Abstract
Objective: This study investigated and analyzed the effect of Thymopentin on immune function and inflammatory levels in end-stage renal disease (ESRD) patients who were undergoing maintenance hemodialysis. Methods: A total of 112 patients with ESRD on regular hemodialysi from May 2018 to October 2019 were chosen and classified into an observation group and a control group by a convenience sampling method, with 56 cases in each group. The control-group was treated with conventional therapy, and the observation-group was treated with thymic pentapeptide based on the conventional treatment. The two groups’ improvements in inflammation level, immunological functioning and living quality before and after treatment were compared. Results: IL-6, IL-8, TNF-α, and hs-CRP levels in the observation group after treatment were (5.52±1.46) ng/L, (18.76±2.83) ng/L, (3.27±1.08) pmol/L and (24.12±2.96) mg/L respectively, which were lower than (6.68±1.51) ng/L, (24.12±2.96) ng/L, (5.13±1.15) pmol/L and (6.46±1.19) mg/L in the control group (t=4.133, 9.795, 8.828, 6.198; P<0.05). After treatment, SOD level in the observation-group was (115.52±9.46) u/mL, which was higher than that of (104.68±9.21) u/mL in the control group (t=6.144, P<0.05); and MDA in the observation-group was (4.06±0.83) u/mL, which was lower than that of (5.22±0.96) u/mL in the control group (t=6.840, P<0.05). In addition, CD3+ (68.25±12.54)%, CD4+ (49.17±6.23)%, and CD4+/CD8+ (1.95±0.37) in the observation group during post-intervention were higher than of the counterparts (62.61±10.23)%, (45.21±5.89)% (1.71±0.32) in the control group (t=2.608, 3.457, 4.807; P<0.05); while CD8+ in the observation group (20.14±5.25)% was lower than that in the control group (25.01±5.47)% (t=3.671; P<0.05). The SF-36 score in the observation group after treatment was (73.43±5.59) points, which was superior to the score (66.06±5.22) in the control group (t=7.211, P<0.05). Conclusion: Thymopentin can greatly improve the micro-inflammatory state of ESRD patients with maintenance hemodialysis, thereby improving the patient’s immune function and living quality.
Keywords: End-stage renal disease, maintenance hemodialysis, thymopentin, immunological functioning, inflammatory cytokines
Introduction
Maintenance hemodialysis (MHD) is a commonly used replacement therapy of renal function for patients with end-stage renal disease (ESRD). The treatment method, along with the increasing iteration of hemodialysis technology, can greatly extend the survival time of patients. However, due to the long course of disease, patient have been affected by urotoxic substances for a long time, and the thymic tissue is atrophied and the secretion of hormones is reduced, resulting in the failure of differentiation and maturation of pre-T lymphocytes produced by the bone marrow. In addition, due to the repeated stimulation by various microorganisms and endotoxins in long-term treatment, the patient’s body presents with a persistent and low-level inflammatory state, resulting in a significant decrease in immune function of the patients [1-3]. As a clinical immunomodulator, Thymopentin has an antigen similar to Mycobacterium tuberculosis, and has better immunological activity than thymosin, which plays a favorable role in regulating cellular immune function [4,5]. Therefore, this study applied thymic pentapeptide to ESRD maintenance hemodialysis patients and explored the curative effect on immune function and inflammatory factors.
Materials and methods
General materials
This study was review and approved by the Medical Ethics Association of our hospital. A total of 112 patients who underwent regular hemodialysis from May 2018 to October 2019 were enrolled as the study subjects. The subjects included 63 males and 49 females, with an average age of 43.25±5.17 years old and dialysis duration of 15.33±6.26 months.
The inclusive criteria were as follows: (1) The patients met the criteria of maintenance hemodialysis; (2) The duration of continuous dialysis treatment was ≥3 months; (3) The patients were informed of the study content and signed an agreement to participate in the research and voluntarily signed the informed consent.
The exclusion criteria included: (1) Patients undergoing peritoneal dialysis or renal transplantation with care that was converted to hemodialysis; (2) Patients with cardiovascular disease, severe infection or malignant tumors; (3) Patients with cognitive impairment or poor compliance.
Methods
The convenience sampling method was adopted to divide the experimental subjects into an observation-group and a control group, with each group of 56 cases. The comparison of the age, dialysis time and primary disease type between the two sets of subjects was statistically comparable (P>0.05), see Table 1.
