Abstract
Objectives: Traditional Chinese medicine has been reported to be effective in the treatment of epidemic diseases. Here, we aimed to investigate the effects of combined therapy of Chinese and western medicine on coronavirus disease 2019 (COVID-19). Methods: A total of 60 patients diagnosed with COVID-19 were enrolled. Both the ordinary and severely affected patients were randomly divided into Groups A-C each with 10 cases each. The patients in Group A-C received Western medicine, Western medicine + traditional Chinese medicine, and Western medicine + traditional Chinese medicine + high dose of vitamin C, respectively. The time of disease recovery, symptoms disappearance, chest CT improvement, and tongue amelioration was recorded. Leukocyte, neutrophil and lymphocyte were monitored, as well as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalitonin (PCT), inflammatory factors, partial pressure of oxygen and carbon dioxide (PaCO2) and oxygenation index (PaO2). Urinary tract stones, liver function, and other side-effects such as gastrointestinal dysfunction were also investigated. Results: Traditional Chinese medicine enhanced the effect of Western medicine, including the reduction of CRP, ESR, PCT, and inflammatory factors, and the increase of leukocyte, neutrophil, and lymphocyte counts, and the improvement of respiratory rate, PaO2, PaCO2, and oxygenation index. Traditional Chinese medicine combined with high-dose Vitamin C therapy more effectively shortened the time of disease recovery, symptom disappearance, chest CT improvement, and tongue amelioration. Conclusions: a combined therapy of Western medicine, traditional Chinese medicine, and high dose of Vitamin C results in a most effective outcome in the treatment of COVID-19.
Keywords: COVID-19, traditional Chinese medicine, oral administration, inhalation, vitamin C
Introduction
COVID-19 is a new coronavirus,that belongs to the genus B coronavirus like SARS-CoV and MERS-CoV. Both SARS-CoV and MERS-CoV have spread globally, and pose a major challenge to clinical management and a great threat to public health. Similar to SARS-CoV and MERS-CoV, COVID-19 has gradually become a threat to global public health [1]. In December 2019, coronavirus disease 2019 (COVID-19) broke out. China has classified COVID-19 as a class B infectious disease, and has taken preventive and control measures based on class A infectious disease. The incubation period of the disease is generally 7 days, the longest is 14 days, but there are some cases whose incubation period is 24 days. Ordinary clinical manifestations include fever, cough, and fatigue [2-4]. So far, although China has announced seven versions of trials and strategies for the diagnosis and treatment of the disease, the focus is still on symptomatic treatment, and there is no specific medicine for the treatment of infection.
After COVID-19 broke out, the National Chinese Medicine Rescue Team rushed to Wuhan to take over Wuhan Jinyintan Hospital and provide Chinese medical assistance. A total of eight patients diagnosed with COVID-19, including 6 critically ill patients, were discharged from hospital after treatment with Chinese medicine or integrated traditional Chinese and Western medicine. Obviously, traditional Chinese medicine has played an important role in preventing and treating COVID-19. In fact, Chinese medicine plays a unique role in the prevention and treatment of emerging infectious diseases. For example, Chinese medicine has showed clinical effects on severe acute respiratory syndrome (SARS), H7N9 avian influenza and Ebola virus disease (EVD) [5].
High dose vitamin C has been confirmed to have therapeutic effects on different types of viral diseases, such as viral pneumonia, Keshan disease, and viral myocarditis [6,7]. In addition, it has been reported that co-treatment with vitamin C and some Western medicines can more effectively relieves some symptoms of COVID-19 than treatment with the Western medicines alone [8]. Vitamin C is not only a necessary nutrient, but also possesses anti-viral properties particularly in high concentrations. Therefore, Vitamin C is commonly recommended to as an adjunctive medicine in the treatment of some viral diseases.
In this study, we aim to analyze whether traditional Chinese medicine alone or in combination with high dose vitamin C can improve the therapeutic effect of Western medicine on COVID-19. The patients with COVID-19 received Western medicine, Western medicine + traditional Chinese medicine, or Western medicine + traditional Chinese medicine + high dose of vitamin C, followed by the evaluation of therapeutic effects and safety. This study was supposed to provide reference for the prevention and treatment of COVID-19.
Patients and methodology
Participants
We designed a prospective study. A total of 60 patients were enrolled in this study between 1 February and 29 February 2020, in Xi’an International Medical Center Hospital (Shaanxi Province, China). This study was approved by the Hospital Ethics Committee of Xi’an International Medical Center (No. 2020001) and the Chinese Ethics Committee of Clinical Trials (Registered number: ChiCTR2000032717). The criteria for severe or critical were defined by the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (5th Interim Edition)” sponsored by the National Health Commission of the People’s Republic of China [9]. The types of traditional Chinese medicine syndrome include cold-dampness-stagnation type, epidemic-toxin-closed lung type, internal closed-out external prolapse type, and lung-spleen-qi deficiency type. The detailed characteristics of patients were described in Table 1.
