Abstract
Objective: To explore the risk factors of postoperative cognitive dysfunction (POCD) in elderly patients with gastric cancer after radical resection and to establish a risk prediction model. Methods: A retrospective analysis of the clinicopathological data of 687 elderly patients who underwent radical gastric cancer surgery from January 2014 to January 2020 in the Third Department of Surgery, Fourth Hospital of Hebei Medical University was conducted. The degree of cognitive impairment was divided into POCD positive group (n=141, 20.52%) and POCD negative group (n=546, 79.48%). The general data of the two groups were compared. Multivariate logistic regression was used to analyze the risk factors for POCD in elderly gastric cancer patients after radical surgery. A risk prediction model was established. The receiver operating characteristic (ROC) curve was used to evaluate the effectiveness of the model. Results: Multivariate logistic regression analysis showed that preoperative ASA classification (OR=4.674, 95% CI: 1.610~12.651, P=0.020), age (OR=3.130, 95% CI: 1.307~8.669, P=0.001), operation time (OR=2.724, 95% CI: 1.232~7.234, P=0.031), preoperative PG-SGA score (OR=4.023, 95% CI: 1.011-10.883, P=0.048), and preoperative hemoglobin (OR=4.158, 95% CI: 2.255~8.227, P=0.001) were independent risk factors for POCD. Intraoperative application of dexmedetomidine (OR=0.172, 95% CI: 0.078~0.314, P=0.002) and maintaining a deeper anesthesia state (OR=0.151, 95% CI: 0.122~0.283, P=0.018) were protective factors. The area under the ROC curve of the POCD risk prediction model for elderly gastric cancer patients after surgery was 0.820 (95% CI: 0.742-0.899) (P<0.01). Conclusion: The occurrence of postoperative POCD in elderly patients with gastric cancer is closely related to a variety of risk factors. By establishing a risk prediction model for the occurrence of POCD, high-risk patients can be effectively identified during the perioperative period, to intervene earlier.
Keywords: Elderly gastric cancer, postoperative cognitive dysfunction, risk factors, risk prediction model
Introduction
Gastric cancer is one of the common malignant tumors of digestive tract in China. It ranks third among all malignant tumors [1]. As the population age increases, elderly patients with gastric cancer establish a slower metabolism and a declined immunity [2]. They are often accompanied with other basic diseases. The reserve function of heart, lung, and brain is poor [3,4]. The surgical risk and the incidence of postoperative complications are higher than those of non-elderly patients with gastric cancer [5-7]. At present, most studies focus on severe complications of patients, including anastomotic leakage, postoperative bleeding, and postoperative intestinal obstruction. Elderly patients with gastric cancer are more likely to have neurological symptoms compared to younger patients. Postoperative cognitive dysfunction is more common.
Postoperative cognitive dysfunction (POCD) refers to the central nervous system complications that occur after anesthesia or surgery with cognitive decline as the main manifestation. It is mainly manifested as the deterioration of mental activity, thinking awareness, personality behavior, and social skills [8-10]. POCD is more common in elderly patients, especially after major abdominal surgery. It can not only cause delays in postoperative recovery, longer hospital stays, and lower quality of life after surgery, but also increase medical expenses, which will affect long-term quality of life. In severe cases, it may even increase postoperative mortality [11,12]. POCD generally begins to appear about 2-7 days after surgery. Its occurrence may be closely related to many factors. The clinical diagnosis lacks specific laboratory indicators [13]. It is particularly important to find a predictive model that can better distinguish the occurrence of POCD in elderly patients with gastric cancer after radical resection.
This study retrospectively analyzed the clinicopathological features of elderly patients for the first time, to find the risk factors of POCD in elderly patients with gastric cancer after a radical operation. A predictive model was established. Its predictive value was tested to provide a reference for clinical prevention and treatment of POCD, to reduce the incidence of POCD in elderly patients with gastric cancer after the operation.
Materials and methods
General information
A retrospective analysis of the clinicopathological data of 687 elderly patients with gastric cancer who underwent radical surgery in the Fourth Hospital of Hebei Medical University from January 2014 to January 2020 was conducted.
Inclusion criteria
(1) Patients who were histopathologically confirmed gastric cancer; (2) Patients without distant organ metastasis as shown in preoperative imaging examination; (3) Patients without surgical contraindications, and were able to tolerate radical gastric cancer surgery; (4) Patients without previous anti-tumor drug treatment before surgery.