Table 1.
The comparison of general information between the two groups of patients
| Item | Observation group (n=56) | Control group (n=56) | χ 2/t | P |
|---|---|---|---|---|
| Gender (number of cases) | ||||
| Male | 34 | 29 | 0.961 | 0.327 |
| Female | 22 | 27 | ||
| Primary disease (cases) | ||||
| Chronic glomerulonephritis | 21 | 25 | 2.094 | 0.553 |
| Chronic interstitial nephritis | 13 | 8 | ||
| Diabetic nephropathy | 10 | 8 | ||
| Others | 12 | 15 | ||
| Age (years, x̅±s) | 44.08±5.52 | 42.58±4.82 | 1.532 | 0.129 |
| Duration of hemodialysis (m, x̅±s) | 15.17±6.20 | 15.49±6.32 | 0.271 | 0.787 |
Both groups were given maintenance hemodialysis by the German Fresenius 4008B hemodialysis machine and bicarbonate dialysate. The dialysate membrane area was 1.5 m2, the dialysate flow was 0.5 L/min, and the blood flow was between 0.2-0.28 L/min. The period of the dialysis was 4 h/time, 3 times/week. Enoxaparin sodium was used in dialysis (Manufacturer: Shenzhen Tiandao Pharmaceutical Co., LTD., Medicine Approval Number: H20056849) to maintain open cardiopulmonary bypass. The control group was given integrated treatments of hypotension, water and salt restriction and anemia correction. The observation group received intravenous drip of 1 mg thymopentin (Manufacturer: Beijing Shuanglu Pharmaceutical, Medicine Approval Number: H20058462) and 250 mg 0.9% sodium chloride once a day based on care given to the control-group. Both groups received the remedy for 12 weeks continuously.
Indexes observation
(1) Measurement of inflammatory factors: including IL-6, IL-8, TNF-α, hs-CRP. Methods: A total of 5 ml of fasting venous blood before and after treatment was collected and placed in EDTA anticoagulant vacuum tubes. The blood was centrifuged for 10 to 15 min with a centrifugal filter to separate the serum. IL-6, IL-8, TNF-α were detected by radioimmunoassay and corresponding test kits, and hs-CRP was detected by Sandwich-ELISA with test kits.
(2) Measurement of oxidative stress indexes: including superoxide dismutase (SOD) and malondialdehyde (MDA). Methods: The activity of SOD was determined by xanthine oxidase. MDA was determined by the thiobarbituric acid method.
(3) Measurement of immune function indicators: including CD3+, CD4+, CD8+, and CD4+/CD8+. Methods: the serum was used to detect the CD3+, CD4+ and CD8+ levels by flow cytometry.
(4) Measurement of living quality: SF-36 scale was employed to evaluate patients’ living quality. The scale covers 8 dimensions and 36 questions, and a 5-level scoring method was used for scoring. A higher score represented superior living quality of patients.
Data statistics
Data analysis was conducted by SPSS 21.0. Enumeration data were represented by (n, %) and verified by χ 2; and measurement data were expressed as (x̅±s) and verified by t-test. P<0.05 was set as the value that denoted statistical significance.
Results
Comparison of serum inflammatory factors between the two groups before and after treatment
Before treatment, the comparison of serum inflammatory factor levels between the two groups of patients was statistically insignificant (P>0.05). The levels of IL-6, IL-8, TNF-α, and hs-CRP in the two groups of patients decreased after treatment, and the levels in the observation group were lower than those in the control group (P<0.05), as shown in Table 2.
Table 2.
