Abstract
Objective: This study was designed to evaluate the efficacy of rectal administration of different doses of Panax notoginseng and Colla Corii Asini (CCA) suppositories in the treatment of ulcerative colitis (UC) and the effect on immune function and recurrence. Methods: Totally 120 UC patients admitted to our hospital from February 2019 to February 2020 were enrolled and randomized into experimental group (n=60) and control group (n=60). The experimental group received rectal administration of a high dose of Panax notoginseng and CCA suppositories, while the control group received a low dose. After three months of treatment, clinical symptom scores, inflammatory factor levels, scores of rectal mucosa, immune function, recurrence rates, adverse reaction rates, and clinical efficacy were compared between the two groups. Results: After treatment, the experimental group obtained significantly lower clinical symptom scores, inflammatory factors, and scores of rectal mucosa than the control group (all P<0.001). The immune function of the observation group was significantly better than that of the control group (P<0.001). At 6, 8, and 12 months after treatment, the recurrence rates in the experimental group were all significantly lower than those in the control group (all P<0.001). The two groups showed no significant difference in the incidence of adverse reaction (P>0.05), and the experimental group obtained a higher clinical efficacy than the control group (P<0.001). Conclusion: For patients with UC, the rectal administration of Panax notoginseng and CCA suppositories can exert positive effects on their inflammatory factors, immune functions, UC severity, clinical symptoms, and recovery. In addition, higher doses were associated with better effects without increased adverse events.
Keywords: Panax notoginseng and Colla Corii Asini suppositories, rectal administration, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a clinically common diffuse nonspecific inflammatory disease of the large intestine, with main manifestations of erosion and ulceration of the rectal mucosal layer, abdominal pain, diarrhea, and purulent and bloody stools [1,2], and systemic symptoms such as fever [3]. Severe lesions may develop to the proximal colon until the involvement of the entire colon, which compromises the physical and mental health of patients [4,5]. Current Western medicine mostly relies on intravenous nutrition, antibiotics, and hormones for treatment, and corticosteroids have captured great attention in clinical application in recent years, with recognized therapeutic effects [6,7]. However, obvious side effects and a high incidence of relapse after discontinuation that compromise long-term efficacy have been reported in these therapies [8]. Clinically, new oral or enema preparations of 5-aminosalicylic acid have been explored and applied in response to the increasingly elevating incidence of UC, but the high price of 5-aminosalicylic acid prevents its extensive application [9,10]. Therefore, it is urgent to explore drugs with a safe and effective profile for the treatment of UC.
Traditional Chinese medicine (TCM) categorizes UC into diarrhea and prolonged dysentery [11], with local identification in the blood secondary to Qi stasis and blood clotting, and emphasizes mitigation on the patient’s inflammatory factor levels to accelerate ulcer healing by hemostasis, heat clearance, and relief of dampness and stasis. Prior research has used Panax notoginseng and Colla Corii Asini (CCA) suppositories in the treatment of UC and found markedly alleviated clinical symptoms of patients after treatment [12], which is ascribed to the excellent effects of Panax notoginseng and CCA suppositories in stopping bleeding, nourishing blood, activating blood circulation, and resolving blood stasis. To date, a conventional dose of Panax notoginseng and CCA suppositories has been well recognized; however, a recent study has revealed that Panax notoginseng, Cortex Phellodendri, Arnebia Root, and Radix Sophorae Flavescentis are all dose-dependent, such as a larger amount of Panax notoginseng saponins in a higher dose of Panax notoginseng [13-15]. The physiological basis of the normal immune response relies on the maintenance of the ratio between the cellular components of the T lymphocyte subpopulations. The disturbance of the ratio between subpopulations of T lymphocytes in response to antigenic stimulation leads to inappropriate enhancement or weakening of the immune response, resulting in tissue damage or persistent inflammation. It is considered as one of the mechanisms of UC pathogenesis and disease activity. Previous research reported a higher proportion of CD3+, CD4+, and CD8+ T lymphocytes in peripheral blood of UC patients than in the healthy population [16]. Interleukin (IL)-6 is an important cytokine closely associated with inflammatory bowel disease (IBD) and the development of other chronic inflammatory diseases and cancers. IL-23 plays a pivotal role in the development and progression of UC. It is mainly produced by activated mononuclear macrophages and B cells and promotes the production of IFN-γ and IL-12 by T cells and antigen-presenting cells, which is closely associated with inflammatory response diseases. High-dose Panax ginseng suppositories may lower the incidence of relapse in patients, with a better maintained promising blood concentration under a longer absorption. Nevertheless, studies on different dose comparisons have yet been reported. Herein, different doses of Rectal administration Panax notoginseng and CAC suppositories were applied in the treatment of UC patients, and the long-term clinical efficacy and effects on inflammatory response and immune function were analyzed and compared, with the goal of providing more evidence-based medical evidence for its clinical application.
