of Gardner's syndrome. Int J Surg Case Rep. 2012;3(5):139–142. doi: 10.1016/j.ijscr.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erguvan-Dogan B, Dempsey PJ, Ayyar G, Gilcrease MZ. Primary desmoid tumor (extraabdominal fibromatosis) of the breast. AJR Am J Roentgenol. 2005;185(2):488–489. doi: 10.2214/ajr.185.2.01850488. [DOI] [PubMed] [Google Scholar]
- 3.Povoski SP, Jimenez RE. Fibromatosis (desmoid tumor) of the breast mimicking a case of ipsilateral metachronous breast cancer. World J Surg Oncol. 2006;4(1):57. doi: 10.1186/1477-7819-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escobar C, Munker R, Thomas JO, Li BD, Burton GV. Update on desmoid tumors. Ann Oncol. 2012;23(3):562–569. doi: 10.1093/annonc/mdr386. [DOI] [PubMed] [Google Scholar]
- 5.Cardenosa G. Wolters Kluwer; Philadelphia: 2017. Breast imaging companion. Fourth edition. [Google Scholar]
- 6.Glazebrook KN, Reynolds CA. Mammary fibromatosis. AJR Am J Roentgenol. 2009;193(3):856–860. doi: 10.2214/AJR.08.1892. [DOI] [PubMed] [Google Scholar]
- 7.Casillas J, Sais GJ, Greve JL, Iparraguirre MC, Morillo G. Imaging of intra- and extraabdominal desmoid tumors. RadioGraphics. 1991;11(6):959–968. doi: 10.1148/radiographics.11.6.1749859. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzen J, Cramer M, Buck N, Friedrichs K, Graubner K, Lühr CS, et al. Desmoid type fibromatosis of the breast: ten-year institutional results of imaging, histopathology, and surgery. Breast Care (Basel) 2021;16(1):77–84. doi: 10.1159/000507842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheer L, Lodi M, Molière S, Kurtz J-E, Mathelin C. Medical treatment of mammary desmoid-type fibromatosis: which benefit? World J Surg Oncol. 2017;15(1):86. doi: 10.1186/s12957-017-1148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]