Exclusion criteria
(1) Patients complicated with preoperative mental and psychological diseases (including depression or Alzheimer’s disease); (2) Patients with language and communication disorders, and those with visual and hearing disorders; (3) Patients with the use of preoperative sedatives, psychiatric drugs such as antidepressants; (4) Patients with severe heart, lung, and kidney dysfunction; (5) Patients who were transferred to the intensive care unit after surgery due to coma or severe infection.
This study complies with the principles of the Declaration of Helsinki and related ethical requirements. All patients and their families signed an informed consent form. This trial was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (approval number: 2019012).
Method
POCD diagnosis
The whole group of patients was provided neuropsychological tests on the subjects 1 day before and 7 days after surgery and recorded the Mini Mental State Scale (MMSE) scores to determine whether the patients had postoperative cognitive dysfunction. The patients were divided into a POCD occurrence group and a POCD non-occurring group according to whether they suffer POCD. The specific content of the MMSE test [14,15] included time and place orientation, language (retelling, naming, and understanding instructions), mental arithmetic, immediate and short-term auditory vocabulary memory, and structural imitation. The full score is 30 points, and it takes 5-10 minutes. Different demarcation points were designated according to the Chinese version of MMSE based on different educational levels: 17 points for the illiterate group, 20 points for the elementary school group, 26 points for the middle school or above group, and cognitive impairment below the threshold. The scoring was done by two technicians who are experts in neuropsychological testing. The final clinical diagnosis was done by doctors who have received formal unified neuropsychological scale training.
Clinicopathological data
The following data were collected from patients: ① General information including gender, age, ECOG score, body mass index (BMI), and education level; ② Preoperative hemoglobin and serum albumin levels; ③ Tumor location and size; ④ Tumor histological type; ⑤ TNM staging; ⑥ Preoperative ASA classification; ⑦ Preoperative morbidity (hypertension, coronary heart disease, diabetes, cerebral infarction, and respiratory diseases); ⑧ Intraoperative operation (including operation method, operation time, whether or not blood transfusion); ⑨ Intraoperative anesthesia (including the depth of anesthesia, preoperative and intraoperative anesthesia related medications).
Statistical processing
The data was analyzed using SPSS21.0 statistical software. The measured data in accordance with the normal distribution was expressed as x±s, and the count data was expressed as the number of cases (percentage). The measured data between groups were compared by t test and the count data was by χ2 test. Single factor and multivariate unconditional Logistic regression analysis were used to screen the risk factors for POCD. P<0.05 indicated that the difference was statistically significant. The Hosmer-Lemeshow test and receiver operating characteristic (ROC) curve were used to detect the discrimination and goodness of fit of the model.
Results
Comparison of clinical data of enrolled patients
The patients in the whole group were 70-93 years old, with a median age of 77 years old. Among them, 453 (65.94%) were males and 234 (34.06%) were females. There were 141 cases (20.52%) that developed POCD on the seventh day after the operation, and 546 cases (79.48%) that did not develop POCD after the operation. The two groups of patients had statistically significant differences in age, education level, NRS2002 score, PG-SGA score, preoperative hemoglobin level, number of preoperative complications, and ASA classification (all P<0.05). There were no statistically significant differences in gender, MMSE score, ECOG score, body mass index, preoperative serum albumin level, pTNM stage, tumor location, tumor size, histological type, surgical method, and preoperative MMSE score (all P>0.05) (Table 1).
Table 1.
