Abstract
Background: Cabazitaxel has been applied to the treatment of castration-resistant prostate cancer (CRPC), but the molecular mechanism remained to be fully understood. Methods: After treatment with Cabazitaxel alone or in combination with ionizing radiation (IR), CRPC cell viability, proliferation and apoptosis were determined by Cell Counting Kit-8 (CCK-8) assay, colony formation, and flow cytometry, respectively. Tumor volume was measured after the establishment of animal xenograft model. Relative expressions of proteins related to apoptosis (B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), and cleaved caspase 3) and phosphoinositide 3-kinase (PI3K)/AKT pathway were measured by Western blot, and the phosphorylated-PI3K/PI3K and p-AKT/AKT ratios were determined as well. Results: Cell viability and proliferation were suppressed, and apoptosis was promoted in CRPC cells after Cabazitaxel treatment alone, accompanied with upregulated expressions of Bax and cleaved caspase 3 and downregulated Bcl-2 expression. Also, a single treatment with Cabazitaxel resulted in suppression of PI3K/AKT pathway activation, along with downregulated expressions of p-PI3K and p-AKT and a reduced ratio of p-PI3K/PI3K to p-AKT/AKT. Meanwhile, Cabazitaxel enhanced the effects of IR on suppressing survival and promoting apoptosis in CRPC cells through downregulating Bcl-2 and upregulating Bax and cleaved caspase 3. However, Cabazitaxel suppressed IR-induced PI3K/AKT pathway activation via downregulating p-PI3K and p-AKT, leading to a reduced ratio of p-PI3K/PI3K to p-AKT/AKT. Furthermore, Cabazitaxel further promoted the effects of IR on suppressing tumor growth in vivo. Conclusion: Cabazitaxel inhibited the proliferation and promoted the apoptosis and radiosensitivity of CRPC cells, which is related to the suppression of PI3K/AKT pathway, providing a therapeutic method for CRPC in clinical practice.
Keywords: Castration resistant prostate cancer, cabazitaxel, proliferation, apoptosis, radiosensitivity, phosphoinositide 3-kinase (PI3K)/AKT pathway
Introduction
At present, prostate cancer has been recognized as one of the most prevalent tumors, with the second highest mortality rate in males among all cancer types over the world [1]. Androgens play a critical role in prostate cancer cell growth, and androgen deprivation therapy (ADT) is a main strategy for the management of advanced prostate cancer treatment [2]. However, after 2-year ADT treatment, patients will generally progress from castration-sensitive prostate cancer (CSPC) into castration-resistant prostate cancer (CRPC) that is characterized by altered expressions of steroidogenic enzymes, which stimulate tumors to produce potent androgens from circulating adrenal androgen precursors [3,4]. Study showed that radiation therapy could be employed for the treatment of metastatic CRPC [5]. However, despite great progress in CRPC treatment, the overall survival rate of CRPC patients is still unfavorable, with the median survival time ranging from 9 to 30 months [6,7]. Therefore, it has become increasingly urgent to develop novel therapeutic strategies for CRPC.
Various biological agents proved to be effective for CRPC treatment have been approved for use with the advent of novel anti-androgen therapeutic methods [8]. Increasing evidence has demonstrated the roles and efficacy of Cabazitaxel in CRPC treatment. Cabazitaxel, a taxane and antineoplastic agent applied in CRPC therapy together with Docetaxel, is another targeted drug for prostate cancer [9]. Previous studies verified the therapeutic effect of Cabazitaxel on anti-proliferation and anti-tumor properties in different tumor xenografts, including CRPC [10]. As reported by Jarvis et al., Cabazitaxel suppressed cell growth and enhanced the anti-tumor properties of pigment epithelium-derived factor (PEDF) [11]. However, the detailed molecular mechanisms remained to be addressed.
Phosphoinositide 3-kinase/AKT (PI3K/AKT) pathway, a cancer regulator, plays a pivotal role in mammalian cells and regulates cell growth, proliferation, migration, and metabolism [12,13]. Recent studies have unveiled the role of PI3K/AKT pathway in the pathogenesis of prostate cancer [14], and indicated that inhibiting PI3K/AKT pathway via the combination therapy of Docetaxel and Thymoquinone could stimulate prostate cancer cell apoptosis [15]. In addition, Cabazitaxel could target PI3K/AKT pathway to promote autophagy, which contributed to the death of lung adenocarcinoma cells [16]. Nevertheless, the interaction between Cabazitaxel and PI3K/AKT pathway in prostate cancer is inadequately understood. Therefore, for bridging the research gap, our current study aimed to examine the role of Cabazitaxel and PI3K/AKT pathway in CRPC, hoping to obtain more evidence on developing a possible therapeutic method for the disease.
Materials and methods
Ethical statement
All animal experiments were performed at Nanjing First Hospital, Nanjing Medical University, strictly following the guidelines of China Council on Animal Care and Use. Our current study has been approved by the Ethics Committee of Experimental Animals of Nanjing First Hospital, Nanjing Medical University (serial number: NK2019100706). Every effort was devoted to minimizing pain and discomfort caused to the animals.
Cell culture and treatment
Human CRPC cell lines 22Rv1 (CRL-2505) and PC-3 (CRL-1435) were ordered from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in a 37°C, 5% CO2 environment. 22Rv1 cells were cultured with RPMI-1640 medium (31870-082, Gibco, Waltham, MA, USA), and PC-3 cells were cultured with Ham’s F12 medium (L0136, BioWest, Riverside, MO, USA) with 10% fetal bovine serum (FBS, 16140-063, Gibco, USA) and 1% penicillin-streptomycin (15070-063, Gibco, USA).
Cabazitaxel (SML2487, chemical structure provided in Figure 1A) was obtained from Sigma-Aldrich (St Louis, MO, USA) to determine the effects of Cabazitaxel and ionizing radiation (IR) on 22Rv1 and PC-3 cells. The two cells were treated with different concentrations of Cabazitaxel (0, 0.003, 0.03, 0.3, 3, and 30 nmol/L) for 72 h. For IR, the 22Rv1 and PC-3 cells were first pretreated with or without Cabazitaxel at 37°C for 24 h, and then given increasing doses of IR (0-8 Gy) using an X-ray linear accelerator (RS2000, RadSource, Suwanee, GA, USA) at a dose rate of 5.2 Gy/minute as previously described [17].
Figure 1.
