Abstract
Background: The pathogenic triggers of diabetic peripheral neuropathy (DPN) mainly include ischemia and hypoxic factors. The combined use of Chinese and Western medicine may be a new perspective for the treatment of DPN. Accordingly, this study explores the clinical efficacy and safety of electro-acupuncture (EA) combined with beraprost sodium (BPS) and α-lipoic acid (α-LA) in the treatment of patients with DPN. Methods: A total of 184 patients with DPN meeting the inclusion criteria were enrolled and divided into electric-acupuncture group (n=54), medication group (n=62) and combination group (n=68), which were treated by EA, BPS+α-LA, and EA+BPS+α-LA, respectively. The three groups were compared with respect to the following factors: clinical efficacy; motor conduction velocities (MCVs) of nervus medianus, nervus peroneus communis and tibial nerve and sensory conduction velocities (SCVs) of nervus medianus, sural nerve and ulnar nerve before and after treatment; the Toronto Clinical Scoring System (TCSS), total symptom score (TSS) and Michigan Diabetes Neuropathy Score (MDNS) before and after treatment; changes of serum homocysteine and cysteine (Cys) levels, oxidative stress indicators and inflammatory factors; incidence of adverse reactions. Results: The overall response rate of the combination group was higher than that of the electric acupuncture group or the medication group. After treatment, the SCV of nervus medianus, sural nerve and ulnar nerve and the MCV of nervus medianus, nervus peroneus communis and tibial nerve were the highest in the combination group among the three groups (P<0.05). After treatment, the scores of TCSS, TSS and MDNS in the combination group was notably lower than those in the medication group and the electric acupuncture group (P<0.05). The amelioration of inflammatory factors in the combination group were the best among the three groups (P<0.05). The incidence of adverse reactions was lower in the combination group compared with the electric acupuncture group and the medication group (P<0.05). Conclusion: EA combined with BPS and α-LA is effective in the treatment of DPN, which can effectively reduce the levels of serum inflammatory factors in patients, with a lower complication rate and higher safety.
Keywords: Electro-acupuncture, beraprost sodium, α-lipoic acid, combined therapy, diabetic peripheral neuropathy, nerve conduction
Introduction
As a metabolic disorder and chronic disease characterized by chronic hyperglycemia, diabetes is increasing worldwide with the changes of people’s lifestyle and diet structure [1]. If left unattended or poorly controlled, it can trigger persistent hyperglycemia, which in turn leads to chronic complications to various tissues such as nerve tissue, glomerulus, retina and blood vessels and even irreversible damage to vital organs [2-5]. Diabetic peripheral neuropathy (DPN), one of the various chronic complications of diabetes, is a disease in which the structure and efficacy of peripheral nerves are impaired during the progression of diabetes, leading to related clinical manifestations and signs [6]. Currently, the mechanism of DPN is complex and remains undefined. However, scholars at home and abroad believe that it is a multifactorial mechanism, which involves abnormal inositol metabolism causing neural cell degeneration [7], advanced glycation end-products damaging nerve function [8], endoplasmic reticulum stress inducing cell apoptosis [9], nerve calcium homeostasis destruction [10], abnormal lipid metabolism [11], metabolic inflammation [12], and insulin resistance [13]. Literature has shown that patients with diabetes have a 10-20% chance of developing DPN at the time of diagnosis [14]. However, the onset of DPN is insidious and hard to detect, and the protective sensation is lost due to peripheral neuropathy, which leads to foot infection and ulceration, and eventually a high risk of disability. The all-cause mortality rate after amputation is as high as 13 percent, according to statistics [15].
Unfortunately, nerve cells are non-dividing cells, and their damage is not reversible and cannot be repaired. So, the current treatment measures for DPN are mainly symptomatic treatment, such as controlling blood sugar, nourishing nerves and improving microcirculation, without an effective treatment [16,17]. In recent years, acupuncture and moxibustion of traditional Chinese medicine has been widely used in the treatment of DPN, rendering some certain benefits [18]. As for drug therapies, lipoic acid drugs are often used alone in clinic, but the efficacy is not ideal. The primary task of treating DPN is to provide enough energy and restore blood supply, so, combined drug therapy may better play a therapeutic role. Many studies have shown that electro-acupuncture (EA) exerts an anti-inflammatory effect on various forms of organ dysfunction, including many diabetic complications [19-21]. Electrical nerve stimulation has been shown to significantly reduce the pain of diabetic patients [22]. It has been recognized by the American Pain Society and the National Center for Complementary and Alternative Medicine as an effective treatment used by millions of people to reduce pain and block inflammation [23]. We hypothesized that the combination of EA, beraprost sodium (BPS), and α-lipoic acid (α-LA) might be ann effective way to treat DPN. Accordingly, we investigated the clinical efficacy and safety of EA combined with BPS and α-LA in the treatment of DPN.
Material and methods
Research participants
The clinical data of 184 patients with DPN treated in the Second Affiliated Hospital of Hainan Medical University from April 2018 to May 2020 were retrospectively analyzed. According to different treatment plans, the patients were divided into the following three groups: electric acupuncture group (n=54), medication group (n=62) and combination group (n=68). Inclusion criteria: All the enrolled patients met the diagnostic criteria for diabetes specified by the American Diabetes Association Standards of Medical Care in Diabetes 2017 [24], with stable and well-controlled blood sugar and no prior treatment before admission. Exclusion criteria included peripheral neuropathy caused by hyperosteogeny, multiple sclerosis, heredity and trauma, drug-induced or alcoholic peripheral neuropathy, severe liver and kidney diseases, and heart diseases. All patients signed an informed consent form. The study was approved by the Institutional Board Review of the Second Affiliated Hospital of Hainan Medical University.