Comparison of basic clinical data of the two groups of patients
| Clinical date | POCD (N=141) | non-POCD (N=546) | X 2/t value | P value |
|---|---|---|---|---|
| Gender | 0.163 | 0.686 | ||
| Male | 95 | 358 | ||
| Female | 46 | 188 | ||
| Age (years old) | 82.5±5.3 | 75.1±4.1 | 9.478 | 0.001 |
| ECOG score (points) | 1.504 | 0.220 | ||
| 0~2 | 111 | 454 | ||
| >2 | 30 | 92 | ||
| BMI (Kg/m2) | 22.9±4.1 | 24.1±3.8 | 1.424 | 0.416 |
| NRS2002 score | 4.1±1.1 | 3.5±0.9 | 5.278 | 0.019 |
| PG-SGA score | 5.8±2.2 | 4.6±1.9 | 6.252 | 0.002 |
| Education level | 17.311 | 0.000 | ||
| illiteracy | 26 | 93 | ||
| primary school | 48 | 286 | ||
| Junior high school and above | 67 | 167 | ||
| Preoperative hemoglobin (g/L) | 99.2±8.2 | 117.1±9.7 | 12.464 | 0.000 |
| Preoperative albumin (g/L) | 39.8±3.2 | 41.1±4.1 | 2.243 | 0.086 |
| Number of concomitant diseases | 19.042 | 0.000 | ||
| 0~1 | 34 | 242 | ||
| ≥2 | 107 | 304 | ||
| Preoperative MMSE score | ||||
| illiteracy | 21.1±2.2 | 20.9±3.1 | 0.624 | 0.516 |
| primary school | 23.9±3.0 | 23.5±4.2 | 0.536 | 0.227 |
| Junior high school and above | 29.2±4.4 | 28.9±3.4 | 0.712 | 0.353 |
| ASA rating | 14.881 | 0.001 | ||
| Class I | 38 | 214 | ||
| Class II | 45 | 194 | ||
| Class III | 58 | 138 | ||
| Tumor location | 2.804 | 0.246 | ||
| Cardia-Fundus of Stomach | 64 | 212 | ||
| Stomach-Antrum | 68 | 281 | ||
| Whole stomach | 9 | 53 | ||
| Tumor size (cm) | 0.495 | 0.482 | ||
| <5 | 48 | 169 | ||
| ≥5 | 93 | 377 | ||
| TNM staging | 5.871 | 0.053 | ||
| I | 16 | 35 | ||
| II | 36 | 181 | ||
| III | 89 | 330 | ||
| Tumor histological type | 1.012 | 0.314 | ||
| High-moderate differentiation | 79 | 280 | ||
| Low-undifferentiated | 62 | 266 | ||
| Surgical approach | 0.760 | 0.383 | ||
| Open surgery | 100 | 407 | ||
| Laparoscopic surgery | 41 | 139 |
Single factor logistic regression analysis of risk factors for POCD in elderly patients with gastric cancer after radical resection
Elderly patients with gastric cancer are more susceptible to POCD under the circumstances of older age, higher education level, larger preoperative BMI, increased preoperative ASA grade, decreased preoperative hemoglobin level, preoperative or intraoperative anesthesia using atropine, intraoperative blood transfusion, prolonged operation time, and shallow depth of anesthesia (higher BIS value). The use of phenobarbital sodium and dexmedetomidine were the protective factors of POCD (Table 2).
Table 2.
Univariate unconditional logistic regression analysis of POCD in elderly patients with gastric cancer after radical resection
| Independent variable (assignment) | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|
| ASA classification (level) | 0.214 | 5.231 | 0.001 | 1.685 | 1.115~2.859 |
| age (years old) | 0.121 | 9.573 | 0.005 | 1.534 | 0.893~2.422 |
| BMI (kg/m2) | 0.188 | 8.153 | 0.005 | 1.597 | 1.323~2.681 |
| Education level (example) | 0.086 | 4.463 | 0.000 | 2.282 | 1.721~3.218 |
| NRS2002 score before operation | 0.286 | 17.216 | 0.029 | 2.148 | 1.622~2.768 |
| Preoperative PG-SGA score | 0.362 | 5.769 | 0.006 | 2.440 | 1.824~2.962 |
| Preoperative hemoglobin (g/L) | 0.581 | 4.415 | 0.034 | 0.687 | 0.423~0.968 |
| Preoperative albumin (g/L) | 0.379 | 6.721 | 0.002 | 1.880 | 1.547~2.539 |
| Number of concomitant diseases before operation (a) | 0.590 | 7.455 | 0.009 | 1.627 | 1.321~2.362 |
| Phenobarbital Sodium (Example) | 0.712 | 4.691 | 0.021 | 0.525 | 0.323~0.728 |
| Atropine (example) | 0.643 | 6.231 | 0.001 | 2.067 | 1.821~2.462 |
| Dexmedetomidine (example) | 0.781 | 11.409 | 0.027 | 0.687 | 0.455~0.997 |
| Depth of Anesthesia (BIS) | 0.378 | 9.781 | 0.001 | 1.861 | 1.514~2.715 |
| Operation time (min) | 0.553 | 12.891 | 0.000 | 1.739 | 1.389~2.555 |
| Whether blood transfusion during the operation | 0.678 | 5.098 | 0.042 | 0.511 | 0.358~0.867 |
Multivariate logistic regression analysis to determine independent risk factors affecting the onset of POCD
Multivariate logistic regression analysis showed that the higher preoperative ASA grade (OR=4.674, 95% CI: 1.610-12.651, P=0.020), the older age (OR=3.130, 95% CI: 1.307~8.669, P=0.001), the longer operation time (OR=2.724, 95% CI: 1.232~7.234, P=0.031), the higher preoperative PG-SGA score (OR=4.023, 95% CI: 1.011-10.883, P=0.048), and preoperative anemia (OR=4.158, 95% CI: 2.255~8.227, P=0.001) were independent risk factors for POCD. Intraoperative application of dexmedetomidine (OR=0.172, 95% CI: 0.078~0.314, P=0.002) and maintaining a deep anesthesia state (OR=0.151, 95% CI: 0.122~0.283, P=0.018) during the operation were protective factors (Table 3).
