Abstract
Recent clinical studies showed that central nervous system (CNS) infection caused by varicella zoster virus (VZV) reactivation was more than previously reported. The clinical manifestations were often diverse and complex, and the outcome often varied among different patients. A systematic study is needed to provide clinical characteristics of the CNS VZV infection to help clinicians with clinical diagnosis and management. Toward that end, we retrospectively analyzed the clinical presentations, laboratory results, imaging findings, treatment and outcomes in74 patients with meningitis or meningoencephalitis caused by VZV reactivation in our center from August 2018 to December 2020. Fever, headache, cranial nerve involvement, cognitive changes, meningeal irritation, nausea, vomiting, and Ramsay-Hunt syndrome (RHS) were the most common clinical manifestations of VZV meningitis or meningoencephalitis. Brain MRI analysis showed no obvious abnormal manifestation. Compared to VZV meningoencephalitis, patients with VZV meningitis were younger (56.9±13.8 vs 66.1±8.5 years; P=0.01), and more likely to develope in winter (P=0.04), had lower cerebrospinal fluid (CSF) glucose content (3.68±0.79 vs 4.21±0.94 mmol/L, P=0.02), and a better outcome at discharge (P=0.00). The outcome at discharge was worse in male patients and when longer than 1.5 days passed between onset of the neurological symptoms to initiation of the antiviral treatment.Early intravenous antiviral treatment for VZV meningitis and meningoencephalitis is important and is expected for a good outcome.
Keywords: Varicella zoster virus, central nervous system infection, treatment, outcome
Introduction
Infection of varicella zoster virus (VZV, also known as human herpesvirus type 3), has the characteristics of neurotropism and skin symptom [1]. After getting infection for the first time, VZV causes chickenpox in children and then lurks to latent state. When stimulated by various inducements, the latent VZV reactivates and replicates, causing immune reaction in the peripheral sensory nerve and the unilateral skin ganglion dominated by the nerve, along with erythema, blisters, neuralgia and other herpes zoster manifestations [2]. Herpes zoster, as a manifestation of VZV reactivation, can occur in both healthy and immunosuppressed people. Most people with healthy immune system have no complications after healing from herpes infection. Studies have shown that some patients with low cellular immune function and even normal immune people may have sequelae of nervous system, such as postherpetic neuralgia (PHN), cranial nerve involvement and other peripheral nervous system symptoms. In some patients, VZV may cause symptoms of central nervous system (CNS) such as encephalitis, meningitis, meningoencephalitis, cerebellitis, myelitis, cerebrovascular disease, and other neural manifestations [3-6]. Meningitis and meningoencephalitis are the main complications of VZV CNS infection [7,8]. Although there are many clinical studies reported CNS infection caused by VZV reactivation, systematic studies were rare. Some retrospective studies suggested that most CNS complications of VZV reactivation had a good outcome but sequelae were also reported in a few cases. Meanwhile, other studies also had different results that a considerable percentage of patients left with persistent (more than 10 years) neurological sequelae [9-12]. In this study, we retrospectively analyzed the clinical presentations, workups, treatment, and outcomes of a relatively large volume of patients with meningitis and meningoencephalitis caused by reactivation of VZV in recent 2.5 years, aiming to provide a reference for clinicians in the diagnosis and treatment of VZV meningitis and meningoencephalitis.
Materials and methods
This is a retrospective clinical-epidemiological descriptive study that included all admitted patients diagnosed with meningitis or meningoencephalitis caused by VZV reactivation in our hospital from August 2018 to December 2020. We followed the criterion of European consensus-based guideline on the management of the herpes zoster to include patients with VZV meningitis and meningoencephalitis [13]. Patients who were older than 14 years were diagnosed of VZV CNS infection based on the presence of a herpes zoster for 4 weeks before or during the onset of the disease, the clinical features of a CNS infection, leukocyte count >5×106/L, protein content >0.45 g/L, or a positive VZV polymerase chain reaction (PCR) in the cerebrospinal fluid (CSF).
Data collection
We collected the clinically related information from the included patients. Medical record (including gender, Age, affected site of herpes zoster, course of disease, comorbid diseases, clinical manifestation, CSF examination, cranial CT/MRI, EEG, days passed between onset of the herpes zoster to onset of the neurological symptoms, days passed between onset of the neurological symptoms to initiation of the antivirus treatment, days of admission, days of intravenous antivirus treatment, etc.) were all collected. Based on the treatment outcome, patients were classified into three subgroups. Patients who were free of complaints were classified into the good prognosis group. Patients with any symptom of pain or cranial nerve involvement were classified into the fair prognosis group. Patients who suffered from cognitive impairment, disturbance of consciousness, multiple cranial nerve involvement or even death were classified into the poor prognosis group. We assessed the outcomes of patients on the day of discharge.