Table 1.
Demographic characteristics of patients with mild or severe COVID-19
| Patients with mild COVID-19 | Patients with severe COVID-19 | Difference (95% CI) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Group A | Group B | Group C | Group A | Group B | Group C | ||
| Gender | |||||||
| Male | 4 | 5 | 6 | 5 | 6 | 5 | P>0.05a |
| Female | 6 | 5 | 4 | 5 | 4 | 5 | |
| Age | 47.7±8.9 | 50.2±9.6 | 47.9±10.1 | 46.1±9.2 | 49.0±7.1 | 50.3±9.5 | P>0.05b |
| Symptom type | |||||||
| Cold-dampness-stagnation type | 5 | 6 | 5 | 3 | 4 | 3 | |
| Epidemic virus closed lung type | 4 | 4 | 5 | 3 | 2 | 3 | |
| Internal closure and external release type | 0 | 0 | 0 | 2 | 3 | 3 | |
| Lung and spleen deficiency syndrome | 1 | 0 | 0 | 2 | 1 | 1 | |
Chi square test;
One way ANOVA.
The enrollment criteria were listed following.
1. Age more than or equal to 18 years old and less than 75 years old; 2. Patients with ordinary or severe pneumonia infected by the new coronavirus; 3. Voluntarily signing written informed consent.
The exclusion criteria were as follows.
1. Critically ill patients and patients with severe complications, those with shock, acute respiratory distress syndrome, and multiple organ failure; 2. Pregnant and lactating women; 3. According to the researcher’s judgment, presence of past or concurrent diseases may affect the patient’s participation in the trial or affect the outcome of the study, including: malignant diseases, autoimmune diseases, severe malnutrition, liver and kidney diseases, blood diseases, nervous system diseases, and endocrine diseases; currently suffering from diseases that seriously affect the immune system, such as: human immunodeficiency virus (HIV) infection, or blood system, or splenectomy, organ transplantation, etc.; 4. People with severe mental illness who cannot cooperate with clinical trials.
The trial was discontinued according to the following criteria.
1. Voluntary patients who withdrew during the clinical trial; 2. Medical examination or clinical manifestations indicate disease progression, treatment failure, and deterioration; 3. Severe adverse reaction or events occuring with no significant improvement after the optimal medical treatment; The detailed reasons for the subject’s withdrawal from the study were recorded, and the adverse events were determined and followed up.
The elimination criteria were as below.
1. Those who did not meet the inclusion criteria after checking in the study because the initial inclusion information was not clear; 2. Any one of the exclusion criteria during the study; 3. Patients were unable to record information about their condition due to special circumstances during the study period; 4. Use of other special treatments due to changes in the condition during treatment.
Study method
Patients were randomized into ordinary A-C and severe A-C groups. Patients in group A received Western medicine. Patients in group B received Western medicine treatment and traditional Chinese medicine. Patients in group C received Western medicine treatment, traditional Chinese medicine, and high-dose vitamin C. Traditional Chinese medicine therapy included the oral administration of herbal decoction, the fumigation of traditional Chinese medicine, plus vitamin C, and the oral administration of vitamin E capsule and folic acid. Vitamin E and folic acid are ordinary nutrients. This study brought them into traditional Chinese medicine for the purpose of the therapy.
Treatments
Western medicine standard therapy
All patients received standard care according to the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (5th Interim Edition) [9]”.
Traditional Chinese medicine therapy
Traditional Chinese medicine based on the overall symptoms of COVID-19 patients was suggested to prescribe a prescription, including Qi-nourishing essence-replenishing decoction, Hu-Huang decoction, and Bai-Mu decoction. The components of Qi-nourishing essence-replenishing decoction, Hu-Huang decoction, and Bai-Mu decoction were listed in Table 2. All herbs were obtained from Xi’an International Medical Center Hospital Pharmacy. The herbs in each prescription were mixed in 1000 mL of H2O, and decocted for 30 min. The supernatant was collected by high-speed centrifugation, followed by concentration into 50 g.
Table 2.