Materials and methods
Research design
Totally 120 UC patients admitted to Tangshan Traditional Chinese Medicine Hospital from February 2019 to February 2020 were enrolled to evaluate the efficacy of rectal administration of different doses of Panax notoginseng and CCA suppositories in the treatment of UC and the effects on immune function and recurrence rate of patients.
Recruitment of research subjects
The inclusion criteria: (1) Patients who met the diagnostic criteria for UC in the latest Guidelines and Consensus of the Chinese Gastrointestinal Diseases [17], with mild or moderate lesions within 15 cm of the anal verge; (2) Patients whose lesions were confirmed in the active stage by colonoscopy, sigmoidoscopy, and pathology; (3) Patients with negative results of stool bacteria culture. The exclusion criteria: (1) Patients without communication ability due to hearing impairment, speech impairment, unconsciousness, or mental illness; (2) Patients with severe UC; (3) Patients with colonic Crohn’s disease, radioactive, or ischemic colitis; (4) Patients with vital organ dysfunctions including abnormal heart, brain, liver, and kidney; (5) Patients with recent use of drugs affecting the treatment of UC within the past 1 month.
Steps
A total of 120 patients were included in the study and assigned to the experimental group (n=60) or the control group (n=60) according to different treatment dose. Their socio-demographic profile and clinical presentation data were collected, and the analysis revealed no statistical differences in the general information of the two groups (P>0.05), as shown in Table 1.
Table 1.
Comparison of general information between the two groups of patients
| Groups | Experimental group (n=60) | Control group (n=60) | X2/t | P-value |
|---|---|---|---|---|
| Gender | 0.033 | 0.855 | ||
| Male | 32 | 31 | ||
| Female | 28 | 29 | ||
| Age (year) | ||||
| Range | 20-64 | 22-62 | ||
| Mean age | 38.65±4.65 | 38.57±4.52 | 0.096 | 0.924 |
| Mean weight (kg) | 57.65±2.45 | 57.68±2.65 | 0.064 | 0.949 |
| BMI (kg/m2) | 22.71±1.23 | 22.69±1.20 | 0.090 | 0.928 |
| Medical payment methods | ||||
| Medical insurance | 35 | 36 | 0.035 | 0.853 |
| Business insurance | 12 | 13 | 0.051 | 0.822 |
| Others | 13 | 11 | 0.208 | 0.648 |
| Marital status | 0.040 | 0.841 | ||
| Married | 42 | 43 | ||
| Unmarried/divorced/widowed | 18 | 17 | ||
| Course of disease (year) | ||||
| Range | 1-8 | 1-7 | 0.096 | 0.924 |
| Mean course of disease | 3.45±0.35 | 3.40±0.36 | 0.771 | 0.442 |
| Place of residence | 0.304 | 0.581 | ||
| Urban | 35 | 32 | ||
| Rural | 25 | 28 | ||
| Monthly income (Yuan (CNY)) | 0.034 | 0.855 | ||
| ≥4000 | 28 | 27 | ||
| <4000 | 32 | 33 | ||
| Living habits | ||||
| Smoking | 26 | 25 | 0.034 | 0.853 |
| Drinking | 28 | 27 | 0.034 | 0.855 |
| Education level | 0.035 | 0.852 | ||
| High school and below | 36 | 37 | ||
| College and above | 24 | 23 |
Ethical considerations
This study complied with the principles of the Declaration of Helsinki [18] and was approved by the review committee of the hospital (Approved no. LC2018-120/56). After enrollment, all patients signed the informed consent form after being fully informed of the purpose, significance, content, and confidentiality of the study.