Statistical analysis
SPSS 20.0 software was used for statistical analysis. Kolmogorov-Smirnov was used to test normality. Measurement data with normality distribution were shown as mean ± standard deviation (x̅±SD). The t test was used to compare data between two groups. The data of abnormal distribution was described by median [M(Q1-Q3)]. Mann-Whitney U test was used to compare these data between two groups. For data that were expressed by frequency and percentage, Chi or Fisher test was used to compare between two groups. Multivariate logistic regression analysis was used to analyze those factors with statistically significant differences in univariate analysis. P<0.05 was considered statistically significant.
Ethical consideration
The study was approved by the research ethics committee of our hospital (Number: 2021KA013). Due to the retrospective nature of data collection and analysis, a waiver to get an informed consent was granted.
Results
Patients characteristics
59 patients met the criteria for clinical diagnosis of VZV meningitis and 15 patients met the criteria of VZV meningoencephalitis. Patients with VZV meningitis and VZV meningoencephalitis had a median age of 56.9±13.8 years (range 26-87 years) and 66.1±8.5 years (range 48-81 years) respectively. Patients with meningitis had a younger median age than those with meningoencephalitis (P=0.01) (Table 1).
Table 1.
Baseline characteristics at admission in patients with VZV meningitis or meningoencephalitis
| varicella zoster virus meningitis (n=59) | varicella zoster virus meningoencephalitis (n=15) | |
|---|---|---|
| Age (mean ± SD) (years) | 56.9±13.8 | 66.1±8.5* |
| Gender | ||
| Male (numbers, %) | 35 (59.3%) | 9 (60.0%) |
| Female (numbers, %) | 24 (40.7%) | 6 (40.0%) |
| Season of onset (cases, %) | ||
| Spring | 15 (25.4%) | 4 (26.7%) |
| Summer | 8 (13.6%) | 5 (33.3%) |
| Autumn | 12 (20.3%) | 4 (26.7%) |
| Winter | 24 (40.7%) | 2 (13.3%)* |
| Comorbidity (cases) | ||
| Coronary heart disease | 6 | 2 |
| COPD | 2 | 2 |
| Hypertension | 13 | 7 |
| Immune diseases | 6 | 2 |
| Use of immune drugs | 4 | 2 |
| Stroke | 0 | 3 |
| Diabetes | 6 | 2 |
| Chronic kidney disease | 2 | 1 |
| Chronic liver disease | 7 | 4 |
| Tumor | 3 | 1 |
| Herpes zoster site (numbers) | ||
| Head and face | 45 (76.3%) | 10 (66.7%) |
| neck | 7 (11.9%) | 0 |
| Chest and back | 12 (20.3%) | 3 (20%) |
| waist | 2 (3.4%) | 2 (13.3%) |
| limb | 3 (5.1%) | 3 (20%) |
| Clinical presentations (cases) | ||
| Headache | 34 (57.6%) | 9 (60%) |
| Fever | 35 (59.3%) | 11 (73.3%) |
| Cranial nerve involvement | 11 (18.6%) | 2 (13.3%) |
| Meningeal irritation | 9 (15.3%) | 3 (20%) |
| Acute cognitive changes | 0 | 5 (33.3%) |
| Nausea and vomiting | 5 (8.5%) | 3 (20%) |
| Vertigo | 7 (11.9%) | 2 (13.3%) |
| Tinnitus | 6 (10.2%) | 1 (6.7%) |
| RHS | 8 (13.6%) | 3 (20%) |
| Seizure | 0 | 1 (6.7%) |
Compared with varicella zoster virus meningitis group.
P<0.05.
In VZV meningitis group, 40.7% patients were female and 59.3% were male. In VZV meningoencephalitis group, 40% were females and 60% males. There was no significant difference in gender between the two groups (P>0.05). There was no significant difference in comorbidity between the two groups either (P>0.05) (Table 1).
Seasonal distribution and herpes zoster site of cases
The seasonal case distribution of VZV meningitis onset was 15 in spring, 8 in summer, 12 in autumn, and 24 in winter. In VZV meningoencephalitis group, 4 patients were admitted in spring, 5 in summer, 4 in autumn, and 2 in winter. Compared to VZV meningoencephalitis group, VZV meningitis were more likely to develop in winter (40.7% vs 13.3%, P<0.05) (Table 1).