Standard formula of traditional Chinese medicine decoction
| Qi-nourishing essence-replenishing decoction | Hu-Huang decoction | Bai-Mu decoction | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| Pharmaceutical | Crude drug content | Pharmaceutical | Crude drug content | Pharmaceutical | Crude drug content |
| Astragalus mongholicus | 30 g | Coptis chinensis | 20 g | Pueraria lobata | 15 g |
| Panax ginseng | 15 g | Rheum officinale | 10 g | Angelica dahurica | 12 g |
| Glycyrrhiza uralensis | 15 g | Scutellaria baicalensis | 10 g | Magnolia sprengeri | 9 g |
| Atractylodes macrocephala | 10 g | Atractylodes lancea | 10 g | Wrightia laevis | 30 g |
| Pericarpium citri reticulatae | 6 g | Aster tataricus | 10 g | Forsythia suspensa | 15 g |
| Angelica sinensis | 10 g | Houttuynia cordata | 10 g | Fritillaria thunbergii | 12 g |
| Zizyphus jujuba | 6 objects | Taraxacum mongolicum | 10 g | ||
| Zingiber officinale | 9 pieces | Polygonum cuspidatum | 10 g | ||
| Bupleurum chinense | 12 g | Astragalus mongholicus | 10 g | ||
| Cimicifuga dahurica | 6 g | ||||
Inhalation of Hu-Huang decoction and vitamin C was innovatively applied. In detail, 50 g of Hu-Huang decoction and 10 g of vitamin C were dissolved in 3000 mL of H2O2, the mixture was boiled into steam, and it was inhaled by the patients with an oxygen tube.
The patients took the traditional Chinese medicine, vitamin E (200 mg each time, 3 times a day) and folic acid (10 mg each time, 3 times a day) tablets orally, and inhaled the steam of Hu-Huang decoction and vitamin C 30-40 min each time, 3-7 times a day.
Treatment with high-dose of Vitamin C
Vitamin C was administered intravenously at a dosage of 10 g/60 kg body weight twice a day, and meanwhile oral vitamin C therapy was administrated at a dosage of 3 g three times a day.
Clinical observation and data collection
Whether the clinical outcomes were markedly improved was estimated based on the following criteria.
1. The time from illness onset to full recovery; 2. The time for remission and disappearance of the main symptoms like fever, debilitation, and cough; 3. The timing and proportion of the conversion from positive results to negative results; 4. The remission time and the remission rate of the patients with advanced CT chest results; 5. The transformation rate of patients with normal symptoms changing to severe or critical symptoms; 6. Significant changes in the tongue color from purple or red to light, as well as tongue fur from yellow to white, thick to thin, and dry to slippery.
In addition, leukocyte content, and proportions of neutrophils and lymphocytes were monitored, as well as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalitonin (PCT), interleukin-6 (IL-6), IL-20, partial pressure of oxygen and carbon dioxide, and oxygenation index.
To evaluate the safety of the therapies in patients, other vital examinations were performed to investigate urinary tract stones, liver function, and other side-effects such as gastrointestinal dysfunction.
Statistical analysis
Statistical analysis was done with IBM SPSS Statistics 21 software (IBM Corporation, Armonk, NY, USA). The data are presented as mean ± standard deviation (SD) if the test statistic followed a normal distribution, otherwise the data are presented as median (QR). Counted data are expressed as a proportion. To compare the differences between paired groups, if the data were in line with normality and the homogeneity of variance, the LSD test was used. If the data did not meet the homogeneity of variance, the Games-Howell test was used for analysis. If there was one or more groups in the data that did not follow normality, one-way analysis of variance (ANOVA) followed by Kruskal-Wallis test was used for analysis. Chi-square test was used for comparisons between taxonomic groups. P-value <0.05 was significant.
Results
Demographic characteristics of patients with ordinary and severe COVID-19
In this study, a total of 60 patient who had received Western medicine standard therapy, traditional Chinese and Western, and high-dose vitamin C therapy between 1 February and 29 February 2020, were screened. Of the 60 subjects, 31 were males, and 29 females. The average age of the patients was from 46.1±9.2 to 50.3±9.5 years. The patients included common cases (n=30) and severe cases (n=30). Patients were randomized into common A-C and severe A-C groups. Statistical analysis indicated no significant difference among different groups in gender and age (P<0.05) (Table 1). According to the traditional Chinese medicine symptom, the patients were classified into three types, cold-dampness-stagnation type, epidemic virus closed lung type, internal closure and external release type, and lung and spleen deficiency syndrome (Table 1).
Analysis of viral clearance after treatment
The average time of disease recovery for the common patients in Group A, was estimated to be 9.11 days, which was remarkably longer than that of patients in Group C (P<0.05); the severe patients in Group C showed shorter disease recovery time compared to that of the patients in Group A and Group B (both P<0.05) (Table 3). The mean symptom disappearance time of the common patients in Group C was found to be obviously different from that in Group A and Group B, of which the results were consistent in the severe patients (both P<0.05). The symptom disappearance time of the common patients and severe patients in Group B was significantly shorter than that of patients in Group A (Table 3). Moreover, the therapy used for the common patients and severe patients in Group B and Group C significantly shortened the mean time of viral clearance, chest CT improvement, and tongue improvement compared with the patients in Group A. The common patients receiving the therapy of Group C showed shorter period of viral clearance and tongue improvement, and the severe patients in Group C suggested shorter chest CT improvement time, compared to the patients of Group A (both P<0.05) (Table 3).
