Abstract
Objective: To investigate the changes and significance of humoral immunity, myocardial damage, trace elements and inflammatory factors levels in children with rotavirus enteritis. Methods: One hundred children with rotavirus enteritis admitted to our hospital from January 2019 to December 2020 were retrospectively selected as the case group, and they were divided into a no dehydration group (33 cases), mild dehydration group (41 cases), and moderate dehydration group (26 cases). Another 100 children with rotavirus-negative enteritis during the same period were selected as the control group. Serum immunoglobulin, cardiac enzyme profile, trace elements, and interleukin-6 (IL-6) levels were compared between the two groups, and among the case groups for different degrees of dehydration. Results: Serum immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), zinc, magnesium, and calcium in the case group were lower than in controls (P<0.05). Serum lactate dehydrogenase (LDH), α-hydroxybutyrate dehydrogenase (α-HBDH), creatine kinase (CK), and creatine kinase isoenzyme (CK-MB) in the case group were higher than in controls (P<0.05). Serum IL-6, interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α) were also higher in cases than controls (P<0.05). Serum IgA, IgG, IgM, zinc, magnesium, and calcium in children with rotavirus enteritis with mild dehydration were lower than those without dehydration, but higher than those with moderate dehydration (P<0.05). Serum LDH, α-HBDH, CK, and CK-MB in children with rotavirus enteritis with mild dehydration were higher than those without dehydration, but lower than those with moderate dehydration (P<0.05). Serum IL-6, IL-8, and TNF-α in children with rotavirus enteritis with mild dehydration were higher than those without dehydration, but lower than those with moderate dehydration (P<0.05). Conclusion: Children with rotavirus enteritis with more severe dehydration exhibited lower levels of humoral immunity and trace elements and greater myocardial damage and inflammatory response. Early detection can accurately assess the condition and provide a reference for clinical treatment.
Keywords: Rotavirus enteritis, humoral immunity, myocardial damage, trace elements, inflammatory factors
Introduction
Enteritis caused by rotavirus is a common disease in infants and children. It has been reported that children under 5 years of age are most prone to infection with rotavirus. Studies have shown that rotavirus enteritis mainly affects infants and children aged 6-24 months, mostly in early spring, late autumn, and winter, with an acute onset and incubation period, accounting for about 40%-60% of childhood enteritis [1]. Data show that the incidence of rotavirus enteritis in community, outpatient and inpatient departments was 10%, 26%, and 46%, respectively [2]. Children with rotavirus enteritis have symptoms such as vomiting, watery diarrhea, and low-grade fever, and most of them are dehydrated due to the large amount of fluid lost by diarrhea.
The children have immature gastrointestinal tracts and immune systems. Rotavirus is highly resistant and multiplies in the cells of the small intestine, causing intestinal mucosal damage and leading to clinical symptoms such as dehydration, diarrhea, and acidosis. In severe cases, rotavirus enteritis can also lead to malnutrition, impede growth and development, and threaten the life and health of the children. When children are infected with rotavirus, their immune function is impaired, leaving non-specific substances in the blood circulation that can damage the function of extraintestinal organs such as the heart, nerves, liver and gallbladder [3,4]. It has been found that about 50%-70% of rotavirus enteritis is accompanied by myocardial damage [5].
The specific pathogenesis of myocardial damage caused by rotavirus has not been fully elucidated. Data suggest that rotavirus infection damages the heart and other organs through viral replication and virus circulation into the bloodstream [6]. It has also been suggested that rotavirus directly induces cellular damage, leading to immunosuppression, toxic killing of cardiac myocytes, and changes in the myocardial enzyme spectrum [7]. Immunosuppression is associated with micronutrient abnormalities, and evidence has shown that most patients with enterocolitis have reduced or disproportionate levels of the micronutrient zinc and certain trace elements [8]. The inflammatory response also plays an important role in the pathogenesis of rotavirus enteritis. However, there are no relevant studies on humoral immunity, myocardial damage, trace elements and inflammatory reaction related to dehydration in rotavirus enteritis patients. Therefore, in this innovative study, children with rotavirus enteritis were selected as the research subjects, and the changes in humoral immunity, myocardial damage, trace elements and inflammatory factors levels in children with varying degrees of dehydration were studied to explore the relationship between these indicators and dehydration, so as to provide a reference for the diagnosis and treatment of children with viral enteritis.
Materials and methods
Baseline data
One hundred children with rotavirus enteritis admitted to our hospital from January 2019 to December 2020 were retrospectively selected as the case group, and they were divided into three subgroups: 33 cases without dehydration, 41 cases with mild dehydration (fluid loss ≤5% of the body weight), and 26 cases with moderate dehydration (5% of the body weight < fluid loss ≤10% of the body weight).