Comparison of serum inflammatory factors before and after treatment
| Group | Number of cases | IL-6 (ng/L) | t | P | IL-8 (ng/L) | t | P | ||
|
|
|
||||||||
| Before treatment | After treatment | Before treatment | After treatment | ||||||
|
| |||||||||
| Observation group | 56 | 8.41±1.53 | 5.52±1.46 | 10.226 | 0.000 | 35.22±3.14 | 18.76±2.83 | 29.139 | 0.000 |
| Control group | 56 | 8.45±1.49 | 6.68±1.51 | 6.244 | 0.000 | 34.39±3.01 | 24.12±2.96 | 18.205 | 0.000 |
| t | 0.140 | 4.133 | 1.428 | 9.795 | |||||
| P | 0.889 | 0.000 | 0.156 | 0.000 | |||||
|
| |||||||||
| Group | Number of cases | TNF-α (pmol/L) | t | P | hs-CRP (mg/L) | t | P | ||
|
|
|
||||||||
| Before treatment | After treatment | Before treatment | After treatment | ||||||
|
| |||||||||
| Observation group | 56 | 7.45±1.51 | 3.27±1.08 | 16.849 | 0.000 | 8.46±1.27 | 5.14±1.06 | 15.019 | 0.000 |
| Control group | 56 | 7.36±1.57 | 5.13±1.15 | 8.575 | 0.000 | 8.21±1.23 | 6.46±1.19 | 7.652 | 0.000 |
| t | 0.309 | 8.828 | 1.058 | 6.198 | |||||
| P | 0.758 | 0.000 | 0.292 | 0.000 | |||||
Comparison of oxidative stress before and after treatment
Before treatment, the comparison of serum SOD and MDA levels in the two groups of patients was insignificant (P>0.05). In post-treatment, the SOD levels in both groups increased, while the MDA level decreased. In addition, the serum SOD level in the observation group was higher than that in the control group, while the MDA level was lower than that in control group (P<0.05). See Table 3.
Table 3.
Comparison of oxidative stress before and after treatment (x̅±s)
| Group | Number of cases | SOD (u/mL) | t | P | MDA (nmol/ml) | t | P | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Before treatment | After treatment | Before treatment | After treatment | ||||||
| Observation group | 56 | 85.21±8.26 | 115.52±9.46 | 18.061 | 0.000 | 6.22±1.14 | 4.06±0.83 | 11.463 | 0.000 |
| Control group | 56 | 84.35±8.13 | 104.68±9.21 | 12.384 | 0.000 | 6.39±1.21 | 5.22±0.96 | 5.669 | 0.000 |
| t | 0.555 | 6.144 | 0.765 | 6.840 | |||||
| P | 0.580 | 0.000 | 0.446 | 0.000 | |||||
Comparison of immune function pre and post treatment
The difference in the levels of CD3+, CD4+, CD8+ and CD4+/CD8+ in the two groups in prior to treatment were insignificant (P>0.05). In post-treatment, the levels of CD3+, CD4+, and CD4+/CD8+ in both groups increased, while CD8+ decreased. In addition, CD3+, CD4+ and CD4+/CD8+ in the observation group were higher than those in the control group, and CD8+ was lower than that in the control group (P<0.05). As shown in Table 4.
Table 4.
Comparison of immune function between the two groups before and after treatment (x̅±s, %)
| Group | Number of cases | CD3+ | t | P | CD4+ | t | P | ||
|
|
|
||||||||
| Before treatment | After treatment | Before treatment | After treatment | ||||||
|
| |||||||||
| Observation group | 56 | 47.35±9.42 | 58.25±12.54 | 5.201 | 0.000 | 40.15±5.46 | 49.17±6.23 | 8.148 | 0.000 |
| Control group | 56 | 47.03±9.55 | 52.61±10.23 | 2.984 | 0.004 | 40.38±5.57 | 45.21±5.89 | 4.459 | 0.000 |
| t | 0.179 | 2.608 | 0.221 | 3.457 | |||||
| P | 0.859 | 0.010 | 0.826 | 0.001 | |||||
|
| |||||||||
| Group | Number of cases | CD8+ | t | P | CD4+/CD8+ | t | P | ||
|
|
|
||||||||
| Before treatment | After treatment | Before treatment | After treatment | ||||||
|
| |||||||||
| Observation group | 56 | 28.03±5.16 | 20.14±5.25 | 8.021 | 0.000 | 1.43±0.28 | 1.95±0.37 | 8.386 | 0.000 |
| Control group | 56 | 28.17±5.38 | 25.01±5.47 | 3.082 | 0.003 | 1.40±0.25 | 1.71±0.32 | 5.713 | 0.000 |
| t | 0.141 | 4.807 | 0.598 | 3.671 | |||||
| P | 0.889 | 0.000 | 0.551 | 0.000 | |||||
Comparison of SF-36 scores pre and post treatment
The comparison of SF-36 scores in the two groups before treatment was insignificantly different (P>0.05). In post-treatment, the SF-36 scores increased in both groups, and the score in the observation-group was higher than that in the control-group (P<0.05) (Table 5 and Figure 1).
Table 5.
