Abstract
We report a case of multifocal cystic meningioangiomatosis (MA), an exceptionally uncommon diagnosis even in patients with type 2 neurofibromatosis (NF2). A 2-year-old male with personal as well as family history of genetically-confirmed NF2 presented with incidental findings of MA after imaging for closed-head injury. Computed tomography and magnetic resonance imaging revealed multifocal subcortical and basal ganglia cysts, enhancing tumor-like vascular encasement, and a cerebellar ependymoma with atypical features. Given the paucity of available literature describing this pathology, imaging findings are discussed to further characterize this elusive disease. Radiologists must keep in mind that children with NF2 may not only present with MA, but also a constellation of MA with classic NF tumors, including ependymoma as in this case.
Keywords: Meningioangiomatosis, Cystic, Neurofibromatosis, Neurofibromatosis type 2, Ependymoma
Case report
Meningioangiomatosis is a complex and heterogeneous entity of both children and adults that can present stochastically but has also been linked to NF2. MA is a proliferative angiomatous lesion originating primarily from the leptomeninges and cerebral cortex. We report a case of multifocal meningioangiomatosis with cystic spaces, intraparenchymal FLAIR changes and calcifications, hyperenhancing tumor-like vascular encasement, and right cerebellar mass with atypical features.
A 2-year-old male presented to an outside facility after falling and hitting his forehead on a tile floor. No loss of consciousness was reported, but his parents noticed new persistently unstable ambulation at home, prompting a visit to his local emergency department. Past medical history was significant for amblyopia and a pathogenic variant of NF2, discovered after targeted genetic testing due to a positive family history, which included his father, paternal half-brother, and paternal grandmother (who passed away at age 40 from intracranial aneurysm).
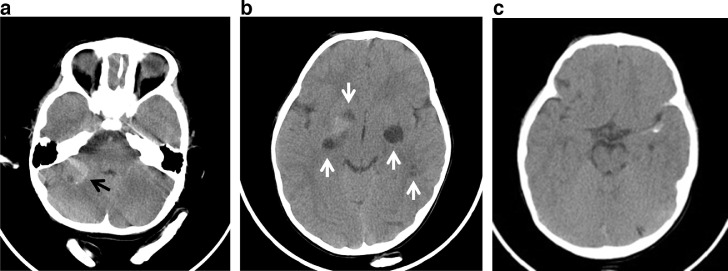
His neurofibromatosis had been managed expectantly, without any symptoms to warrant prior neurological imaging. Radiologic evaluation in the ER initially consisted of head CT for CHI. He was then referred to a level 1 pediatric center for higher level of care given multiple incidental intracranial abnormalities on CT, including a right posterior fossa hyperdense mass, linear hyperdensity encasing the left middle cerebral artery (MCA), and supratentorial hypodense lesions (Fig. 1).
Fig. 1.
Axial CT (A) Heterogeneous, predominantly hyperdense mass in the right posterior fossa (black arrow) centered over the right cerebellar hemisphere, not the cerebellopontine or cerebellomedullary angles. There are cystic changes within the mass and punctate peripheral calcification. (B) Cystic changes in the bilateral basal ganglia, extending into the temporal stem on the left, and right frontal operculum. Diffuse calcification in the right basal ganglia extends to the superior aspect of the medial temporal lobe. (C) Linear calcification along the margins of left MCA.
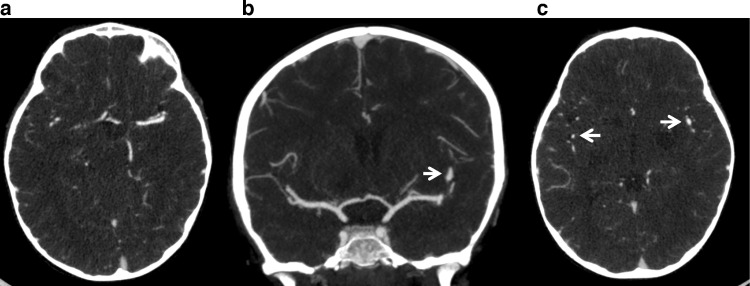
Upon transfer, physical examination was positive for bruised right forehead contusion, as well as unsteady gait and tendency to fall to the left side. Head and neck CT angiography was obtained to rule out possible MCA aneurysm. Findings were negative for focal aneurysm but did show some underlying irregularity; there was mild ectasia of the left MCA M2 segment within the sylvian fissure (Fig. 2).