Methods
The experimental group was given a high dose of Panax notoginseng and CCA suppositories (Preparation Room, Tangshan Hospital of Traditional Chinese Medicine, Hebei Province, China, Ji Pharmaceutical System Z20050846). The recipe consists of 36 g Panax notoginseng, 36 g CCA, 120 g Cortex Phellodendri, 120 g Radix Sanguisorbae, 120 g Arnebia Root, 120 g Radix Pulsatillae, 120 g Rhizoma Coptidis, 120 g Cortex Fraxini, 120 g Rhizoma Corydalis, 72 g Pericarpium Papaveris, 72 g Radix Sophorae Flavescentis, and 72 g Rhizoma Bletillae. Panax ginseng and CCA were ground into fine powders, and the other herbs were decocted twice with water to obtain the filtrate which was then concentrated into a thick paste. The paste was mixed with the fine powder, dried, and transferred into a bolus mold, with each suppository containing 1.0 g raw medicine. All ingredients of Traditional Chinese medicine were provided by Beijing Tong Ren Tang Co., Ltd.
The control group received a low dose of Panax notoginseng and CCA suppositories. Each suppository containing 0.8 g raw medicine (80% compared with experimental group).
Patients in both groups were administered rectally, with 2 suppositories in the morning after defecation and at night before bedtime during the first month of treatment, 1 suppository in the morning after defecation and at night before bedtime during the second month of treatment, and only 1 suppository at night before bedtime during the third month of treatment. The suppositories were placed at 5 cm inside the rectum.
Observation indicators
Primary outcomes
(1) Clinical efficacy: With reference to the Consensus on Diagnosis and Management of Inflammatory Bowel Disease [20], the criteria for efficacy evaluation were formulated: ① Normal: The patient experienced a normal frequency of bowel movements, without blood in stool, and normal rectal mucosa was observed under the endoscope. ② Markedly effective: The patient experienced more bowel movements per day than normal conditions by 1-2 times and had bowel movements with bloody stools less than 50% of the total number of bowel movements. ③ Effective: The patient experienced more bowel movements per day than normal conditions by 3-4 times, with frequent occurrence of bloody stool. ④ Ineffective: The patient had more bowel movements per day than normal condition by over 5 times, with persistent bleeding.
(2) Clinical symptom scores: Diarrhea and purulent and bloody stool of the two groups of patients before and after treatment were observed and scored with reference to the diagnosis and treatment plan of UC [19]: ① Diarrhea: A score of 0 points indicates a normal condition without diarrhea. A score of 3 points indicates mild diarrhea with a daily diarrhea frequency ≤4 times. A score of 6 points indicates moderate diarrhea with a daily diarrhea frequency between 4 and 6 times. A score of 9 points indicates severe diarrhea with a daily diarrhea frequency >6 times. ② Purulent and bloody stool: A score of 0 point indicates a normal condition without a purulent and bloody stool. A score of 3 points indicates a mild condition with a small amount of blood and pus. A score of 6 points indicates a moderate condition with a large amount of purulent and bloody stool. A score of 9 points indicates a severe condition with blood and pus in all stool samples.
Secondary outcomes
(1) Inflammatory factors: Morning fasting venous blood (5 ml) was collected from each patient before and after treatment. Enzyme-linked immunosorbent assay (ELISA) was adopted to determine the levels of IL-6 (Human IL-6 ELISA Kit, MultiSciences, Co., LTD, Cat. No. 70-EK106/2-96) and IL-23 (Human IL-23 ELISA Kit, MultiSciences, Co., LTD, Cat. No. 70-EK123-96).
(2) Scores of rectal mucosa: The rectal mucosal conditions of the two groups of patients were evaluated under an endoscope and scored with reference to the UC diagnosis and treatment protocol: ① A score of 0 points indicates normal rectal mucosa; ② A score of 1 point indicates rectal mucosal hyperemia with edema, vascular blurring, and mildly brittle mucosa; ③ A score of 2 points indicates brittle rectal mucosa with contact bleeding; ④ A score of 3 points indicates rough rectal mucosa with spontaneous bleeding; ⑤ A score of 4 points indicates rectal mucosa with diffuse and multiple erosions and ulcers, covered with purulent blood secretions on the surface.
