Abstract
Vascular barrier dysfunction is considered as the initial and critical event in atherosclerosis progression. Recent studies have revealed that treatment with piceatannol (PIC) alleviates both acute and chronic responses to vascular injury. We investigated whether PIC treatment would have beneficial effects on glucolipotoxicity-induced endothelial barrier dysfunction. Target proteins of PIC were identified from several online databases. Then, we confirmed the effect of PIC on endothelial barrier function. PIC treatment mitigated the impairment of endothelial cell motility, adhesion and migration ability associated with high glucose/lipid stimulation. PIC stabilized cytoskeletal reorganization and expression of cell cytoskeletal associated proteins GTPase. PIC reversed changes in critical vascular junction proteins and thus preserved endothelial barrier function and permeability. Finally, we confirmed that reducing of nuclear factor kappa B (NF-κB)/p65 activation and elimination of reactive oxygen species (ROS) were involved in the protective effect of PIC against glucolipotoxicity-induced vascular barrier injury. We identify PIC as a promising therapeutic strategy for glucolipotoxicity-induced endothelial barrier injury.
Keywords: Glucolipotoxicity, endothelial barrier dysfunction, piceatannol
Introduction
Vascular injury is responsible for atherosclerosis associated high rates of morbidity and mortality [1]. Endothelial barrier injury and vascular hyper-permeability always occur at the initial stage of atherosclerosis, which are closely related to endothelium dysfunction [1].
It is necessary to develop new agents to prevent endothelial barrier dysfunction in the development of atherosclerosis. Piceatannol (trans-3, 4, 3’, 5’-tetrahydroxy-stilbene or 3, 3’ 4, 5’-tetrahydroxy-trans-stilbene, PIC) is a polyphenolic stilbene phytochemical that present in seeds of Euphorbia lagascae [2] and a variety of natural foods [3]. PIC is an analog and metabolite of resveratrol (trans-3, 5, 4’-trihydroxystilbene, RES), but has higher radical scavenging activity [4] because it has an additional aromatic hydroxy group [5]. PIC shows a wide range of protective activities, including anti-tumor [5-7], anti-mutagenic [8], anti-bacterial [9], anti-inflammatory and anti-obesity activities [10-12]. PIC has been shown to have protective activities in ischemic reperfusion injury of rats; heart and anti-arrhythmic activity [13-15]. Inflammatory activation in human umbilical vein endothelial cells (HUVECs) was also inhibited by PIC through the Nrf2/HO-1 pathway [16].
However, the effect of PIC on glucolipotoxicity-induced endothelial barrier injury remains unknown. This study was performed to explore the effect of PIC on glucolipotoxicity-induced endothelial barrier injury using bioinformatics and experimental analyses, and the results confirmed its protective effects in vitro.
Materials and methods
Cell cultures
HUVECs were purchased from ScienCell Research Laboratories, Inc. (San Diego, CA, USA) and grown in endothelial cell medium (ECM; ScienCell Research Laboratories). All experiments were performed with these primary HUVECs between passage 2 and 6. The human monocytic cell line, THP-1, was purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China) and grown in RPMI 1640 medium (Gibco-BRL, Grand Island, NY, USA).
Treatment of HUVECs
For glucolipotoxicity treatment, HUVECs were cultured in ECM containing 33 mmol/L glucose and 200 μmol/L of saturated free fatty acid palmitate for 24 hours, with or without 10 μM PIC or vehicle (dimethyl sulfoxide, DMSO; final concentration, 1%).
Reagents and antibodies
PIC (purity > 98%) obtained from Sigma Chemical Co. (St. Louis, MO, USA) was dissolved in DMSO as a 100 mM stock solution. Primary antibodies against Cdc42, Rac1, VE-cadherin, ZO-1, Cx-43, eNOS and p-eNOS were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-GAPDH antibody was obtained from Abcam (Cambridge, UK) and goat anti-rabbit and anti-mouse IgG-HRP secondary antibodies were obtained from Cell Signaling Technology.
Bioinformatic analysis
We searched for PIC targets using DrugBank, STITCH and SwissTargetPrediction databases. Data were integrated into a PIC target network and analyzed with Cytoscape (version 3.6.0). Then, GO and KEGG function and pathway enrichment analyses were carried out with ClusterProfiler in R studio.
Cytotoxicity tests
We evaluated the cytotoxicity of PIC in HUVECs by CCK-8 assay (Dojindo, Tokyo, Japan). The optical density was determined at a wavelength of 450 nm with a spectrophotometer (ScienCell Research Laboratories).
Monocyte-endothelial cell adhesion assay
HUVECs grown in 6-well plates were washed with phosphate-buffered saline (PBS). Calcein-AM pre-stained THP-1 cells were added at a ratio of 1:3 (HUVEC: THP-1) and incubated for 1 hour. After incubation, the medium containing monocytes was aspirated off and HUVECs were washed three times to remove non-adhering THP-1 cells. Adherence of THP-1 to endothelial cells was expressed as fluorescence intensity.
Endothelial cell monolayer permeability
Endothelial cell permeability was tested with Evans blue-labelled BSA according to the protocol. Briefly, HUVECs were grown to confluence on tissue culture inserts (3 µm pore size membrane filters; Corning Inc., Corning, NY, USA) in 24-well plates. Endothelial cell monolayer permeability was examined by testing the diffusion of the Evans blue-BSA complex.
Electrical resistance
To measure the endothelial cell monolayer permeability, TER was determined with a Millicell-ERS meter (Millipore Corp., Bedford, MA, USA). HUVECs were grown to confluence on tissue culture inserts and TER was examined at various times according to the manufacturer’s instructions.
Trypan blue exclusion assay
The viability of HUVECs was measured by the trypan blue exclusion method. Briefly, HUVECs were plated in 24-well plates (5×104 cells per well). At various time points, cells were rinsed, trypsinised and stained with trypan blue dye (Invitrogen, Carlsbad, CA, USA). The number of live cells was counted using a cell counting chamber slide (Invitrogen) with an automated cell counter (Invitrogen).
EdU incorporation assay
Cell proliferation was measured using an EdU Apollo in vitro kit (RiboBio, Guangzhou, China) according to the manufacturer’s protocol. Cells were imaged under a fluorescence microscope (Nikon, Melville, NY, USA) and counted using ImageJ software (NIH, Bethesda, MD, USA).
Boyden chamber cell migration assay
The migration assay was performed using a Transwell chamber (8 µm pore size membrane filter, 24-well plates; Corning Inc.). Cells (2×104) were grown in the upper chamber in ECM supplemented with 0.5% FBS. The medium in the lower chamber consisted of ECM supplemented with 10% FBS. Following incubation, the cells that migrated to the bottom surface of the filter were fixed and the membranes were mounted on slides using mounting medium. The number of migrating cells was quantified from microscopic images of Transwell membranes.