Fig. 2.
Axial (A, C) and coronal (B) CT angiogram of the head are negative for focal aneurysm or perivascular contrast enhancement. Mild ectasia of the M2 segment of the left MCA compared to right within the Sylvian fissure (arrows).
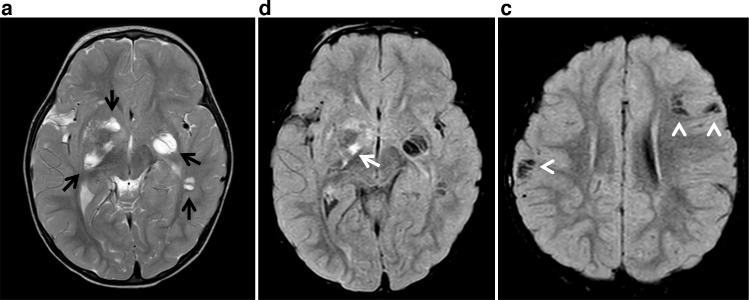
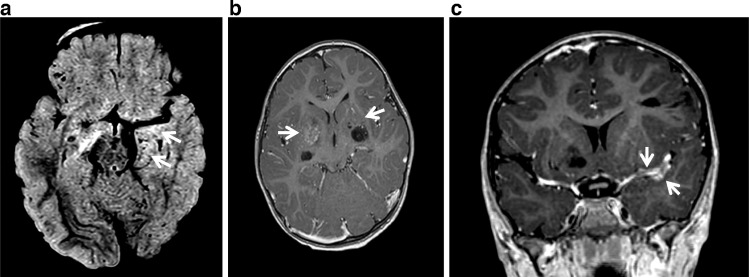
MR analysis of the brain revealed multiple supratentorial cystic spaces, which followed cerebrospinal fluid signal on all fluid-weighted sequences. These spaces exhibited variable enhancement and perilesional hyperintensity on FLAIR sequence, involving the bilateral basal ganglia, left inferior frontal gyrus, right frontal operculum, left superomedial temporal and posterior frontal cortex abutting the left MCA course (Fig. 3). FLAIR hyperintensity and linear enhancement was noted in medial and frontal lobe cortex abutting the left MCA M1 segment extending into the Sylvian fissure (Figs. 3 and 4). There was also enhancement in the inferior regions of the bilateral basal ganglia with crowding of lenticulostriate vessels (Fig. 4).
Fig. 3.
Axial T2WI (A) and axial FLAIR (B, C) demonstrate cystic changes in the bilateral basal ganglia, extending into the temporal stem on the left, and right frontal operculum (black arrows). There is prominent parenchymal FLAIR hyperintensity associated with the right basal ganglia lesion (white arrow) and additional cystic spaces involving the right inferior parietal lobule as well as left inferior frontal gyrus subcortical white matter with complete FLAIR suppression (arrowheads).
Fig. 4.
Axial FLAIR (A) shows subtle FLAIR hyperintensities also noted at the inferior aspect of the left basal ganglia cystic lesion and along the left MCA. Axial (B) postcontrast T1WI demonstrates enhancement in the inferior regions of the bilateral basal ganglia with crowding of lenticulostriate vessels, more pronounced on the right. Coronal (C) postcontrast T1WI demonstrates linear enhancement in the left inferomedial frontal lobe and left superomedial temporal lobe abutting the M1 segment of the left MCA extending to the sylvian fissure.
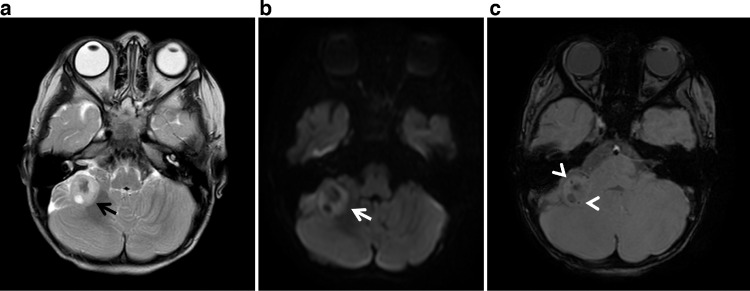
Multisequence MRI of the lateral right cerebellar mass revealed a peripheral rind of enhancement as well as reduced diffusivity, punctate calcification, and cyst formation (Figs. 5 and 6). MR spectroscopy displayed a nonspecific high choline peak (Fig. 6). Given its atypical features, prospective differential at that time included peripheral medulloblastoma, aggressive form of meningioangiomatosis, or intraparenchymal ependymoma. No nerve sheath tumor or typical meningioma was identified. MR investigation of the spine was negative for drop metastasis or spinal nerve sheath tumor.