(3) Immune function: Morning fasting venous blood (5 ml) was collected from each patient before and after treatment, and the peripheral blood T lymphocyte subpopulation levels (CD3+, CD4+, CD4+/CD8+) were determined using the flow cytometry (Mouse anti-Human CD4/CD8/CD3 mAb, FITC/PE/PE-Cy5, Sizhengbai Biological Co., LTD, Cat. No. FHT001-050).
(4) Recurrence rate: The recurrence rates of both groups at 6, 8, and 12 months after treatment were recorded.
(5) Incidence of adverse reactions: The number of patients with adverse reactions including dizziness, headache, nausea and vomiting, and stomach discomfort was counted.
Statistical analyses
In this research, the data were processed by SPSS20.0, and visualized into required graphics by GraphPad Prism 7 (GraphPad Software, San Diego, USA). The count data were expressed by [n (%)] and analyzed using the chi-square test, and the measurement data were expressed by (x̅±s) and the intra-group comparison before and after treatment was analyzed using the paired t-test, and inter-group comparison was analyzed using a two-group t-test. P<0.05 indicated that the difference was statistically significant.
Results
Comparison of general information
The two groups showed no significant difference in terms of general information (P>0.05). See Table 1.
Comparison of clinical symptom scores
Markedly lower clinical symptom scores were observed in the experimental group than those in the control group after treatment (all P<0.05). See Figure 1.
Figure 1.
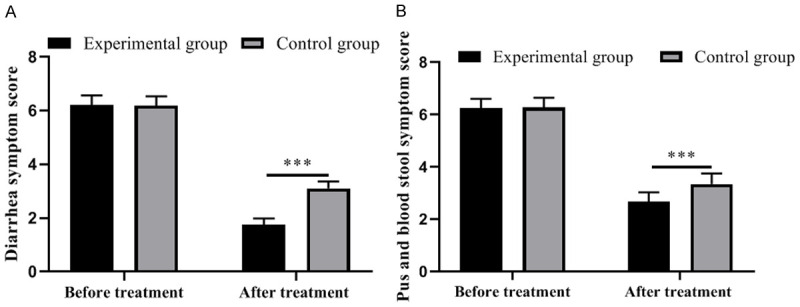
Comparison of clinical symptom scores between the two groups of patients. A: Diarrhea symptom scores; B: Purulent and bloody stool symptom scores. ***, P<0.001.
Comparison of inflammatory factors
The experimental group showed remarkably lower levels of inflammatory factors than the control group (all P<0.001). See Figure 2.
Figure 2.
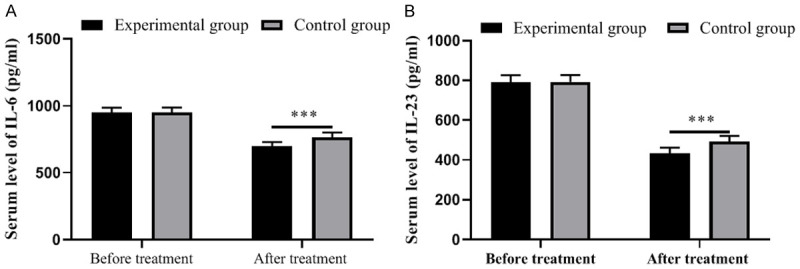
Comparison of inflammatory factors between the two groups of patients. A: Serum level of IL-6; B: Serum level of IL-23. ***, P<0.001.
Comparison of rectal mucosa scores
The score of rectal mucosal condition in the experimental group was significantly lower than that in the control group (P<0.001). See Figure 3.
Figure 3.
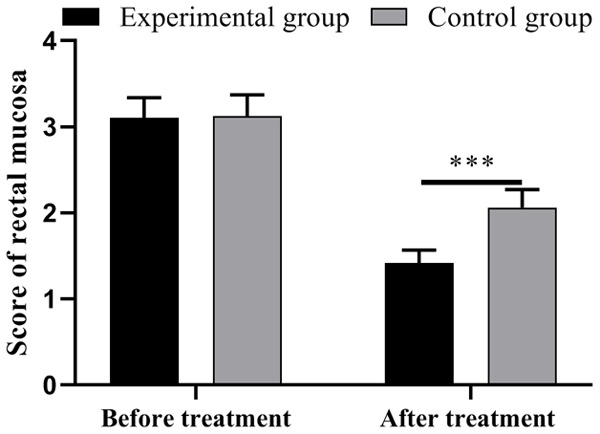
Comparison of scores of rectal mucosa between the two groups of patients. Notes: ***, P<0.001.
