Abstract
Objective: The aim of this prospective study was to explore the clinical efficacy of Weisu granules combined with Weifuchun tablets in the treatment of chronic atrophic gastritis and its effect on serum gastrin-17 (G-17), pepsinogen I (PG I), and II (PG II) levels. Methods: Totally, 120 patients with chronic atrophic gastritis admitted to our hospital from February 2019 to February 2020 were enrolled and randomized into a control group (n=60) treated with Weifuchun tablets, and a experimental group given Weisu granules. Serum G-17, PG I, and PG II levels, inflammatory factor levels, TCM syndrome scores, gastric mucosa pathological scores, and clinical efficacy were compared between the two groups. Gastric tissue changes were observed using gastroscopy and HE staining. Results: After treatment, the levels of serum G-17, PG I, and PG II of the experimental group were significantly better than those of the control group (P<0.001). The levels of inflammatory factors, traditional Chinese medicine (TCM) syndrome scores, and gastric mucosal pathology scores of the experimental group were significantly lower than those of the control group (P<0.001). The overall response rate of the experimental group was significantly higher than that of the control group (P<0.05). The experimental group showed a lower HP positive result and a higher HP negative conversion ratio than the control group (all P<0.05). HE staining results revealed that after treatment, the number of glands was basically restored to the level of normal gastric mucosa, and the improvement of inflammatory cell infiltration in the experimental group was significantly better than that in the control group. Conclusion: Weisu granule combined with Weifuchun tablets can ameliorate serum G-17, PG I, and PG II levels in patients with chronic atrophic gastritis, relieve inflammatory responses and clinical symptoms, and improve the treatment effect, which is worth promoting in clinical practice. Clinical trial registration: Chinese Registry of Clinical Trials. Trial Registration Number: ChiCTR200002548416. Trial URL: http://www.chictr.org.cn/showproj.aspx?proj=26516901.
Keywords: Weisu granules, Weifuchun tablets, chronic atrophic gastritis
Introduction
Chronic atrophic gastritis is pathologically characterized by atrophy of glands of the gastric mucosa, occasionally accompanied by intestinal metaplasia and dysplasia. The clinical manifestations are swelling and pain in the upper abdomen and loss of appetite, and improper treatment may result in increasingly thinner gastric mucosa with less protective functions. Chronic atrophic gastritis has been classified as a precancerous disease by the World Health Organization (WHO) [1]. Severe dysplasia may contribute to an increase in the canceration rate of the disease to more than 80% [2], which seriously endangers the life and health of patients. Clinical studies have shown that the holistic treatment and syndrome differentiation of traditional Chinese medicine (TCM) have unique advantages in the treatment of digestive system diseases [3-5]. Helicobacter pylori (HP) infection is a major cause for chronic atrophic gastritis, for which quadruple therapy is a common treatment method. However, the increase in HP drug resistance has led to an increasing number of adverse reactions, which reduces the eradication rate of HP and fails to ensure a promising treatment effect [6]. In TCM, chronic atrophic gastritis belongs to the category of “stomach illness”. In recent years, with the constant advancement of TCM, a variety of traditional Chinese herbal preparations have been used in the treatment of digestive system diseases, with the advantages of fewer adverse reactions and treatment of both symptoms and root causes. Reportedly, Weifuchun tablets, composed of red ginseng, isodoma methystoides, and citrus aurantium, can mitigate the levels of serum inflammatory factors in patients [7], and regulate the secretion of gastrointestinal hormones, which is conducive to alleviating the clinical symptoms of patients. In addition to Weifuchun tablets, the latest research has found that Weisu granules can enhance gastric mucosal secretion function, alleviate the pathological damage of gastric mucosa in patients, and accelerate the recovery of impairedgastric mucosa [8]. G-17 is a gastrointestinal hormone secreted by G cells of the digestive tract, which regulates the function of the digestive tract. It is a commonly used biochemical method for evaluating gastric function and is of great significance in the diagnosis of precancerous lesions. PG I and PG II are aspartic proteases secreted by gastric cells, which reflect the morphology and function of the gastric mucosa [9]. This study aimed to explore whether the combination of Weisu granules can increase the curative effect of gastric rejuvenation.
Materials and methods
Research design
As a prospective study, the study was conducted in our hospital from February 2019 to February 2020 to explore the clinical efficacy of Weisu Granules combined with Weifuchun tablets in the treatment of chronic atrophic gastritis and its effects on serum gastrin-17 (G-17), pepsinogen I (PG I), and II (PG II) levels.