Monolayer wound healing experiments
HUVECs were grown to 80-90% confluence in 6-well plates and then starved of serum for 24 hours. One scratch was then made in each well using a 100-1000-μL pipette tip. After rinsing twice with PBS, HUVECs were incubated in ECM. Images of the same regions were taken at 0 and 24 hours after stimulation.
Filamentous actin (F-actin) staining
Cytoskeletal F-actin was stained with Acti-stain 488 phalloidin according to the manufacturer’s instructions (Yesen, Shanghai, China).
Western blotting
Proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Merck Millipore, Billerica, MA, USA). After blocking with 5% non-fat milk for 1 hour, the membranes were incubated with primary antibody overnight at 4°C. The membranes were incubated with secondary antibodies for 1 hour at room temperature, then visualize the membranes using Electro-chemiluminescence Plus Reagent (Merck Millipore), and the band intensity was quantified using a Chemiluminescence Imaging System (Shenhua Science Technology Co., Ltd., Hangzhou, China).
Detection of intracellular ROS
Intracellular ROS production was assessed using the cell-permeable oxidation-sensitive fluorogenic probe, DCFH-DA (Beyotime Biotechnology, Shanghai, China). Confluent HUVECs were cultured in 6-well plates and incubated with 10 μM DCFH-DA for 1 hour, and then measurement of ROS was performed using a fluorescence microscope (EVOS™ FL Auto 2; Invitrogen).
Immunofluorescence staining
To analyze the activation of p-NF-κB, HUVECs were fixed with 4% paraformaldehyde for 15 minutes at room temperature, and then rinsed twice with PBS. The cell membranes were permeabilized by treatment with 0.1% Triton X-100 for 5 minutes. Non-specific antibody binding sites were blocked by incubation with 5% BSA for 1 hour at room temperature. The cells were then incubated with the primary antibody overnight at 4°C, washed three times with PBS and then incubated with fluorophore-conjugated secondary antibody for 1 hour at room temperature while protected from light with aluminum foil. HUVECs were rinsed twice with PBS, and then nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI, blue) for 10 minutes. The samples were rinsed again with PBS and finally observed using a confocal microscope.
Statistical analysis
All data were presented as the mean ± standard deviation (SD). Means of two groups were compared using Student’s t-test (unpaired, two-tailed) and one-way analysis of variance was used for comparison among multiple groups. Statistical analyses were performed using SPSS 20.0 statistical software (IBM Corp., Armonk, NY, USA). In all analyses, P < 0.05 indicates statistical significance.
Results
Proteins related to endothelial barrier function and inflammation were identified as functional targets of PIC
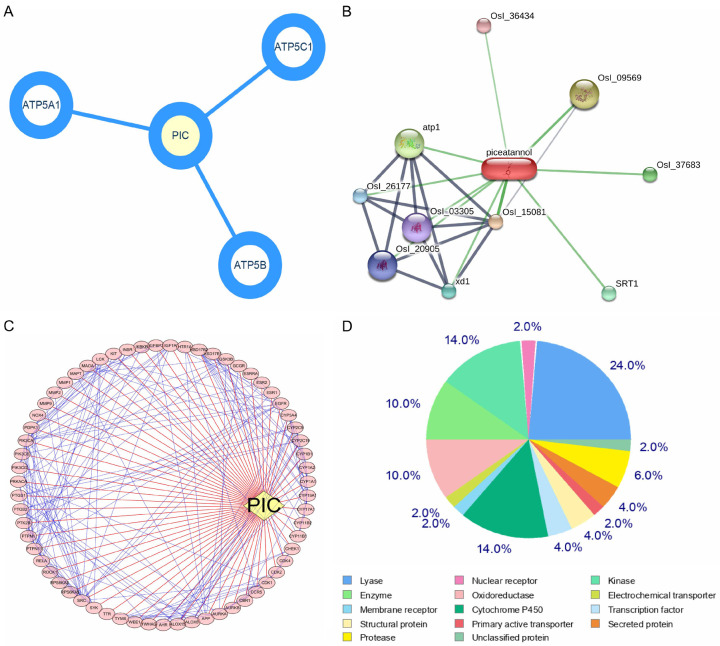
According to Drug-Bank, the targets of PIC can regulate endothelial proliferation, redox reactions, and cell motility (Figure 1A and Table 1). The target genes determined using STITCH were similar to those identified in Drug-Bank (Figure 1B and Table 2). The Swiss Target Prediction web-based tool predicted 102 target genes (Figure 1C, 1D). GO and KEGG function enrichment analysis showed that a number of these target genes played roles in functions that were closely related to vascular function, such as cell growth, cell cytoskeleton-related proteins and inflammation. The results of KEGG pathway analysis of these genes are shown in Figure 2.
Figure 1.
Drug-target predictions for PIC. PIC targets were identified using DrugBank, STITCH and SwissTargetPrediction database, and analysed using Cytoscape: A. PIC targets obtained from the DrugBank database; B. PIC targets obtained from STITCH database; C. PIC targets predicted based on its structure in the SwissTargetPrediction web-based tool and analysed using Cytoscape; D. Distribution range of PIC targets in the SwissTargetPrediction web-based tool.
Table 1.
PIC targets according to Drug-Bank database
| Compound | Target | Related-process |
|---|---|---|
| PIC | ATP synthase subunit alpha | Endothelial cell proliferation/Respiratory electron transport chain |
| PIC | ATP synthase subunit beta | Cell adhesion/Respiratory electron transport chain |
| PIC | ATP synthase subunit gamma | Respiratory electron transport chain |
Table 2.
PIC targets in STITCH database
| Compound | Target | Related-process |
|---|---|---|
| PIC | ATP synthase beta chain mitochondrial | Cell adhesion/Respiratory electron transport chain |
| PIC | ATP synthase F0 subunit 1 | Endothelial cell proliferation/Respiratory electron transport chain |
| PIC | SIRT1 | Cell senescence/Cell growth |
Figure 2.