In terms of herpes zoster sites, 45 cases (76.3%) were found in the head and face, 12 cases (20.3%) in the chest and back, 7 cases (11.9%) in the neck, 2 cases (3.4%) in the waist, and 3 cases (5.1%) in the limbs in the VZV meningitis group. In the VZV meningoencephalitis group, herpes zoster was mainly distributed in the head and face in 10 cases (66.7%), followed by chest back in 3 cases (20%), waist in 2 cases (13.3%), and limbs in 3 cases (20%). There was no significant difference in the site of herpes zoster between the two groups (P>0.05) (Table 1).
Clinical presentations
The clinical symptomatology in 59 meningitis and 15 meningoencephalitis cases were summarized in Table 1. At admission, fever (35/59, 59.3%), headache (34/59, 57.6%), cranial nerve involvement (11/59, 18.6%), meningeal irritation (9/59, 15.3%), and Ramsay-Hunt syndrome (RHS) (8/59, 13.6%) were the five most common symptoms of VZV meningitis. However, fever (11/15, 73.5%), headache (9/15, 60%), acute cognitive changes (5/15, 33.3%), meningeal irritation (3/15, 20%), and RHS (3/15, 20%) were the most common symptoms of VZV meningoencephalitis. No obvious clinical presentations preponderance was found between the two groups (P>0.05).
CSF finding
There was no significant difference in the leukocyte count, protein content, chloride concentration, LDL, and ADA between the two groups. However, the glucose level was observed to be lower in VZV meningitis group than that in VZV meningoencephalitis group (3.68±0.79 vs 4.21±0.94, P=0.02), although the CSF glucose level of the two groups was normal (normal: 2.5-4.5 mmol/L) (Table 2). Further analysis of blood glucose levels found no significant difference between two groups (P>0.05).
Table 2.
Cerebrospinal fluid analysis, treatment and outcome in patients with VZV meningitis or meningoencephalitis
| Varicella zoster virus meningitis (n=59) | Varicella zoster virus meningoencephalitis (n=15) | P value | |
|---|---|---|---|
| Cerebrospinal fluid analysis | |||
| Leukocyte count (×106/L) | 13 (3-87) | 6.5 (2-27) | 0.33 |
| Protein (mg/ml) | 70.56±36.42 | 74.52±32.74 | 0.36 |
| CSF chlorine (mmol/L) | 123.6±4.2 | 123.1±7.09 | 0.37 |
| LDH (U/L) | 24.78±9.28 | 27.29±11.11 | 0.19 |
| CSF glucose (mmol/L) | 3.68±0.79 | 4.21±0.94 | 0.02 |
| ADA (U/L) | 3.30±5.42 | 2.71±1.87 | 0.35 |
| Serum analysis | |||
| IgG (g/L) | 11.53±2.16 | 10.96±3.19 | 0.45 |
| IgA (g/L) | 2.26±0.79 | 2.26±0.93 | 0.98 |
| Days passed between onset of the herpes zoster to onset of neurological symptoms [median (Q1-Q3)] | 3 (1-6) | 3 (1-9) | 0.16 |
| Days passed between onset of the neurological symptoms to initiation of the antivirus treatment [median (Q1-Q3)] | 2 (1-4) | 4 (1-5) | 0.03 |
| Days of admission | 17.1±3.9 | 20.2±8.6 | 0.04 |
| Days of intravenous antivirus treatment | 14.9±3.4 | 16.7±4.8 | 0.09 |
| Outcome at discharge | |||
| good | 51 | 7 | 0.00 |
| fair | 8 | 4 | 0.22 |
| poor | 0 | 4 |
The VZV DNA in CSF was detected in 6 out of 20 patients tested using PCR (6/20, 30%). All 20 patients with PCR tests were additionally tested and found negative in enteroviruses, herpes simplex virus (l+ll), cytomegalovirus, Epstein-Barr virus, and Japanese encephalitis virus (JEV-RNA).
Cranial CT/MRI imaging and EEGs findings
Cranial CT/MRI was performed in all patients. Forty-three out of 59 patients with VZV meningitis (12 cases with contrast) and 14 out of 15 patients with VZV meningoencephalitis (7 cases with contrast) received cranial MRI examination on the day of the admission or within 4 days of the admission. Sixteen of 59 patients with VZV meningitis and 1 out of 15 patients with VZV meningoencephalitis received cranial CT examination and no obvious abnormal signs were found in these patients. Three cases of VZV meningoencephalitis were found with abnormal cranial MRI: 1 case showed new infarction in right lateral ventricle and brainstem, 1 patient presented with acute lacunar infarction in the right occipital lobe, and 1 patient was detected ischemic changes in the left temporal occipital parietal junction for possible lacunar infarction but no abnormality was found using enhanced cranial MRI. The third patient still had obvious headache one month after discharge and re-examination with enhanced cranial MRI showed obvious meningeal enhancement.