Treatment methods
All patients received conventional therapy to control blood sugar and blood pressure within a reasonable range, with the fasting blood glucose of 5.6-7.0 mmol/L, blood pressure within 130/80 mmHg, and HbA1c of 6.5%-7.0%.
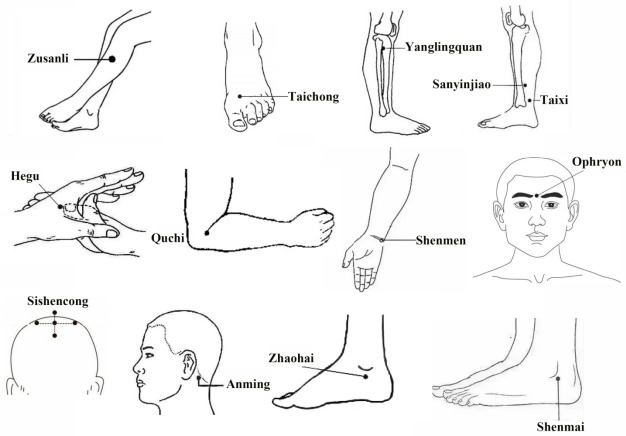
Electric acupuncture (EA) group: patients were treated with electroacupuncture apparatus (6805-AII, (Yue) Medical Device Approval No. 20172260590) and Hwato stainless steel filiform needles (Suzhou Medical Appliance Factory, specification 0.3×40 mm). Acupoints selection: Acupoints Zusanli, Taichong, Yanglingquan, Sanyinjiao, Hegu, Quchi, Shenmen, Anmian Zhaohai Shenmai on both sides and Ophryon, Sishencong were selected. The locations of the acupoints are shown in Figure 1. Communication was conducted before acupuncture to relieve the anxiety and tension of the patient. In a quiet treatment area, the patient was instructed to lie supine on the treatment bed to fully expose the above acupoints, and the corresponding obvious sites were marked with a marker pen. After conventional local disinfection with 75% alcohol, the above acupoints were needled with the neutral supplementation and draining method, with Zusanli, Yanglingquan and Sanyinjiao 1.0-1.5 Cun, Anmian, Hegu and Quchi 0.5-1.0 Cun, Shenmai and Shenmen 0.3-0.5 Cun, Zhaohai and Taichong 0.5-0.8 Cun through perpendicular insertion, Ophryon 0.3-0.5 Cun through oblique insertion, and Sishencong 0.5-0.8 Cun through a horizontal insertion. Each acupoint was applied with manipulations until the arrival of Qi. Then, the single-side Quchi-Neiguan and Yanglingquan-Taichong acupoints were electrified for 30 min (disperse-dense wave, 4 Hz of disperse wave, 20 Hz of dense wave, and 6 s of disperse-dense cycle). During needle retaining, the patient was asked about how he/she felt, and the procedure was stopped immediately if there was any special discomfort (such as fainting during acupuncture). Patients were treated once a day for 2 weeks as a course of treatment for 2 continuous courses, with 2 days’ rest between courses.
Figure 1.
Location of acupoints.
Medication group: patients were treated with α-LA+BPS. BPS tablets (Beijing Taide Pharmaceutical Co., Ltd., SFDA Approval No. H20083589, specifications 40 μg × 10 tablets) were given, per os, 3 times a day, 40 μg/time. In addition, α- LA injection (Jiangsu Shenlong Pharmaceutical Co. LTD, SFDA Approval No.H20059737, specification: 12 mL: 0.3 g) were added into 100 ml 0.9% sodium chloride injection, administered intravenously at 300 mg/d. The treatment lasted for 2 courses, with 2 weeks as a course of treatment.
Combination group: The above-mentioned EA and medication were used for combined treatment. Acupoint selection, operation, dosage, course of treatment, and medical advice were exactly the same as those in EA group and medication group. The treatment time was reasonably arranged.
Endpoints
(1) Electromyography: The motor conduction velocity (MCV) of tibial nerve, nervus medianus and nervus peroneus communis and the sensory conduction velocity (SCV) of nervus medianus, ulnar nerve and sural nerve were measured by an electromyography machine (MY01400a, Noraxon, USA) at room temperature using surface electrode stimulation. The mean of SCV and MCV was taken as the final measurement result.
(2) Clinical efficacy: Ineffective: the patient’s symptoms did not change or even worsened, with no change in SCV and MCV before and after treatment. Effective: the patient’s symptoms were resolved, with SCV and MCV increased by >3 m/s. Markedly effective: the symptoms of the patient resolved obviously, with normal MCV and SCV. Overall response rate ORR was calculated as (markedly effective + effective) cases/total number of cases × 100%.
(3) Toronto Clinical Scoring System (TCSS) score [25]: The TCSS can reflect the presence and severity of diabetic peripheral sensorimotor polyneuropathy in patients. The system consists of nerve reflex, nerve symptoms, and sensory test, with a total score of 0-19 points. The lower the score, the milder the symptoms.