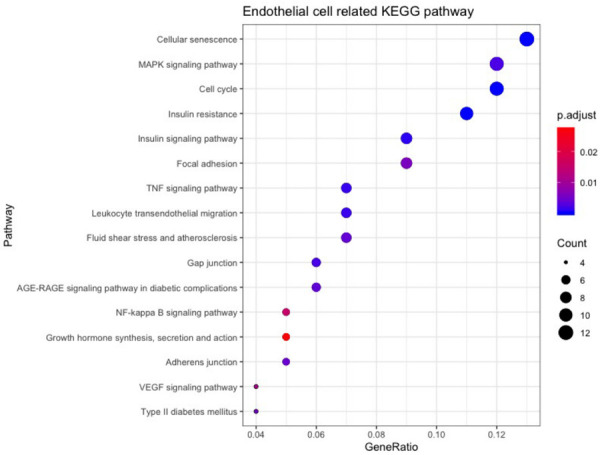
Bioinformatics analysis of endothelial cell-related functions in PIC target genes. KEGG pathway enrichment analysis of PIC targets associated with endothelial cells.
Toxicity of PIC in endothelial cell
The molecular structure of PIC has four hydroxyl groups, a characteristic of stilbenes (Figure 3A). To study the cytotoxic effects of PIC on HUVECs, cell viability was assessed by cell counting kit-8 (CCK-8) assay after incubation of cells with different concentrations of PIC (0, 10, 25, 50 and 100 μM) for 8, 24 or 48 hours. PIC significantly reduced cell viability at 100 μM after 8 hours as well as at 50 μM after 24 hours (Figure 3B-D). However, cell viability was unaffected by 25 μM or 10 μM for 24 or 48 hours (Figure 3B-D). Therefore, cells were incubated with 10 μM PIC for 24 hours in the subsequent experiments.
Figure 3.
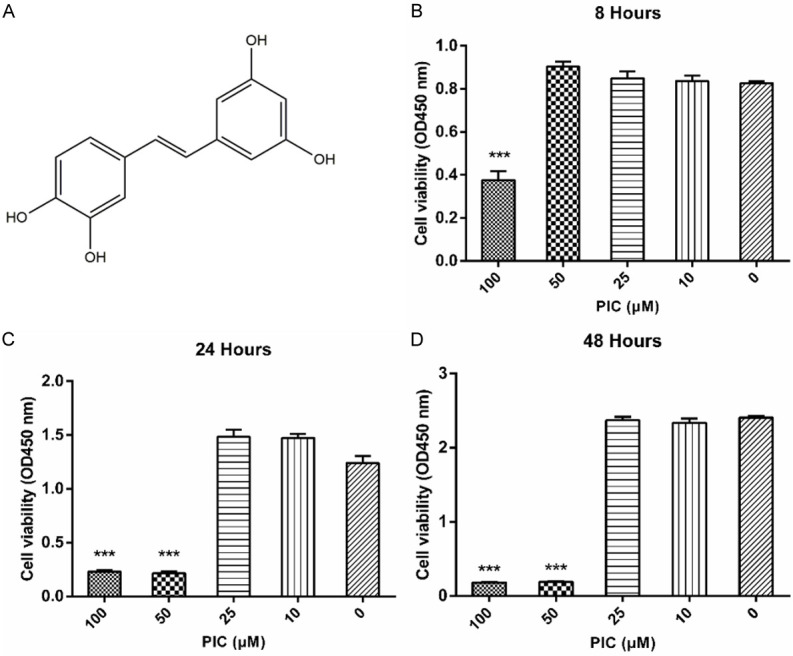
Structure and toxicity of PIC. A. Chemical structure of PIC. B-D. Cytotoxicity of PIC in HUVECs determined by CCK-8 assay at different concentrations for 8, 24 or 48 hours. PIC was not toxic to HUVECs at 10 or 25 μM. Values are expressed as means ± SD ***P < 0.05, vs. control group (0 μM).
PIC inhibited glucolipotoxicity-induced monocyte adhesion to endothelial cells and inflammation
Monocyte adhesion to the endothelium is a critical process in the initiation of atherosclerosis. When the vascular barrier is damaged, circulating monocytes would bind to the damaged endothelium and transmigrate into the vascular endothelium where they transform into lipid-laden foam cells. Compared to the control group, high glucose/lipid (HG/HL) treatment promoted monocytes adhesion to HUVECs. However, PIC treatment decreased the percentage of monocytes adhering to the HUVECs (Figure 4A, 4B). ICAM-1 is known to mediate monocytes adhesion to the endothelium during inflammation. PIC was shown to prevent the change in ICAM-1 protein level associated with HG/HL treatment (Figure 4E).
Figure 4.
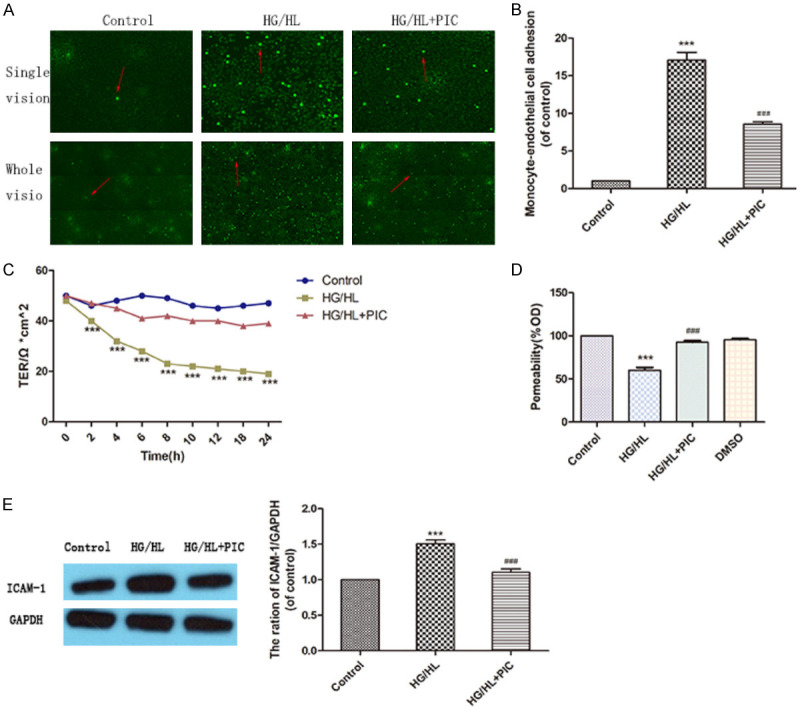
PIC attenuated HG/HL-induced monocyte adhesion to endothelial cells by decreasing endothelial permeability under glucolipotoxicity conditions. A, B. Effects of HG/HL or HG/HL+PIC on monocyte (THP-1) adherence to HUVECs. The arrow indicates adherent monocytes (THP-1) in HUVECs. C. TER assay showing an increase in endothelial cell permeability at 2 hours following HG/HL stimulation; this increase persisted through 24 hours and PIC inhibited glucolipotoxicity-induced endothelial hyperpermeability. D. Evans Blue-labelled BSA diffusion assay confirming the significant protective effect of PIC against endothelial cell permeability. E. Western blotting analysis demonstrating the effects of PIC on ICAM-1 levels following HG/HL. The ICAM-1 protein level is shown relative to GAPDH. Values are shown as means ± SD. ***P < 0.05, vs. control group; ###P < 0.05, vs. HG/HL treatment group. Scale bar: 200 μm for 4×.