During hospitalization, 13 patients completed one or two EEG examinations and 2 patients were found to have transient diffuse electroencephalographic abnormalities (including 1 patient presenting with seizures). However, no EEG abnormality was found in patients after discharge.
Treatments
All patients received intravenous antiviral therapy and 27/74 (36.5%) patients were treated with intravenous corticosteroid for 3-5 days (methylprednisolone 40-80 mg/d). The average course of antiviral treatment was 14.9±3.4 days in VZV meningitis group and 16.7±4.8 days (P=0.09) in VZV meningoencephalitis group. The average admission days in meningitis group was 17.1±3.9 days, which was significantly shorter than that of meningoencephalitis group (20.2±8.6 days) (P=0.04) (Table 2).
Outcome
The days between onset of the neurological symptoms to antivirus initiation was 4 (1-5) days in VZV meningoencephalitis group which was significantly longer than that of meningitis group [2 (1-4) days] (P=0.03). However, there was no statistical difference in the days passed between onset of the herpes zoster to onset of neurological symptoms between the two groups: 3 (1-6) days vs 3 (1-9) days, P=0.16 (Table 2).
At discharge, 51 patients (86.4%) in VZV meningitis group had good prognosis and 8 patients (13.6%) had fair prognosis with cranial nerve involvement. In VZV meningoencephalitis group, 7 patients (46.7%) had good, 4 patients (26.7%) had fair, and 4 patients (26.7%) had poor prognosis. Compared to VZV meningoencephalitis, VZV meningitis patients have a better prognosis at the time of discharge (P=0.00) (Table 2).
In univariate analysis, age, gender, and the days passed between onset of the neurological symptoms to initiation of the antivirus treatment were correlated with the outcome of patients with VZV meningitis/meningoencephalitis at discharge (P<0.05, Table 3); Other factors including days of admission, days of intravenous antivirus treatment, days passed between onset of the herpes zoster to onset of the neurological symptoms, and CSF test results did not show any correlation with the patient’s outcome at discharge (P>0.05, Table 3).
Table 3.
Univariate analysis of various factors and outcome of VZV meningitis/meningoencephalitis at discharge
| variable | Z/χ2 value | P value |
|---|---|---|
| Age | 7.90 | 0.02 |
| Gender | 10.04 | 0.01 |
| Days passed between onset of the herpes zoster to onset of the neurological symptoms | 0.42 | 0.81 |
| Days passed between onset of the neurological symptoms to initation of the antivirus treatment | 11.00 | 0.00 |
| Days of intravenous antivirus treatment | 0.46 | 0.80 |
| Days of admission | 0.89 | 0.64 |
| CSF Leukocyte count | 2.09 | 0.35 |
| CSF glucose | 0.56 | 0.76 |
| CSF chlorine | 2.24 | 0.33 |
| CSF ADA | 1.61 | 0.45 |
| CSF Protein | 3.51 | 0.17 |
| CSF LDH | 0.79 | 0.68 |
The outcomes of patients at discharge were used as the dependent variable, and the age, gender and the days passed between onset of the neurological symptoms to initiation of the antivirus treatment were used as independent variables to perform a multivariate logistic regression analysis. The results showed that gender (male) and the days passed between onset of the neurological symptoms to initiation of the antivirus treatment were independent risk factors for the outcome of patients at discharge (P<0.05, Table 4). The area under curve (AUC) of the days passed between onset of the neurological symptoms to initiation of the antivirus treatment was 0.7785 (Figure 1). The best cut-off interval was 1.5 days, with the sensitivity and specificity were 78.4% and 68.9%, respectively.
Table 4.
Multivariate logistic regression analysis of the outcomes at discharge and various factors in patients with VZV meningitis/meningoencephalitis
| Variable | β value | SE value | Wald | OR value | 95% CI | P value |
|---|---|---|---|---|---|---|
| Age | 0.03 | 0.03 | 1.02 | 1.03 | 0.98-1.08 | 0.31 |
| Gender (Male) | 2.50 | 1.10 | 5.15 | 12.22 | 1.41-106.04 | 0.02 |
| Days passed between onset of the neurological symptoms to initiation of the antivirus treatment | 0.79 | 0.18 | 2.78 | 1.34 | 1.05-1.89 | 0.03 |
Figure 1.