Recruitment of research subjects
Totally, 120 patients with chronic atrophic gastritis admitted to our hospital from February 2019 to February 2020 were selected and randomized into a control group (n=60) treated with Weifuchun tablets and the experimental group given additional Weisu granules. Inclusion criteria: (1) Patients were diagnosed with chronic atrophic gastritis by gastroscopy, pathological examinations, and were in line with the Guiding Principles for Clinical Research of New Chinese Medicines (2002) [10] and the diagnostic criteria for chronic atrophic gastritis established by the 2000 Jinggangshan National Chronic Gastritis Symposium [11], with the carbon-14 (14C) urea breath test results showing Helicobacter pylori (HP) positive; (2) Patients aged 18-50 years; (3) Patients had complete clinical data. Exclusion criteria: (1) Patients were unable to communicate due to hearing impairment, language impairment, unconsciousness, or mental illness; (2) Patients withdrew from treatment, died, had the change of treatment plan, or lost follow-up; (3) Patients had incomplete clinical data; (4) Patients had severe organic disease, accompanied by severe gastric ulcer and gastric mucosal damage; (5) Patients had atypical hyperplasia or suspected cancer of the gastric mucosa; (6) Patients had recent use of glucocorticoid therapy; (7) Patients had a history of gastrointestinal surgery; (8) Patients had allergies to the studied drugs.
Steps
The sociodemographic data and clinical performance data of patients were collected on the day they agreed to participate in the study. After analysis, no statistical difference was found in general information between the two groups of patients (P>0.05), Table 1.
Table 1.
Comparison of general information of patients
| Groups | Experimental group (n=60) | Control group (n=60) | χ2/t | P |
|---|---|---|---|---|
| Gender | 0.034 | 0.855 | ||
| Male | 28 | 27 | ||
| Female | 32 | 33 | ||
| Age (year) | ||||
| Range | 30-56 | 32-54 | ||
| Mean age | 42.68±2.10 | 42.54±2.11 | 0.364 | 0.716 |
| Mean weight (kg) | 56.26±2.15 | 56.28±2.10 | 0.052 | 0.959 |
| BMI (kg/m2) | 21.99±1.98 | 22.00±1.68 | 0.030 | 0.976 |
| Disease course (year) | ||||
| range | 2-6 | 2-5 | ||
| Mean disease course | 3.97±1.12 | 3.92±0.90 | 0.270 | 0.788 |
| Atrophy degree | ||||
| Mild | 20 | 21 | 0.037 | 0.847 |
| Moderate | 22 | 23 | 0.036 | 0.850 |
| Severe | 18 | 16 | 0.164 | 0.685 |
| Marital status | 0.261 | 0.609 | ||
| Single | 10 | 8 | ||
| Married | 50 | 52 | ||
| Place of residence | 0.134 | 0.715 | ||
| Urban area | 32 | 30 | ||
| Rural area | 28 | 30 | ||
| Monthly income (yuan) | 0.133 | 0.715 | ||
| ≥4000 | 29 | 31 | ||
| <4000 | 31 | 29 | ||
| Living habit | ||||
| History of smoking | 32 | 30 | 0.134 | 0.715 |
| History of drinking | 35 | 34 | 0.034 | 0.853 |
| Educational background | 0.033 | 0.855 | ||
| High school and below | 32 | 31 | ||
| University and above | 28 | 29 |
Ethics statement
This study complies with the principles of the Declaration of Helsinki [12] and was approved by the ethical committee of the hospital. After the patients were recruited, the research team explained to them the purpose, significance, content, and confidentiality of the study, and asked them to sign an informed consent form. The protocol of this study was approved by the ethics committee of Nanjing University of Chinese Medicine Hospital (Approved No. of the ethics committee: EC-2018-1209).
Withdrawal criteria
Patients with the following situations were judged to be unsuitable for future experiments. In such a case, the medical records of patients were retained, but not analyzed: (1) adverse events or serious adverse events; (2) disease exacerbations during the experiment; (3) some serious complications; (4) unwilling to continue the clinical trial, and ask the research team for withdrawal from the study.
Methods
The control group was treated with Weifuchun tablets. The patients took Weifuchun tablets (Hangzhou Huqing Yutang Pharmaceutical Co., Ltd.; SFDA Approval No.: Z20040003) three times a day, 4 tablets each time, for 6 weeks.
The experimental group was additionally given Weisu granules. The patients took Weisu granules (Yangtzejiang Pharmaceutical Group Jiangsu Pharmaceutical Co., Ltd.; SFDA Approval No.: Z10930002) three times a day, 15 g each time, for 6 weeks.
Outcome measures
(1) General information: The general information, including the number of hospitalizations, name, gender, age, average body weight, BMI, course of disease, degree of shrinkage, marital status, place of residence, monthly income, living habits, and education level, was collected from all the patients.
(2) Serum G-17, PG II, and PG II levels: Fasting venous blood (5 mL) was drawn from each patient before and after treatment and centrifuged at 3500 r/min for 10 min to obtain the serum. The serum levels of G-17, PG I, and PG IIwere determined by enzyme-linked immunosorbent assay (ELISA, Enzyme-linked Biotechnology Co., Ltd., Shanghai). The product No. of the G-17 ELISA kit was ML037633, the product No. of the PG I ELISA kit was ML037658, and the product No. of PG II ELISA kit was ML037635.
(3) Inflammatory factor levels: Morning fasting venous blood (5 mL) was collected from each patient before and after treatment, and centrifuged at 3500 r/min for 10 min to obtain the serum. ELISA kits (Enzyme-linked Biotechnology Co., Ltd., Shanghai) were used to determine the serum levels of interleukin 1β (IL-1β, product No. ML064299), interleukin 6 (IL-6, product No. ML058097), and C-reactive protein (CRP, product No. ML220482).