PIC alleviated endothelial cell hyperpermeability during glucolipotoxicity injury
Endothelial permeability is evaluated based on the results of transepithelial electrical resistance (TER) assay of the cell monolayer and Evans blue-labelled bovine serum albumin (BSA) diffusion assay. The results confirmed the protective effect of PIC against glucolipotoxicity-induced endothelial cell hyperpermeability (Figure 4D). The results of TER assay showed that endothelial cell permeability increased at 2 hours following HG/HL stimulation, and this increase persisted through 24 hours. PIC prevented this vascular injury, indicating that it effectively inhibited glucolipotoxicity-induced endothelial hyperpermeability (Figure 4C).
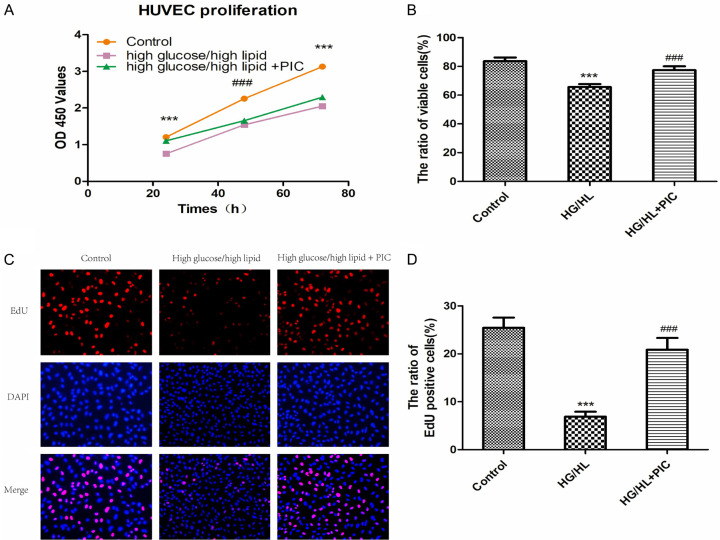
PIC attenuated glucolipotoxicity-induced HUVEC proliferation disability
Both CCK-8 and trypan blue exclusion assays showed that incubation of cells with HG/HL resulted in a significant decrease in cell viability and proliferation compared to normal control medium (5.5 mM dextrose). This reduction of proliferative capacity was ameliorated by pre-treatment of the cells with PIC (Figure 5A, 5B). Consistent with these results, 5-ethynyl-2’-deoxyuridine (EdU) fluorescence staining also revealed that HG/HL significantly reduced the percentage of EdU-positive cells (red), and pre-treatment with PIC mitigated the anti-proliferation effect of HG/HL on HUVECs (Figure 5C, 5D).
Figure 5.
PIC attenuated the HG/HL-induced decrease in HUVEC proliferation. A. Cell proliferation of HUVECs treated with d-glucose (33 mM)/palmitic acid (200 μM) for 72 hours alone or in combination with 10 μM PIC for 24 hours based on CCK-8 assay. B. HUVEC cell viability under different conditions based on trypan blue exclusion assay. C. Cell proliferation based on active DNA synthesis using the EdU incorporation assay. D. Ratio of EdUration based on active DNA synthesis using the E Values are expressed as means ± SD. ***P < 0.05, vs. control group; ###P < 0.05, vs. HG/HL treatment group. Scale bar: 200 μm for 4×.
PIC protected against glucolipotoxicity-induced endothelial motility dysfunction
HG/HL reduced the migration of HUVECs, as revealed by scratch wound-healing assay (Figure 6A, 6B). Treatment with PIC moderately promoted the return to confluence of cells in the scratch wound-healing assay (Figure 6D). We then performed Transwell migration assay to exclude the proliferation effect of PIC from the wound-healing assay and to further investigate its role in chemotactic migration. The results of the Transwell migration assay revealed that HG/HL significantly decreased the ratio of migrating cells, while PIC ameliorated this inhibition and increased cell migration (Figure 6C, 6E).
Figure 6.
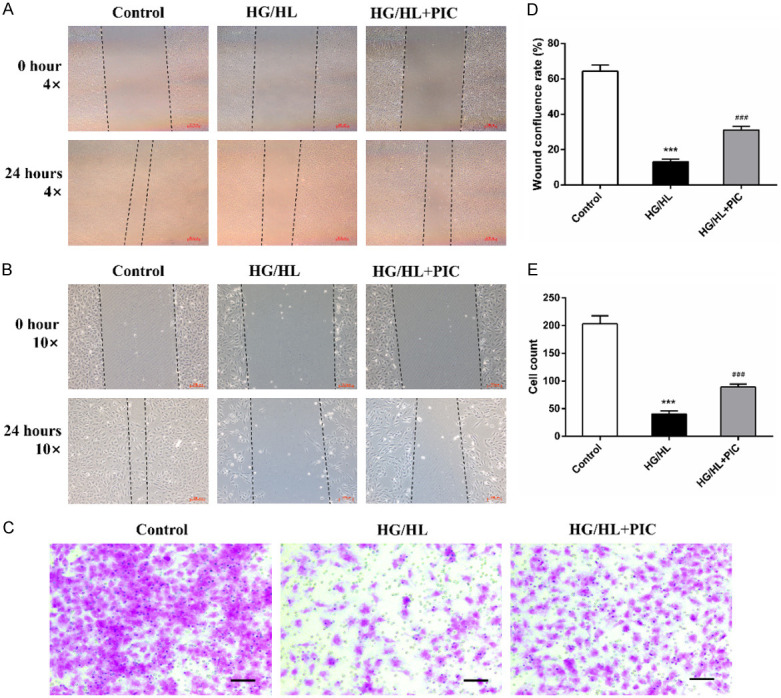
PIC protected HUVECs against HG/HL-induced reduction of migratory capacity. A, B, D. Effects of HG/HL or HG/HL+PIC stimulation on scratch wound healing in cultured HUVECs at different magnifications. A-C. Representative images. D. Quantification of confluence rate at 24 hours. C-E. Migration ability of HUVECs following HG/HL or HG/HL+PIC treatment based on the Transwell assay. E. Quantification of cell migration after 24 hours. Values are expressed as means ± SD. ***P < 0.05, vs. control group; ###P < 0.05, vs. HG/HL treatment group. Scale bar: 200 μm for 4× and 100 μm for 10×.