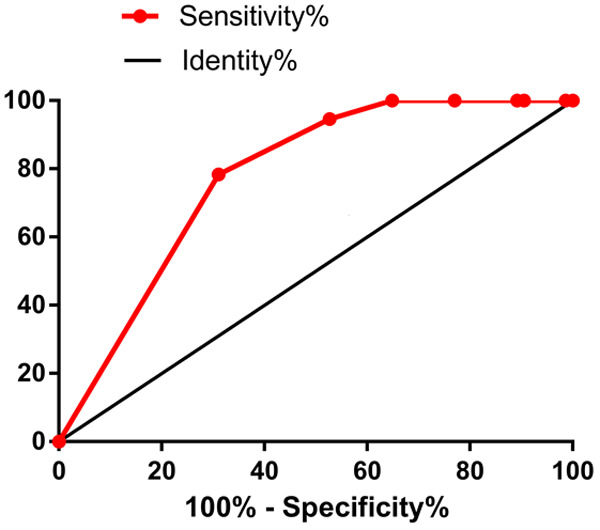
The area under curve of the days passed between onset of the neurological symptoms to initiation of the antivirus treatment could predict the outcome of VZV meningitis/meningoencephalitis at discharge. The cut-off value was 1.5 days, AUC=0.7785, with sensitivity and specificity of 78.4% and 68.9%, respectively.
Discussion
In this study, we analyzed a large group of patients with CNS involvement caused by VZV reactivation in a tertiary hospital with a well-known dermatology department in China and a large volume of patient populations with VZV. VZV is a double strand, unique human DNA virus with high affinity for skin, mucous membrane and nerve tissue. It can be transmitted to human being through respiratory tract, skin and mucous membrane. The first infection usually occurs in children and causes chickenpox which is mainly manifested as herpes zoster distributed along the peripheral nerve, then the virus lurks in the peripheral ganglion, cerebral ganglion, the posterior root ganglion of spinal cord, and autonomic ganglion along the nerve axis [14]. Under the circumstance of fatigue, cold, and decreased body immunity, the virus revives to cause reinfection. The reactivated VZV invades the ectodermal tissue and reaches the innervated area along the nerve axons that cause a variety of nervous system damage in peripheral and central nervous system. The main manifestations are herpetic neuralgia, cranial neuropathy, meningitis, meningoencephalitis, encephalitis, and even myelopathy and cerebrovascular disease. Among them, meningitis and meningoencephalitis are the most common complications of VZV CNS infection. The detection of VZV DNA and/or anti-VZV antibody in CSF was an important criteria for the diagnosis of central VZV infection. In our study, VZV DNA was tested positive in CSF of 30% (6 of 20) of the patients. Before the diagnosis of VZV infection, other CSF inflammation and infection, such as immune-mediated nonspecific inflammation, tuberculosis, and fungal infection were ruled out. The number of leukocyte count in CSF increased significantly, especially when herpes zoster appeared 4 weeks before or during the onset of the disease. Therefore, the diagnosis of VZV CNS infection in these patients was established. Thus, we believe it is suitable to ascertain the actual presentation of VZV meningitis/meningoencephalitis in clinical settings.
In this study, we found that 62.2% of the VZV meningitis/meningoencephalitis patients had fever, 56.8% of the patients had headache, and 17.6% of the patients had cranial nerve involvement. In addition, meningeal irritation and cognitive changes were common symptoms. Our study further confirmed the diversity and variability of neurological symptoms of VZV central infection, which is easy to be ignored and misdiagnosed in the early stage and leads to serious or even life-threatening complications. Especially for patients with zoster sine herpete VZV central infection, the diagnosis is more difficult for clinicians. Previous reports suggested that the probability of central infection for segmental herpes zoster was low and lumbar puncture was not necessary [15]. However, our study found that active CSF examination was useful for early diagnosis and effective treatment as well as prevention from further complications when the patients with herpes zoster had clinical manifestations such as fever, headache, meningeal irritation, cranial nerve involvement and even cognitive changes. No other obvious abnormalities were observed with the cranial MRI and EEG, which further suggests the importance of early CSF examination.