(4) TCM syndrome scoring: Based on the Guiding Principles for Clinical Research of New Chinese Medicines [13], the patients’ chronic atrophic gastritis symptoms before and after treatment were quantitatively scored. According to the scores, the severity was divided into none (0 points), mild (1 point), moderate (2 points), and severe (3 points). A higher score indicates more severe symptoms. The main evaluation items include epigastric pain, bitter and dry mouth, stomach noises, and belching pantothenic acid.
(5) Gastric mucosal pathology score: The gastroscopy and pathological examination were performed before treatment and 1 week after treatment. The biopsy sites were the lesser curvature and the greater curvature of the gastric antrum, the angle of the stomach, and the lesser curvature of the lower part of the gastric corpus. According to the scores, the severity was divided into none (0 points), mild (1 point), moderate (2 points), and severe (3 points). Higher scores indicate more severe symptoms.
(6) Clinical efficacy: The treatment effect of patients was evaluated based on the Guiding Principles for Clinical Research of New Chinese Medicines (2002). The treatment outcome was determined to be cured if the patient’s physical signs disappeared after treatment, the mucosal inflammation turned to mild after gastroscopy, and the symptoms of glands atrophy, intestinal metaplasia, and dysplasia returned to normal or disappeared confirmed by histopathological examination. If the patient’s physical signs disappeared by more than 2/3, the mucosal inflammation was significantly alleviated after gastroscopy, and histopathological examination confirmed that the symptoms of gland atrophy, intestinal metaplasia, and intestinal dysplasia were reduced by 2 grades, it was classified as markedly effective. If the patient’s physical signs disappeared by more than 1/3, the scope of mucosal lesions on gastroscopy was reduced by more than 1/2, and the symptoms of gland atrophy, intestinal metaplasia, and dysplasia were reduced by one grade by histopathological examination, it was considered as effective. Ineffective was indicated as the failure to meet the above criteria.
Statistical analysis
The data analysis was performed using SPSS 20.0, and image rendering was conducted by GraphPad Prism 7 (GraphPad Software, San Diego, USA). The count data and measurement data were presented as (n, %) and (x̅±sd). Chi-squared test and t-test were adopted for analysis, respectively. P value <0.05 indicated that the difference was statistically significant.
Results
Comparison of general patient data
The general information, including gender, age, weight, body weight index (BMI), disease course, marital status, residence, income, living habits, and educational background were collected in all patients. For atrophy degree, there were 20 cases of mild, 22 cases of moderate, and 18 cases of severe in the experimental group, and 21, 23, and 26 cases in the control group, respectively. There was no statistical difference in general information and atrophy degree between the two groups (P>0.05), as shown in Table 1.
Comparison of serum G-17, PG I, and PG II levels in patients
The serum G-17 level was comparable between the experimental group and the control group before treatment (270.68±12.10 vs. 269.68±14.20, P>0.05); after treatment, it was significantly higher in the experimental group (382.68±15.68 vs. 321.68±16.98, P<0.001), as shown in Figure 1. The serum PG I level was comparable between the experimental group and the control group before treatment (56.12±5.68 vs. 55.96±5.12, P>0.05); after treatment, it was significantly higher in the experimental group (90.68±4.68 vs. 69.24±4.35, P<0.001), as shown in Figure 2. The serum PG II level was comparable between the experimental group and the control group before treatment (15.98±1.12 vs. 16.00±1.14, P>0.05); after treatment, it was significantly higher in the experimental group (11.10±1.10 vs. 13.42±1.23, P<0.001), as shown in Figure 3.
Figure 1.
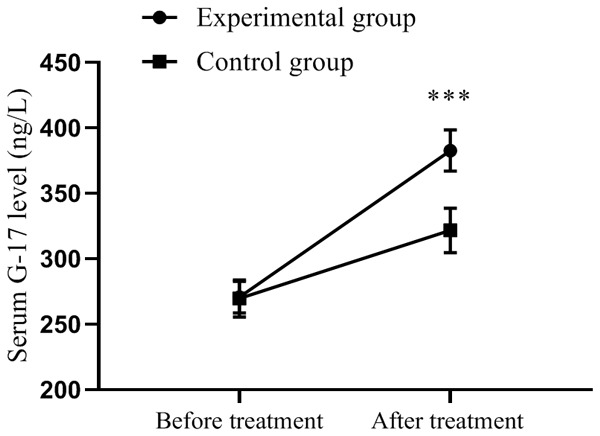
Comparison of serum G-17 levels in patients (x̅±sd, ng/L). Note: The horizontal axis indicates before treatment and after treatment, and the vertical axis is serum G-17 level (ng/L). The dotted line represents the experimental group, and the square line represents the control group. *** indicates P<0.001, experimental group vs. control group by two independent sample t-test.
Figure 2.