(4) Total symptom score (TSS) [26]: The TSS was used to evaluate the conscious symptoms of neuropathy in patients. The questionnaire covers four symptoms of pain, burning, paresthesia, and numbness, which are scored according to the severity and frequency of symptoms. Each score ranges from 0 to 3.66 points, with a total score of 14.64 points. The higher the score, the more serious the clinical symptoms.
(5) Michigan Diabetes Neuropathy Score (MDNS) [27]: As we know, scoring patients over a period of time can assess the progression of a disease. In this study, the MDNS was used to determine the presence of peripheral neuropathy. The score includes vibration sensation at the dorsum of the great toe, muscle strength of distal extremities and tendon reflex scores, with a total score of 0-46 points.
(6) Serum-related indexes: Serum levels of homocysteine (Hcy) and cysteine (Cys) and inflammatory factors high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) in patients before and after treatment were detected by special instruments and equipment in the clinical laboratory.
(7) Adverse reactions: Adverse reactions such as diarrhea, nausea, retching, and pruritus were recorded.
Statistical processing
Graphpad Prism 8.0 software was used to analyze the data statistically and draw charts. Continuous variables were expressed as mean ± standard deviation. The difference between two groups was compared using two-tailed Student’s t-tests and that among multiple groups was compared using one-way ANOVA. Bonferroni’s correction was used for post-hoc multiple comparisons. Categorical variables were described as percentage (%) and analyzed by Chi-square test. P<0.05 indicated a significant difference.
Results
General data of patients in the three groups
The general data were similar in the three groups, which were comparable. See Table 1.
Table 1.
General information
| Electric acupuncture group (n=54) | Medication group (n=62) | Combination group (n=68) | F | P | |
|---|---|---|---|---|---|
| Gender [n (%)] | 0.8031 | 0.6693 | |||
| Male | 36 (66.7) | 38 (61.3) | 40 (58.8) | ||
| Female | 18 (33.3) | 24 (38.7) | 28 (41.2) | ||
| Age (years) | 49.84±6.84 | 51.28±7.37 | 50.65±7.51 | 0.5665 | 0.5685 |
| BMI (kg/m2) | 23.41±3.15 | 24.08±3.84 | 24.41±4.25 | 1.0510 | 0.3516 |
| Course of diabetes mellitus (year) | 9.25±2.45 | 8.84±2.68 | 9.01±3.06 | 0.3186 | 0.7276 |
| HbA1c (%) | 8.56±1.97 | 9.12±2.30 | 8.89±2.18 | 0.9716 | 0.3804 |
| SBP (mmHg) | 127.19±15.84 | 128.31±14.97 | 127.55±15.23 | 0.0823 | 0.9211 |
| DBP (mmHg) | 78.84±5.17 | 79.74±4.92 | 79.16±5.08 | 0.4795 | 0.6199 |
| TC (mmol/L) | 4.45±0.87 | 4.71±1.06 | 4.67±0.99 | 1.1590 | 0.3161 |
| TG (mmol/L) | 1.65±0.43 | 1.72±0.57 | 1.63±0.69 | 0.4159 | 0.6603 |
| Cr (μmol/L) | 70.25±8.94 | 69.49±9.15 | 71.44±9.08 | 0.7664 | 0.4662 |
| Vb12 (pmol/L) | 242.84±35.78 | 248.25±40.64 | 240.73±41.68 | 0.6088 | 0.5451 |
Comparison of clinical efficacy
The overall response rate was 92.7% in the combination group, which was higher than the overall response rate of 70.4% in the electric acupuncture group and 80.7% in the medication group (P<0.05, Table 2).
Table 2.
Clinical efficacy
| Markedly effective | Effective | Ineffective | Overall response rate | |
|---|---|---|---|---|
| Electric acupuncture group (n=54) | 17 (31.5) | 21 (38.9) | 16 (29.6) | 38 (70.4) |
| Medication group (n=62) | 28 (45.2) | 22 (35.5) | 12 (19.3) | 50 (80.7) |
| Combination group (n=68) | 45 (66.2) | 18 (26.5) | 5 (7.3) | 63 (92.7)*,# |
| Medication group vs Electric acupuncture group | χ2=1.6641, P=0.1971 | |||
| Combination group vs Electric acupuncture group | χ2=10.4810, P=0.0012 | |||
| Combination group vs Medication group | χ2=4.1101, P=0.0426 | |||
P<0.05 vs Electric acupuncture group;
P<0.05 vs Medication group.
SCVs of nervus medianus, sural nerve, and ulnar nerve before and after treatment
The SCV of nervus medianus, sural nerve, and ulnar nerve was not statistically different among the three groups before treatment (P>0.05). After treatment, the SCV of nervus medianus, sural nerve, and ulnar nerve increased in all the three groups, and the increase was most evident in the combination group, followed by the medication group and the electric acupuncture group successively (P<0.05). See Table 3.
Table 3.