Table 3.
Multivariate unconditional logistic regression analysis of POCD in elderly patients with gastric cancer after radical resection
| Clinical factors | SE | Wald | P value | OR | 95% CI |
|---|---|---|---|---|---|
| ASA classification | 1.309 | 5.401 | 0.020 | 4.674 | 1.610~12.651 |
| age | 0.081 | 11.020 | 0.001 | 3.130 | 1.307~8.669 |
| PG-SGA score | 0.806 | 3.317 | 0.048 | 4.023 | 1.011~10.883 |
| hemoglobin (g/L) | 0.911 | 10.750 | 0.001 | 4.158 | 2.255~8.227 |
| Dexmedetomidine | 1.421 | 6.578 | 0.002 | 0.172 | 0.078~0.314 |
| (BIS) | 0.096 | 8.042 | 0.018 | 0.151 | 0.122~0.283 |
| Operation time | 0.013 | 5.735 | 0.031 | 2.724 | 1.232~7.234 |
Establishment and verification of risk prediction scoring model
A risk prediction equation was establish based on the results of multi-factor Logistic regression: logit(p) = -7.638+1.672×X1+1.454×X2+1.673×X3+1.585×X4-1.722×X5-1.884×X6+1.212×X7. Hosmer-Lemeshow test was used to detect the goodness of fit of the regression equation (P=0.794). Each factor X is a binomial assignment (0 or 1), among which X1-X7 are ASA classification (level III was assigned to 1), age (≥80 years was assigned to 1), preoperative PG-SGA score (≥4 was assigned to 1), Preoperative hemoglobin (≤110 g/L was assigned to 1), dexmedetomidine (not used during surgery was assigned to 1), depth of anesthesia (BIS>40 was assigned to 1), operation time (≥4 h was assigned to 1). The area under the ROC curve of established model to predict postoperative POCD in elderly patients with gastric cancer was 0.820 (95% CI: 0.742-0.899) (P<0.01) (Figure 1). According to the regression coefficient of the multi-factor Logistic regression equation, the risk factors of postoperative POCD were scored (Table 4), and the parameter model was established. Using the established risk prediction scoring model analysis, the probability of postoperative POCD in elderly gastric cancer patients with a score ≥4 points was 42.55%, and the probability of a patient with a score less than 4 points was 5.24%.
Figure 1.
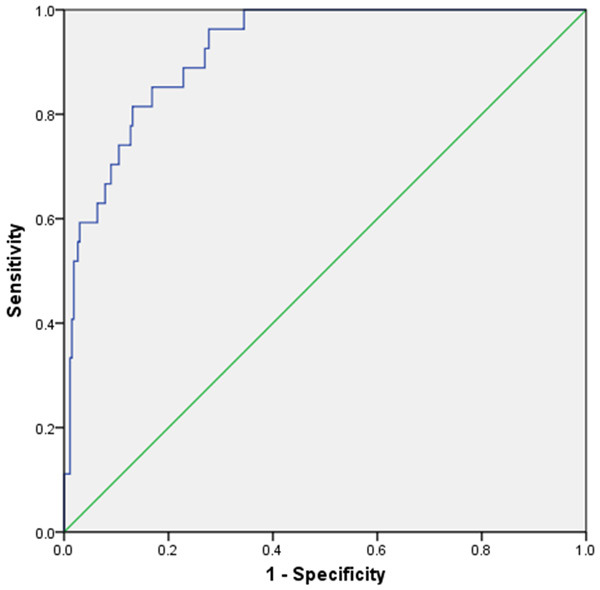
Receiver operating characteristic curve of POCD risk prediction model for elderly patients with gastric cancer after surgery.