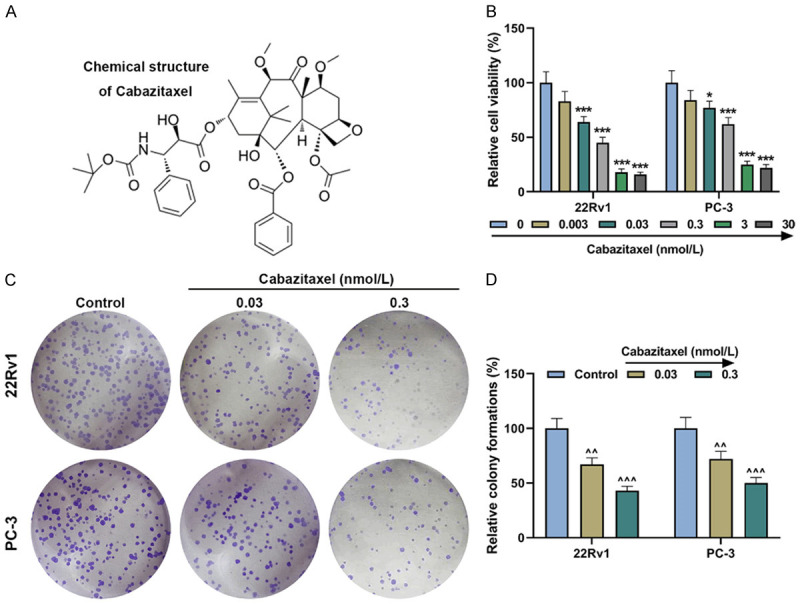
Cabazitaxel suppressed the viability and proliferation of CRPC cells. A. Chemical structure of Cabazitaxel. B. Relative viability of CRPC 22Rv1 and PC-3 cells after treatment with different concentrations of Cabazitaxel was determined by CCK-8 assay. C, D. Relative colony formation rate of CRPC cells after treatment with different concentrations of Cabazitaxel was measured by colony formation assay. All experiments have been performed in triplicate and data were expressed as mean ± standard deviation (SD). *P<0.05, ***P<0.001, vs. 0 nmol/L. ^^P<0.01, ^^^P<0.001, vs. Control. CRPC: Castration Resistant Prostate Cancer; CCK-8: Cell Counting Kit-8.
Cell counting Kit-8 (CCK-8) assay
The 22Rv1 and PC-3 cells were grown in 96-well plates at a density of 5 × 103 cells/well at 37°C with 5% CO2 and then treated with different concentrations of Cabazitaxel (0, 0.003, 0.03, 0.3, 3, and 30 nmol/L) for 72 h. Next, 10 µL of CCK-8 reagent (B34302, Bimake, Houston, TX, USA) and 100 µL of serum-free medium were added to incubate the cells at 37°C for 4 h. OD values for absorbance at 450 nm were determined using an iMark Microplate Absorbance Reader (Bio-Rad, Hercules, CA, USA).
Colony formation assay
The 22Rv1 and PC-3 cells (1 × 103 cells/well) were cultured in 6-well plates at 37°C with 5% CO2 after treatment with increasing doses of IR and different concentrations of Cabazitaxel. After two-week culture, the cells were fixed with methanol (M116122, Aladdin, Shanghai, China) for 15 min and stained using crystal violet (C196471, Aladdin, China) for 30 min. The fixed colonies were observed and photographed under a stereo microscope (SZ61, Olympus, Tokyo, Japan) to calculate the colony formation rate and the surviving fraction.
Flow cytometry
The apoptosis of 22Rv1 and PC-3 cells was detected with an Annexin V-FITC cell apoptosis kit (APOAF, Sigma-Aldrich, USA) based on the manufacturer’s instruction. 22Rv1 and PC-3 cells (1 × 105 cells/well) were treated with Annexin V and propidium iodide (PI) (5 μL for each) together for 15 min at room temperature in the dark. The two cells were then collected and washed twice with 400 μL of cold phosphate buffered saline (PBS, C0221A, Beyotime, Shanghai, China). Apoptosis was detected using a cell apoptosis detection kit and a NovoCyte 2090V flow cytometer (ACEA Biosciences, San Diego, CA, USA), and the data were analyzed by Kaluza C Analysis Software (version 2.1, Beckman Coulter, Indianapolis, IN, USA).
Western blot
Protein expressions were determined by Western blot as previously described [18]. After cell collection, protein extraction was conducted using RIPA lysis buffer (89900, Thermo Fisher Scientific, Waltham, MA, USA), and the concentration of the extracted protein was quantified using a Bicinchoninic acid (BCA) protein assay kit (23225, Thermo Fisher Scientific, USA). 20 μg of protein lysates were electrophoresed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; P0012A, Beyotime, China), and transferred onto a polyvinylidene fluoride (PVDF) membrane (FFP33, Beyotime, China). Then the membrane was blocked by fat-free milk (5%) for 2 h and incubated with the following primary antibodies: anti-B-cell lymphoma 2 (Bcl-2) antibody (ab59348, 1:1000, Abcam, Cambridge, UK), anti-Bcl-2-associated X protein (Bax) antibody (ab32503, 1:10000, Abcam, UK), anti-cleaved Caspase 3 antibody (ab2302, 1:500, Abcam, UK), anti-phosphorylated-PI3K (p-PI3K) antibody (#4228, 1:1000, Cell Signaling Technology (CST), Danvers, MA, USA), anti-PI3K antibody (#4292, 1:1000, CST, USA), anti-phosphorylated-AKT (p-AKT) antibody (ab38449, 1:1000, Abcam, UK), anti-AKT antibody (ab8805, 1:500, Abcam, UK), and anti-β-actin antibody (ab8226, 1:2000, Abcam, UK) at 4°C overnight, with β-actin as an internal control. After that, the membrane was incubated with secondary HRP-conjugated antibodies goat anti-rabbit IgG H&L (1:2000, #7074, CST, USA) and goat anti-mouse IgG H&L (1:2000, A0216, Beyotime, China) at room temperature for 1 h and then washed by tris-buffer saline tween (TBST, T196393, Aladdin, China) for three times. After collecting protein bands, an enhanced chemiluminescence (ECL) kit (P0018FS, Beyotime, China) was used for visualization. The data were analyzed using iBright CL1500 Imaging System (A44240, Thermo Fisher Scientific, USA) and grey values were calculated using ImageJ (version 5.0, Bio-Rad, Hercules, CA, USA).
Animal xenograft model
Animal xenograft model was constructed as previously described [19]. Male BALB/C nude mice (5 weeks old, 20-25 g, n=12) were obtained from Orient Bio (Seongnam, Korea) and kept in specific non-pathogen cages under specific conditions (12-hour day/night cycle, 21-23°C and 50-60% humidity). All the mice were provided with free access to a standard experimental diet and sterile tap water throughout the entire experiment.
For subsequent study, the mice were randomly assigned to the following groups: Control, Cabazitaxel, IR and IR+Cabazitaxel groups (n=3 for each group). The mice in the Cabazitaxel, IR and IR+Cabazitaxel groups received subcutaneous injection of 2 × 106 PC-3 cells at the same side of the posterior flank, while those in the Control group only received intraperitoneal injection of an equivalent volume of saline (B020, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). For the Cabazitaxel group, the mice were injected with Cabazitaxel (33 mg/kg) via the tail vein, whereas those in the IR group were given 5 Gy of single dose irradiation with an X-ray linear accelerator. Also, the mice in the IR+Cabazitaxel group first received tail vein injection of Cabazitaxel (33 mg/kg) and then were subjected to 5 Gy of single dose irradiation with an X-ray microirradiator. All the mice were sacrificed on day 52 via inhalation of 5% isoflurane (YZ-1349003, Solarbio, China) and the tumor volume was measured by the formula: Tumor volume (mm3) = 0.5 × Width2 × Length/2.