VZV is characterized by short replication cycle, rapid intracellular diffusion and long-term incubation in ganglion. VZV only infects humans and most animal derived neuronal models cannot be used for study [16,17]. So far, the exact mechanism of CNS complications caused by VZV reactivation remains unclear. Previous studies speculated that the following factors may be involved in the process of CNS complications following VZV reactivation. First, the re-activated VZV hidden in the ganglion spreads to adventitia of artery wall to thicken the intima of the blood vessels and cause micro-infarction of small blood vessels, which leads to ischemia and demyelination [18]. Second, re-activated VZV directly invades the central nervous system. Third, re-activated VZV directly destroys the elastic membrane of blood vessels, resulting in the lack of smooth muscle cells which cause aneurysms and bleeding [18]. Fourth, re-activated VZV can induce the aggregation of inflammatory cells (such as CD4+ and CD8+ T cells, CD68 macrophages, etc.) and produce corresponding neurological symptoms [19]. Lastly, the hematogenous invasion of VZV may also play a role in CNS infections. Latest studies supported that VZV CNS infection was caused by vasculitis [20-22]. In a group of 15 patients with VZV meningoencephalitis, 3 cases were found to have acute vascular disease (ischemic stroke), which was consistent with early studies that central infection was closely related to cerebral vascular disease.
The cellular immunity of patients is gradually decreased with the increase in age. It is reported that older patients had more chance of getting herpes zoster [5,23], with more serious damage [24]. Therefore, VZV meningoencephalitis damages meninges and brain parenchyma that results in a worse outcome. However, VZV meningitis in younger patients may only damage the meninges, which is similar to result of previous studies that patients with VZV meningitis were younger with a better prognosis [7,9,10,25]. Compared with VZV meningoencephalitis, the CSF glucose content of VZV meningitis is lower. However, there is no significant difference in blood glucose content between the two groups, indicating that the lower CSF glucose content of VZV meningitis is not caused by the difference in blood glucose level. It may be related to the higher number of leukocytes in CSF of VZV meningitis (13×106/L VS 6.5×106/L) (although there was no significant difference between two groups), which possibly made the fermentation speed of glucose too fast and then lead to the lower glucose content in CSF.
According to previous reports, there was a debate about whether the incidence of herpes zoster is seasonal [26-29]. In our study, we found that VZV meningitis are more likely to occur in winter, which is different from previous reports that VZV CNS infection has nothing to do with season [30]. However, future epidemiological studies are necessary to confirm whether the difference is caused by the number of patients we included or due to a short follow-up time.
Previous studies have categorized prevalent VZV strains around the world into different genotypes or genetic branches. The VZV genotype that prevails in China is mainly clade 2, but other different types of clades also exist in some areas [31-33]. It is of high clinical value to explore the relationship between VZV genotypes and clinical manifestations as well as the pathogenesis of VZV CNS infection. In some clinical studies, single nucleotide polymorphism analysis was used to detect different VZV strains of VZV meningitis/encephalitis patients [34,35]. It is unclear whether there are differences in the pathogenesis between VZV meningitis and meningoencephalitis, and whether there are special genotypes of VZV strains. The mechanism of CNS infection caused by VZV reactivation is worthy of further exploration. Clarifying its pathogenesis will be conducive to an early prevention and treatment of VZV CNS infection.
In this study, 74 patients with VZV meningitis/meningoencephalitis had a good prognosis at discharge. The sequelae rate was 5.4% (4/74), which was significantly lower than 50% reported by Persson et al. [10]. This may benefit from a longer course of intravenous antiviral treatments in our cohort. Other possibilities are that we screened CNS infection when herpes zoster patients had neurological symptoms and we used antiviral drugs intravenously in the early stage of the disease as well as corticosteroids in some patients. Antiviral drugs can inhibit the synthesis and replication of viral DNA and corticosteroids can eliminate neuroedema and reduce denervation. Our study also found that the days passed between onset of the neurological symptoms to initiation of the antiviral treatment were significantly correlated with the outcome at discharge. Similar to previous reports by Arruti et al. [7], a longer delay in antiviral treatment led to a worse outcome. We also found that the best cut-off value was 1.5 days. Therefore, early use of intravenous antiviral drugs is very important for patients with VZV meningitis/meningoencephalitis. In addition, we found male patients had a worse prognosis compared to females. A future study is worth exploring if hormone levels cause the difference in prognosis.
The present study has several limitations. First, data were evaluated retrospectively in single center. The clinical descriptions and available information may be lack of uniformity. Second, CSF VZV DNA with PCR or intrathecal antibody examinations were not routinely performed for many patients, as only 20 patients (less than one third of patients) were tested for PCR in the present study. Third, there was no follow-up investigation of the patients in this study.
In conclusion, we enrolled a relatively large number of VZV meningitis/meningoencephalitis patients over a period of 2.5 years for the analysis. The clinical manifestations of meningitis/meningoencephalitis caused by VZV reactivation are various, and the clinical symptoms and imaging manifestations are not specific. Early CSF examination is very important. The outcome was worse in male patients and those who received antiviral treatment longer than 1.5 days after onset of the neurological symptoms. Early intravenous antiviral treatment for VZV meningitis/meningoencephalitis has a good outcome. Further study with a prospective design is necessary.