Comparison of immune function
The experimental group outperformed the control group in terms of immune function (P<0.001). See Table 2. The typical FCM plot figure was shown in Figure 4.
Table 2.
Comparison of immune function between the two groups of patients (x̅±s, %)
| Indicators | Experimental group | Control group | t | P-value | ||
|---|---|---|---|---|---|---|
| CD3+ | Before treatment | 70.26±5.65 | Before treatment | 70.30±5.40 | 0.040 | 0.968 |
| After treatment | 57.65±4.68 | After treatment | 63.58±5.20 | 6.566 | <0.001 | |
| t | 13.314 | t | 6.943 | |||
| P | <0.001 | P | <0.001 | |||
| CD4+ | Before treatment | 30.98±3.51 | Before treatment | 31.01±3.20 | 0.049 | 0.961 |
| After treatment | 40.98±3.68 | After treatment | 35.41±3.98 | 7.959 | <0.001 | |
| t | 15.231 | t | 6.674 | |||
| P | <0.001 | P | <0.001 | |||
| CD4+/CD8+ | Before treatment | 1.08±0.12 | Before treatment | 1.06±0.14 | 0.840 | 0.403 |
| After treatment | 1.72±0.23 | After treatment | 1.42±0.25 | 6.841 | <0.001 | |
| t | 19.109 | t | 9.732 | |||
| P | <0.001 | P | <0.001 |
Figure 4.
Immune function determined using the flow cytometry.
Comparison of recurrence rates
At 6, 8, and 12 months after treatment, the recurrence rates in the experimental group were all remarkably lower than those in the control group (all P<0.001). See Table 3.
Table 3.
Comparison of recurrence rates between the two groups of patients [n (%)]
| Groups | n | 6 months | 8 months | 12 months |
|---|---|---|---|---|
| Experimental group | 60 | 3 (5.0) | 5 (8.3) | 6 (10.0) |
| Control group | 60 | 20 (33.3) | 24 (40.0) | 28 (46.7) |
| X2 | 15.545 | 16.415 | 19.863 | |
| P-value | <0.001 | <0.001 | <0.001 |
Comparison of adverse reaction rate
The two groups showed no significant difference in adverse reaction rate (P>0.05). See Table 4.
Table 4.
Comparison of adverse reaction rates between the two groups of patients [n (%)]
| Groups | n | Dizziness | Headache | Nausea and vomiting | Gastrointestinal discomfort | Total incidence |
|---|---|---|---|---|---|---|
| Experimental group | 60 | 2 (3.33) | 2 (3.33) | 3 (5.00) | 3 (5.00) | 10 (16.67) |
| Control group | 60 | 1 (1.67) | 2 (3.33) | 2 (3.33) | 3 (5.00) | 8 (13.33) |
| χ2 | 0.261 | |||||
| P-value | 0.609 |
Comparison of clinical efficacy
There was no death, hospital referral or discontinuation of treatment in both groups of patients. The experimental group showed a higher clinical efficacy compared with the control group (P=0.015). See Table 5.
Table 5.