Comparison of SF-36 scores between the two groups before and after treatment
| Group | Number of cases | SF-36 scores | t | P | |
|---|---|---|---|---|---|
|
| |||||
| Before treatment | After treatment | ||||
| Observation group | 56 | 55.97±4.26 | 73.43±5.59 | 18.591 | 0.000 |
| Control group | 56 | 56.54±4.48 | 66.06±5.22 | 10.357 | 0.000 |
| t | 0.690 | 7.211 | |||
| P | 0.492 | 0.000 | |||
Figure 1.
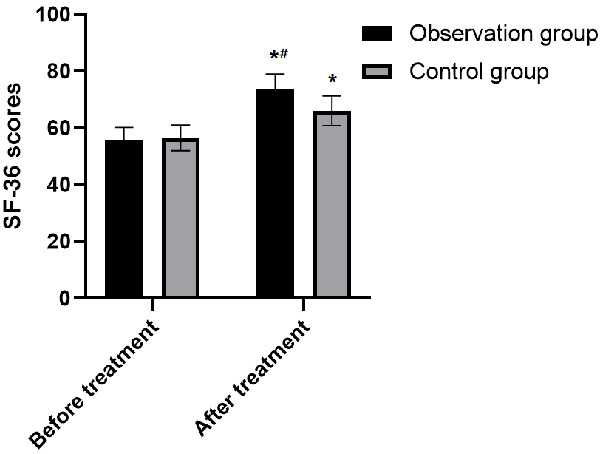
Comparison of SF-36 scores between the two groups before and after treatment. Note: Compared with before treatment, *P<0.05; Compared with control group, #P<0.05.
Discussion
An epidemiological survey showed that in the treatment of renal replacement, 89.5% of patients received hemodialysis and 10.5% received peritoneal dialysis [6]. In recent years, the survival rate of hemodialysis patients has been significantly improved with the progressive development of hemodialysis techniques and the maturity of dialysis materials. However, due to the long treatment cycle, the patient’s body is susceptible to repeated stimulation by various microorganisms, endotoxins, etc., which results in a persistent, low-level inflammatory state. The continuous micro-inflammatory response can stimulate the immune system, leading to a remarkable decline of immune function in the body [7-9]. Therefore, it is of the key importance to reduce the micro-inflammatory response and improve the nutritional status of the body to promote the patient’s disease prognosis.
Thymopentin is a polypeptide substance extracted from the thymus, which has the function of immune stimulation. Thymopentin can selectively induce transformation of cellular Thy-1-, increase the level of intracellular cAMP, and thus induce a series of intracellular reactions [10,11]. Clinical studies have shown that Thymopentin can promote the proliferation of T lymphocytes, inhibit allergic reactions to a certain extent, and ultimately plays a part in enhancing the ability to resist viruses and bacteria in the body [12]. Studies have also shown that Thymopentin can reduce phosphorylated proteins in monocytes in the peripheral blood, inhibit the activation of it, thereby reducing the release of pro-inflammatory factors and inhibiting an acute phase response induced by ESRD [13]. The results show that IL-6, IL-8, TNF- and hs-CRP in the observation-group after treatment were less than those before treatment and also those the post-intervention in the control-group (P<0.05), indicating that Thymopentin can notably reduce the inflammatory response in the body.
The activity of antioxidants in the plasma of patients with ESRD undergoing maintenance hemodialysis decreases significantly. When the generation of free radicals and the metabolic balance of the body are not in balance, damage of target tissues will occur, the level of unsaturated fatty acids will be reduced, and a large amount of lipid peroxides will be produced, which results in a state of oxidative stress [14,15]. SOD is a major antioxidant enzyme in the body, it can inhibit the accumulation of lipid peroxides, prevent damage to the body caused by oxygen free radicals, thereby protecting tissue cells [16]. MDA is a marker of oxidative stress and a synthetic product of lipid peroxidation. MDA will aggravate damage of cellular membranes and is an indirect indicator used to assess the degree of tissue and cellular damage [17]. The post-intervention SOD level in the observation-group was higher than that in the control-group, while the MDA level was lower than in the control group (P<0.05), indicating that Thymopentin can remarkably improve the state of oxidative stress in the body.