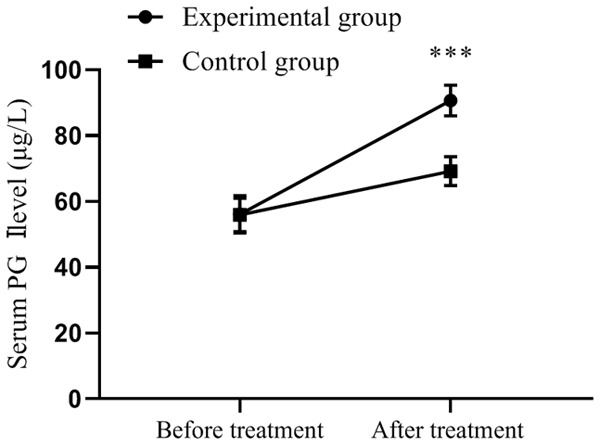
Comparison of serum PG I levels in patients (x̅±sd, μg/L). Note: The horizontal axis from left to right represents before and after treatment, and the vertical axis is serum PG I level (μg/L). The dotted line represents the experimental group, and the square line represents the control group. *** indicates P<0.001, experimental group vs. control group by two independent sample t-test.
Figure 3.
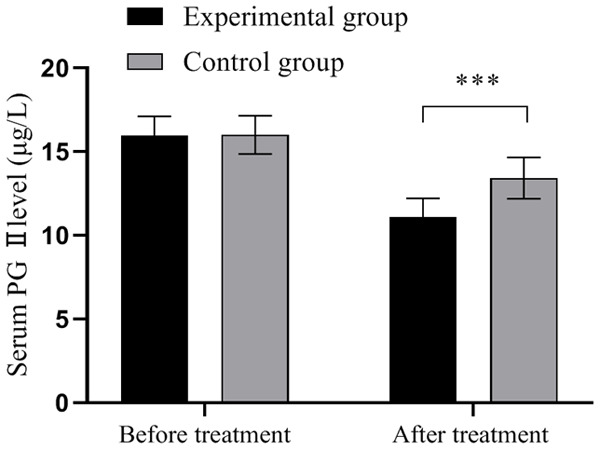
Comparison of serum PG II levels in patients (x̅±sd, μg/L). Note: The horizontal axis is from left to right before and after treatment, and the vertical axis is the serum PG II level (μg/L). The black area represents the experimental group, and the gray area represents the control group. *** indicates P<0.001, experimental group vs. control group by two independent sample t-test.
Comparison of the levels of inflammatory factors in patients
Before treatment, the serum levels of IL-1β, IL-6, and CRP were comparable between the two groups (all P>0.05). After treatment, the above items were decreased in all patients and significantly lower in the experimental group than those in the control group (P<0.001), as shown in Table 2.
Table 2.
Comparison of the levels of inflammatory factors in patients (x̅±sd)
| Groups | Experimental group (n=60) | Control group (n=60) | t | P |
|---|---|---|---|---|
| IL-1β (ng/L) | ||||
| Before treatment | 130.68±10.52 | 131.25±10.54 | 0.296 | 0.767 |
| After treatment | 28.21±4.23 | 70.68±5.68 | 46.451 | <0.001 |
| IL-6 (ng/L) | ||||
| Before treatment | 152.14±12.24 | 152.68±12.10 | 0.243 | 0.808 |
| After treatment | 35.98±3.58 | 69.12±5.41 | 39.570 | <0.001 |
| CRP (mg/dl) | ||||
| Before treatment | 10.12±1.05 | 10.15±1.06 | 0.156 | 0.877 |
| After treatment | 2.41±0.65 | 5.89±0.41 | 35.076 | <0.001 |
Comparison of TCM syndrome scores of patients
Before treatment, the TCM syndrome scores of pain in the gastric cavity, bitter and dry mouth, stomach noises, and belching pantothenic acid were comparable between the two groups (all P>0.05). All the TCM syndrome scores of the above items were decreased and the experimental group yielded significantly lower scores than those of the control group (P<0.001), as shown in Table 3.
Table 3.
Comparison of TCM syndrome scores of patients (x̅±sd)
| Groups | Experimental group (n=60) | Control group (n=60) | t | P |
|---|---|---|---|---|
| Pain in the gastric cavity | ||||
| Before treatment | 2.89±0.10 | 2.88±0.11 | 0.521 | 0.603 |
| After treatment | 0.98±0.12 | 2.10±0.11 | 53.293 | <0.001 |
| Bitter and dry mouth | ||||
| Before treatment | 2.05±0.15 | 2.03±0.12 | 0.806 | 0.422 |
| After treatment | 0.54±0.08 | 1.80±0.10 | 76.212 | <0.001 |
| Stomach noises | ||||
| Before treatment | 1.98±0.12 | 1.96±0.13 | 0.876 | 0.383 |
| After treatment | 0.41±0.08 | 1.00±0.56 | 8.079 | <0.001 |
| Belching pantothenic acid | ||||
| Before treatment | 2.54±0.12 | 2.56±0.13 | 0.876 | 0.383 |
| After treatment | 0.68±0.09 | 1.72±0.12 | 53.705 | <0.001 |
Comparison of gastric mucosal pathological scores of patients
Before treatment, there were no significant differences in pathological scores of the gastric mucosa of atrophy, intestinal metaplasia, and dysplasia between the two groups (all P>0.05). After treatment, the gastric mucosal pathology scores were decreased in the two groups, and were lower in the experimental group than those of the control group (P<0.001), as shown in Table 4. Gastroscopic observation results revealed a thinner gastric mucosa with reduced glandular atrophy and alleviated inflammation before treatment (Figure 4A, 4C). After treatment, the atrophy of gastric mucosal glands was relieved (Figure 4B, 4D). The improvement of gastric mucosal glandular atrophy was significantly better in the experimental group than that in the control group after treatment. HE staining results revealed loosely arranged tissue with inflammatory cell infiltration before treatment (Figure 5A, 5C). After treatment, the number of glands was restored to the level of the normal gastric mucosa (Figure 5B, 5D). The improvement of inflammatory cell infiltration in the experimental group was significantly better than that in the control group after treatment.