Comparison of clinical efficacy between the two groups of patients [n (%)]
| Groups | n | Normal | Markedly effective | Effective | Ineffective | Total effective rate |
|---|---|---|---|---|---|---|
| Experimental group | 60 | 18 (30.00) | 30 (50.00) | 10 (16.67) | 2 (3.33) | 58 (96.67) |
| Control group | 60 | 12 (20.00) | 24 (40.00) | 14 (23.33) | 10 (16.67) | 50 (83.33) |
| χ2 | 5.926 | |||||
| P-value | 0.015 |
Discussions
Ulcerative colitis, or chronic non-specific UC, is a common gastrointestinal disease featured by chronic inflammation of the colonic mucosa and mainly manifested as diarrhea, mucoid degeneration, and tenesmus. In this study, the better treatment efficacy in the experimental group than the control group after treatment may be associated with the dose-dependence of drugs in the suppositories. In TCM, UC has been classified as diarrhea and prolonged dysentery and referred to Changpi (acute and severe intestinal disease) [21,22] in The Internal Canon of Medicine. It is related to the dysfunctional disorder of the spleen and stomach where the disease stems from dampness and heat from fatty, sweet, and thick foods, which results in stagnation of Qi and blood, unfavorable conduction, and eventually internal ulcers. Accordingly, the treatment of the disease is to stop bleeding, clear heat and dampness, and invigorate Qi and blood circulation. The Panax notoginseng and CCA suppositories selected for this study have been verified in many publications to be effective in alleviating the clinical symptoms of patients with UC. This formula mainly includes Panax notoginseng with a positive effect of invigorating blood circulation and removing blood stasis, CCA that stops bleeding and nourishes the blood, and Rhizoma Bletillae that diminishes swelling and promotes muscle growth, which are sovereign drugs contributing to the acceleration of ulcer healing. The formula also contains minister drugs including Radix Pulsatillae, Rhizoma Coptidis, Cortex Phellodendri, Cortex Fraxini, Radix Sanguisorbae, Arnebia Root, Radix Sophorae Flavescentis, which serve to clear heat and dampness and detoxify stasis. In addition, Pericarpium Papaveris is an assistant drug for relieving diarrhea and promoting the production of body fluid, and Rhizoma Corydalis as a guide drug is to dispel Qi and relieve pain. A proper combination of the above four types of drugs delivers a desirable effect of clearing heat and relieving dampness.
In this study, the experimental group got a lower score of rectal mucosa than the control group after treatment (P<0.001). In addition, the immune function of the experimental group after treatment was significantly better than that of the control group (P<0.001). Prior research has shown that a high dose of Panax notoginseng can exert strong therapeutic effects on patients with cerebral hemorrhage [23], as the drug dramatically increases the number of platelets in a short period of time while elevating the prothrombin content. Its higher concentration accelerates the release of hemostatic active substances such as ADP and calcium ions from platelets and subsequently exerts an outstanding hemostatic effect. Furthermore, the Panax notoginseng saponins in Panax notoginseng regulates the expression of apoptosis-related proteins in human bone marrow hematopoietic cells, so high doses of Panax notoginseng sufficiently attenuate the apoptosis of hematopoietic cells while upregulating transcription factors such as NFkB and accelerating cell proliferation. In addition, research by Zhu Z et al. [24] demonstrated that gavage administration of Rhizoma Coptidis and Huang Lian Jiedu Decoction (Rhizoma Coptidis - Radix Scutellariae - Cortex Phellodendri) to rats alleviated gastric mucosal injury in a dose-dependent manner, suggesting a close correlation between the preventive effect of Rhizoma Coptidis on gastric ulcer damage and the dosage, with 41.7% gastric ulcer inhibition for high doses of Rhizoma Coptidis. As mentioned above, Arnebia Root in Panax notoginseng and CCA suppositories is highly dose-dependent. Consequently, the suppositories at a high dose may enhance the immune function the humoral and cellular immunity of immunocompromised mice [25].
Accordingly, the advantages of the higher concentration account for significantly lower recurrence rates in the experimental group than those in the control group at 6, 8, and 12 months after treatment in this study (all P<0.001). There are two modes of administration for UC, namely, oral administration and topical delivery, in which oral administration is mainly adopted for aminosalicylates medication. However, oral administration fails to deliver an effective drug concentration of local administration, so rectal administration is more advocated in academic consensus. Herein, the experimental group obtained better therapeutic effects, which may be ascribed to the higher concentration of the drug that prolongs the local effective drug concentrations and enhances long-term efficacy. At the current stage, no controlled study is available on the effect of high-dose Panax notoginseng and CCA suppositories on the recurrence rate of patients with UC. Moreover, the recurrence of UC is associated with multiple factors, which requires a more in-depth analysis of the mechanisms of action. The limitations of this study maybe that it is a single-center study without a double-blind approach, and a control group for conventional Western medicine treatment is absent, which prevents the determination of the effect of comparison with conventional treatment methods. In the future, further strategies of multicenter, large sample, double-blind randomized controlled studies would be needed for the treatment of UC patients.
Conclusion
For patients with UC, the rectal administration of Panax notoginseng and CCA suppositories can exert positive effects on their inflammatory factors, immune functions, UC severity, clinical symptoms, and recovery. In addition, higher doses were associated with better effects without increased adverse events.