Fig. 5.
Axial T2WI (A), axial DWI (B) and SWI (C) demonstrate a heterogenous intra-axial mass in the right cerebellar hemisphere with peripheral T2 hyperintensity and focal cystic change (black arrow). A peripheral rind of reduced diffusivity (white arrow) and punctate calcification (arrowheads) are also noted.
Fig. 6.
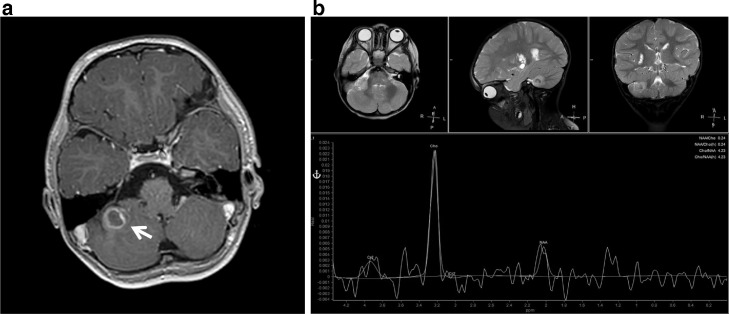
Axial postcontrast T1WI (A) and MR spectroscopy (B) demonstrate a right cerebellar mass with peripheral rind of enhancement. The lesion has a high choline peak, small lactate peak, and decreased NAA peak.
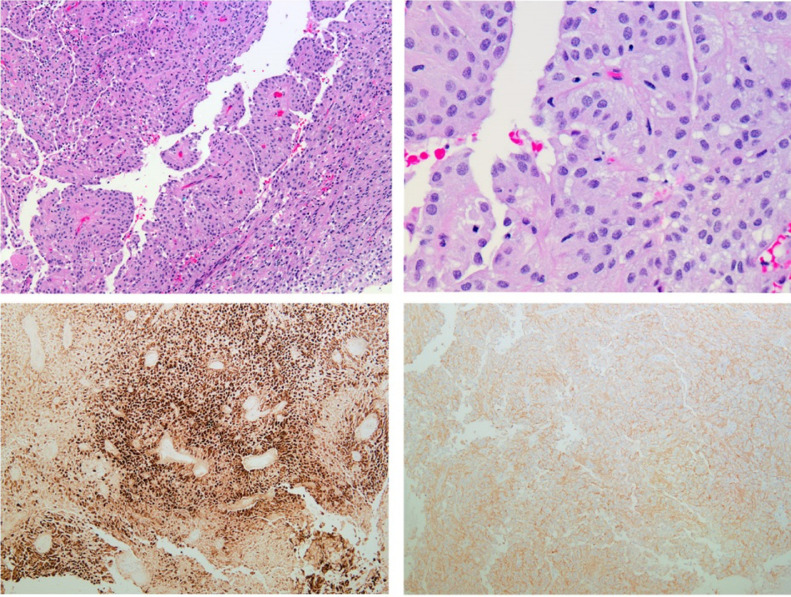
Neurosurgery performed a right-sided retrosigmoid craniotomy for cerebellar tumor resection. Histopathology and immunoprofile were compatible with a posterior fossa ependymoma, WHO grade II. H3k27me3 genotyping was positive, consistent with PFE-B, which would be quite rare in this age group (Fig. 7). The tumor had a low MIB-1 index at 2%-5%, indicating that it was not rapidly dividing. At 6-month follow-up, he continued to be a happy and playful 2-year-old boy.
Fig. 7.
Cerebellar ependymoma. Microscopic examination demonstrates a papillary neoplasm composed of tumor cells with relatively uniform round to oval nuclei and abundant eosinophilic cytoplasm (A, B). The tumor cells show patchy strong immunoreactivity for GFAP (C). CD99 stain demonstrates a membranous staining pattern (D).
Discussion
NF2 is a neurocutaneous disorder with a birth incidence of 1 in 27,956 and prevalence of 1 in 50,000. Affected individuals have a predisposition for developing schwannomas, meningiomas and ependymomas. Other described features include ocular lesions (cataracts, retinal detachment), meningeal angiomatosis, and glial hamartomas.