Studies have confirmed [18,19] that most patients with ESRD undergoing maintenance hemodialysis have weakened immune function. It is suspected that the long-term effects of urotoxic substances in ESRD patients results in thymus tissue atrophy and decreased secretion of related hormones; moreover, the pre-T lymphocytes in the bone marrow cannot differentiate and mature, resulting in a decrease in the stimulation and transformation of inhibitory T lymphocytes and monocytes, thus promoting cell differentiation. The CD3+ and CD4+ lymphocytes decrease and CD8+ lymphocytes increase, which results in the reduction of immune function in the body. According to the test results, CD3+, CD4+, and CD4+/CD8+ in the observation-group post-intervention were higher than in the control group; while the observation group had lower levels of CD8+ than the control group, indicating that Thymopentin significantly improved the cellular immune function of patients with ESRD undergoing maintenance hemodialysis, thus improving the therapeutic effect. In addition, patients with ESRD undergoing maintenance hemodialysis are often accompanied by uremic symptoms such as vomiting and edema, which lead to a lack of L-carnitine in their body and adverse reactions such as spasms and arrhythmia, affecting their living quality [20,21]. The results of this study indicated that SF-36 scores in the observation-group after treatment were higher than that in control group, indicating that Thymopentin can improve the living quality of patients with ESRD undergoing maintenance hemodialysis.
In summary, Thymopentin can remarkably improve the micro-inflammation status in patients with ESRD undergoing maintenance hemodialysis, and improve their immune function and living quality. However, there were certain limitations that existed in this study. The included research subjects were all from the same hospital, which lacks a level of representativeness; and the intervention duration only lasted 12 weeks, which may cause some deviation in the long term results. Therefore researchers may need to increase the source of subjects in further research.
Disclosure of conflict of interest
None.
References
- 1.Allawi AAD. Malnutrition, inflamation and atherosclerosis (MIA syndrome) in patients with end stage renal disease on maintenance hemodialysis (a single centre experience) Diabetes Metab Syndr. 2018;12:91–97. doi: 10.1016/j.dsx.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Lu W, Ren C, Han X, Yang X, Cao Y, Huang B. The protective effect of different dialysis types on residual renal function in patients with maintenance hemodialysis: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e12325. doi: 10.1097/MD.0000000000012325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen HJ, Wang YF, Qi R, Schoepf UJ, Varga-Szemes A, Ball BD, Zhang Z, Kong X, Wen J, Li X, Lu GM, Zhang LJ. Altered amygdala resting-state functional connectivity in maintenance hemodialysis end-stage renal disease patients with depressive mood. Mol Neurobiol. 2017;54:2223–2233. doi: 10.1007/s12035-016-9811-8. [DOI] [PubMed] [Google Scholar]
- 4.El-Mashad GM, Omar ZA, Seif ES. Evaluation of dialysis practice patterns in children having end-stage renal disease on maintenance hemodialysis at a pediatric nephrology unit. Saudi J Kidney Dis Transpl. 2019;30:615–627. doi: 10.4103/1319-2442.261334. [DOI] [PubMed] [Google Scholar]
- 5.Minami Y, Kajimoto K, Sato N, Hagiwara N, Takano T ATTEND study investigators. End-stage renal disease patients on chronic maintenance hemodialysis in a hospitalized acute heart failure cohort: prevalence, clinical characteristics, therapeutic options, and mortality. Int J Cardiol. 2016;224:267–270. doi: 10.1016/j.ijcard.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Shibiru T, Gudina EK, Habte B, Derbew A, Agonafer T. Survival patterns of patients on maintenance hemodialysis for end stage renal disease in Ethiopia: summary of 91 cases. BMC Nephrol. 2013;14:127. doi: 10.1186/1471-2369-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soltani P, Ketabi Moghaddam P, Haghverdi F, Cheraghi A. A randomized clinical trial of the effect of pentoxifylline on C-reactive protein level and dialysis adequacy in end-stage renal disease patients on maintenance hemodialysis. Iran J Kidney Dis. 2016;10:299–303. [PubMed] [Google Scholar]