Table 3.
Clinical characteristics of 60 patients with COVID-19 before and after treatment
| Common patients | Severe patients | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Group A | Group B | Group C | Group A | Group B | Group C | |
| Disease recovery time (day) | 9.11±1.25 | 8.20±1.14 | 7.00±0.94* | 18.45±3.12 | 15.89±4.06 | 13.45±3.11*,# |
| Symptom disappearance time (day) | 7.40±1.26 | 5.70±1.16* | 4.10±0.88*,# | 15.20±2.49 | 13.40±2.76* | 10.20±1.75*,# |
| Viral clearance time (day) | 7.50±0.97 | 6.30±0.95* | 5.10±1.06*,# | 15.10±2.38 | 11.30±4.03* | 9.70±1.49* |
| Chest CT improvement time (day) | 7.50±1.08 | 6.00±1.05* | 5.30±1.83* | 17.50±3.72 | 14.50±2.45* | 11.00±3.06*,# |
| Tongue improvement time (day) | 7.70±2.00 | 5.90±1.10* | 4.50±0.97*,# | 16.10±3.96 | 12.50±3.63* | 11.80±2.87* |
Asterisks indicated statistical comparisons to Group A (* P<0.05), and pound sign indicated statistical comparisons to Group B (# P<0.05). Comparison was carried out by the ANOVA followed by Kruskal-Wallis.
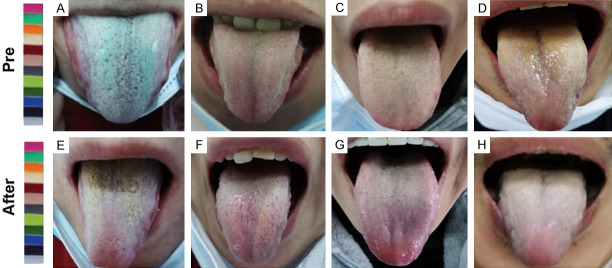
As indicated in Figure 1A-D, 4 male patients with COVID-19 showed bilateral ground-glass opacities prior to the treatment. However, the patients showed improved chest CT images after the treatment (Figure 1E-H). In addition, the patients possessed purple or red tongue, yellow tongue fur, and thick coating before the treatment, as suggested in Figure 2A-D. The patients receiving the treatment showed a light tongue, white tongue fur, and thin coating on the tongue (Figure 2E-H).
Figure 1.
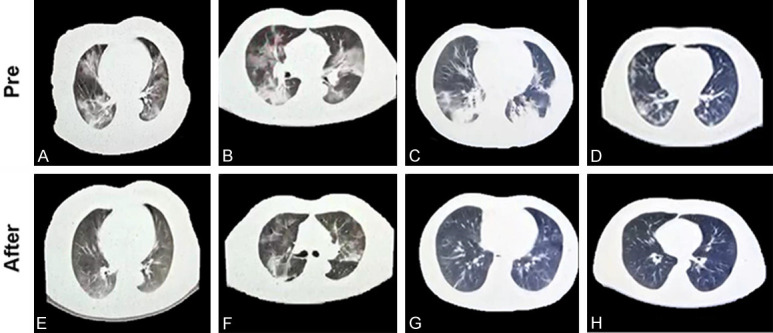
A-D. Axial CT images obtained from 4 male patients with COVID-19 showed bilateral ground-glass opacities prior to the Western medicine standard therapy, or the combined therapy of Chinese and Western, and high-dose vitamin C therapy. E-H. After the treatment, the patients showed improved chest CT images.
Figure 2.
Tongue characteristics of patients with COVID-19. A-D. The patients showed purple or red tongue, yellow tongue fur, and thick coating on the tongue before the treatment. E-H. After the treatment, the patients indicated light tongue, white tongue fur, and thin coating on the tongue.