Statistical analysis
Each experiment was independently performed for more than three times. The data were expressed as mean ± standard deviation (SD) and analyzed using SPSS 20.0 software (SPSS, Chicago, IL, USA). Statistical significance was determined by one-way ANOVA followed by Tukey post hoc test and student t test, and P<0.05 was considered statistically significant.
Results
Cabazitaxel suppressed the viability and proliferation of CRPC cells
To confirm the effects of Cabazitaxel on CRPC cells, CCK-8 assay was conducted to measure CRPC cell viability after treatment with different concentrations of Cabazitaxel (0, 0.003, 0.03, 0.3, 3 and 30 nmol/L). In Figure 1B, it could be observed that the viability of both 22Rv1 and PC-3 cells was decreased after Cabazitaxel treatment (Figure 1B, P<0.05). As the viability of both 22Rv1 and PC-3 cells was closer to 50% after treatment with 0.03 or 0.3 nmol/L Cabazitaxel, 0.03 and 0.3 nmol/L Cabazitaxel were used in subsequent studies.
The effects of Cabazitaxel on CRPC cell proliferation were analyzed by colony formation assay. As displayed in Figure 1C, 1D, colony formation was decreased in both 22Rv1 and PC-3 cells after Cabazitaxel treatment (Figure 1C, 1D, P<0.01), which suggested that Cabazitaxel could suppress CRPC cell proliferation.
Cabazitaxel promoted CRPC cell apoptosis
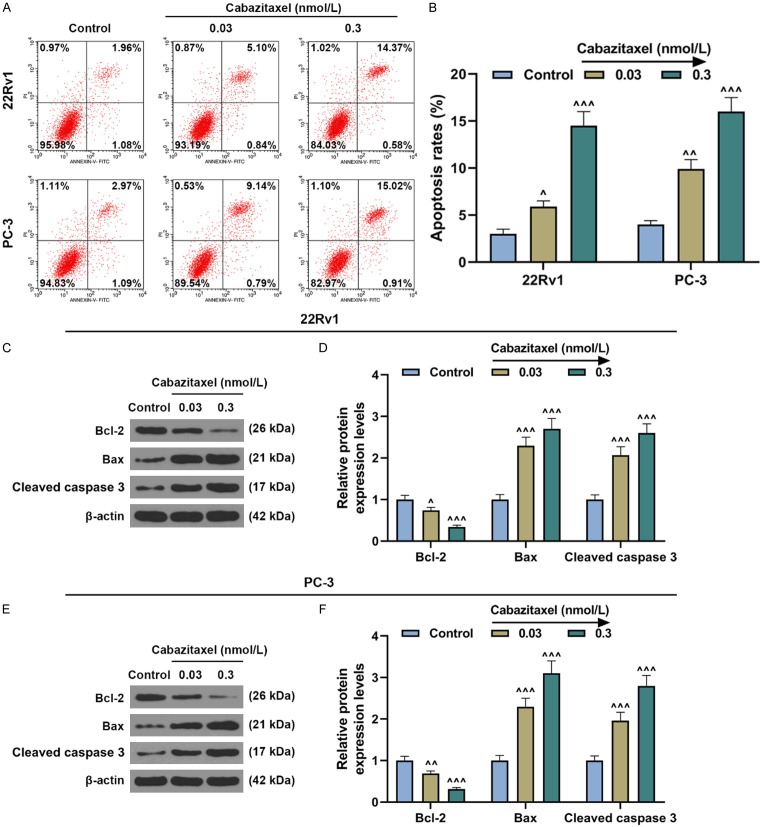
The results from flow cytometry demonstrated that the apoptosis of CRPC cells (22Rv1 and PC-3 cells) was evidently increased after Cabazitaxel treatment (Figure 2A, 2B, P<0.05), indicating that Cabazitaxel could promote CRPC cell apoptosis. In order to further confirm our results, Western blot was performed to measure the protein expressions of apoptosis-related factors. As shown in Figure 2C-F, Bcl-2 expression was downregulated after Cabazitaxel treatment, while those of Bax and cleaved caspase 3 were upregulated (Figure 2C-F, P<0.05), which further proved that Cabazitaxel could promote CRPC cell apoptosis.
Figure 2.
Cabazitaxel promoted CRPC cell apoptosis. A, B. Apoptosis of CRPC 22Rv1 and PC-3 cells after treatment with different concentrations of Cabazitaxel was detected by flow cytometry. C-F. Relative protein/β-actin expressions of apoptosis-related factors (Bcl-2, Bax, and cleaved caspase 3) after treatment with different concentrations of Cabazitaxel were quantified by Western blot. β-actin was used as internal control. All experiments have been performed in independent triplicates and the data were expressed as mean ± standard deviation (SD). ^P<0.05, ^^P<0.01, ^^^P<0.001, vs. Control. Bcl-2: B-cell lymphoma 2; Bax: Bcl-2-associated X protein.
Cabazitaxel suppressed PI3K/AKT pathway activation in CRPC cells
PI3K/AKT pathway-related protein expressions in CRPC cells were quantified by Western blot, and the ratios of p-PI3K/PI3K and p-AKT/AKT were also determined. It was discovered that both the protein expressions of p-PI3K and p-AKT were decreased after Cabazitaxel treatment, and that ratios of p-PI3K/PI3K and p-AKT/AKT dropped as well (Figure 3A-H, P<0.05). These results showed that Cabazitaxel could suppress PI3K/AKT pathway activation in the CRPC cells.
Figure 3.