Acknowledgements
This work was supported by grants from the Medical Key Subject Construction Project of Hangzhou (2020-2024), Hangzhou City Science and Technology Committee (Grant No. 20181228Y05), and Key Projects of Hangzhou Health Science and Technology Plan (ZD20210010).
Disclosure of conflict of interest
None.
References
- 1.Kennedy PG, Rovnak J, Badani H, Cohrs RJ. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96:1581–1602. doi: 10.1099/vir.0.000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilden D, Nagel M, Cohrs R, Mahalingam R, Baird N. Varicella zoster virus in the nervous system. F1000Res. 2015;4:1356. doi: 10.12688/f1000research.7153.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635–645. doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- 4.Grahn A, Studahl M. Varicella-zoster virus infections of the central nervous system-prognosis, diagnostics and treatment. J Infect. 2015;71:281–293. doi: 10.1016/j.jinf.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Nagel MA, Gilden D. Neurological complications of varicella zoster virus reactivation. Curr Opin Neurol. 2014;27:356–360. doi: 10.1097/WCO.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagel MA, Niemeyer CS, Bubak AN. Central nervous system infections produced by varicella zoster virus. Curr Opin Infect Dis. 2020;33:272–278. doi: 10.1097/QCO.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 7.Arruti M, Piñeiro LD, Salicio Y, Cilla G, Goenaga MA, López de MA. Incidence of varicella zoster virus infections of the central nervous system in the elderly: a large tertiary hospital-based series (2007-2014) J Neurovirol. 2017;23:451–459. doi: 10.1007/s13365-017-0519-y. [DOI] [PubMed] [Google Scholar]
- 8.Herlin LK, Hansen KS, Bodilsen J, Larsen L, Brandt C, Østergaard Andersen C, Hansen BR, Lüttichau HR, Helweg-Larsen J, Wies L, Storgaard M, Nielsen H, Mogensen TH. Varicella zoster virus encephalitis in Denmark from 2015 to 2019 - a nationwide prospective cohort study. Clin Infect Dis. 2021;72:1192–1199. doi: 10.1093/cid/ciaa185. [DOI] [PubMed] [Google Scholar]
- 9.Chamizo FJ, Gilarranz R, Hernández M, Ramos D, José Pena M. Central nervous system infections caused by varicella-zoster virus. J Neurovirol. 2016;22:529–532. doi: 10.1007/s13365-016-0422-y. [DOI] [PubMed] [Google Scholar]
- 10.Persson A, Bergström T, Lindh M, Namvar L, Studahl M. Varicella-zoster virus CNS disease-viral load, clinical manifestations and sequels. J Clin Virol. 2009;46:249–253. doi: 10.1016/j.jcv.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Lozano Becerra JC, Sieber R, Martinetti G, Costa ST, Meylan P, Bernasconi E. Infection of the central nervous system caused by varicella zoster virus reactivation: a retrospective case series study. Int J Infect Dis. 2013;17:e529–534. doi: 10.1016/j.ijid.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Corral C, Quereda C, Muriel A, Martínez-Ulloa P, González-Gómez FJ, Corral Í. Clinical spectrum and prognosis of neurological complications of reactivated varicella-zoster infection: the role of immunosuppression. J Neurovirol. 2020;26:696–703. doi: 10.1007/s13365-020-00872-x. [DOI] [PubMed] [Google Scholar]
- 13.Werner RN, Nikkels AF, Marinović B, Schäfer M, Czarnecka-Operacz M, Agius AM, Bata-Csörgő Z, Breuer J, Girolomoni G, Gross GE, Langan S, Lapid-Gortzak R, Lesser TH, Pleyer U, Sellner J, Verjans GM, Wutzler P, Dressler C, Erdmann R, Rosumeck S, Nast A. European consensus-based (S2k) guideline on the management of herpes zoster-guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), Part 1: Diagnosis. J Eur Acad Dermatol Venereol. 2017;31:9–19. doi: 10.1111/jdv.13995. [DOI] [PubMed] [Google Scholar]
- 14.Sauerbrei A. Diagnosis, antiviral therapy, and prophylaxis of varicella-zoster virus infections. Eur J Clin Microbiol Infect Dis. 2016;35:723–734. doi: 10.1007/s10096-016-2605-0. [DOI] [PubMed] [Google Scholar]