SCVs of nervus medianus, ulnar nerve, and sural nerve
| Nervus medianus | Sural nerve | Ulnar nerve | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Electric acupuncture group (n=54) | 39.51±2.58 | 47.45±3.25 | 37.56±2.28 | 45.84±2.79 | 38.94±2.97 | 50.87±4.04 |
| Medication group (n=62) | 38.94±2.84 | 53.84±3.16* | 38.07±2.11 | 51.07±2.64* | 39.48±3.23 | 56.81±3.61* |
| Combination group (n=68) | 40.08±3.76 | 59.07±3.55*,# | 37.84±2.03 | 56.71±2.94*,# | 39.04±3.17 | 60.97±3.77*,# |
| Medication group vs Electric acupuncture group | t=1.1249, | t=10.7207, | t=1.2507, | t=10.3651, | t=0.9323, | t=8.3627, |
| P=0.2630 | P<0.0001 | P=0.2136 | P<0.0001 | P=0.3532 | P<0.0001 | |
| Combination group vs Electric acupuncture group | t=0.9379, | t=18.2864, | t=0.6997, | t=20.3387, | t=0.1745, | t=13.9207, |
| P=0.3503 | P<0.0001 | P=0.4855 | P<0.0001 | P=0.8618 | P<0.0001 | |
| Combination group vs Medication group | t=1.9050, | t=8.6678, | t=0.6185, | t=11.2390, | t=0.7655, | t=6.2755, |
| P=0.0591 | P<0.0001 | P=0.5374 | P<0.0001 | P=0.4454 | P<0.0001 | |
P<0.05 vs Electric acupuncture group;
P<0.05 vs Medication group.
MCVs of nervus medianus, nervus peroneus communis, and tibial nerve before and after treatment
The MCV of nervus medianus, nervus peroneus communis and tibial nerve differed insignificantly among the three groups before treatment (P>0.05). After treatment, the MCV of nervus medianus, nervus peroneus communis and tibial nerve elevated, and the increase was most evident in the combination group, followed by the medication group and the electric acupuncture group successively (P<0.05). See Table 4.
Table 4.
MCVs of nervus medianus, nervus peroneus communis, and tibial nerve
| Nervus medianus | Nervus peroneus communis | Tibial nerve | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Electric acupuncture group (n=54) | 41.64±3.74 | 43.58±2.84 | 40.15±3.06 | 42.97±2.74 | 40.47±1.84 | 43.84±3.68 |
| Medication group (n=62) | 42.06±3.87 | 46.84±3.09* | 39.94±3.23 | 45.05±2.37* | 40.01±1.76 | 46.71±3.19* |
| Combination group (n=68) | 41.94±3.51 | 49.87±2.67*,# | 40.87±3.47 | 48.94±2.88*,# | 39.87±2.26 | 50.38±3.07*,# |
| Medication group vs Electric acupuncture group | t=0.5922, | t=5.8843, | t=0.3579, | t=4.3844, | t=1.3747, | t=4.4998, |
| P=0.5549 | P<0.0001 | P=0.7211 | P<0.0001 | P=0.1719 | P<0.0001 | |
| Combination group vs Electric acupuncture group | t=0.4454, | t=12.2865, | t=1.1772, | t=11.3904, | t=1.5532, | t=10.4340, |
| P=0.6569 | P<0.0001 | P=0.2415 | P<0.0001 | P=0.1231 | P<0.0001 | |
| Combination group vs Medication group | t=0.1808, | t=5.8422, | t=1.5447, | t=8.2122, | t=0.3848, | t=6.5271, |
| P=0.8567 | P<0.0001 | P=0.1250 | P<0.0001 | P=0.7010 | P<0.0001 | |
P<0.05 vs Electric acupuncture group;
P<0.05 vs Medication group.
TCSS, TSS and MDNS scores before and after treatment
Before treatment, there was no significant difference in TCSS, TSS and MDNS scores among the three groups (P>0.05). After treatment, all the three scores decreased, and the decreases were more significant in the medication group and the combination group compared with the electric acupuncture group (P<0.05). See Table 5.
Table 5.
TCSS, TSS and MDNS scores
| TCSS scores | TSS scores | MDNS scores | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Electric acupuncture group (n=54) | 10.64±2.15 | 8.57±1.13 | 9.74±1.46 | 8.09±1.18 | 28.84±4.12 | 21.26±3.14 |
| Medication group (n=62) | 11.08±2.64 | 5.84±1.38* | 10.24±1.33 | 5.46±1.29* | 28.24±4.61 | 18.27±3.05* |
| Combination group (n=68) | 10.87±2.39 | 5.31±1.51*,# | 9.98±1.21 | 5.03±1.01*,# | 28.57±4.43 | 17.31±1.14*,# |
| Medication group vs Electric acupuncture group | t=0.9749, | t=11.5493, | t=1.9298, | t=11.3939, | t=0.7344, | t=5.1948, |
| P=0.3316 | P<0.0001 | P=0.0561 | P<0.0001 | P=0.4642 | P<0.0001 | |
| Combination group vs Electric acupuncture group | t=0.5416, | t=13.0046, | t=0.9679, | t=15.0498, | t=0.3382, | t=9.2358, |
| P=0.5892 | P<0.0001 | P=0.3351 | P<0.0001 | P=0.7358 | P<0.0001 | |
| Combination group vs Medication group | t=0.4643, | t=2.0401, | t=1.1386, | t=1.8744, | t=0.4065, | t=2.3215, |
| P=0.6432 | P=0.0434 | P=0.2571 | P=0.0409 | P=0.6851 | P=0.0219 | |
P<0.05 vs Electric acupuncture group;
P<0.05 vs Medication group.
Serum Hcy and CysC levels before and after treatment
Hcy and CysC were not statistically different among the three groups before treatment (P>0.05). After treatment, Hcy and CysC decreased, and the decreases were most significant in the combination group, followed in a descending order by the medication group and the electric acupuncture group (P<0.05). See Figure 2.