Analysis of changes in clinical observation and laboratory examinations
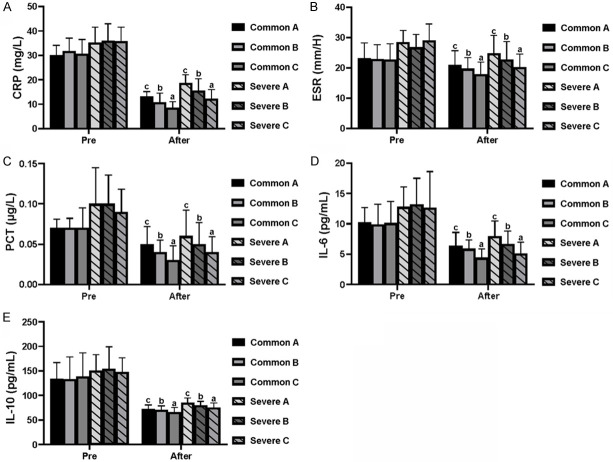
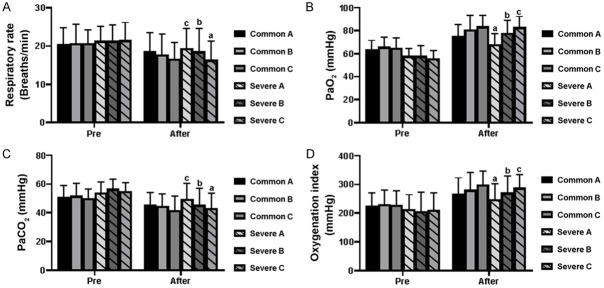
Before treatment, there was no significant difference in leukocyte count (Figure 3A), neutrophil proportion (Figure 3B), lymphocyte proportion (Figure 3C), CRP (Figure 4A), ESR (Figure 4B), PCT (Figure 4C), IL-6 (Figure 4D), IL-10 (Figure 4E), respiratory frequency (Figure 5A), PaO2 (Figure 5B), PaCO2 (Figure 5C), and oxygenation index (Figure 5D), between the common patients and severe patients in Group A-C (both P>0.05). The common and severe patients in Group B and Group C showed increased leukocytes, neutrophils, and lymphocyte proportions, compared with that of Group A (both P<0.05) (Figure 3A-C). As for CRP, ESR, PCT, IL-6, and IL-10, the patients in Group B and Group C showed a decreasing trend in comparison to Group A, and the therapy used for the patients in Group C produced the best results among these three treatments (both P<0.05) (Figure 4A-E), which was further evidenced by the changes in respiratory rate, PaO2, PaCO2, and oxygenation index (both P<0.05) (Figure 5A-D).
Figure 3.
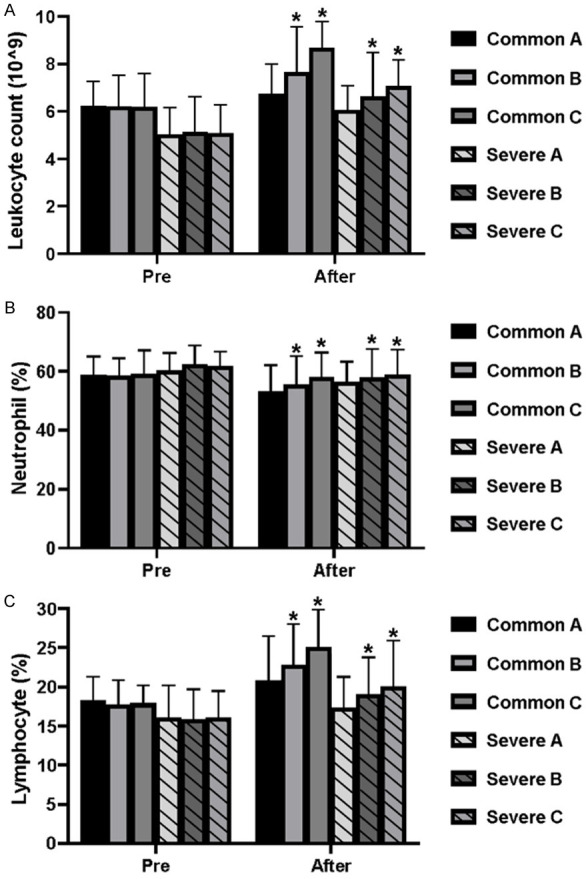
Characteristics of leukocyte count, neutrophil proportion, and lymphocyte ratio in the patients with COVID-19 before and after treatment. (A) Leukocyte count, (B) neutrophil proportion, and (C) lymphocyte ratio were increased in the common and severe patients with COVID-19 after the treatment. Bars in each column represent standard deviation. Asterisks indicate a statistical difference among common patients (*P<0.05 vs. group A) and severely affected patients (*P<0.05 vs. group A) after treatment. Comparison was carried out by the ANOVA followed by Kruskal-Wallis.
Figure 4.
Alteration in arterial blood gas parameters of the patients before and after treatment. (A) CRP, (B) ESR, (C) PCT, (D) IL-6, and (E) IL-10 were decreased in the common and severely affected patients with COVID-19 after the treatment. Different letters (a, b and c) above the bar indicate statistical difference among common patients (P<0.05) and severely affected patients (P<0.05) after the treatments. Comparison was carried out by the LSD test. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PCT, procalitonin; IL, interleukin.