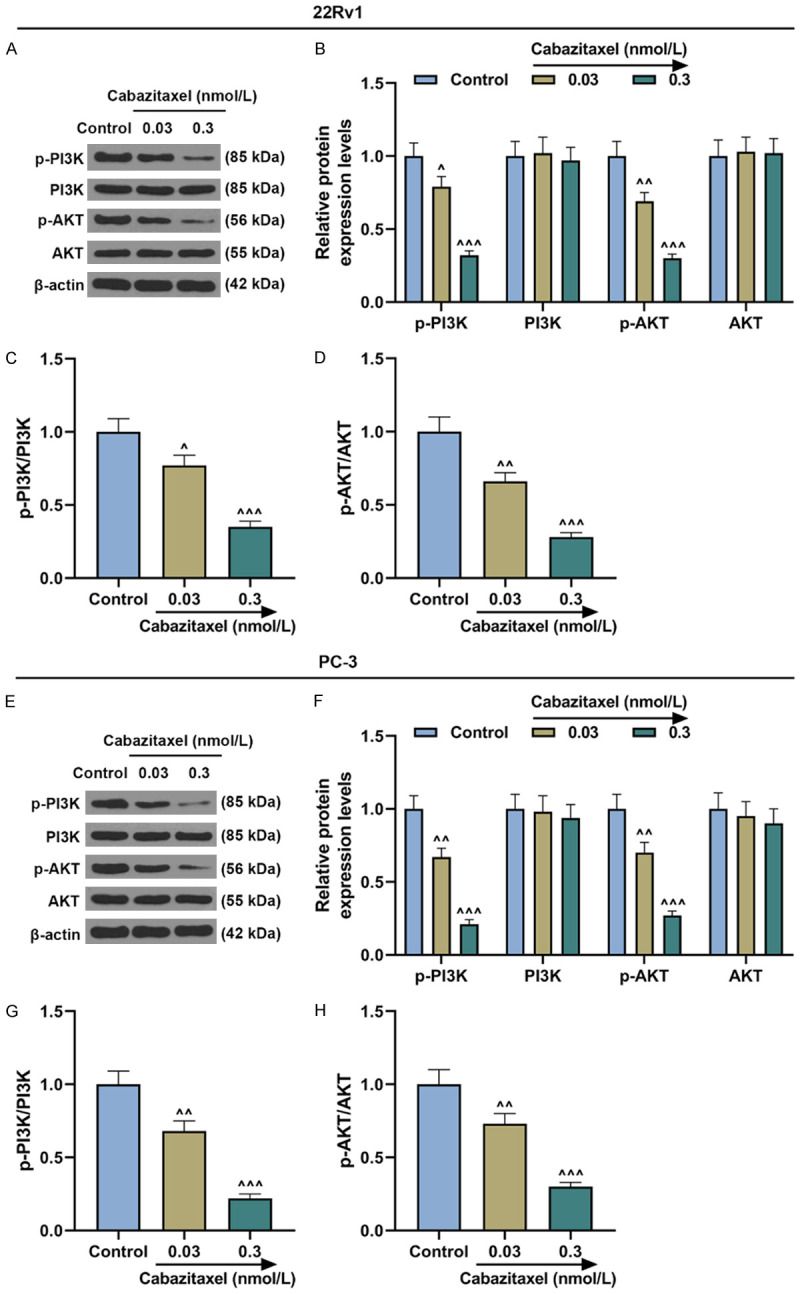
Cabazitaxel suppressed PI3K/AKT pathway activation in CRPC cells. (A, B) Relative protein/β-actin expressions of PI3K/AKT pathway-related proteins after treatment of 22Rv1 cells with different concentrations of Cabazitaxel were measured by Western blot. β-actin was used as internal control. (C, D) Ratios of p-PI3K/PI3K (C) and p-AKT/AKT (D) were measured. (E, F) Relative protein/β-actin expressions of PI3K/AKT pathway-related proteins after treatment of PC-3 cells with different concentrations of Cabazitaxel were measured by Western blot. β-actin was used as internal control. (G, H) Ratios of p-PI3K/PI3K (G) and p-AKT/AKT (H) were quantified. All the experiments have been performed in independent triplicates and the data were expressed as mean ± standard deviation (SD). ^P<0.05, ^^P<0.01, ^^^P<0.001, vs. Control.
Cabazitaxel enhanced the effects of IR on suppressing CRPC cell survival
In order to determine the effect of IR on CRPC cells, colony formation assay was used to measure the surviving fraction of both 22Rv1 and PC-3 cells after treatment with different doses of IR. As shown in Figure 4A-D, IR treatment resulted in a decrease in the surviving fraction of both 22Rv1 and PC-3 cells; Cabazitaxel further promoted the effects of IR, and the effect of Cabazitaxel was the strongest at 4 Gy (Figure 4A-D, P<0.05). Thus, it could be summarized that Cabazitaxel combined with IR suppressed CRPC cell survival.
Figure 4.
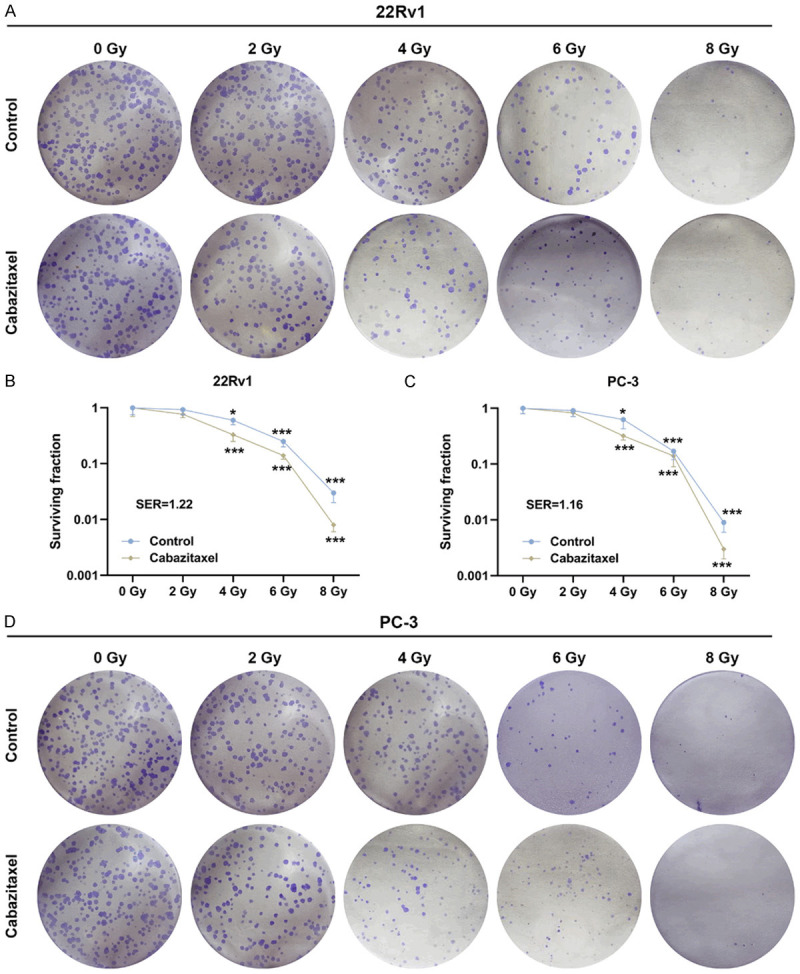
Cabazitaxel enhanced the suppressive effects of IR on CRPC cell survival. (A-D) Colony formation assay was used to determine the effects of Cabaziatxel and IR on the survival of CRPC 22Rv1 (A, B) and PC-3 (C, D) cells. All the experiments were performed in triplicates and the data were expressed as mean ± standard deviation (SD). *P<0.05, **P<0.01, ***P<0.001, vs. Control. IR: ionizing radiation; SER: sensitivity enhancement ratio.