Inclusion criteria: Children who met the diagnostic criteria of rotavirus enteritis, with rotavirus-positive confirmed by fecal colloidal gold test; children with ≥5 times of loose or yellow watery stools; children aged ≤2 years; and children with diarrhea and vomiting. All guardians of children signed the informed consent.
Exclusion criteria: Children with gastrointestinal diseases, viral or bacterial infections; those with severe complications such as shock or severe dehydration; those who had received relevant treatment; those with organ insufficiency or immunodeficiency diseases; and those with concomitant malignant tumors.
Another 100 children with rotavirus-negative enteritis during the same period were selected as the control group. Written informed consent was obtained from the guardians of the subjects prior to the beginning of the trial. The trial was approved by the Medical Ethics Committee of Affiliated Shenzhen Maternity & Child Healthcare Hospital, Southern Medical University (Approval No. NCT05368428).
Methods
In both groups, 3 mL of fasting venous blood was taken on the 2nd day of admission, followed by centrifugation for 10 min at 1500 rpm, and the supernatant was stored at -80°C for testing. Immunoglobulins A, G and M (IgA, IgG, IgM) were detected by radioimmunoassay (Shanghai Hengyuan Biological Co. Ltd). Creatine kinase (CK), creatine kinase isoenzyme (CK-MB), lactate dehydrogenase (LDH) and α-hydroxybutyrate dehydrogenase (α-HBDH) were detected by BIOBASE automatic biochemical analyzer (Shandong Boke Biological Industry Co. Ltd.). The trace elements including zinc, iron, copper, lead, magnesium and calcium were detected using iCE 3300 AAS atomic absorption spectrometer (Thermo Fisher Scientific (China) Co.). Interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α) were measured by enzyme-linked immunosorbent assay (ELISA), and the kits were purchased from Shanghai Hengyuan Biological Co. Ltd.
Statistical methods
All data were processed by SPSS 22.0 statistical software and plotted on GraphPad Prism 8 software. Enumeration data were expressed as (n, %) and examined by χ2 test. Data conforming to a normal distribution were represented by (x̅±s), and intergroup comparison was performed by independent samples t-test. Data not conforming to a normal distribution such as IgA and IgM were expressed as median (upper and lower quartiles) [M (P25 to P75)] and examined by Mann-Whitney U test. One-way ANOVA was used for comparison among multiple groups, and the least significant difference (LSD) test was used for pairwise comparison between groups. Significance was set at P<0.05.
Results
Baseline data
Baseline data such as gender, age, and duration of disease were compared between the two groups, exhibiting no significant difference (P>0.05, Table 1).
Table 1.
Comparison of baseline data (x̅±s; n, %)
| Baseline data | Case group (n=100) | Control group (n=100) | t/χ2 | P | |
|---|---|---|---|---|---|
| Age (months) | 12.64±3.45 | 12.31±3.57 | 0.529 | 0.598 | |
| Gender | Male | 53 (53) | 55 (55) | 0.081 | 0.777 |
| Female | 47 (47) | 45 (45) | |||
| Duration of illness (d) | 4.17±0.38 | 4.18±0.41 | 0.179 | 0.858 | |
Humoral immunity, myocardial damage
Serum IgA, IgG, and IgM in the case group were significantly lower than those of the control group (P<0.05), whereas serum LDH, α-HBDH, CK, and CK-MB in the case group were significantly higher than in the control group (P<0.05) (Table 2). Serum IgA, IgG, and IgM in children with rotavirus enteritis with mild dehydration were lower than those without dehydration, but higher than those with moderate dehydration (P<0.05). Serum LDH, α-HBDH, CK, CK-MB in children with rotavirus enteritis with mild dehydration were higher than those without dehydration, but lower than those with moderate dehydration (P<0.05, Table 3).
Table 2.
Comparison of immunoglobulin, and cardiac enzyme spectrum between groups (n, %)
| Value | Case group (n=100) | Control group (n=100) | Z/t | P | |
|---|---|---|---|---|---|
| Immunoglobulin | IgA (g/L) | 0.70 (0.60 to 0.80) | 1.30 (0.60 to 2.00) | 5.331 | <0.001 |
| IgG (g/L) | 7.82±2.55 | 9.26±2.96 | 3.686 | <0.001 | |
| IgM (g/L) | 1.00 (0.33 to 2.00) | 1.30 (0.40 to 2.10) | 0.621 | 0.534 | |
| Cardiac enzyme spectrum | LDH (U/L) | 256.34±36.07 | 236.87±32.41 | 4.015 | <0.001 |
| α-HBDH (U/L) | 250.58±47.13 | 187.64±33.84 | 10.848 | <0.001 | |
| CK (U/L) | 317.04±45.68 | 111.74±29.85 | 37.623 | <0.001 | |
| CK-MB (U/L) | 86.99±9.48 | 21.28±6.82 | 56.267 | <0.001 | |
Table 3.