Figure 2.
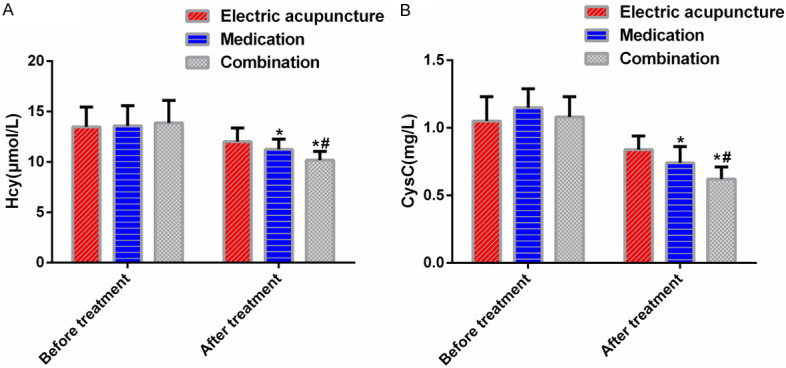
Serum Hcy and Cys levels. A: Hcy levels; B: CysC levels; *P<0.05 vs electric acupuncture group; #P<0.05 vs medication group.
Oxidative stress before and after treatment
The oxidative stress indexes superoxide dismutase (SOD) and glutathione (GSH) were not significantly different among the three groups before treatment (P<0.05). After treatment, SOD and GSH increased, and the increases were most distinct in the combination group, followed in a descending order by the medication group and the electric acupuncture group (P<0.05). See Table 6.
Table 6.
Oxidative stress indicators
| SOD (U/L) | GSH (ng/L) | |||
|---|---|---|---|---|
|
|
|
|||
| Before treatment | After treatment | Before treatment | After treatment | |
| Electric acupuncture group (n=54) | 30.54±4.19 | 34.81±3.85 | 79.84±5.14 | 84.64±6.01 |
| Medication group (n=62) | 31.05±4.42 | 36.71±3.48* | 80.51±5.69 | 87.04±5.39* |
| Combination group (n=68) | 30.87±4.37 | 39.48±3.04*,# | 80.14±5.53 | 89.47±5.51*,# |
| Medication group vs Electric acupuncture group | t=0.6350, | t=2.7915, | t=0.6615, | t=2.2673, |
| P=0.5267 | P=0.0062 | P=0.5096 | P=0.0253 | |
| Combination group vs Electric acupuncture group | t=0.4135, | t=7.2925, | t=0.3011, | t=4.5144, |
| P=0.6800 | P<0.0001 | P=0.7638 | P<0.0001 | |
| Combination group vs Medication group | t=0.2280, | t=4.7202, | t=0.3672, | t=2.4824, |
| P=0.8200 | P<0.0001 | P=0.7141 | P=0.0144 | |
P<0.05 vs Electric acupuncture group;
P<0.05 vs Medication group.
Serum inflammatory factors before and after treatment
hs-CRP, TNF-α, and IL-6 levels were not statistically different among the three groups before treatment (P>0.05). After treatment, the three indexes reduced, and the reductions were most significant in the combination group, followed by the medication group and the electric acupuncture group successively (P<0.05). See Figure 3.
Figure 3.
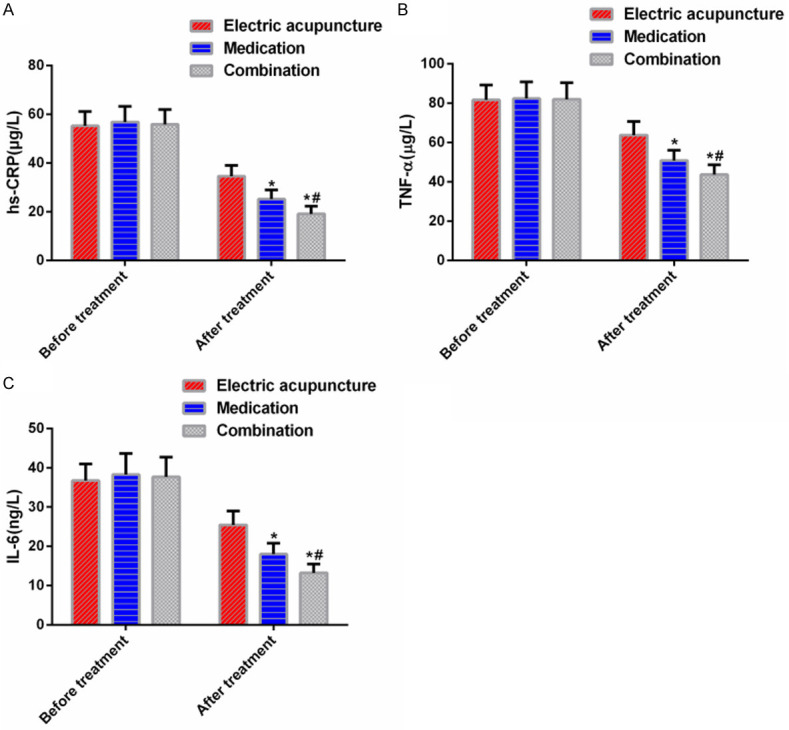
Serum inflammatory factor levels. A: hs-CRP levels; B: TNF-α levels; C: IL-6 levels; *P<0.05 vs electric acupuncture group; #P<0.05 vs medication group.