Figure 5.
Changes in (A) respiratory rate, (B) PaO2, (C) PaCO2, and (D) oxygenation index of the patients after the treatment. Bars in each column represent the standard deviation. Different letters (a, b and c) above the bar indicate a difference among severely affected patients (P<0.05) after the treatments. Comparison was carried out by the LSD test.
Adverse events after treatment
During this study, no serious adverse reactions occurred in any patients. One severe patient developed mild dizziness, headache and discomfort on the 5th day after treatment. After symptomatic treatment, the symptoms were relieved, and the evaluation did not affect the results of this study. The treatment of this patient was continued.
Discussion
‘Syndrome Differentiation and Treatment’, which is treatment according to syndrome differentiation of patients, is one of the most important guidelines of traditional Chinese therapy. Most COVID-19 patients with fever have internal resistance to damp-heat. Based on the theory of traditional Chinese medicine, this study used Hu-Huang decoction, and Bai-Mu decoction to clear away heat and relieve the ‘fire’. Hu-Huang decoction is composed of multiple Chinese herbs, such as Houttuynia cordata, Scutellaria baicalensis, Taraxacum mongolicum, Aster tataricus and Rheum officinale. It has been proved that Houttuynia cordata, Scutellaria baicalensis and Taraxacum mongolicum possess the ability to inhibit a variety of viruses [10-12]. Scutellaria baicalensis, meanwhile, has anti-inflammatory effects, such as inducing G2/M cell cycle arrest of immune cells, suppressing Th1 polarization and inhibiting the production of the inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α [13-15]. Aster tataricus not only suppresses inflammation in lung tissue, but also inhibits tracheal ring contraction and expands the trachea [16]. Taraxacum mongolicum can suppress mucus hypersecretion by goblet cells in the airway [17]. In this study, after receiving traditional Chinese medicine treatment, the ESR, CRP, and PCT levels of ordinary and severe patients were significantly reduced, and the treatment effects of group B and C were significantly better than those of group A. The method of aerosolized inhalation of traditional Chinese medicine allows the bioactive components of traditional Chinese medicine to be directly inhaled into the lungs through the respiratory tract of the patient in the form of steam, and directly functions on the lesions, thereby improves the therapeutic effect.
The prescription of Bai-Mu decoction contains Angelica dahurica, Magnolia sprengeri, Forsythia suspense, Fritillaria thunbergii and other herbal medicines. Most of the components have been confirmed to have anti-inflammatory and anti-oxidant effects [18-20], which may be helpful to clear away heat and relieve the ‘fire’ of the patients. Of note, Angelica dahurica can inhibit the production of IL-6 and weaken its biological activity in the human body [21,22]. In this study, traditional Chinese medicine enhanced the effect of Western medicine on decreasing the levels of IL-6 in both ordinary and severe patients. Furanocoumarins isolated from Angelica dahurica has been confirmed to have antiviral activity against influenza caused by viruses H1N1 and H9N2 [23]. Fritillaria thunbergii also has antiviral function during influenza virus type A (H1N1) infection and has not toxicity in studies in vitro and in vivo [24]. Two compounds (beta-sitosterol and pelargonidin) of Fritillaria thunbergii and nine target genes (BCL2, CASP3, HSP90AA1, ICAM1, JUN, NOS2, PPARG, PTGS1, PTGS2) are probably associated to the antiviral function [25].
In the later stage of COVID-19, the main manifestation is deficiency of lung and spleen ‘Qi’. Although the nucleic acid test results of some patients are negative, symptoms related to pneumonia have been alleviated, and their body temperature has returned to normal, patients still show symptoms such as fatigue, shortness of breath, loss of appetite, and anxiety. Qi-nourishing essence-replenishing decoction can nourish the ‘middle’ and ‘Qi’ in the spleen and lungs, balance the body’s ‘Yin and Yang’, improve immunity, and restore organ function. In clinical practice, Qi-nourishing essence-replenishing decoction significantly increases the white blood cell and lymphocyte counts, enhances the phagocytic ability of lymphocytes, and improves the body’s immunity, which has been widely used in tumor chemotherapy/radiotherapy, leukopenia, and postoperative recovery period [26]. Normal or reduced neutrophil counts and a general decrease in lymphocytes are the characteristics of COVID-19 patients. The lymphocyte count and white blood cell count of severely affected patients are significantly reduced compared with ordinary patients, indicating that it is related to the severity of the disease. Traditional Chinese medicine enhanced the effect of Western medicine on increasing the white blood cell count and lymphocyte count.