Comparison of immunoglobulin and cardiac enzyme in case group with different degrees of dehydration (x̅±s)
| Value | No dehydration (n=33) | Mild dehydration (n=41) | Moderate dehydration (n=26) | F | P | |
|---|---|---|---|---|---|---|
| Immunoglobulin | IgA (g/L) | 0.84±0.13 | 0.70±0.14 | 0.50±0.16 | 41.555 | <0.001 |
| IgG (g/L) | 9.38±1.53 | 7.70±1.19 | 6.43±1.11 | 38.849 | <0.001 | |
| IgM (g/L) | 1.75±0.25 | 1.22±0.25 | 0.84±0.27 | 95.466 | <0.001 | |
| Cardiac enzyme spectrum | LDH (U/L) | 233.71±28.51 | 250.13±24.09 | 294.81±27.91 | 40.213 | <0.001 |
| α-HBDH (U/L) | 229.74±25.04 | 251.89±26.99 | 291.00±31.85 | 35.868 | <0.001 | |
| CK (U/L) | 287.28±35.72 | 313.61±33.56 | 360.35±34.65 | 32.842 | <0.001 | |
| CK-MB (U/L) | 77.54±8.27 | 87.73±7.88 | 97.61±7.60 | 46.797 | <0.001 | |
Micronutrients
Serum trace elements zinc, magnesium and calcium in the case group were significantly lower than those in the control group (P<0.05), but the serum trace elements iron, copper and lead were not significantly different between the two groups (P>0.05, Table 4). Serum trace elements zinc, magnesium and calcium in children with rotavirus enteritis with mild dehydration were lower than those without dehydration, but higher than those with moderate dehydration (P<0.05). Serum trace elements iron, copper and lead in children with rotavirus enteritis with different levels of dehydration showed no significant difference (P>0.05, Table 5).
Table 4.
Comparison of serum trace elements between two groups (x̅±s; U/L)
| Trace element | Case group (n=100) | Control group (n=100) | t | P |
|---|---|---|---|---|
| Zinc (μmol/L) | 65.78±10.54 | 76.89±7.85 | 8.454 | <0.001 |
| Iron (mmol/L) | 8.05±0.68 | 7.94±0.67 | 1.152 | 0.251 |
| Copper (μmol/L) | 22.32±1.96 | 22.69±3.09 | 1.011 | 0.313 |
| Lead (μg/L) | 30.38±5.23 | 30.15±5.75 | 0.296 | 0.768 |
| Magnesium (mmol/L) | 1.56±0.16 | 1.64±0.23 | 2.855 | 0.005 |
| Calcium (mmol/L) | 1.91±0.15 | 1.97±0.19 | 2.479 | 0.014 |
Table 5.
Comparison of serum trace elements in case groups with different degrees of dehydration (x̅±s)
| Trace element | No dehydration (n=33) | Mild dehydration (n=41) | Moderate dehydration (n=26) | F | P |
|---|---|---|---|---|---|
| Zinc (μmol/L) | 73.26±7.37 | 64.90±7.24 | 57.78±6.36 | 35.449 | <0.001 |
| Iron (mmol/L) | 7.89±0.83 | 8.27±1.01 | 8.20±0.86 | 1.688 | 0.190 |
| Copper (μmol/L) | 22.48±2.38 | 22.64±1.94 | 22.05±2.55 | 0.554 | 0.577 |
| Lead (μg/L) | 30.33±9.30 | 30.46±9.32 | 30.30±9.20 | 0.003 | 0.997 |
| Magnesium (mmol/L) | 1.78±0.04 | 1.62±0.05 | 1.45±0.06 | 319.473 | <0.001 |
| Calcium (mmol/L) | 2.02±0.06 | 1.93±0.07 | 1.78±0.05 | 109.492 | <0.001 |
IL-6, IL-8, and TNF-α levels
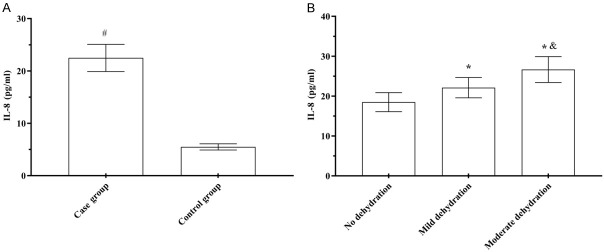