Table 4.
The risk factors score of POCD after radical gastrectomy for gastric cancer
| clinical pathological factors | b | score |
|---|---|---|
| ASA classification | 1.672 | 1 |
| Age | 1.454 | 1 |
| PG-SGA score | 1.673 | 1 |
| Hemoglobin (g/L) | 1.585 | 1 |
| Dexmedetomidine | -1.722 | -1 |
| (BIS) | -1.884 | -1 |
| Operation time | 1.212 | 1 |
Discussion
Gastric cancer is one of the common malignant tumors of the digestive tract in China. Its incidence ranks third among all malignant tumors. Its tumor-related mortality ranks second [16]. According to authoritative epidemiological research reports, with the emergence of the problem of an aging society in China, the incidence of elderly patients with gastric cancer has shown an upward trend each year. The incidence and mortality rates are 21% and 30% respectively [17]. Previous related studies [18] have shown that, compared with the best conservative treatment, radical surgery in elderly patients with gastric cancer can effectively improve survival and improve patient prognosis. Elderly patients with gastric cancer often suffer from related complications after the operation due to the slow metabolism and declined immunity, accompanied with underlying diseases [19]. POCD is a complication related to cognitive dysfunction that occurs after surgery. It is a relatively common complication in elderly patients. The incidence is significantly higher than that of non-elderly patients with gastric cancer. The results of this study show that the incidence of POCD in elderly patients with gastric cancer (≥70 years old) on the seventh day after radical gastric cancer surgery under total intravenous anesthesia was 20.47%, which was slightly lower than the 25.8% released by the International POCD Research Collaboration Group [20]. The reasons for the difference are mainly related to the sample size, the different diagnostic criteria of POCD, the age of the research subjects, the method of anesthesia, and the depth of anesthesia.
This study conducted univariate and multivariate logistic regression analysis for the risk factors that may affect the occurrence of POCD. The results found that the higher preoperative ASA grade, older age, longer operation time, higher preoperative PG-SGA score, and pre-existed anemia were independent risk factors for POCD. Intraoperative application of dexmedetomidine and maintenance of a deep anesthetic state during surgery were protective factors, which are consistent with the results of related studies in many countries [21-24]. The older the patient, the greater the risk of POCD after surgery. The analysis may be related to the following factors [25-27]: ① The function of the central nervous system in elderly patients gradually declines with age, mainly manifested by the decreases in nerve cells, the number of synapses, and the number of neurotransmitters and neurons, which leads to a decrease in the functional activity of the central nervous system; ② In elderly patients, due to cerebrovascular sclerosis, cerebral blood flow, and the ability to control cerebral hemodynamics are reduced, which leads to decreased brain function and metabolism rate; ③ Elderly patients have poor reserve function of heart, lung, and kidney organs, especially the decline in liver and kidney function which leads to the weakening of the body’s drug detoxification ability, slower metabolism, and excretion of drugs from the body; ④ Due to long-term drug consumption in elderly patients, the muscle content in the body decreases, and the proportion of fat tissue in body mass increases, resulting in the distribution of fat-soluble drugs and the prolongation of the half-life, which increases the action time of anesthetics in the body. The general condition and nutritional status of the patient before surgery are also risk factors for the occurrence of POCD. The reason may be that the patient does not correct the general status in time before surgery. This can lead to intraoperative hypovolemic hypotension, which causes the brain to be in a state of hypoperfusion. Continuous insufficient blood supply to the brain causes ischemic damage to brain tissue, which in turn affects brain function [28,29]. Surgical trauma stimulation, excessive operation time, and shallow depth of anesthesia will aggravate the damage caused by insufficient blood supply to the brain. This can cause neuronal degeneration and axonal rupture in the brain and induce intracellular mitochondrial transport and functional impairment, affecting memory and cognitive function [30].