Vitamin C is a strong antioxidant. In the treatment of influenza and bronchial pneumonia, high doses of vitamin C have been reported to have therapeutic effects [27]. Compelling evidence suggests that using a high dose of vitamin C (1.5 g/kg body weight) to combat COVID-19 can achieve effective clinical outcomes in suppressing inflammatory conditions [28]. In this study, patients received vitamin C through intravenous drip and oral intake. During the treatment, no serious complications such as dizziness, nausea, vomiting, and diarrhea, occurred. The urinary system was checked at the end of the treatment, and no stone formation was found. However, there are clinical reports of anaphylactic shock caused by intravenous infusion of Vitamin C, so before using super-high dose treatment, one must make sure that the patient is not allergic to the product [29]. Similar to vitamin C, vitamin E and folic acid are also have anti-inflammatory and anti-oxidant effects. In this study, vitamin E and folic acid were added for partial substitution of vitamin C, and the alleviation of side-effects of vitamin C.
Conclusions
Treatment with traditional Chinese medicine alone or in combination with high-dose of vitamin C improved the therapeutic effect of Western medicine on COVIP-19, such as the reduction of disease period, CRP, ESR, PCT, and inflammatory indicators, the improvement of the patient’s lung ventilation, and respiratory rate.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 81771349) and Shaanxi Administration of Traditional Chinese Medicine (No. 2020-YJ012).
Disclosure of conflict of interest
None.
References
- 1.Rabaan AA, Al-Ahmed SH, Haque S, Sah R, Tiwari R, Malik YS, Dhama K, Yatoo MI, Bonilla-Aldana DK, Rodriguez-Morales AJ. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez Med. 2020;28:174–184. [PubMed] [Google Scholar]
- 2.Halaji M, Farahani A, Ranjbar R, Heiat M, Dehkordi FS. Emerging coronaviruses: first SARS, second MERS and third SARS-CoV-2: epidemiological updates of COVID-19. Infez Med. 2020;28:6–17. [PubMed] [Google Scholar]
- 3.Nascimento Junior JAC, Santos AM, Quintans-Junior LJ, Walker CIB, Borges LP, Serafini MR. SARS, MERS and SARS-CoV-2 (COVID-19) treatment: a patent review. Expert Opin Ther Pat. 2020;30:567–579. doi: 10.1080/13543776.2020.1772231. [DOI] [PubMed] [Google Scholar]
- 4.Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai H, Wu X, Qi M, Zhang L, Hu H, Zhao Q, Zhao J, Liu H. Study on the mechanisms of active compounds in traditional Chinese medicine for the treatment of influenza virus by virtual screening. Interdiscip Sci. 2018;10:320–328. doi: 10.1007/s12539-018-0289-0. [DOI] [PubMed] [Google Scholar]
- 7.Hoang BX, Shaw G, Fang W, Han B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J Glob Antimicrob Resist. 2020;23:256–262. doi: 10.1016/j.jgar.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Zhao W, Zhang B, Jia Y, Wu S, Zhong B, Yu X, Wang X, Hao Y, Wang H, Zhao Y, Mizuno K, Bu H, Tseng Y. Clinical effect of intravenous vitamin C on viral myocarditis in children: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:3082437. doi: 10.1155/2019/3082437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Zhu Y, Zhang J, Li Y, Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10:e039519. doi: 10.1136/bmjopen-2020-039519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia YY, Guan RF, Wu YH, Yu XP, Lin WY, Zhang YY, Liu T, Zhao J, Shi SY, Zhao Y. Taraxacum mongolicum extract exhibits a protective effect on hepatocytes and an antiviral effect against hepatitis B virus in animal and human cells. Mol Med Rep. 2014;9:1381–1387. doi: 10.3892/mmr.2014.1925. [DOI] [PubMed] [Google Scholar]
- 11.Chiow KH, Phoon MC, Putti T, Tan BK, Chow VT. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac J Trop Med. 2016;9:1–7. doi: 10.1016/j.apjtm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Q, Gao L, Fu X, Meng Q, Lu Z. Scutellaria baicalensis inhibits coxsackievirus B3-induced myocarditis via AKT and p38 pathways. J Microbiol Biotechnol. 2019;29:1230–1239. doi: 10.4014/jmb.1904.04050. [DOI] [PubMed] [Google Scholar]