Acknowledgements
This study was supported by National Natural Science Foundation of China [Grants no.81704059].
Disclosure of conflict of interest
None.
References
- 1.Graziano F, Macaluso FS, Cassata N, Citrano M, Orlando A. Pyoderma gangrenosum in an UC pediatric patient during vedolizumab therapy successfully treated with oral cyclosporine. Inflamm Bowel Dis. 2021;27:e110–e111. doi: 10.1093/ibd/izab106. [DOI] [PubMed] [Google Scholar]
- 2.Bossuyt P, Vermeire S, Bisschops R. Assessing disease activity in UC using artificial intelligence: can “equally good” be seen as “better”? Gastroenterology. 2020;159:1625–1626. doi: 10.1053/j.gastro.2020.03.090. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann P, Globig AM, Thomann AK, Grigorian M, Krisam J, Hasselblatt P, Reindl W, Gauss A. Tofacitinib in treatment-refractory moderate to severe UC: real-world experience from a retrospective multicenter observational study. J Clin Med. 2020;9:2177. doi: 10.3390/jcm9072177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fudman DI, Sattler L, Feuerstein JD. Inpatient management of acute severe UC. J Hosp Med. 2019;14:766–773. doi: 10.12788/jhm.3207. [DOI] [PubMed] [Google Scholar]
- 5.Kotwani P, Terdiman J, Lewin S. Tofacitinib for rescue therapy in acute severe uc: a real-world experience. J Crohns Colitis. 2020;14:1026–1028. doi: 10.1093/ecco-jcc/jjaa018. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Cao N, Wang Y, Wang Y, Wu C, Cheng X, Wang C. Improvement of oxazolone-induced UC in rats using andrographolide. Molecules. 2019;25:76. doi: 10.3390/molecules25010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clement E, Dang J, Laffin M, Wang H. Incidence of venous thromboembolism following proctectomy is greater in UC than in malignancy or Crohn’s disease. J Gastrointest Surg. 2020;24:2664–2666. doi: 10.1007/s11605-020-04738-9. [DOI] [PubMed] [Google Scholar]
- 8.Luttropp K, Dalén J, Svedbom A, Dozier M, Black CM, Puenpatom A. Real-world patient experience of switching biologic treatment in inflammatory arthritis and UC - a systematic literature review. Patient Prefer Adherence. 2020;14:309–320. doi: 10.2147/PPA.S238843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura M, Yamamura T, Maeda K, Sawada T, Mizutani Y, Ishikawa E, Ohashi A, Kajikawa G, Furukawa K, Ohno E, Honda T, Kawashima H, Ishigami M, Fujishiro M. Refractory UC improved by scheduled combination therapy of vedolizumab and granulocyte and monocyte adsorptive apheresis. Intern Med. 2020;59:3009–3014. doi: 10.2169/internalmedicine.5302-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordes F, Foell D, Ding JN, Varga G, Bettenworth D. Differential regulation of JAK/STAT-signaling in patients with UC and Crohn’s disease. World J Gastroenterol. 2020;26:4055–4075. doi: 10.3748/wjg.v26.i28.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantavou K, Yiallourou AI, Piovani D, Evripidou D, Danese S, Peyrin-Biroulet L, Bonovas S, Nikolopoulos GK. Efficacy and safety of biologic agents and tofacitinib in moderate-to-severe UC: a systematic overview of meta-analyses. United European Gastroenterol J. 2019;7:1285–1303. doi: 10.1177/2050640619883566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negoro A, Takano T, Tajiri H, Nezu R, Kawamura N, Brooks S. A role of colectomy in immune thrombocytopenic purpura associated with UC: a case report and a review of the literature. Int J Colorectal Dis. 2014;29:1179–80. doi: 10.1007/s00384-014-1903-1. [DOI] [PubMed] [Google Scholar]