Once POCD occurs in elderly patients with gastric cancer after surgery, it will cause serious consequences. If it is not handled properly, it will increase the risk of postoperative death and increase hospitalization costs, resulting in a decline in the quality of life after surgery and affecting the long-term survival of the patient. A simple and effective scoring model can be established for the prediction of POCD in elderly patients with gastric cancer in advance. This will be of guiding significance for patients with high risk to carry out active intervention before surgery. We conducted a multi-factor analysis on the risk factors that may be related to the occurrence of POCD in elderly patients and established the Logistic risk prediction equation. The Hosmer-Lemeshow test was used to detect the goodness of fit and the ROC curve to prove that the model fit well, indicating that the prediction equation has good clinical practical value. Analysis based on the prediction equation found that the probability of POCD in elderly patients with scores ≥4 points was 42.55%, and the probability of POCD in patients with scores <4 points was 5.24%.
With the improvement of anesthesia and surgical techniques, elderly patients are included as surgical objects. The clinical incidence of postoperative POCD is gradually increasing, and is still an unavoidable problem after radical gastric cancer surgery in elderly patients. The prediction of POCD during the perioperative period in elderly patients who underwent surgery has positive clinical guiding significance. For high-risk patients in the perioperative period, the following targeted interventions can be done: preoperative assessments, improvement of general conditions, correction of nutritional status, prediction of surgical difficulty and risk in advance, reduction of surgical time, and appropriate intraoperative amounts the use of dexmedetomidine will have important clinical significance in reducing the incidence of POCD in elderly patients with gastric cancer.
Our research still has some limitations. This study is a single-center retrospective study with a limited number of cases and certain limitations. We only investigated the clinicopathological features of the patients, without imaging examination and molecular mechanism research. It is necessary to carry out a multi-center prospective study, combined with imaging examinations and new molecules to predict the occurrence of POCD in patients.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Joharatnam-Hogan N, Shiu KK, Khan K. Challenges in the treatment of gastric cancer in the older patient. Cancer Treat Rev. 2020;85:101980. doi: 10.1016/j.ctrv.2020.101980. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto T, Kurokawa Y, Mikami J, Takahashi T, Miyazaki Y, Tanaka K, Makino T, Yamasaki M, Motoori M, Kimura Y, Nakajima K, Mori M, Doki Y. Postoperative long-term outcomes in elderly patients with gastric cancer and risk factors for death from other diseases. World J Surg. 2019;43:2885–2893. doi: 10.1007/s00268-019-05109-5. [DOI] [PubMed] [Google Scholar]
- 4.Wakahara T, Ueno N, Maeda T, Kanemitsu K, Yoshikawa T, Tsuchida S, Toyokawa A. Impact of gastric cancer surgery in elderly patients. Oncology. 2018;94:79–84. doi: 10.1159/000481404. [DOI] [PubMed] [Google Scholar]
- 5.Saif MW, Makrilia N, Zalonis A, Merikas M, Syrigos K. Gastric cancer in the elderly: an overview. Eur J Surg Oncol. 2010;36:709–17. doi: 10.1016/j.ejso.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Yamada H, Kojima K, Inokuchi M, Kawano T, Sugihara K. Laparoscopy-assisted gastrectomy in patients older than 80. J Surg Res. 2010;161:259–63. doi: 10.1016/j.jss.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 8.Alalawi R, Yasmeen N. Postoperative cognitive dysfunction in the elderly: a review comparing the effects of desflurane and sevflurane. J Perianesth Nurs. 2018;33:732–740. doi: 10.1016/j.jopan.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Kotekar N, Shenkar A, Nagaraj R. Postoperative cognitive dysfunction-current preventive strategies. Clin Interv Aging. 2018;13:2267–2273. doi: 10.2147/CIA.S133896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasan TF, Kelley RE, Cornett EM, Urman RD, Kaye AD. Cognitive impairment assessment and interventions to optimize surgical patient outcomes. Best Pract Res Clin Anaesthesiol. 