Cabazitaxel enhanced the effects of IR on promoting CRPC cell apoptosis
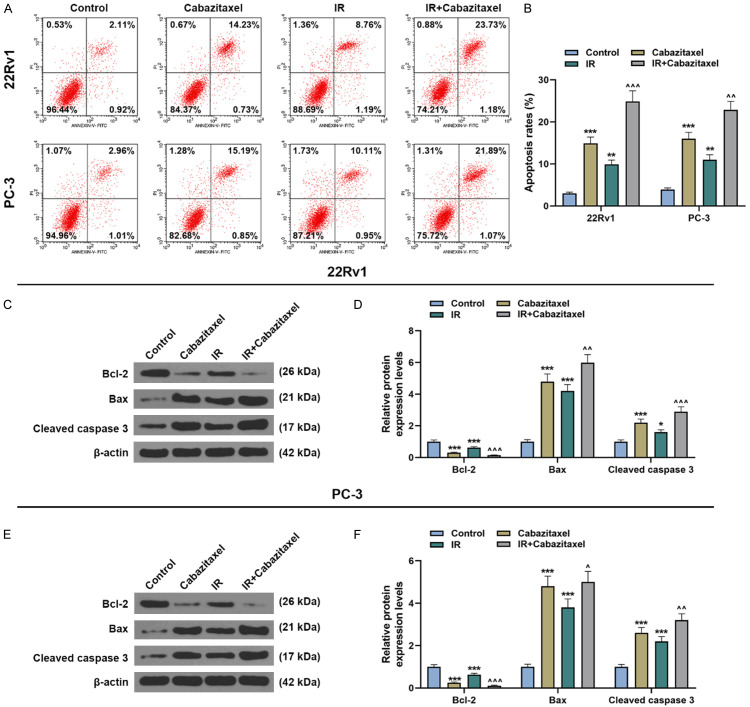
The results of flow cytometry showed that after sole treatment with IR or Cabazitaxel, the apoptosis rate of CRPC cells was increased (Figure 5A, 5B, P<0.01). Also, the combined treatment with Cabazitaxel and IR further promoted cell apoptosis (Figure 5A, 5B, P<0.01). Meanwhile, the protein expressions of apoptosis-related factors were measured with Western blot. In Figure 5C-F, it could be observed that after treatment with Cabazitaxel or IR alone, both the protein expressions of Bax and cleaved caspase 3 were up-regulated, whereas that of Bcl-2 was down-regulated (Figure 5C-F, P<0.05). Higher protein expressions of Bax and cleaved caspase 3 and lower Bcl-2 expression were detected after combined treatment with Cabazitaxel and IR (Figure 5C-F, P<0.05). These results showed that Cabazitaxel enhanced the effects of IR on promoting CRPC cell apoptosis.
Figure 5.
Cabazitaxel enhanced the effects of IR on promoting CRPC cell apoptosis. A, B. Apoptosis of CRPC 22Rv1 and PC-3 cells after Cabazitaxel and IR treatment was detected by flow cytometry. C-F. Relative protein/β-actin expressions of apoptosis-related factors (Bcl-2, Bax, and Cleaved caspase 3) after Cabazitaxel and IR treatment were quantified by Western blot. β-actin was used as internal control. All the experiments have been performed in independent triplicates and the data were expressed as mean ± standard deviation (SD). *P<0.05, **P<0.01, ***P<0.001, vs. Control; ^P<0.05, ^^P<0.01, ^^^P<0.001, vs. IR.
Cabazitaxel suppressed IR-induced PI3K/AKT pathway activation in CRPC cells
Then, after sole treatment with Cabazitaxel or IR or combined treatment with Cabazitaxel and IR, PI3K/AKT pathway-related protein expressions in CRPC cells were measured by Western blot, and the ratio of p-PI3K/PI3K to p-AKT/AKT was measured as well. It was found that after Cabazitaxel treatment alone, the protein expressions of p-PI3K and p-AKT were down-regulated, and the ratios of p-PI3K/PI3K and p-AKT/AKT dropped, whereas IR treatment alone up-regulated p-PI3K and p-AKT expressions and the ratios of p-PI3K/PI3K and p-AKT/AKT (Figure 6A-H, P<0.05). These results showed that Cabazitaxel could suppress IR-induced PI3K/AKT pathway activation in CRPC cells.
Figure 6.
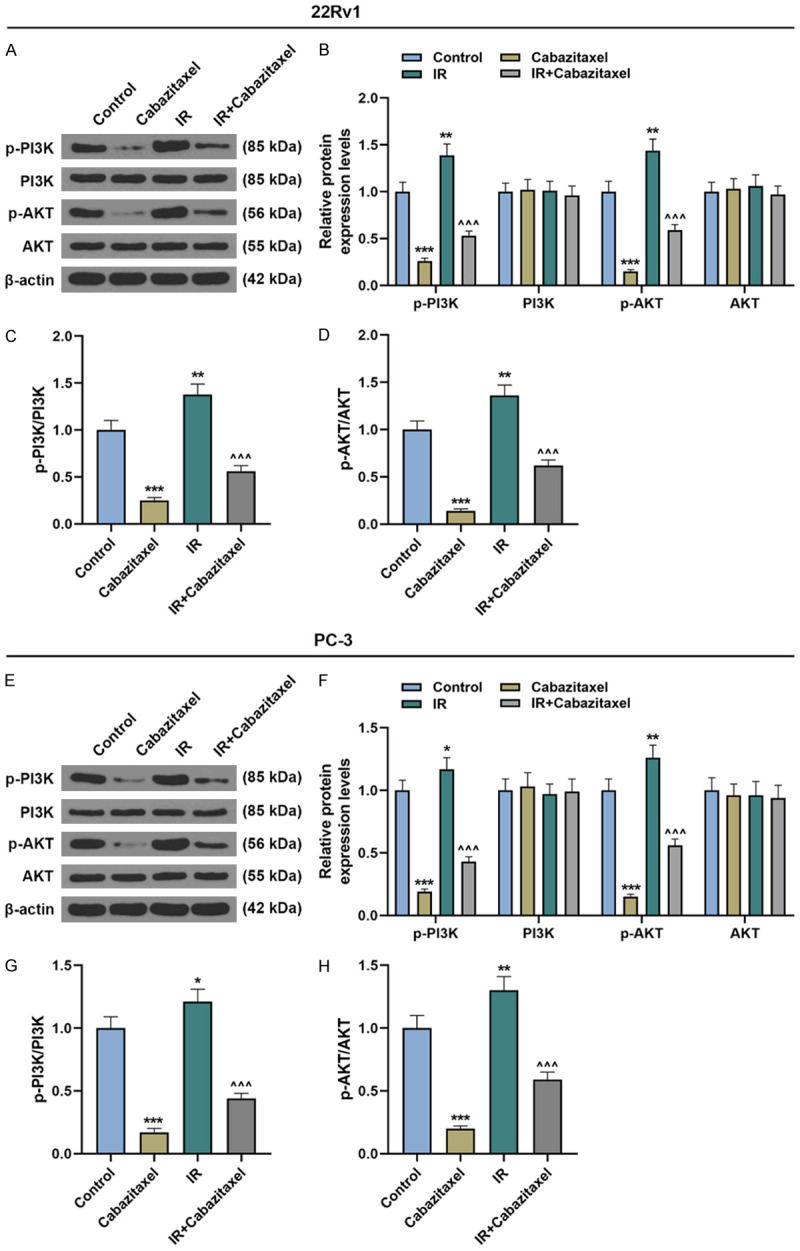
Cabazitaxel suppressed IR-induced PI3K/AKT pathway activation in CRPC cells. (A, B) Relative protein/β-actin expressions of PI3K/AKT pathway-related proteins after treatment of 22Rv1 cells with Cabazitaxel and IR were measured by Western blot. β-actin was used as internal control. (C, D) Ratios of p-PI3K/PI3K (C) and p-AKT/AKT (D) were measured. (E, F) Relative protein/β-actin expressions of PI3K/AKT pathway-related proteins after treatment of PC-3 cells with Cabazitaxel and IR were measured by Western blot. β-actin was used as internal control. (G, H) Ratios of p-PI3K/PI3K (G) and p-AKT/AKT (H) were quantified. All the experiments have been performed in independent triplicates and the data were expressed as mean ± standard deviation (SD). *P<0.05, **P<0.01, ***P<0.001, vs. Control; ^^^P<0.001, vs. IR.