- 13.Xue NN, He M, Li Y, Wu JZ, Du WW, Wu XM, Yang ZZ, Zhang CG, Li QY, Xiao H. Periplaneta americana extract promotes intestinal mucosa repair of UC in rat. Acta Cir Bras. 2020;35:e202001002. doi: 10.1590/s0102-865020200100000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parian AM, Limketkai BN, Chowdhury R, Brewer GG, Salem G, Falloon K, Selaru F, Melia J, Lazarev MG. Serrated epithelial change is associated with high rates of neoplasia in UC patients: a case-controlled study and systematic review with meta-analysis. Inflamm Bowel Dis. 2021;27:1475–1481. doi: 10.1093/ibd/izaa312. [DOI] [PubMed] [Google Scholar]
- 15.Park SH, Jeong SK, Lee JH, Rhee KH, Kim YH, Hong SN, Kim KH, Seo SI, Cha JM, Park SY, Park H, Kim JS, Im JP, Yoon H, Kim SH, Jang J, Kim JH, Suh SO, Kim YK, Ye BD, Yang SK Songpa-Kangdong Inflammatory Bowel Disease (SK-IBD) Study Group. Clinical characteristics and long-term prognosis of elderly-onset UC in a population-based cohort in the songpa-kangdong district of Seoul, Korea. Gut Liver. 2021;15:742–751. doi: 10.5009/gnl20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon JR, Lee CK, Hong SN, Im JP, Ye BD, Cha JM, Jung SA, Lee KM, Park DI, Jeen YT, Park YS, Cheon JH, Kim H, Seo B, Kim Y, Kim HJ MOSAIK study group of the Korean Association for Study of Intestinal Diseases. Unmet psychosocial needs of patients with newly diagnosed UC: results from the nationwide prospective cohort study in Korea. Gut Liver. 2020;14:459–467. doi: 10.5009/gnl19107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge CY, Wei LY, Tian Y, Wang HH. A seven-NF-κB-related gene signature may distinguish patients with UC-associated colorectal carcinoma. Pharmgenomics Pers Med. 2020;13:707–718. doi: 10.2147/PGPM.S274258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First- and second-line pharmacotherapies for patients with moderate to severely active UC: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020;18:2179–2191. e6. doi: 10.1016/j.cgh.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Østvik AE, Svendsen TD, Granlund AVB, Doseth B, Skovdahl HK, Bakke I, Thorsvik S, Afroz W, Walaas GA, Mollnes TE, Gustafsson BI, Sandvik AK, Bruland T. Intestinal epithelial cells express immunomodulatory ISG15 during active UC and Crohn’s disease. J Crohns Colitis. 2020;14:920–934. doi: 10.1093/ecco-jcc/jjaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh SJ, Choi Y, Kim CR, Park JH, Lee JH, Lee DY. An atypical extraintestinal manifestation in a child with UC: cutaneous leukocytoclastic vasculitis. Ann Dermatol. 2020;32:90–91. doi: 10.5021/ad.2020.32.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuwahara R, Ikeuchi H, Bando T, Sasaki H, Goto Y, Horio Y, Minagawa T, Uchino M. Clinical results of one-stage restorative proctocolectomy with J-pouch anal anastomosis in 300 UC patients. J Anus Rectum Colon. 2020;4:181–185. doi: 10.23922/jarc.2020-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammed Vashist N, Samaan M, Mosli MH, Parker CE, MacDonald JK, Nelson SA, Zou GY, Feagan BG, Khanna R, Jairath V. Endoscopic scoring indices for evaluation of disease activity in UC. Cochrane Database Syst Rev. 2018;1:CD011450. doi: 10.1002/14651858.CD011450.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X, Li ZC, Chen PP, Xie RF. Primary mechanism study of Panax notoginseng flower (Herb) on myocardial infarction in rats. Cardiol Res Pract. 2019;2019:8723076. doi: 10.1155/2019/8723076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu JG, Huang ZY, Shen QN, Li L, Wang QH, Wang L, Lu TL, Mao CQ. Studies on fingerprints and efficacy-related substance of classical prescription Zhuru decoction. Zhongguo Zhong Yao Za Zhi. 2020;45:5599–5606. doi: 10.19540/j.cnki.cjcmm.20200921.301. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida A, Matsuoka K, Ueno F, Morizane T, Endo Y, Hibi T. Serum PR3-anca is a predictor of primary nonresponse to anti-TNF-α agents in patients with UC. Inflamm Intest Dis. 2021;6:117–122. doi: 10.1159/000515361. [DOI] [PMC free article] [PubMed] [Google Scholar]