- 8.Müller D, Goldstein SL. Hemodialysis in children with end-stage renal disease. Nat Rev Nephrol. 2011;7:650–658. doi: 10.1038/nrneph.2011.124. [DOI] [PubMed] [Google Scholar]
- 9.Mandel-Shorer N, Tzvi-Behr S, Harvey E, Revel-Vilk S. Central venous catheter-related venous thrombosis in children with end-stage renal disease undergoing hemodialysis. Thromb Res. 2018;172:150–157. doi: 10.1016/j.thromres.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Sexton DJ, Reule S, Solid CA, Chen SC, Collins AJ, Foley RN. End-stage renal disease from hemolytic uremic syndrome in the United States, 1995-2010. Hemodial Int. 2015;19:521–530. doi: 10.1111/hdi.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapolyai MB, Faludi M, Berta K, Szarvas T, Lengvárszky Z, Molnar MZ, Dossabhoy NR, Fülöp T. The effect of ambient temperature and humidity on interdialytic weight gains in end-stage renal disease patients on maintenance hemodialysis. Int Urol Nephrol. 2016;48:1171–1176. doi: 10.1007/s11255-016-1297-9. [DOI] [PubMed] [Google Scholar]
- 12.Pai SM, Chaikin P, Berg JK. Pharmacokinetics of lapatinib, a nonrenally cleared drug, in patients with end-stage renal disease on maintenance hemodialysis. J Clin Pharmacol. 2019;59:1379–1383. doi: 10.1002/jcph.1430. [DOI] [PubMed] [Google Scholar]
- 13.Nakao T, Kanazawa Y, Takahashi T. Once-weekly hemodialysis combined with low-protein and low-salt dietary treatment as a favorable therapeutic modality for selected patients with end-stage renal failure: a prospective observational study in Japanese patients. BMC Nephrol. 2018;19:151. doi: 10.1186/s12882-018-0941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lufiyani I, Zahra AN, Yona S. Factors related to insomnia among end-stage renal disease patients on hemodialysis in Jakarta, Indonesia. Enferm Clin. 2019;29:331–335. [Google Scholar]
- 15.Rivara MB, Adams SV, Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, Cheung AK, Katz R, Molnar MZ, Ravel V, Soohoo M, Streja E, Himmelfarb J, Mehrotra R. Extended-hours hemodialysis is associated with lower mortality risk in patients with end-stage renal disease. Kidney Int. 2016;90:1312–1320. doi: 10.1016/j.kint.2016.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Königsbrügge O, Lorenz M, Auinger M, Schmaldienst S, Klauser-Braun R, Kletzmayr J, Grilz E, Posch F, Antlanger M, Pabinger I, Säemann M, Ay C. Venous thromboembolism and vascular access thrombosis in patients with end-stage renal disease on maintenance hemodialysis: cross-sectional results of the Vienna InVestigation of AtriaL fibrillation and thromboembolism in patients on hemoDIalysis (VIVALDI) Thromb Res. 2017;158:59–64. doi: 10.1016/j.thromres.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Nakamoto H, Kobayashi T, Noguchi T, Kusano T, Ashitani K, Imaeda H, Maezono M. Prevalence and severity of itching in patients with end-stage renal disease: treatment with nalfurafine hydrochloride. Blood Purif. 2019;47:45–49. doi: 10.1159/000496637. [DOI] [PubMed] [Google Scholar]
- 18.Wang SY, Zang XY, Fu SH, Bai J, Liu JD, Tian L, Feng YY, Zhao Y. Factors related to fatigue in Chinese patients with end-stage renal disease receiving maintenance hemodialysis: a multi-center cross-sectional study. Ren Fail. 2016;38:442–450. doi: 10.3109/0886022X.2016.1138819. [DOI] [PubMed] [Google Scholar]
- 19.Santosh S, Chu C, Mwangi J, Narayan M, Mosman A, Nayak R, Philipneri M. Changes in pulmonary artery systolic pressure and right ventricular function in patients with end-stage renal disease on maintenance dialysis. Nephrology (Carlton) 2019;24:74–80. doi: 10.1111/nep.13183. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen TL, Plesner LL, Warming PE, Pallisgaard JL, Dalsgaard M, Schou M, Høst U, Rydahl C, Brandi L, Køber L, Johansen JS, Kastrup J, Iversen KK. YKL-40 in patients with end-stage renal disease receiving haemodialysis. Biomarkers. 2018;23:357–363. doi: 10.1080/1354750X.2018.1428359. [DOI] [PubMed] [Google Scholar]
- 21.Nakamoto H, Kobayashi T, Noguchi T, Kusano T, Ashitani K, Imaeda H, Maezono M. Prevalence and severity of itching in patients with end-stage renal disease: treatment with nalfurafine hydrochloride. Blood Purif. 2019;47:45–49. doi: 10.1159/000496637. [DOI] [PubMed] [Google Scholar]