- 15.Skripuletz T, Pars K, Schulte A, Schwenkenbecher P, Yildiz Ö, Ganzenmueller T, Kuhn M, Spreer A, Wurster U, Pul R, Stangel M, Sühs KW, Trebst C. Varicella zoster virus infections in neurological patients: a clinical study. BMC Infect Dis. 2018;18:238. doi: 10.1186/s12879-018-3137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahalingam R, Messaoudi I, Gilden D. Simian varicella virus pathogenesis. Curr Top Microbiol Immunol. 2010;342:309–321. doi: 10.1007/82_2009_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12:197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagel MA, Traktinskiy I, Azarkh Y, Kleinschmidt-DeMasters B, Hedley-Whyte T, Russman A, VanEgmond EM, Stenmark K, Frid M, Mahalingam R, Wellish M, Choe A, Cordery-Cotter R, Cohrs RJ, Gilden D. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011;77:364–370. doi: 10.1212/WNL.0b013e3182267bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagel MA, Traktinskiy I, Stenmark KR, Frid MG, Choe A, Gilden D. Varicella-zoster virus vasculopathy: immune characteristics of virus-infected arteries. Neurology. 2013;80:62–68. doi: 10.1212/WNL.0b013e31827b1ab9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagel M, Gilden D. Editorial commentary: varicella zoster virus infection: generally benign in kids, bad in grown-ups. Clin Infect Dis. 2014;58:1504–1506. doi: 10.1093/cid/ciu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagel MA, Gilden D. Developments in varicella zoster virus vasculopathy. Curr Neurol Neurosci Rep. 2016;16:12. doi: 10.1007/s11910-015-0614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, Choi SM, Kim BC, Choi KH, Nam TS, Kim JT, Lee SH, Park MS, Kim SJ. Risk factors for aseptic meningitis in herpes zoster patients. Ann Dermatol. 2017;29:283–287. doi: 10.5021/ad.2017.29.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81:928–930. doi: 10.1212/WNL.0b013e3182a3516e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy PGE, Gershon AA. Clinical features of varicella-zoster virus infection. Viruses. 2018;10:609. doi: 10.3390/v10110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Science M, MacGregor D, Richardson SE, Mahant S, Tran D, Bitnun A. Central nervous system complications of varicella-zoster virus. J Pediatr. 2014;165:779–785. doi: 10.1016/j.jpeds.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26–33. doi: 10.1016/s1473-3099(03)00857-0. [DOI] [PubMed] [Google Scholar]
- 27.Toyama N, Shiraki K Society of the miyazaki prefecture dermatologists. Epidemiology of herpes zoster and its relationship to varicella in Japan: a 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J Med Virol. 2009;81:2053–2058. doi: 10.1002/jmv.21599. [DOI] [PubMed] [Google Scholar]
- 28.Korostil IA, Regan DG. Varicella-zoster virus in Perth, Western Australia: seasonality and reactivation. PLoS One. 2016;11:1–13. doi: 10.1371/journal.pone.0151319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berlinberg EJ, Kim E, Deiner MS, Patterson C, Porco TC, Acharya NR. Seasonality of herpes zoster and herpes zoster ophthalmicus. J Clin Virol. 2020;126:104306. doi: 10.1016/j.jcv.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gil A, San-Martín M, Carrasco P, González A. Epidemiology of severe varicella-zoster virus infection in Spain. Vaccine. 2004;22:3947–3951. doi: 10.1016/j.vaccine.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Xu S, Chen M, Zheng H, Wang H, Chen M, Zhou J, Shuang W, Yu P, Ma C, He J, Feng D, Zhen Z, Yan Z, Naiying M, Cui A, Wu Q, Qi M, Li C, Xu X, Xu W. Nationwide distribution of varicella-zoster virus clades in China. BMC Infect Dis. 2016;16:542. doi: 10.1186/s12879-016-1863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Wang M, Gan L, Yang S, Chen J. Genotyping of clinical varicella-zoster virus isolates collected in China. J Clin Microbiol. 2009;47:1418–1423. doi: 10.1128/JCM.01806-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B, Xu J, Song X, Wang T, Quan Z, Qian M, Liu W, Song N. Characterization and comparison of genetic variation in clinical varicella-zoster virus isolates collected from Shanghai and Urumqi, China. Jpn J Infect Dis. 2020;73:226–230. doi: 10.7883/yoken.JJID.2019.280. [DOI] [PubMed] [Google Scholar]
- 34.Pahud BA, Glaser CA, Dekker CL, Arvin AM, Schmid DS. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316–323. doi: 10.1093/infdis/jiq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levin MJ, DeBiasi RL, Bostik V, Schmid DS. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444–1447. doi: 10.1086/592452. [DOI] [PubMed] [Google Scholar]