PIC attenuated glucolipotoxicity-induced cytoskeletal reorganization and dysfunction of cytoskeleton-associated Rho GTPases family proteins
Considering the protective effects of PIC on vascular barrier and endothelial cell motility function, we explored the effects of PIC on cytoskeleton rearrangement as well as stress fibre formation induced by HG/HL in HUVECs. Cytoskeletal proteins play central roles in endothelial barrier function and cell motility. HG/HL stimulation caused actin cytoskeleton remodeling, as demonstrated by thickening of the filamentous actin (F-actin) band and the presence of F-actin bundles within the cells. PIC ameliorated this actin cytoskeleton remodeling, resulting in the maintenance of cell morphology (Figure 7A). The Rho GTPase family is vital for endothelial cell morphology and motility, as members of this family regulate cytoskeleton reorganization. Glucolipotoxicity induces decreases in the expression of Rac1 and Cdc42 as well as the effector protein, IQ Motif Containing GTPase Activating Protein 1 (IQGAP1). PIC treatment recovered the decreases in Rac1/Cdc42 and IQGAP1 expression, as well as cytoskeleton rearrangement induced by HG/HL (Figure 7B-E).
Figure 7.
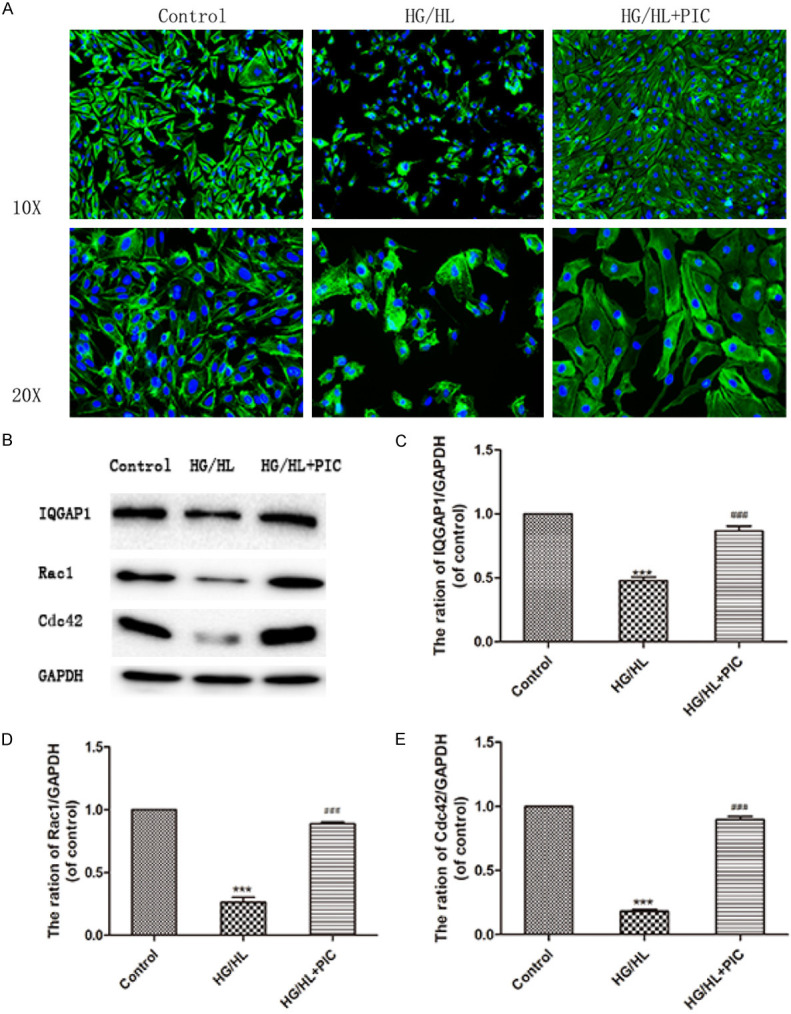
PIC rescued HG/HL-induced cytoskeletal reorganisation and dysfunction of cytoskeleton-associated protein GTPases (Rac1, Cdc42) and IQGAP1. A. Immunofluorescence staining of F-actin showing that HG/HL treatment resulted in a thickened F-actin band and the presence of F-actin bundles inside the cells. PIC ameliorated the actin cytoskeleton remodelling and maintained cell morphology. B. Western blotting analysis showing the expression of cytoskeleton-associated proteins, Rac1/Cdc42, and their effector, IQGAP1. C-E. The levels of these proteins relative to GAPDH. Values are expressed as means ± SD. ***P < 0.05, vs. control group; ###P < 0.05, vs. HG/HL treatment group.
PIC prevented glucolipotoxicity-induced reduction of endothelial cell junction protein expression
To assess the expression of endothelial cell junction proteins following glucolipotoxicity stimulation with HG/HL and PIC treatment, we measured the protein levels of vascular endothelial (VE)-cadherin, zonula occludens-1 (ZO-1) and connexin-43 (Cx-43). HG/HL compromised endothelial barrier function by reducing VE-cadherin and ZO-1 levels, while increasing Cx-43 protein in HUVECs. PIC effectively ameliorated these changes in VE-cadherin, ZO-1 and Cx-43 protein levels, thereby maintaining endothelial cell barrier function (Figure 8A-D).
Figure 8.
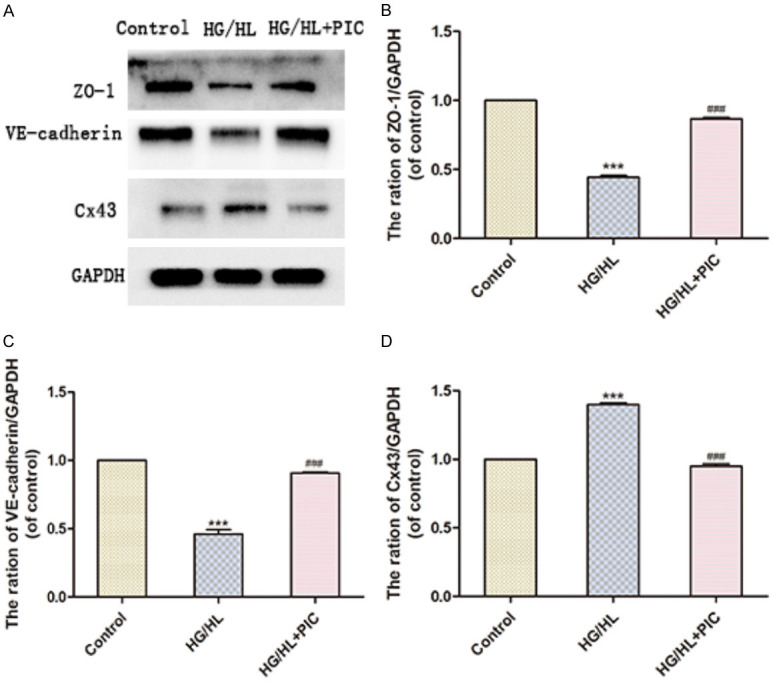
PIC attenuated the HG/HL-induced decrease in endothelial junction protein levels. A. Western blotting analysis showing the expression of ZO-1 (TJ protein), VE-cadherin (AJ protein) and Cx-43 (GJ protein). B-D. ZO-1, VE-cadherin and Cx-43 protein levels relative to GAPDH. Values are expressed as means ± SD. ***P < 005, vs. control group; ###P < 0.05, vs. HG/HL treatment group.