Cabazitaxel enhanced the effects of IR on suppressing tumor growth
Finally, to investigate the efficacy of Cabazitaxel and IR in vivo, the Cabazitaxel-treated PC-3 cells were injected into the nude mice, some of which were further treated with IR. Tumor volume was subsequently measured, and the results showed that tumor volume was decreased after treatment with Cabazitaxel or IR alone, and the combined treatment with Cabazitaxel and IR further reduced tumor volume (Figure 7A, 7B, P<0.001). These results suggested that Cabazitaxel enhanced the effects of IR on suppressing tumor growth in vivo.
Figure 7.
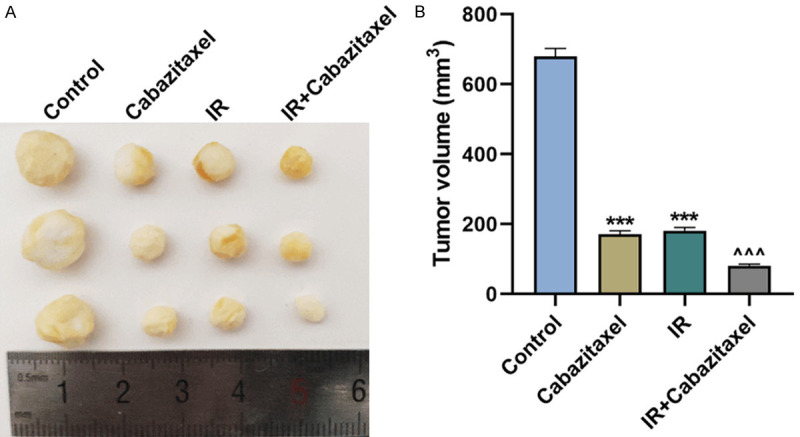
Cabazitaxel enhanced the inhibitory effects of IR on tumor growth. A, B. Tumor volume was measured after injection of the PC-3 cells treated with Cabazitaxel and IR into the nude mice. All the experiments have been performed in independent triplicates and the data were expressed as mean ± standard deviation (SD). ***P<0.001, vs. Control; ^^^P<0.001, vs. IR.
Discussion
At present, a better understanding of the mechanisms related to CRPC development and progression has been the focus of clinical cancer research [20]. Therefore, it has become increasingly important to further discover an effective therapeutic target for CPRC treatment. Drug resistance is an obstacle hindering the effective and efficient treatment of cancer, yet a variety of agents have been found to be able to prevent cancer patients from developing drug resistance. Cabazitaxel is a semisynthetic taxoid that restores the efficacy of chemotherapy by overcoming drug resistance [21]. Accumulating evidence showed that Cabazitaxel could ameliorate CRPC and increase the overall survival rate when compared with Mitoxantrone [22,23]. Besides, Cabazitaxel was previously observed to produce anti-proliferative effects [11]. Nevertheless, the mechanisms of the effects of Cabazitaxel on CRPC cells remained vague. In our study, we found that Cabazitaxel, alone or in combination with IR, suppressed CRPC cell viability and proliferation in vitro and tumor growth in vivo and promoted cell apoptosis. Moreover, PI3K/AKT pathway activation was inhibited after Cabazitaxel treatment, suggesting that Cabazitaxel exerted its effects via suppressing PI3K/AKT pathway.
Proliferation of cancer cells, which has been recognized as a key part in cancer development, is reflected by altered expression and/or activity of cell cycle-related proteins. Deregulated apoptotic cell death is another main characteristic of cancer, and apoptosis alteration is responsible for tumor progression and tumor resistance against therapy. Thus, understanding the mechanisms of apoptosis is of great significance to improve the efficacy of cancer-related therapy and bypassing resistance [24,25]. Some member proteins of the Bcl-2 family, such as Bax and Bcl-2, are related to cell apoptosis, and regulate cell life and the mitochondrial suicide program of apoptosis [26,27]. Bax can prevent the inhibitory effect of the anti-apoptotic protein Bcl-2 on apoptosis via heterodimer formation with Bcl-2 [28,29]. Caspases are a family of originally produced inactive monomeric proteases which need to be dimerized or cleaved to be activated [30]. Also, some caspases are involved in cell apoptosis, among which caspase 3 is a key effector caspase triggering apoptosis [31]. Suppressing cleaved caspase 3 activation could lead to apoptosis suppression [32]. In our study, we found that Cabazitaxel inhibited CRPC cell proliferation and promoted apoptosis, accompanied with upregulated expressions of Bax and cleaved caspase 3 and downregulated Bcl-2 expression. Nevertheless, the detailed molecular mechanism remained poorly understood.
Radiosensitivity refers to the relative susceptibility of cells, tissues, organs, organisms or other substances to radiation, which can be achieved by targeting signaling pathways, gene transcriptions, and non-coding RNAs (including microRNAs and long non-coding RNAs). Radioresistance is a process where tumor cells or tissues adapt to changes induced by radiotherapy and develop resistance to IR, which will cause failure of radiotherapy and poor prognosis of cancer patients [33-35]. Recent evidence suggested that cancer stem cells (CSCs) are involved in and connected to radio sensitivity [36]. CSCs are also responsible for the development of cancer radioresistance during treatment, because they could overcome the anti-cancer effects of radiotherapy and contribute to the production of radioresistant heterogeneous tumor cell population [37]. Study showed that microtubule stabilization, which is induced by Cabazitaxel, could enhance the radiosentivity of ovarian cancer cells [38]. In our study, we found that the survival fraction of CRPC cells was decreased and their apoptosis rate was increased after Cabazitaxel or IR treatment alone, and moreover, Cabazitaxel could enhance the effect of IR on suppressing the survival and promoting the apoptosis of CRPC cells, indicating that Cabazitaxel could possibly enhance the radiosentivity of CRPC cells. However, the detailed molecular mechanism remained to be further elucidated.