Table 4.
Comparison of pathological scores of gastric mucosa of patients (x̅±sd)
| Groups | Experimental group (n=60) | Control group (n=60) | t | P |
|---|---|---|---|---|
| Atrophy | ||||
| Before treatment | 4.89±0.54 | 4.90±0.56 | 0.100 | 0.921 |
| After treatment | 1.98±0.12 | 3.42±0.45 | 23.950 | <0.001 |
| Intestinal metaplasia | ||||
| Before treatment | 2.98±0.35 | 2.96±0.30 | 0.336 | 0.737 |
| After treatment | 0.99±0.12 | 1.89±0.23 | 26.873 | <0.001 |
| Dysplasia | ||||
| Before treatment | 3.12±0.23 | 3.10±0.25 | 0.456 | 0.649 |
| After treatment | 0.96±0.08 | 2.10±0.20 | 40.994 | <0.001 |
Figure 4.
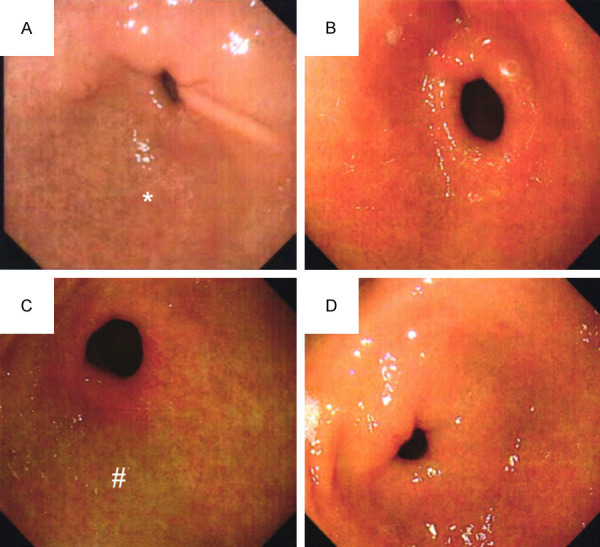
Typical pictures of gastroscopy. Note: (A) Indicated gastroscopy picture of the experimental group before treatment, while (B) Indicated gastroscopy picture of the experimental group after treatment. (C) Indicated gastroscopy picture of the control group before treatment, while (D) Indicated a gastroscopy picture of the control group after treatment. * Mucosa atrophy and submucosal vascular penetration, # The surface of mucous membrane is rough and uneven, and erosion is visible.
Figure 5.
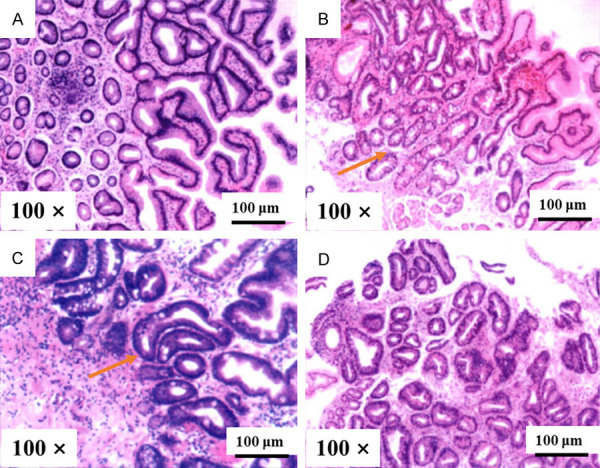
Typical pathological pictures of the gastric mucosa (HE stain, Magnified ×100). Note: (A) Indicated a pathological picture of the experimental group before treatment, while (B) Indicated a pathological picture of the experimental group after treatment. (C) Indicated a pathological picture of the control group before treatment, while (D) Indicated a pathological picture of the control group after treatment. The red arrows indicated markedly reduced glands.
Comparison of clinical efficacy of patients
In the experimental group, there were 16 cases of cured, 24 cases of markedly effective, 18 cases of effective, and 2 cases of ineffective, with an overall response rate of 96.7% (58/60). In the control group, there were 10 cases of cured, 12 cases of markedly effective, 26 cases of effective, and 12 cases of ineffective, with an overall response rate of 80.0% (48/60). The overall response rate of the experimental group was significantly higher than that of the control group (P<0.05), as shown in Table 5.