PIC suppressed glucolipotoxicity-stimulated oxidative stress and NF-kappa B activation
As determined by a dichlorodihydrofluorescein diacetate (DCFH-DA) assay, HG/HL stimulation induced excess probe-associated fluorescence and intracellular ROS production. Fluorescence was diminished in the PIC treatment group, suggesting that PIC may contribute to the scavenging of ROS induced by HG/HL (Figure 9A, 9B). ROS plays a critical role in NF-κB activation. We performed immunofluorescence staining of NF-κB p65 to evaluate its nuclear translocation during NF-κB activation. Under resting conditions, NF-κB localized in the cytoplasm. After HG/HL stimulation, intense staining for p65 was detected in the nucleus, while there was no staining in the cytoplasm, indicating nuclear translocation of p65 and enhancement of NF-κB activation. However, PIC attenuated the nuclear translocation of NF-κB and significantly suppressed NF-κB activation (Figure 9C, 9D). These results demonstrated that PIC reduced the nuclear translocation and activation of NF-κB and thus protected endothelial barrier function.
Figure 9.
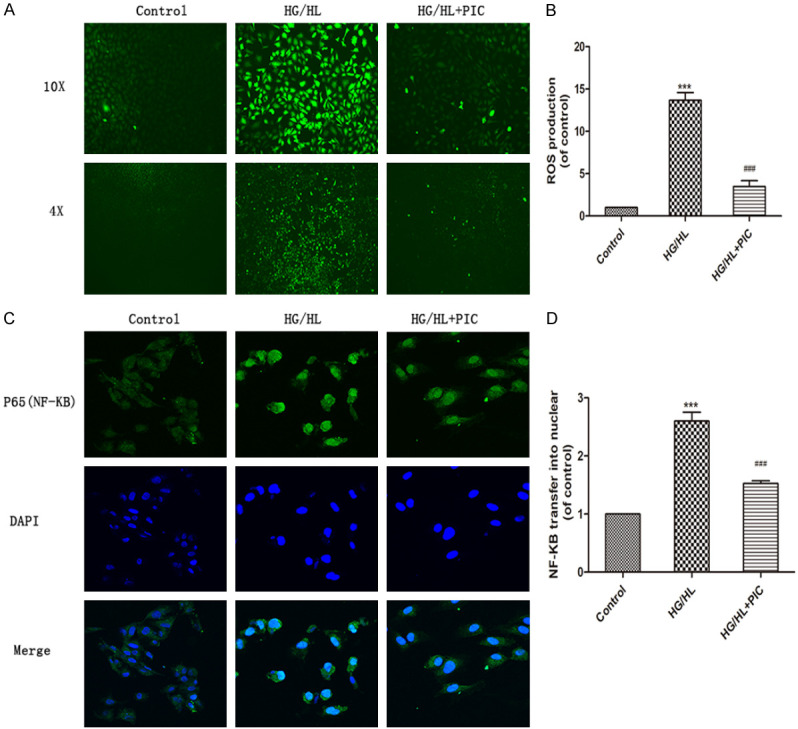
Effects of PIC on HG/HL-stimulated NF-κB translocation and ROS production. (A) Intracellular ROS production in HUVECs based on the fluorescent probe, DCFH-DA, and (B) fluorescence intensity analysed using ImageJ. (C) NF-κB nuclear translocation during its activation evaluated using immunofluorescence staining, and (D) fluorescence intensity analysed using ImageJ. Values are expressed as means ± SD. ***P < 0.05, vs. control group; ###P < 0.05, vs. HG/HL treatment group. Scale bar: 200 μm for 4×, 100 μm for 10× and 50 μm for 20×.
Discussion
This study was performed to examine the effects of PIC on glucolipotoxicity-induced vascular barrier injury in atherosclerosis. The results presented here demonstrated the protective effect of PIC on glucolipotoxicity-induced vascular barrier injury at several levels.
When endothelial barrier function is damaged, circulating leukocytes, especially monocytes, would adhere to endothelium and migrate into the vascular intima, passing through the damaged endothelium. These monocytes ultimately lead to the development of atherosclerosis [17]. Notably, PIC treatment decreased the glucolipotoxicity-induced adhesion of monocytes to endothelial cells.
Endothelial barrier function is precisely regulated through cytoskeletal organization and proteins involved in cell-cell junctions, i.e., adherens junctions (AJs), gap junctions (GJs) and tight junctions (TJs). In AJs, VE-cadherin [18-20] is an important contributor to vascular barrier integrity and permeability due to its intercellular domain, which is connected to the actin cytoskeleton [21]. Cx43, a GJ protein, participates in the initial and progression of atherosclerosis. Further, enhanced expression of Cx43 plays a critical role in the development of atherosclerosis during the early phases [22,23]. In the meanwhile, the TJ protein, ZO-1, connects junctional structures with the cytoskeleton and is downregulated in diabetes and obesity [24]. Glucolipotoxicity leads to an increase in CX43 and decrease of ZO1 and VE-cadherin in endothelial cells. However, the results revealed that PIC effectively prevented changes in the levels of these endothelial junction proteins in response to glucolipotoxicity.
Endothelial barrier damage is also accompanied by cytoskeleton polymerization, proliferation dysfunction and reduced migration of endothelial cells [25-27]. The proliferation and migration of endothelial cells play a critical role in vascular repair and integrity [28,29]. Coordinated rearrangement of actin filaments and microtubules is required for cell migration. Hyperglycemia leads to serious injuries, affecting endothelial cell survival and migration [30-32]. Data demonstrated that PIC promoted endothelial cell proliferation and migration following HG/HL stimulation by ameliorating the rearrangement of actin filaments, which enhances vascular barrier integrity.