Incidence of adverse reactions
The numbers of cases developing diarrhea, nausea, retching and pruritus in the electric acupuncture group were 2, 2, 1, and 4 respectively, and that in the medication group were 2, 2, 2, and 3, respectively. In the combination group, there were 0 case of diarrhea, and 1 case each of nausea, retching, and pruritus. The incidence of adverse reactions in the combination group (4.5%) was significantly lower than that in the electric acupuncture group (16.7%) and the medication group (14.4%) (P<0.05). See Table 7.
Table 7.
Incidence of adverse reactions
| Diarrhea | Nausea | Retching | Pruritus | Total incidence | |
|---|---|---|---|---|---|
| Electric acupuncture group (n=54) | 2 (3.7) | 2 (3.7) | 1 (1.9) | 4 (7.4) | 9 (16.7) |
| Medication group (n=62) | 2 (3.2) | 2 (3.2) | 2 (3.2) | 3 (4.8) | 9 (14.4) |
| Combination group (n=68) | 0 (0) | 1 (1.5) | 1 (1.5) | 1 (1.5) | 3 (4.5)*,# |
| Medication group vs Electric acupuncture group | χ2=0.1018, P=0.7497 | ||||
| Combination group vs Electric acupuncture group | χ2=5.0971, P=0.0240 | ||||
| Combination group vs Medication group | χ2=3.9521, P=0.0468 | ||||
P<0.05 vs Electric acupuncture group;
P<0.05 vs Medication group.
Discussion
The main symptoms of DPN patients are numbness and pain in the distal limbs, which adversely affect the lives of patients. At present, the treatment of DPN mainly includes antioxidant drugs, vasodilators, nutraceuticals, and analgesics [28], but the treatment effect is not ideal. Therefore, a search for effective treatment regimens to control DPN is necessary.
In this study, the clinical efficacy and safety of EA combined with BPS and α-LA in the treatment of DPN were investigated. The results indicated that the overall response rate of the combination group was higher than that of the electric acupuncture group or the medication group. Long-term hyperglycemia and blood lipid metabolism abnormalities will significantly reduce the free radical scavenging rate of the body, which leads to excessive production of free radicals, resulting in damage to neuronal DNA, proteins, and lipids of patients and hindering axonal transport and signal transmission. Oxidative stress can also reduce the level of neurotrophins and weaken the repair and regeneration ability of damaged nerve fibers [29,30]. α-LA is a multifunctional antioxidant, which can not only effectively remove oxygen free radicals in the body and delay cell senescence, but also increase the blood flow of blood vessels of trophic nerves [31]. As for BPS, it is an activated PGI2 analogue that improves the vasodilation function of blood vessels with weakened diastolic function [32]. The function of EA is that current passing through specific acupoints can make local muscle tissues in a long-term excited state, thus enhancing the clinical effect [33]. After treatment, the SCV of nervus medianus, ulnar nerve, sural nerve, and the MCV of nervus medianus, nervus peroneus communis and tibial nerve in the combination group were the best among the three groups, followed in descending order by the medication group and the electric acupuncture group. The scores of TCSS, TSS and MDNS, moreover, were the lowest in the combination group. The underlying reason may be that after α-LA scavenged the pathogenic free radicals, the lipid oxidation in nerve tissue isreduced, the function of vascular endothelial cells in trophic nerves is improved. The recovery of endothelial cell function improved the nutritional status of peripheral nerves, which in turn improved the nerve conduction velocity. In addition, BPS tablets can validly increase the blood flow of trophic nerves of lower limbs, improve local microcirculation, thus ensuring the homeostasis of the distal end of the affected limb. The effect of EA may lie in activating the central descending inhibitory system and inhibiting the central sensitization of spinal dorsal neurons. Therefore, the effect of the three combined is better. Further, we observed that the ameliorateion of inflammatory factors hs-CRP, TNF-α, IL-6 and serum Hcy and Cys in the combination group was the most significant among the three groups, followed by the medication group and the electric acupuncture group successively. Hcy is an α amino acid, which is not one of the 20 amino acids that constitute proteins needed by the human body, but an intermediate metabolite produced after the conversion of methionine to methyl. Studies [34,35] have found that Hcy can destroy vascular endothelial cells, induce proliferation of smooth muscle cells, produce oxidative stress, and damage coagulation and lipid metabolism, ultimately leading to vascular damage. In addition, evidence has shown that Hcy is an independent risk factor for DPN [36]. Cystatin C (CysC), also known as γ-microprotein, is a member of the Cys, which can inhibit cysteine activity. Almost all nucleated cells can consistently produce CysC, which is metabolically cleared in the kidney and is a recognized marker of kidney injury [37]. Correlated with Hcy, CysC can increase the level of Hcy and lead to vascular and nerve injury [38]. Also, the pathogenesis of DPN is complex, which may be related to abnormal inflammatory response and other factors. The inhibition of vascular endothelial inflammatory response caused by abnormal level of inflammatory factors due to long-term hyperglycemia plays an important role in its release process [39]. hs-CRP [40] is an acute phase protein synthesized by hepatocytes. It is a non-specific inflammatory response marker and is widely used in the detection of inflammatory diseases. TNF-α [41] is an inflammatory factor produced and released by macrophages and lymphocytes. IL-6 [42] is a prototypical cytokine for maintaining homeostasis. Therefore, decreasing serum hs-CRP, TNF-α, and IL-6 levels and inhibiting vascular endothelial inflammatory response is a new way to treat DPN. In our study, EA combined with drugs can reduce the levels of both and protect the patient’s neurological function. Finally, the lowest incidence of adverse reactions was determined in combination group, indicating that the combined treatment had better safety.