2020;34:225–253. doi: 10.1016/j.bpa.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer ST, Koenigsperger S, Olotu C, Saller T. Biomarkers and postoperative cognitive function: could it be that easy? Curr Opin Anaesthesiol. 2019;32:92–100. doi: 10.1097/ACO.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Li J, Huang X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry. 2012;12:156. doi: 10.1186/1471-244X-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen HB, Zhang ZX, Niu FS, Li L. The application of Montreal cognitive assessment in urban Chinese residents of Beijing. Zhonghua Nei Ke Za Zhi. 2008;47:36–39. [PubMed] [Google Scholar]
- 14.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 15.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 16.Wei W, Zeng H, Zheng R, Zhang S, An L, Chen R, Wang S, Sun K, Matsuda T, Bray F, He J. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21:e342–e349. doi: 10.1016/S1470-2045(20)30073-5. [DOI] [PubMed] [Google Scholar]
- 17.Nunobe S, Oda I, Ishikawa T, Akazawa K, Katai H, Isobe Y, Miyashiro I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, Suzuki S, Kakeji Y Registration Committee of the Japanese Gastric Cancer. Surgical outcomes of elderly patients with Stage I gastric cancer from the nationwide registry of the Japanese Gastric Cancer Association. Gastric Cancer. 2020;23:328–338. doi: 10.1007/s10120-019-01000-3. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Huang LY, Xue HP. Comparison of prognostic factors in different age groups and prognostic significance of neutrophil-lymphocyte ratio in patients with gastric cancer. World J Gastrointest Oncol. 2020;12:1146–1166. doi: 10.4251/wjgo.v12.i10.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 20.Yong R, Meng Y. Preoperative neutrophil-lymphocyte ratio, an independent risk factor for postoperative cognitive dysfunction in elderly patients with gastric cancer. Geriatr Gerontol Int. 2020;20:927–931. doi: 10.1111/ggi.14016. [DOI] [PubMed] [Google Scholar]
- 21.Feinkohl I, Winterer G, Spies CD, Pischon T. Cognitive reserve and the risk of postoperative cognitive dysfunction. Dtsch Arztebl Int. 2017;114:110–117. doi: 10.3238/arztebl.2017.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotekar N, Shenkar A, Nagaraj R. Postoperative cognitive dysfunction-current preventive strategies. Clin Interv Aging. 2018;13:2267–2273. doi: 10.2147/CIA.S133896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L, Hu M, Lu Y, Cao Y, Chang Y, Dai Z. The protective effects of dexmedetomidine on ischemic brain injury: a meta-analysis. J Clin Anesth. 2017;40:25–32. doi: 10.1016/j.jclinane.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Silverstein JH, Timberger M, Reich DL, Uysal S. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology. 2007;106:622–8. doi: 10.1097/00000542-200703000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Kawada T. Postoperative cognitive decline in older patients. Psychogeriatrics. 2021;21:139. doi: 10.1111/psyg.12597. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Liu L, Zhao B, Wang Y, Yu S, Wang H. Effect of general and non-general anesthesia on postoperative cognitive dysfunction. J Coll Physicians Surg Pak. 2020;30:407–411. doi: 10.29271/jcpsp.2020.04.407. [DOI] [PubMed] [Google Scholar]
- 27.Kwon DH, Kim BS, Chang H, Kim YI, Jo SA, Leem YH. Exercise ameliorates cognition impairment due to restraint stress-induced oxidative insult and reduced BDNF level. Biochem Biophys Res Commun. 2013;434:245–51. doi: 10.1016/j.bbrc.2013.02.111. [DOI] [PubMed] [Google Scholar]
- 28.Hussein M, Fathy W, Nabil T, Abd Elkareem R. Postoperative cognitive dysfunction and the possible underlying neurodegenerative effect of anaesthesia. Int J Neurosci. 2019;129:729–737. doi: 10.1080/00207454.2018.1561451. [DOI] [PubMed] [Google Scholar]
- 29.Fan D, Li J, Zheng B, Hua L, Zuo Z. Enriched environment attenuates surgery-induced impairment of learning, memory and neurogenesis possibly by preserving BDNF expression. Mol Neurobiol. 2016;53:344–54. doi: 10.1007/s12035-014-9013-1. [DOI] [PubMed] [Google Scholar]
- 30.Liang F, Xu X, Liang B. Comparison of intraoperative indicators and postoperative efficacy in treatment of benign ovarian tumor: laparoscopy versus open surgery. Am J Ther. 2017;24:e681–e688. doi: 10.1097/MJT.0000000000000367. [DOI] [PubMed] [Google Scholar]