Cytoskeleton polymerization and endothelial migration are largely orchestrated by the Rho family of GTPases and their effector, IQGAP1. The Rho family GTPase have also emerged as regulators of endothelial junction proteins, mediating vascular barrier integrity via such interaction. Rac1/Cdc42 is thought to spatially influence the forming of filopodia and lamellipodia, which can promote vascular barrier development [33-36]. They also promoted endothelial cells migration and adhesion through the assembly and organization of the actin cytoskeleton [37]. The present study indicated that PIC enhanced the expression of the Rho family GTPases (Rac1, Cdc42 but not RhoA) and their effector protein, IQGAP1, in endothelial cells during HG/HL stimulation, thereby influencing cell proliferation, migration and the expression of several junction proteins, which in turn enhanced vascular barrier repair as well as vascular permeability.
Oxidative stress, increased ROS production, and dysregulated redox balance contribute to dysfunction of endothelial barrier. ROS can not only increase endothelial permeability through directly damaged endothelium junction protein components, such as AJ proteins, leading to endothelial barrier dysfunction, but may also cause endothelial hyperpermeability by promoting NF-kappa B activation. During cancer and lipopolysaccharide induced toxemia, NF-kappa B activation alters the levels of vascular junction proteins such as ZO-1 and ZO-2, inducing endothelial hyperpermeability separately from pro-inflammatory gene expression [38]. In the present study, PIC decreased HG/HL induced intracellular ROS production and NF-kappa B activation, protecting endothelial barrier function.
However, this study had some limitations. We did not confirm the protective effects of PIC in a clinical study, because this compound is not approved for human trials. With the development of nanoparticle technology [39] and the murine endothelial cells single-cell transcriptome atlas [40], we plan to create PIC-incorporated albumin nanoparticles, thereby enhancing its solubility and targeting potential, and will study the effects of PIC on endothelial dysfunction in vivo in future.
Conclusions
Glucolipotoxicity causes endothelial ROS accumulation and NF-κB activation, inducing severe endothelial barrier dysfunction. PIC ameliorates the glucolipotoxicity-induced endothelial barrier injury following HG/HL stimulation through inhibition of the ROS/NF-κB signaling pathway. PIC is a promising therapeutic agent for glucolipotoxicity-induced endothelial barrier injury.
Acknowledgements
This work was supported by the National Key R&D Program of China (Grant No. 2016YFC1301003); the Natural Science Foundation of Zhejiang Province, Zhejiang, China, Grant/Award Number: LQ19H070002. All authors have read and agreed with the submission of the manuscript. This manuscript has not been published or presented elsewhere in part or in entirety.
Disclosure of conflict of interest
None.
Abbreviations
- AJ
adherens junction
- BSA
bovine serum albumin
- CCK-8
cell counting kit-8
- Cx-43
connexin-43
- DAPI
4’,6-diamidino-2-phenylindole
- DCFH-DA
dichlorodihydrofluorescein diacetate
- DMSO
dimethyl sulfoxide
- ECM
endothelial cell medium
- EdU
5-ethynyl-2’-deoxyuridine
- F-actin
filamentous actin
- GJ
gap junction
- HG/HL
high glucose/lipid
- HUVECs
human umbilical vein endothelial cells
- NF-κB
nuclear factor-kappa B
- PBS
phosphate-buffered saline
- PIC
piceatannol
- ROS
reactive oxygen species
- TER
transepithelial electrical resistance
- TJ
tight junction
- VE-cadherin
vascular endothelial-cadherin
- ZO-1
zonula occludens-1
References
- 1.An X, Li L, Chen Y, Luo A, Ni Z, Liu J, Yuan Y, Shi M, Chen B, Long D, Cheng J, Lu Y. Mesenchymal stem cells ameliorated glucolipotoxicity in HUVECs through TSG-6. Int J Mol Sci. 2016;17:483. doi: 10.3390/ijms17040483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrigni NR, McLaughlin JL, Powell RG, Smith CR Jr. Use of potato disc and brine shrimp bioassays to detect activity and isolate piceatannol as the antileukemic principle from the seeds of Euphorbia lagascae. J Nat Prod. 1984;47:347. doi: 10.1021/np50032a019. [DOI] [PubMed] [Google Scholar]
- 3.Viñas P, Martínez-Castillo N, Campillo N, Hernández-Córdoba M. Directly suspended droplet microextraction with in injection-port derivatization coupled to gas chromatography-mass spectrometry for the analysis of polyphenols in herbal infusions, fruits and functional foods. J Chromatogr A. 2011;1218:639–646. doi: 10.1016/j.chroma.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Fauconneau B, Waffo-Teguo P, Huguet F, Barrier L, Decendit A, Merillon JM. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from vitis vinifera cell cultures using in vitro tests. Life Sci. 1997;61:2103–2110. doi: 10.1016/s0024-3205(97)00883-7. [DOI] [PubMed] [Google Scholar]
- 5.Potter GA, Patterson LH, Wanogho E, Perry PJ, Butler PC, Ijaz T, Ruparelia KC, Lamb JH, Farmer PB, Stanley LA, Burke MD. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br J Cancer. 2002;86:774–778. doi: 10.1038/sj.bjc.6600197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banik K, Ranaware AM, Harsha C, Nitesh T, Girisa S, Deshpande V, Fan L, Nalawade SP, Sethi G, Kunnumakkara AB. Piceatannol: a natural stilbene for the prevention and treatment of cancer. Pharmacol Res. 2020;153:104635. doi: 10.1016/j.phrs.2020.104635. [DOI] [PubMed] [Google Scholar]
- 7.Aljabali AAA, Bakshi HA, Hakkim FL, Haggag YA, Al-Batanyeh KM, Al Zoubi MS, Al-Trad B, Nasef MM, Satija S, Mehta M, Pabreja K, Mishra V, Khan M, Abobaker S, Azzouz IM, Dureja H, Pabari RM, Dardouri AAK, Kesharwani P, Gupta G, Dhar Shukla S, Prasher P, Charbe NB, Negi P, Kapoor DN, Chellappan DK, Webba da Silva M, Thompson P, Dua K, McCarron P, Tambuwala MM. Albumin nano-encapsulation of piceatannol enhances its anticancer potential in colon cancer via downregulation of nuclear p65 and HIF-1α. Cancers (Basel) 2020;12:113. doi: 10.3390/cancers12010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larrosa M, Tomás-Barberán FA, Espín JC. Grape polyphenol resveratrol and the related molecule 4-hydroxystilbene induce growth inhibition, apoptosis, S-Phase arrest, and upregulation of cyclins A, E, and B1 in humanSK-Mel-28 melanoma cells. J Agric Food Chem. 2003;51:4576–4584. doi: 10.1021/jf030073c. [DOI] [PubMed] [Google Scholar]
- 9.Docherty JJ, McEwen HA, Sweet TJ, Bailey E, Booth TD. Resveratrol inhibition of Propionibacterium acnes. J Antimicrob Chemother. 2007;59:1182–1184. doi: 10.1093/jac/dkm099. [DOI] [PubMed] [Google Scholar]