- 13.Pan TL, Wang PW, Leu YL, Wu TH, Wu TS. Inhibitory effects of Scutellaria baicalensis extract on hepatic stellate cells through inducing G2/M cell cycle arrest and activating ERK-dependent apoptosis via Bax and caspase pathway. J Ethnopharmacol. 2012;139:829–837. doi: 10.1016/j.jep.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Kim ME, Kim HK, Park HY, Kim DH, Chung HY, Lee JS. Baicalin from scutellaria baicalensis impairs Th1 polarization through inhibition of dendritic cell maturation. J Pharmacol Sci. 2013;121:148–156. doi: 10.1254/jphs.12200fp. [DOI] [PubMed] [Google Scholar]
- 15.Liao H, Ye J, Gao L, Liu Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: a comprehensive review. Biomed Pharmacother. 2021;133:110917. doi: 10.1016/j.biopha.2020.110917. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Wu H, Li Y, Liu J, Jia Z, Xu W, Xiao H, Wang W. Aster tataricus attenuates asthma efficiently by simultaneously inhibiting tracheal ring contraction and inflammation. Biomed Pharmacother. 2020;130:110616. doi: 10.1016/j.biopha.2020.110616. [DOI] [PubMed] [Google Scholar]
- 17.Chu X, Wei M, Yang X, Cao Q, Xie X, Guan M, Wang D, Deng X. Effects of an anthraquinone derivative from rheum officinale baill, emodin, on airway responses in a murine model of asthma. Food Chem Toxicol. 2012;50:2368–2375. doi: 10.1016/j.fct.2012.03.076. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Lee H, Kim MH, Choi YY, Ahn KS, Um JY, Lee SG, Yang WM. Angelica dahurica ameliorates the inflammation of gingival tissue via regulation of pro-inflammatory mediators in experimental model for periodontitis. J Ethnopharmacol. 2017;205:16–21. doi: 10.1016/j.jep.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Shao SY, Song XY, Xia CY, Yang YN, Zhang PC, Chen NH. Protective effects of Forsythia suspense extract with antioxidant and anti-inflammatory properties in a model of rotenone induced neurotoxicity. Neurotoxicology. 2016;52:72–83. doi: 10.1016/j.neuro.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Kim M, Hong S, Kwon B, Song MW, Song K, Kim EY, Jung HS, Sohn Y. Anti-inflammatory effects of fritillaria thunbergii miquel extracts in LPS-stimulated murine macrophage RAW 264.7 cells. Exp Ther Med. 2021;21:429. doi: 10.3892/etm.2021.9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, Smith AD. Vitamin C-an adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients. 2020;12:3760. doi: 10.3390/nu12123760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Zhang C, Wu Z, Wang G, Zhao H. The mechanism and clinical outcome of patients with corona virus disease 2019 whose nucleic acid test has changed from negative to positive, and the therapeutic efficacy of favipiravir: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:488. doi: 10.1186/s13063-020-04430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee BW, Ha TKQ, Cho HM, An JP, Kim SK, Kim CS, Kim E, Oh WK. Antiviral activity of furanocoumarins isolated from angelica dahurica against influenza a viruses H1N1 and H9N2. J Ethnopharmacol. 2020;259:112945. doi: 10.1016/j.jep.2020.112945. [DOI] [PubMed] [Google Scholar]
- 24.Kim M, Nguyen DV, Heo Y, Park KH, Paik HD, Kim YB. Antiviral activity of fritillaria thunbergii extract against human influenza virus H1N1 (PR8) in vitro, in ovo and in vivo. J Microbiol Biotechnol. 2020;30:172–177. doi: 10.4014/jmb.1908.08001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim M, Kim YB. A network-based pharmacology study of active compounds and targets of Fritillaria thunbergii against influenza. Comput Biol Chem. 2020;89:107375. doi: 10.1016/j.compbiolchem.2020.107375. [DOI] [PubMed] [Google Scholar]
- 26.Gong Y, Xu Z, Jin C, Deng H, Wang Z, Zhou W, Zhang M, Zhao X, Wang L. Treatment of advanced non-small-cell lung cancer with Qi-nourishing essence-replenishing Chinese herbal medicine combined with chemotherapy. Biol Proced Online. 2018;20:9. doi: 10.1186/s12575-018-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colunga Biancatelli RML, Berrill M, Marik PE. The antiviral properties of vitamin C. Expert Rev Anti Infect Ther. 2020;18:99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- 28.Farjana M, Moni A, Sohag AAM, Hasan A, Hannan MA, Hossain MG, Uddin MJ. Repositioning vitamin C as a promising option to alleviate complications associated with COVID-19. Infect Chemother. 2020;52:461–477. doi: 10.3947/ic.2020.52.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang ZW, Xu XC, Liu T, Yuan S. Mitochondrion-permeable antioxidants to treat ROS-burst-mediated acute diseases. Oxid Med Cell Longev. 2016;2016:6859523. doi: 10.1155/2016/6859523. [DOI] [PMC free article] [PubMed] [Google Scholar]