Table 5.
Comparison of clinical efficacy of patients [n (%)]
| Groups | Cured | Markedly effective | Effective | Ineffective | Overall response rate |
|---|---|---|---|---|---|
| Experimental group | 16 (26.7) | 24 (40.0) | 18 (30.0) | 2 (3.3) | 58 (96.7) |
| Control group | 10 (16.7) | 12 (20.0) | 26 (43.3) | 12 (20.0) | 48 (80.0) |
| χ2 | 1.768 | 5.714 | 2.297 | 8.086 | 8.086 |
| P | 0.184 | 0.017 | 0.130 | 0.004 | 0.004 |
Comparison of HP cure rate of patients
Before treatment, there were 38 HP positive cases and 22 HP negative cases in the experimental group, while 34 HP positive cases and 26 HP negative cases were in the control group. There was no difference in the HP infection rate between the two groups (P>0.05). After treatment, there were 4 HP positive cases and 56 HP negative cases in the experimental group, while 12 HP positive cases and 48 HP negative cases were in the control group. The experimental group had a lower HP positive result and a higher HP negative conversion ratio than the control group (all P<0.05). See Table 6.
Table 6.
Comparison of HP cure rate of patients
| Groups | Beforetreatment | Aftertreatment | HP negative conversion ratio | ||
|---|---|---|---|---|---|
|
|
|
||||
| HP positive | HP negative | HP positive | HP negative | ||
| Experimental group (n=60) | 38 | 22 | 4 | 56 | 34/38 |
| Control group (n=60) | 34 | 26 | 12 | 48 | 22/34 |
| χ2 | 0.556 | 4.615 | 6.369 | ||
| P | 0.456 | 0.032 | 0.012 | ||
Discussion
Chronic atrophic gastritis refers to the atrophy of the inherent glands of gastric mucosa after repeated injury of the gastric mucosa epithelium, with clinical presentations of gastric distending pain, acid reflux, heartburn, loss of appetite, and the pathological manifestations of decreased gastric mucosal thickness and gastric acid secretion. Some patients also experience intestinal metaplasia and dysplasia [14,15]. The WHO classified chronic atrophic gastritis as a precancerous lesion of gastric cancer. Studies have shown that the incidence of canceration in simple chronic atrophic gastritis is 0.2% and that in patients with dysplasia ranges from 2.5% to 83.0% [16-18], which seriously endangers the lives and health of patients. Currently, with the popularization of gastroscopy, the clinical detection rate of chronic atrophic gastritis has increased, accounting for about 10.0% of the total gastroscopy [19]. However, the complicated pathogenesis hinders the improvement of the treatment effect on the disease. Although the academic community has not yet fully clarified its pathogenic factors, it is believed that biological factors, physical factors, chemical factors, and immune factors are all predisposing factors [20]. From the perspective of TCM, the onset of chronic atrophic gastritis is mainly ascribed to the weakness of the spleen and stomach, and poor transport and transformation, resulting in internal resistance to damp turbidity, stagnation of qi, and stasis induced by chronic illness [21]. Therefore, the treatment should be aimed at invigorating the spleen, replenishing qi, promoting blood circulation, and detoxification, to mitigate the deficiency, toxin, and blood stasis. The main ingredients of Weifuchun tablets selected in this study are red ginseng, isodoma methystoides, and citrus aurantium. Red ginseng, the monarch drug, can nourish qi and invigorate the spleen, contribute to the digestion of intake, and regulate the circulation of qi, which can stabilize the acquired foundation of patients. Isadora methystoides is a ministerial drug, which has the effects of clearing heat and regulating qi, promoting blood circulation, and removing blood stasis. Citrus aurantium can relieve vomiting and invigorate the stomach, which is beneficial to alleviate the symptoms of patients [22]. From the perspective of modern pharmacology, red ginseng is rich in ginseng total saponins, ginseng monomer saponins CK, Rd, and red ginseng acid polysaccharides [21], which can inhibit leukopenia, enhance the activity of interleukins, and improve the immune function of patients. In addition, the methanol extract from the whole herb of isodoma methystoides possesses a strong inhibitory effect on the NO and prostaglandin E2 produced by RAW264.7 cells induced by lipopolysaccharides [22], and its diterpene components can also inhibit the amino acid end. Askinase serves to regulate the inflammation of the nervous system, and isodoma methystoides have a strong anti-inflammatory effect. Overall, Weifuchun tablets can accelerate the rate of gastric mucosal regeneration, regulate blood circulation at the lesion site, and prevent the accumulation of inflammatory factors [23]. The study found that the TCM syndrome scores were improved in both groups of patients after treatment, indicating that chronic atrophic gastritis was alleviated.