- 10.Kwon JY, Seo SG, Heo YS, Yue S, Cheng JX, Lee KW, Kim KH. Piceatannol, natural polyphenolic stilbene, inhibits adipogenesis via modulation of mitotic clonal expansion and insulin receptor-dependent insulin signaling in early phase of differentiation. J Biol Chem. 2012;287:11566–11578. doi: 10.1074/jbc.M111.259721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djoko B, Chiou RY, Shee JJ, Liu YW. Characterization of immunological activities of peanut stilbenoids, arachidin-1, piceatannol, and resveratrol on lipopolysaccharide-induced inflammation of RAW 264.7 macrophages. J Agric Food Chem. 2007;55:2376–2383. doi: 10.1021/jf062741a. [DOI] [PubMed] [Google Scholar]
- 12.Jin C, Moon D, Lee K, Kim M, Lee J, Choi Y, Park Y, Kim G. Piceatannol attenuates lipopolysaccharide-induced NF-κB activation and NF-κB-related proinflammatory mediators in BV2 microglia. Pharmacol Res. 2006;54:461–467. doi: 10.1016/j.phrs.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Chen WP, Hung LM, Hsueh CH, Lai LP, Su MJ. Piceatannol, a derivative of resveratrol, moderately slows I (Na) inactivation and exerts antiarrhythmic action in ischaemia-reperfused rat hearts. Br J Pharmacol. 2009;157:381–391. doi: 10.1111/j.1476-5381.2008.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou CC, Chang PC, Wen MS, Lee HL, Wo HT, Yeh SJ, Wu D. Piceatannol facilitates conduction block and ventricular fibrillation induction in ischemia-reperfused rabbit hearts with pacing-induced heart failure. Int J Cardiol. 2014;171:250–258. doi: 10.1016/j.ijcard.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Chou CC, Chang PC, Wen MS, Lee HL, Wo HT, Yeh SJ, Wu D. Piceatannol facilitates conduction block and ventricular fibrillation induction in ischemia-reperfused rabbit hearts with pacing-induced heart failure. Int J Cardiol. 2014;171:250–258. doi: 10.1016/j.ijcard.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 16.Jeong SO, Son Y, Lee JH, Cheong YK, Park SH, Chung HT, Pae HO. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol Med Rep. 2015;12:937–944. doi: 10.3892/mmr.2015.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 18.Gavard J. Endothelial permeability and VE-cadherin. Cell Adh Migr. 2014;8:158–164. doi: 10.4161/cam.29026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Developmental Cell. 2013;26:441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Developmental Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackburn JP, Peters NS, Yeh HI, Rothery S, Green CR, Severs NJ. Upregulation of connexin43 gap junctions during early stages of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1219–1228. doi: 10.1161/01.atv.15.8.1219. [DOI] [PubMed] [Google Scholar]
- 23.Morel S, Chanson M, Nguyen TD, Glass AM, Richani Sarieddine MZ, Meens MJ, Burnier L, Kwak BR, Taffet SM. Titration of the gap junction protein Connexin43 reduces atherogenesis. Thromb Haemost. 2014;112:390–401. doi: 10.1160/TH13-09-0773. [DOI] [PubMed] [Google Scholar]
- 24.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 25.Bermejo-Martin JF, Martín-Fernandez M, López-Mestanza C, Duque P, Almansa R. Shared features of endothelial dysfunction between sepsis and its preceding risk factors (aging and chronic disease) J Clin Med. 2018;7:400. doi: 10.3390/jcm7110400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mundi S, Massaro M, Scoditti E, Carluccio MA, van Hinsbergh VWM, Iruela-Arispe ML, De Caterina R. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res. 2017;114:35–52. doi: 10.1093/cvr/cvx226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chistiakov DA, Orekhov AN, Bobryshev YV. Endothelial barrier and its abnormalities in cardiovascular disease. Front Physiol. 2015;6:365. doi: 10.3389/fphys.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CH, Shieh YS, Hsiao FC, Kuo FC, Lin CY, Hsieh CH, Hung YJ. High glucose induces human endothelial dysfunction through an Axl-dependent mechanism. Cardiovasc Diabetol. 2014;13:53. doi: 10.1186/1475-2840-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen J, DiCorleto PE. ADP stimulates human endothelial cell migration via P2Y1 nucleotide receptor-mediated mitogen-activated protein kinase pathways. Circ Res. 2008;102:448–456. doi: 10.1161/CIRCRESAHA.107.165795. [DOI] [PubMed] [Google Scholar]
- 30.Zanetti M, Zwacka R, Engelhardt J, Katusic Z, O’Brien T. Superoxide anions and endothelial cell proliferation in normoglycemia and hyperglycemia. Arterioscler Thromb Vasc Biol. 2001;21:195–200. doi: 10.1161/01.atv.21.2.195. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Duong MN, Psaltis PJ, Bursill CA, Nicholls SJ. High-density lipoproteins attenuate high glucose-impaired endothelial cell signaling and functions: potential implications for improved vascular repair in diabetes. Cardiovasc Diabetol. 2017;16:121. doi: 10.1186/s12933-017-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Duong MN, Psaltis PJ, Bursill CA, Nicholls SJ. High-density lipoproteins attenuate high glucose-impaired endothelial cell signaling and functions: potential implications for improved vascular repair in diabetes. Cardiovasc Diabetol. 2017;16:121. doi: 10.1186/s12933-017-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 34.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost. 2010;103:40–55. doi: 10.1160/TH09-06-0403. [DOI] [PubMed] [Google Scholar]
- 35.Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 36.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 37.Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161:429–439. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kisseleva T, Song L, Vorontchikhina M, Feirt N, Kitajewski J, Schindler C. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest. 2006;116:2955–2963. doi: 10.1172/JCI27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kugelberg E. Neutrophils: nanoparticles targeting the bad guys. Nat Rev Immunol. 2014;14:214. doi: 10.1038/nri3648. [DOI] [PubMed] [Google Scholar]
- 40.Kalucka J, de Rooij L, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, García-Caballero M, Khan S, Geldhof V, Sokol L, Chen R, Treps L, Borri M, de Zeeuw P, Dubois C, Karakach TK, Falkenberg KD, Parys M, Yin X, Vinckier S, Du Y, Fenton RA, Schoonjans L, Dewerchin M, Eelen G, Thienpont B, Lin L, Bolund L, Li X, Luo Y, Carmeliet P. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180:764–779. e720. doi: 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]