To sum up, EA combined with BPS and α-LA can effectively restore the nerve conduction velocity of patients with DPN, improve their neurological function, and reduce the inflammatory response, with higher efficacy and safety, which is a feasible scheme worthy of clinical use.
This study still has some limitations due to its design. The retrospective nature of analysis does not allow randomization of patients in each group so that the similarity of patients in groups is jeopardized. In addition, the sample size may be too small to detect a difference between groups. Therefore, a well-designed, randomized, and controlled trial with prospective data collection and sample size calculation is needed to confirm the findings in our study and to examine the efficacy and safety of EA combined with BPS and α-LA in the treatment of DPN.
Disclosure of conflict of interest
None.
References
- 1.Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 3.Kurniawan AH, Suwandi BH, Kholili U. Diabetic Gastroenteropathy: a complication of diabetes mellitus. Acta Med Indones. 2019;51:263–271. [PubMed] [Google Scholar]
- 4.Graves LE, Donaghue KC. Vascular complication in adolescents with diabetes mellitus. Front Endocrinol (Lausanne) 2020;11:370. doi: 10.3389/fendo.2020.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Zhang J, Yu J, Liu S, Zhang R, Ma X, Yang Y, Wang P. Diagnostic accuracy of monofilament tests for detecting diabetic peripheral neuropathy: a systematic review and meta-analysis. J Diabetes Res. 2017;2017:8787261. doi: 10.1155/2017/8787261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 8.Lupachyk S, Watcho P, Stavniichuk R, Shevalye H, Obrosova IG. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes. 2013;62:944–952. doi: 10.2337/db12-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamagishi S, Nakamura N, Suematsu M, Kaseda K, Matsui T. Advanced glycation end products: a molecular target for vascular complications in diabetes. Mol Med. 2015;21(Suppl 1):S32–S40. doi: 10.2119/molmed.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandramoorthy HC, Bin-Jaliah I, Karari H, Rajagopalan P, Ahmed Shariff ME, Al-Hakami A, Al-Humayad SM, Baptain FA, Ahmed HS, Yassin HZ, Haidara MA. MSCs ameliorates DPN induced cellular pathology via [Ca2+] i homeostasis and scavenging the pro-inflammatory cytokines. J Cell Physiol. 2018;233:1330–1341. doi: 10.1002/jcp.26009. [DOI] [PubMed] [Google Scholar]
- 11.Eid S, Sas KM, Abcouwer SF, Feldman EL, Gardner TW, Pennathur S, Fort PE. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019;62:1539–1549. doi: 10.1007/s00125-019-4959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stino AM, Rumora AE, Kim B, Feldman EL. Evolving concepts on the role of dyslipidemia, bioenergetics, and inflammation in the pathogenesis and treatment of diabetic peripheral neuropathy. J Peripher Nerv Syst. 2020;25:76–84. doi: 10.1111/jns.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman AM, Pennings N. Insulin resistance. StatPearls [Internet] 2021;7:10. [PubMed] [Google Scholar]
- 14.Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbaek A. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes care. 2011;34:2244–2249. doi: 10.2337/dc11-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uccioli L, Izzo V, Meloni M, Vainieri E, Ruotolo V, Giurato L. Non-healing foot ulcers in diabetic patients: general and local interfering conditions and management options with advanced wound dressings. J Wound Care. 2015;24(Suppl):35–42. doi: 10.12968/jowc.2015.24.Sup4b.35. [DOI] [PubMed] [Google Scholar]
- 16.Karonova T, Stepanova A, Bystrova A, Jude EB. High-dose vitamin D supplementation improves microcirculation and reduces inflammation in diabetic neuropathy patients. Nutrients. 2020;12:2518. doi: 10.3390/nu12092518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, Tesfaye S. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7:938–948. doi: 10.1016/S2213-8587(19)30081-6. [DOI] [PubMed] [Google Scholar]
- 18.Jiang HL, Jia P, Fan YH, Li MD, Cao CC, Li Y, Du YZ. Manual acupuncture or combination with vitamin B to treat diabetic peripheral neuropathy: a systematic review and meta-analysis of randomized controlled trials. Biomed Res Int. 2020;2020:4809125. doi: 10.1155/2020/4809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang CL, Tsai PS, Wang TY, Yan LP, Xu HZ, Huang CJ. Acupuncture stimulation of ST36 (Zusanli) attenuates acute renal but not hepatic injury in lipopolysaccharide-stimulated rats. Anesth Analg. 2007;104:646–654. doi: 10.1213/01.ane.0000255288.68199.eb. [DOI] [PubMed] [Google Scholar]