In this study, the levels of inflammatory factors in the experimental group were significantly lower than those in the control group, suggesting that Weisu granules can alleviate the inflammatory response of patients, thereby inhibiting the progression of the disease and reducing the damage to the gastric mucosa. In the experimental group, Weisu granules, mainly composed of perilla stem, Cyperusrotundus, tangerine peel, citron, Fructus Citri Sarcodactylis, citrus aurantium, betel nut, and stir-fried chicken’s gizzard-membrane, were added based on Weifuchun tablets. Perilla stem can soothe the liver and promote blood circulation, Cyperusrotundus can regulate the qi in a wide range, the tangerine peel can invigorate the spleen and stomach, and Fructus Citri Sarcodactylis can reduce phlegm and swelling. The combination of the above various herbal medicines can regulate qi [24]. The research of Abdulhassan et al. [24] suggests that perilla stems are rich in oleanolic acid, a broad-spectrum antibacterial drug, which can effectively treat diseases such as bronchitis and acute gastroenteritis, and is in line with the present study. In this study, the gastric mucosal pathology score of the experimental group was significantly lower than that of the control group. Previous studies pointed out that the Cyperusrotundus in Weisu granules can exert the oil’s effect on the isolated intestinal tube and reduce the intestinal smooth muscle spasm of patients, thereby alleviating the clinical symptoms of patients after treatment. Zhe et al. treated patients with HP in the research group with Weiss granules and found a higher HP eradication rate of patients in this group after treatment [25], indicating that Weisu granules can regulate gastric secretion and accelerate intestinal peristalsis. The present study also found that serum G-17, PG I, and PG II levels as well as gastric mucosal pathology scores of the experimental group after treatment were significantly better than those of the control group, confirming an improved gastric mucosal secretion function of the patients. G-17 is affected by the HP value of patients. In those with atrophy of the gastric corpus, the oxyntic glands are reduced and the stomach is in a state of low acidity, resulting in abnormal G-17 levels. In general, patients with early gastric cancer have aberrant levels of-17. PG I and PG II are also common indicators of gastric function, which are closely related to the degree of inflammatory in the patients. The improvement of PG I and PG II levels indicates an ameliorated gastric mucosal secretion function of patients and alleviated pathological conditions. Therefore, the comprehensive curative effect of the experimental group is significantly better than that of the control group. There were still the following limitations identified in the study. No control group was established for conventional western medical treatment. It is a monocentric study with a small number of participants and a short follow-up. A randomized controlled study with a large sample is needed to confirm this conclusion further.
In summary, the combination of Weisu granules and Weifuchun tablets can ameliorate serum G-17, PG I, and PG II levels in patients with chronic atrophic gastritis, alleviate inflammatory responses and clinical symptoms, and improve the treatment effect.
Disclosure of conflict of interest
None.
References
- 1.Holleczek B, Schttker B, Brenner H. Helicobacter pylori infection, chronic atrophic gastritis and risk of stomach and esophagus cancer: results from the prospective population-based ESTHER cohort study. Int J Cancer. 2020;146:2773–2783. doi: 10.1002/ijc.32610. [DOI] [PubMed] [Google Scholar]
- 2.Kanai M, Togo R, Ogawa T, Haseyama M. Chronic atrophic gastritis detection with a convolutional neural network considering stomach regions. World J Gastroenterol. 2020;26:3650–3659. doi: 10.3748/wjg.v26.i25.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian G, Wu C, Li J, Liang B, Zhang F, Fan X, Li Z, Wang Y, Li Z, Liu D, Lai-Han Leung E, Chen J. Network pharmacology based investigation into the effect and mechanism of modified sijunzi decoction against the subtypes of chronic atrophic gastritis. Pharmacol Res. 2019;144:158–166. doi: 10.1016/j.phrs.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Woodford AM, Chaudhry R, Conte GA, Gupta V, Anne M. Chronic atrophic gastritis presenting as hemolytic anemia due to severe vitamin B12 deficiency. Case Rep Hematol. 2021;2021:9571072. doi: 10.1155/2021/9571072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong S, Ye F, Dang Y, Hua Y, Zhang G. Association of MTHFR C677T polymorphism with severity and localization of chronic atrophic gastritis patients without Helicobacter pylori infection: a case control study. BMC Cancer. 2020;20:725. doi: 10.1186/s12885-020-07208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda K, Ohishi W, Cullings H, Fujiwara S, Suzuki G, Hayashi T, Mitsui F, Hida A, Ozasa K, Ito M, Chayama K, Tahara E. Modifying effect of chronic atrophic gastritis on radiation risk for noncardia gastric cancer according to histological type. Radiat Res. 2020;194:180–187. doi: 10.1667/RR15482.1. [DOI] [PubMed] [Google Scholar]