PI3K/AKT pathway is one of the most significant intracellular pathways with regulatory effects on basic intracellular functions, such as growth, motility, survival, metabolism and angiogenesis [39]. Activation of PI3K/AKT pathway contributes to tumor development and tumor resistance to anti-cancer therapies and treatment [40]. Previous studies have confirmed the association between the inhibition of PI3K/AKT pathway and the increased radiosentivity of different cancer cells, such as oesophageal carcinoma cells, laryngeal squamous cell carcinoma (LSCC) cells, and ovarian cancer cells [41-43]. It has also been unveiled that Cabazitaxel could result in the death of lung adenocarcinoma cells by inducing autophagy via targeting PI3K/AKT pathway [16]. Nevertheless, the interaction between Cabazitaxel and PI3K/AKT pathway in CRPC remained to be addressed. In our study, we discovered that both the phosphorylation levels of PI3K and AKT in CRPC cells were decreased after Cabazitaxel treatment, and furthermore, Cabazitaxel could also down-regulate the expressions of p-PI3K and p-AKT which were previously up-regulated by IR induction. These results suggest that Cabazitaxel could exert its effects on CRPC cells, which might be related to the suppression of PI3K/AKT pathway.
To conclude, this study found that Cabazitaxel could suppress the proliferation and survival and promote apoptosis of CRPC cells, and it enhanced the effects of IR on CRPC cells, which might be related to the suppression of PI3K/AKT pathway. Our study provided novel lines of evidence supporting the efficacy of Cabazitaxel and the involvement of PI3K/AKT pathway in CRPC cells. The limitation of this study was the lack of detecting how Cabazitaxel affected PI3K/AKT pathway activation, either directly or indirectly, and whether Cabazitaxel can be a therapeutic method for CRPC in clinical practice, which would be studied in future. We hope that our current findings can contribute to the understanding of the effectiveness of Cabazitaxel in CRPC treatment and to the development of therapeutic methods for CRPC.
Acknowledgements
This work was supported by the Jiangsu Provincial Project for Young Medical Talents [QNRC2016072].
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bonfill X, Arevalo-Rodriguez I, Martinez Garcia L, Quintana MJ, Buitrago-Garcia D, Lobos Urbina D, Cordero JA. Intermittent androgen deprivation therapy: recommendations to improve the management of patients with prostate cancer following the GRADE approach. Cancer Manag Res. 2018;10:2357–2367. doi: 10.2147/CMAR.S164856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Q, Song Z, Ruan H, Wang C, Yang X, Bao L, Wang K, Cheng G, Xu T, Xiao W, Xiong Z, Liu D, Yang M, Zhou D, Yang H, Chen K, Zhang X. Targeting the KIF4A/AR axis to reverse endocrine therapy resistance in castration-resistant prostate cancer. Clin Cancer Res. 2020;26:1516–1528. doi: 10.1158/1078-0432.CCR-19-0396. [DOI] [PubMed] [Google Scholar]
- 4.Barnard M, Mostaghel EA, Auchus RJ, Storbeck KH. The role of adrenal derived androgens in castration resistant prostate cancer. J Steroid Biochem Mol Biol. 2020;197:105506. doi: 10.1016/j.jsbmb.2019.105506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, Kopka K, Apostolidis C, Haberkorn U, Morgenstern A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Liu J, Cheng L, Li C, Zhang Z, Bai Y, Wang R, Han T, Huang C, Kong Y, Feng F, Liu X. Inhibition of noncanonical Wnt pathway overcomes enzalutamide resistance in castration-resistant prostate cancer. Prostate. 2020;80:256–266. doi: 10.1002/pros.23939. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida S, Takahara T, Arita Y, Ishii C, Uchida Y, Nakagawa K, Toda K, Sakamoto T, Kijima T, Yokoyama M, Ishioka J, Matsuoka Y, Saito K, Yoshimura R, Fujii Y. Progressive site-directed therapy for castration-resistant prostate cancer: localization of the progressive site as a prognostic factor. Int J Radiat Oncol Biol Phys. 2019;105:376–381. doi: 10.1016/j.ijrobp.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Chen J, Liu W, Liang C, Hu H, Huang J. Targeting androgen receptor-independent pathways in therapy-resistant prostate cancer. Asian J Urol. 2019;6:91–98. doi: 10.1016/j.ajur.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abidi A. Cabazitaxel: a novel taxane for metastatic castration-resistant prostate cancer-current implications and future prospects. J Pharmacol Pharmacother. 2013;4:230–237. doi: 10.4103/0976-500X.119704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vrignaud P, Semiond D, Benning V, Beys E, Bouchard H, Gupta S. Preclinical profile of cabazitaxel. Drug Des Devel Ther. 2014;8:1851–1867. doi: 10.2147/DDDT.S64940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarvis C, Nelius T, Martinez-Marin D, Sennoune SR, Filleur S. Cabazitaxel regimens inhibit the growth of prostate cancer cells and enhances the anti-tumor properties of PEDF with various efficacy and toxicity. Prostate. 2018;78:905–914. doi: 10.1002/pros.23647. [DOI] [PubMed] [Google Scholar]
- 12.Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res. 2015;5:1602–1609. [PMC free article] [PubMed] [Google Scholar]
- 13.Pompura SL, Dominguez-Villar M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J Leukoc Biol. 2018 doi: 10.1002/JLB.2MIR0817-349R. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Zhou L, Wu X, Li R, Wen J, Sha J, Wen X. The PI3K/AKT pathway in the pathogenesis of prostate cancer. Front Biosci (Landmark Ed) 2016;21:1084–1091. doi: 10.2741/4443. [DOI] [PubMed] [Google Scholar]