We report a case of a young patient with NF2, who presented with findings of cystic meningioangiomatosis, enhancing plaque-like lesion circumferentially encasing the left MCA, and a right cerebellar WHO grade II ependymoma. Our patient did not exhibit intracranial schwannomas or meningiomas. It has been observed that a preponderance of young children does not present with bilateral vestibular schwannomas, which are traditionally considered pathognomonic for this neurocutaneous disorder.
Meningioangiomatosis is a rare—and historically poorly-understood—disorder of the brain, typically involving the leptomeninges and subjacent cortex [1]. However, lesions have also been shown to arise from the cerebellum, subcortical white matter, and deep gray matter, albeit to a lesser extent [2], [3], [4]. This meningovascular process can be sporadic or in conjunction with NF2. In particular, NF-associated MA often presents asymptomatically and is only discovered as an incidental finding, as in this case [[1], [2],5]. Medically refractory seizures and headache can be presenting symptoms of sporadic MA, but epileptogenic lesions are seldom reported with underlying NF [6], [7]. NF-related MA especially tends to manifest as a nonprogressive disease with a low proliferation index, likely contributing to a high level of functionality in these patients [5,7].
Imaging characteristics of MA are nonspecific and highly variable [1], [2]. Nonetheless, the literature does delineate several overarching themes. For example, MA in the NF population tends to be multifocal or diffuse with predilection for the frontal, temporal and parietal lobes [[1], [2],[5], [6], [7]]. This appears to be substantiated in our case, which highlights multifocal cystic lesions in a frontotemporoparietal distribution (Figs. 3 and 4).
The vast majority of abnormalities on head CT are hypodense (Fig. 1). A first-line modality in many instances, CT has also been validated in as high as 90% of MA reports for identifying calcifications, ranging in morphology from psammomatous to a dense osteoid type [2,7]. In our patient, punctate calcifications were noted on CT in the right basal ganglia as well as right cerebellar mass, further supported on SWI (Figs 1 and 5).
Despite being an angiomatous disease, cerebral angiography is likely to be unremarkable, but there have been scarce reports of hypervascularity or even vascular malformations [2]. Our case was also an exception to the rule, as CT angiogram and postcontrast T1-weighted imaging detected asymmetric ectasia of the left MCA at its M2 branch (Figs. 2 and 4).
Another prevailing hallmark of meningioangiomatosis is heterogenous gadolinium enhancement, although a specific pattern is discrepant in the literature [2,5]. Subtle enhancement was seen in the left frontal and temporal lobes along the proximal course of the left MCA (Fig. 4). These findings appear to closely parallel a case reported by Jamil et al., in which a 3-year-old girl underwent left frontotemporal craniotomy for left Sylvian fissure meningioangiomatosis [8].
In our patient, numerous cystic foci were cortical-based but others were also found in subcortical white matter and deep motor nuclei. These likely represent Virchow-Robin spaces, similar to findings in a study by Fedi et al. with pathologically-confirmed perivascular cysts [7]. These enlarged spaces gain their morphology when plaque-like meningothelial and fibroblastic cells proliferate in an angiocentric fashion along leptomeningeal and intracortical blood vessels [1], [2].
One proposed mechanism describes an obstructive process in which aberrant arachnoid and vascular cells become ensnared in cortical sulci, allowing cerebrospinal fluid to slowly accumulate over time, forming cysts within trapped tissue [7]. In general, there is precedent in the literature that histopathology will favor either a predominant cellular or vascular component [1], [2]. MR imaging in our case also demonstrated regional crowding of lenticulostriate vessels adjacent to these cysts in the basal ganglia, which recapitulates the architectural distortion described on a cellular level (Fig. 4).
MA has been diagnosed concurrent with CNS tumors, frequently meningioma but also schwannoma (especially in NF-associated cases), oligodendroglioma, and primary fibrosarcoma of the CNS [2,5]. Remarkably, a literature search of case reports did not reveal any evidence of MA with ancillary findings of a cerebellar ependymoma (Figs. 5 and 6). Cerebellar MA has been reported by Bulut et al., in which a mass in the left cerebellar hemisphere was intra-axial without dural connection, as in our case. However, histopathology in their case was positive for fibrous tissue and hyalinized cerebellar vasculature coated with proliferating spindle cells and excessive psammoma bodies [3].