- 7.Pritchard DM, Crabtree JE. Helicobacter pylori and gastric cancer. Curr Opin Gastroenterol. 2006;22:620–5. doi: 10.1097/01.mog.0000245539.50765.f6. [DOI] [PubMed] [Google Scholar]
- 8.Pellicano R. Is chronic atrophic gastritis the missing link between atrophic glossitis, gastric parietal cell antibody positivity and hematological deficiencies? J Formos Med Assoc. 2020;119:1004–1005. doi: 10.1016/j.jfma.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Piazuelo MB, Camargo MC, Mera RM, Delgado AG, Peek RM Jr, Correa H, Schneider BG, Sicinschi LA, Mora Y, Bravo LE, Correa P. Eosinophils and mast cells in chronic gastritis: possible implications in carcinogenesis. Hum Pathol. 2008;39:1360–9. doi: 10.1016/j.humpath.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botezatu A, Bodrug N. Chronic atrophic gastritis: an update on diagnosis. Med Pharm Rep. 2021;94:7–14. doi: 10.15386/mpr-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kluger N, Mähönen K, Aitkoski A, Hiltunen E, Pankakoski A, Panelius J, Lappalainen K. Bullous pemphigoid-associated chronic atrophic gastritis. Dermatol Ther. 2020;33:e13671. doi: 10.1111/dth.13671. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhou A, Liu Y, Zhao Y, Zhang L, Sun L, Du S, Yang Q, Song X, Liang C, Ding X. Exploratory factor analysis for validating traditional Chinese syndrome patterns of chronic atrophic gastritis. Evid Based Complement Alternat Med. 2016;2016:6872890. doi: 10.1155/2016/6872890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Li C, Sun S, Cao Z, Chen J, Xiang H, Song L. Screening and identification of molecular targets involved in preventing gastric precancerous lesions in chronic atrophic gastritis by Qilianshupi decoction. Evid Based Complement Alternat Med. 2019;2019:5804710. doi: 10.1155/2019/5804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shafaie E, Saberi S, Esmaeili M, Karimi Z, Najafi S, Tashakoripoor M, Abdirad A, Hosseini ME, Mohagheghi MA, Khalaj V, Mohammadi M. Multiplex serology of Helicobacter pylori antigens in detection of current infection and atrophic gastritis-a simple and cost-efficient method. Microb Pathog. 2018;119:137–144. doi: 10.1016/j.micpath.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Jamil O, Sarwar S, Hussain Z, Fiaz RO, Chaudary RD. Association between functional dyspepsia and severity of depression. J Coll Physicians Surg Pak. 2016;26:513–6. [PubMed] [Google Scholar]
- 16.Fallahi P, Ferrari SM, Ruffilli I, Antonelli A. Reversible normalisation of serum TSH levels in patients with autoimmune atrophic gastritis who received L-T4 in tablet form after switching to an oral liquid formulation: a case series. BMC Gastroenterol. 2016;16:22. doi: 10.1186/s12876-016-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suna N, Etik D, Öcal S, Gündüz C, Açıkgöz A, Bildik İ, Gürsoy A, Kaşgöz İ, Tüleylioğlu H, Boyacıoğlu A. The effect of helicobacter pylori eradication on atrophic gastritis and intestinal metaplasia: a retrospective single center research. Acta Gastroenterol Belg. 2020;83:381–384. [PubMed] [Google Scholar]
- 18.Mattar R, Marques SB, Ribeiro IB, Visconti TAC, Funari M, DE Moura EGH. Diagnostic accuracy of gastropanel® for atrophic gastritis in Brazilian subjects and the effect of proton pump inhibitors. Arq Gastroenterol. 2020;57:154–160. doi: 10.1590/S0004-2803.202000000-29. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Jiang A, Qi B, Ma Z, Xiong Y, Dou J, Wang J. Resveratrol protects against helicobacter pylori-associated gastritis by combating oxidative stress. Int J Mol Sci. 2015;16:27757–69. doi: 10.3390/ijms161126061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong Y, Wang R, Liu X, Tian M, Wang Y, Cui Y, Zou W, Zhao Y. Zuojin pill ameliorates chronic atrophic gastritis induced by MNNG through TGF-β1/PI3K/Akt axis. J Ethnopharmacol. 2021;271:113893. doi: 10.1016/j.jep.2021.113893. [DOI] [PubMed] [Google Scholar]
- 21.Porter KM, Hoey L, Hughes CF, Ward M, Clements M, Strain J, Cunningham C, Casey MC, Tracey F, O’Kane M, Pentieva K, McAnena L, McCarroll K, Laird E, Molloy AM, McNulty H. Associations of atrophic gastritis and proton-pump inhibitor drug use with vitamin B-12 status, and the impact of fortified foods, in older adults. Am J Clin Nutr. 2021;114:1286–1294. doi: 10.1093/ajcn/nqab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papakonstantinou IP, Karakousis ND, Andreadis EA. Gastric neuroendocrine tumour, atrophic gastritis and autoimmune haemolyticanaemia: a case report and review. Scott Med J. 2019;64:154–158. doi: 10.1177/0036933019867574. [DOI] [PubMed] [Google Scholar]
- 23.Cittolin-Santos GF, Khalil S, Bakos JK, Baker K. Chronic atrophic gastritis with negative intrinsic factor and parietal cell antibody presenting as a severe hemolytic anemia. Case Rep Hematol. 2020;2020:8697493. doi: 10.1155/2020/8697493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila) 2009;2:353–60. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 25.Huang X, Lu B, Zhang S, Meng LN, Chen BB, Zhao M. Effect of Weifuchun on inhibiting inflammation of helicobacter pylori-infected GES-1 cells and NF-kappaB signaling pathway. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:450–4. [PubMed] [Google Scholar]