- 20.Firouzjaei A, Li GC, Wang N, Liu WX, Zhu BM. Comparative evaluation of the therapeutic effect of metformin monotherapy with metformin and acupuncture combined therapy on weight loss and insulin sensitivity in diabetic patients. Nutr Diabetes. 2016;6:e209. doi: 10.1038/nutd.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan D, Xu N, Sun J, Li Z, Liao R, Zhang H, Liang X, Yi W. Electroacupuncture mitigates endothelial dysfunction via effects on the PI3K/Akt signalling pathway in high fat diet-induced insulin-resistant rats. Acupunct Med. 2018;36:162–169. doi: 10.1136/acupmed-2016-011253. [DOI] [PubMed] [Google Scholar]
- 22.Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, Zochodne D American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–65. doi: 10.1212/WNL.0b013e3182166ebe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulloa L, Quiroz-Gonzalez S, Torres-Rosas R. Nerve stimulation: immunomodulation and control of inflammation. Trends Mol Med. 2017;23:1103–1120. doi: 10.1016/j.molmed.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marathe PH, Gao HX, Close KL. American Diabetes Association standards of medical care in diabetes 2017. J Diabetes. 2017;9:320–324. doi: 10.1111/1753-0407.12524. [DOI] [PubMed] [Google Scholar]
- 25.Bril V, Perkins BA. Validation of the Toronto clinical scoring system for diabetic polyneuropathy. Diabetes Care. 2002;25:2048–2052. doi: 10.2337/diacare.25.11.2048. [DOI] [PubMed] [Google Scholar]
- 26.Cornblath D, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, Joh T. Total neuropathy score: validation and reliability study. Neurology. 1999;53:1660–4. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]
- 27.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 28.Khdour MR. Treatment of diabetic peripheral neuropathy: a review. J Pharm Pharmacol. 2020;72:863–872. doi: 10.1111/jphp.13241. [DOI] [PubMed] [Google Scholar]
- 29.Papanas N, Ziegler D. Efficacy of α-lipoic acid in diabetic neuropathy. Expert Opin Pharmacother. 2014;15:2721–2731. doi: 10.1517/14656566.2014.972935. [DOI] [PubMed] [Google Scholar]
- 30.Bartkoski S, Day M. FPIN’s help desk answers: alpha-lipoic acid for treatment of diabetic peripheral neuropathy. Am Fam Physician. 2016;93:786. [PubMed] [Google Scholar]
- 31.Solmonson A, DeBerardinis RJ. Lipoic acid metabolism and mitochondrial redox regulation. J Biol Chem. 2018;293:7522–7530. doi: 10.1074/jbc.TM117.000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang XF, Zhang BH, Lu XQ, Wang P. Beraprost sodium, a stable analogue of PGI2, inhibits the renin-angiotensin system in the renal tissues of rats with chronic renal failure. Kidney Blood Press Res. 2018;43:1231–1244. doi: 10.1159/000492405. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Liu J, Sun M, Liu W, Han J, Wang H. Acupuncture for type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2019;36:100–112. doi: 10.1016/j.ctcp.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Tchantchou F, Goodfellow M, Li F, Ramsue L, Miller C, Puche A, Fiskum G. Hyperhomocysteinemia-induced oxidative stress exacerbates cortical traumatic brain injury outcomes in rats. Cell Mol Neurobiol. 2021;41:487–503. doi: 10.1007/s10571-020-00866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C, Cai Y, Adachi MT, Oshiro S, Kitajima S, Aso T, Kaufman RJ. Homocysteine induces programmed cell death in human vascular endothelial cells through activation of the unfolded protein response. J Biol Chem. 2001;276:35867–35874. doi: 10.1074/jbc.M100747200. [DOI] [PubMed] [Google Scholar]
- 36.Anan F, Masaki T, Umeno Y, Yonemochi H, Eshima N, Saikawa T, Yoshimatsu H. Correlations between homocysteine levels and atherosclerosis in Japanese type 2 diabetic patients. Metabolism. 2007;56:1390–1395. doi: 10.1016/j.metabol.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 37.de Vries AP, Rabelink TJ. A possible role of cystatin C in adipose tissue homeostasis may impact kidney function estimation in metabolic syndrome. Nephrology Dialysis Transplantation. 2013;28:1628–1630. doi: 10.1093/ndt/gfs571. [DOI] [PubMed] [Google Scholar]
- 38.Xu LL, Gao W, Chen ZM, Shao KK, Wang YG, Cui LL, Guo NZ. Relationships between diabetic nephropathy and insulin resistance, inflammation, Trx, Txnip, CysC and serum complement levels. Eur Rev Med Pharmacol Sci. 2020;24:11700–11706. doi: 10.26355/eurrev_202011_23815. [DOI] [PubMed] [Google Scholar]
- 39.Angeli F, Reboldi G, Poltronieri C, Lazzari L, Sordi M, Garofoli M, Bartolini C, Verdecchia P. Hyperglycemia in acute coronary syndromes: from mechanisms to prognostic implications. Ther Adv Cardiovasc Dis. 2015;9:412–424. doi: 10.1177/1753944715594528. [DOI] [PubMed] [Google Scholar]
- 40.Deme D, Telekes A. Prognostic importance of plasma C-reactive protein (CRP) in oncology. Orv Hetil. 2017;158:243–256. doi: 10.1556/650.2017.30646. [DOI] [PubMed] [Google Scholar]
- 41.Idriss HT, Naismith JH. TNFα and the TNF receptor superfamily: Structure-function relationship (s) Microsc Res Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) immunotherapy. Cold Spring Harb Perspect Biol. 2018;10:a028456. doi: 10.1101/cshperspect.a028456. [DOI] [PMC free article] [PubMed] [Google Scholar]