Among over 200 reported studies of MA up to this point, the literature has put forth a myriad of hypotheses, ranging from developmental, dysplastic, hamartomatous, and reactive etiologies [2]. In NF-related MA, it is reasonably conjectured that the inherited tumor suppressor gene pathway is a causative factor [4,6]. MA has been demonstrated to mimic neoplasms, tumefactive demyelination, neuroinflammatory lesion and vasculitis. Moreover, it can even imitate a progressive tumor with clinical and imaging recurrence [5].
Preoperative identification can be challenging due to its rarity and variable appearance, and often a definitive diagnosis is not possible radiologically without biopsy confirmation [3,6]. In cases of epilepsy-related disease, surgical excision is essential not only for pathological diagnosis but also for seizure control. Notably, meningioangiomatosis will not typically recur after surgical resection and usually does not necessitate adjuvant treatment.
Given that NF-related MA can be benign and indolent, an asymptomatic patient could present for the first time, without any known history of NF2. For this reason, it is imperative that radiologists are well-versed in imaging findings of MA. Familiarity with characteristic features can increase interobserver specificity and lend greater confidence in recognition of this extremely rare condition. Theoretically, an experienced imager could recognize MA and raise clinical suspicion, prompting a genetic workup for underlying NF. We report this case of cystic MA to further elucidate common radiological findings, with intent of improving detection and patient care.
Patient consent
Patient consent was obtained.
Footnotes
Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2022.01.050.
Appendix. Supplementary materials
References
- 1.Wiebe S., Munoz D.G., Smith S., Lee D.H. Meningioangiomatosis. A comprehensive analysis of clinical and laboratory features. Brain. 1999;122(Pt 4):709–726. doi: 10.1093/brain/122.4.709. PMID: 10219783. [DOI] [PubMed] [Google Scholar]
- 2.Tomkinson C., Lu J.Q. Meningioangiomatosis: A review of the variable manifestations and complex pathophysiology. J Neurol Sci. 2018;392:130–136. doi: 10.1016/j.jns.2018.07.018. Epub 2018 Jul 24. PMID: 30056201. [DOI] [PubMed] [Google Scholar]
- 3.Bulut E., Mut M., Soylemezoglu F., Oguz K.K. Meningioangiomatosis of the cerebellum: radiopathologic characteristics of a case. Acta Neurochir (Wien) 2015;157(8):1371–1372. doi: 10.1007/s00701-015-2487-4. Epub 2015 Jul 2. PMID: 26126763. [DOI] [PubMed] [Google Scholar]
- 4.Omeis I., Hillard V.H., Braun A., Benzil D.L., Murali R., Harter D.H. Meningioangiomatosis associated with neurofibromatosis: report of 2 cases in a single family and review of the literature. Surg Neurol. 2006;65(6):595–603. doi: 10.1016/j.surneu.2005.09.034. PMID: 16720184. [DOI] [PubMed] [Google Scholar]
- 5.Roux A., Zanello M., Mancusi R.L., Still M.E.H., Nascimento F.A., Tauziede-Espariat A., et al. Meningioangiomatosis: multimodal analysis and insights from a systematic review. Neurology. 2021;96(6):274–286. doi: 10.1212/WNL.0000000000011372. Epub 2020 Dec 22. PMID: 33361266. [DOI] [PubMed] [Google Scholar]
- 6.Oyedokun K., Agabna M.M., Israni A., du Plessis D. Meningioangiomatosis: an uncommon cause of focal epilepsy with characteristic neuroimaging and neuropathology. BMJ Case Rep. 2021;14(6) doi: 10.1136/bcr-2021-242953. PMID: 34117000; PMCID: PMC8201970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedi M., Kalnins R.M., Shuey N., Fitt G.J., Newton M., Mitchell L.A. Cystic meningioangiomatosis in neurofibromatosis type 2: an MRI-pathological study. Br J Radiol. 2009;82(979):e129–e132. doi: 10.1259/bjr/56536580. PMID: 19541939. [DOI] [PubMed] [Google Scholar]
- 8.Jamil O., Ramkissoon S., Folkerth R., Smith E. Multifocal meningioangiomatosis in a 3-year-old patient. J Neurosurg Pediatr. 2012;10(6):486–489. doi: 10.3171/2012.9.PEDS1224. Epub 2012 Sep 28. PMID: 23020197; PMCID: PMC3762590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.