- 15.Singh SK, Apata T, Gordetsky JB, Singh R. Docetaxel combined with thymoquinone induces apoptosis in prostate cancer cells via inhibition of the PI3K/AKT signaling pathway. Cancers (Basel) 2019;11:1390. doi: 10.3390/cancers11091390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huo R, Wang L, Liu P, Zhao Y, Zhang C, Bai B, Liu X, Shi C, Wei S, Zhang H. Cabazitaxel-induced autophagy via the PI3K/Akt/mTOR pathway contributes to A549 cell death. Mol Med Rep. 2016;14:3013–3020. doi: 10.3892/mmr.2016.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskra JN, Schlicht MJ, Bosland MC. Effects of black raspberries and their ellagic acid and anthocyanin constituents on taxane chemotherapy of castration-resistant prostate cancer cells. Sci Rep. 2019;9:4367. doi: 10.1038/s41598-019-39589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao J, Jin H, Li S, Xu L, Peng Z, Wei G, Long J, Guo Y, Kuang M, Zhou Q, Peng S. Apatinib potentiates irradiation effect via suppressing PI3K/AKT signaling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:454. doi: 10.1186/s13046-019-1419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Bezawy R, Tinelli S, Tortoreto M, Doldi V, Zuco V, Folini M, Stucchi C, Rancati T, Valdagni R, Gandellini P, Zaffaroni N. miR-205 enhances radiation sensitivity of prostate cancer cells by impairing DNA damage repair through PKCε and ZEB1 inhibition. J Exp Clin Cancer Res. 2019;38:51. doi: 10.1186/s13046-019-1060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, De Bernardis G, Losa M, Mirenda M, Pasquini E, Rinaldi A, Sumanasuriya S, Lambros MB, Neeb A, Lucianò R, Bravi CA, Nava-Rodrigues D, Dolling D, Prayer-Galetti T, Ferreira A, Briganti A, Esposito A, Barry S, Yuan W, Sharp A, de Bono J, Alimonti A. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559:363–369. doi: 10.1038/s41586-018-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villanueva C, Bazan F, Kim S, Demarchi M, Chaigneau L, Thiery-Vuillemin A, Nguyen T, Cals L, Dobi E, Pivot X. Cabazitaxel: a novel microtubule inhibitor. Drugs. 2011;71:1251–1258. doi: 10.2165/11591390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wülfing C, Kramer G, Eymard JC, Bamias A, Carles J, Iacovelli R, Melichar B, Sverrisdóttir Á, Theodore C, Feyerabend S, Helissey C, Ozatilgan A, Geffriaud-Ricouard C, Castellano D. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506–2518. doi: 10.1056/NEJMoa1911206. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T, Armstrong AJ. The who, what, and how of cabazitaxel treatment in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2017;35:3175–3177. doi: 10.1200/JCO.2017.74.7931. [DOI] [PubMed] [Google Scholar]
- 24.Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J, Marino M, Martinez-Chantar ML, Nawroth R, Sanchez-Garcia I, Sharma D, Saxena NK, Singh N, Vlachostergios PJ, Guo S, Honoki K, Fujii H, Georgakilas AG, Bilsland A, Amedei A, Niccolai E, Amin A, Ashraf SS, Boosani CS, Guha G, Ciriolo MR, Aquilano K, Chen S, Mohammed SI, Azmi AS, Bhakta D, Halicka D, Keith WN, Nowsheen S. Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin Cancer Biol. 2015;35(Suppl):S25–S54. doi: 10.1016/j.semcancer.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 2016;8:603–619. doi: 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warren CFA, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10:177. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edlich F. BCL-2 proteins and apoptosis: recent insights and unknowns. Biochem Biophys Res Commun. 2018;500:26–34. doi: 10.1016/j.bbrc.2017.06.190. [DOI] [PubMed] [Google Scholar]
- 28.Tirapelli D, Lustosa IL, Menezes SB, Franco IM, Rodrigues AR, Peria FM, Marinho A, Serafini LN, Carlotti CG Jr, Tirapelli LF. High expression of XIAP and Bcl-2 may inhibit programmed cell death in glioblastomas. Arq Neuropsiquiatr. 2017;75:875–880. doi: 10.1590/0004-282X20170156. [DOI] [PubMed] [Google Scholar]
- 29.Kaya-Aksoy E, Cingoz A, Senbabaoglu F, Seker F, Sur-Erdem I, Kayabolen A, Lokumcu T, Sahin GN, Karahuseyinoglu S, Bagci-Onder T. The pro-apoptotic Bcl-2 family member Harakiri (HRK) induces cell death in glioblastoma multiforme. Cell Death Discov. 2019;5:64. doi: 10.1038/s41420-019-0144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuo J, Haga S, Hashimoto K, Okubo T, Ozawa T, Ozaki M, Yamaguchi H. Activation of caspase-3 during chlamydia trachomatis-induced apoptosis at a late stage. Can J Microbiol. 2019;65:135–143. doi: 10.1139/cjm-2018-0408. [DOI] [PubMed] [Google Scholar]
- 32.Xiong Y, Jin E, Yin Q, Che C, He S. Boron attenuates heat stress-induced apoptosis by inhibiting endoplasmic reticulum stress in mouse granulosa cells. Biol Trace Elem Res. 2020;199:611–621. doi: 10.1007/s12011-020-02180-1. [DOI] [PubMed] [Google Scholar]
- 33.Kesäniemi J, Lavrinienko A, Tukalenko E, Boratyński Z, Kivisaari K, Mappes T, Milinevsky G, Møller AP, Mousseau TA, Watts PC. Exposure to environmental radionuclides associates with tissue-specific impacts on telomerase expression and telomere length. Sci Rep. 2019;9:850. doi: 10.1038/s41598-018-37164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Q, Ma J, Sun J, Yang L, Yang F, Zhang W, Li R, Wang L, Wang Y, Wang H. Genistein and AG1024 synergistically increase the radiosensitivity of prostate cancer cells. Oncol Rep. 2018;40:579–588. doi: 10.3892/or.2018.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang L, Wei F, Wu Y, He Y, Shi L, Xiong F, Gong Z, Guo C, Li X, Deng H, Cao K, Zhou M, Xiang B, Li X, Li Y, Li G, Xiong W, Zeng Z. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J Exp Clin Cancer Res. 2018;37:87. doi: 10.1186/s13046-018-0758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang L, Graham P, Hao J, Ni J, Deng J, Bucci J, Malouf D, Gillatt D, Li Y. Cancer stem cells and signaling pathways in radioresistance. Oncotarget. 2016;7:11002–11017. doi: 10.18632/oncotarget.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi J, Yoon YN, Kim N, Park CS, Seol H, Park IC, Kim HA, Noh WC, Kim JS, Seong MK. Predicting radiation resistance in breast cancer with expression status of phosphorylated S6K1. Sci Rep. 2020;10:641. doi: 10.1038/s41598-020-57496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunos CA, Stefan T, Jacobberger JW. Cabazitaxel-induced stabilization of microtubules enhances radiosensitivity in ovarian cancer cells. Front Oncol. 2013;3:226. doi: 10.3389/fonc.2013.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125–132. doi: 10.1016/j.semcancer.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Yang XX, Ma M, Sang MX, Zhang XY, Liu ZK, Song H, Zhu SC. BMI-1 suppression increases the radiosensitivity of oesophageal carcinoma via the PI3K/Akt signaling pathway. Oncol Rep. 2018;39:667–678. doi: 10.3892/or.2017.6136. [DOI] [PubMed] [Google Scholar]
- 42.Tang T, Xiao ZY, Shan G, Lei HB. Descending-SHIP2-mediated radiosensitivity enhancement through PI3K/Akt signaling pathway in laryngeal squamous cell carcinoma. Biomed Pharmacother. 2019;118:109392. doi: 10.1016/j.biopha.2019.109392. [DOI] [PubMed] [Google Scholar]
- 43.Deng J, Bai X, Feng X, Ni J, Beretov J, Graham P, Li Y. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer. 2019;19:618. doi: 10.1186/s12885-019-5824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]