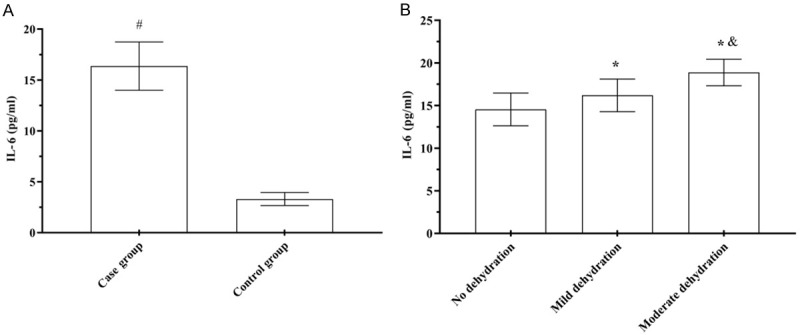
IL-6, IL-8, and TNF-α levels in the case group were (16.36±2.37) pg/mL, (22.49±2.59) pg/mL, and (4.68±0.92) μg/L, respectively, which was significantly higher than (3.30±0.64) pg/mL, (6.49±0.59) pg/mL, and (2.13±0.52) μg/L, respectively, in the control group (t=53.184, 40.797, 15.028, P<0.001). IL-6, IL-8 and TNF-α levels in children with rotavirus enteritis with mild dehydration were (16.20±1.91) pg/mL, (22.12±2.54) pg/mL, and (4.82±0.95) μg/L, respectively, higher than those without dehydration [(14.55±1.92) pg/mL, (18.49±2.38) pg/mL, and (4.04±0.88) μg/L, respectively], but lower than those with moderate dehydration [(18.88±1.56) pg/mL, (26.67±3.25) pg/mL, and (5.53±0.98) μg/L, respectively], showing significant three-fold differencs (t=41.071, 67.096, 18.656, P<0.001) (Figures 1, 2 and 3).
Figure 1.
Comparison of serum IL-6 levels. A: Serum IL-6 levels; B: Serum IL-6 levels. Compared to the control group, #P<0.05; compared to children without dehydration, *P<0.05; compared to children with mild dehydration, &P<0.05.
Figure 2.
Comparison of serum IL-8 levels. A: Serum IL-8; B: Serum IL-8 levels. Compared to the control group, #P<0.05; compared to children without dehydration, *P<0.05; compared to children with mild dehydration, &P<0.05.
Figure 3.
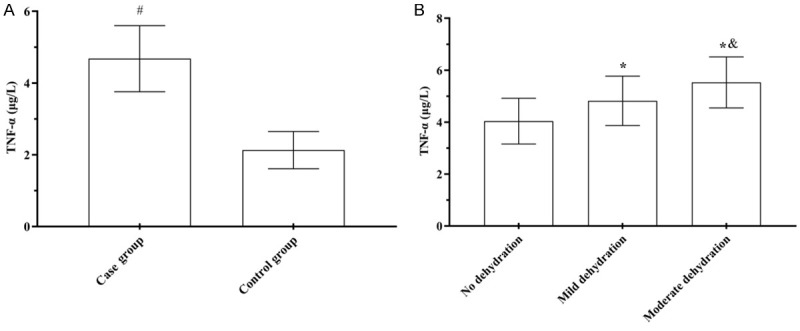
Comparison of serum TNF-α levels. A: Serum TNF-α levels; B: Serum TNF-α levels. Compared to the control group, #P<0.05; compared to children without dehydration, *P<0.05; compared to children with mild dehydration, &P<0.05.
Discussion
The pathogenesis of rotavirus enteritis remains unclear, and some studies suggest that rotavirus enteritis is mainly caused by the invasion of the small intestine by rotavirus, resulting in atrophy and swelling of the small intestinal villi, along with proliferation of cells in the lamina propria, leading to dysfunction of secretion and absorption and thus inducing diarrhea [9-11]. If rotavirus enteritis is left untreated, it can lead to varying degrees of dehydration and even death. Humoral immunity is also called antibody-mediated immunity. With assistance from helper T cells, B cells differentiate into plasma B cells, which can produce antibodies against specific antigens [12,13]. Humoral immunity defends against and monitors viruses and plays an important role in organism immunity. IgA, IgG, and IgM are all anti-infective antibodies, of which IgG is associated with most antitoxin, antibacterial, and antiviral antibodies. Children with rotavirus enteritis suffer from immune dysfunction, with weakened function of T cells and B cells, and reduced conversion of Ig classes in B cells, which delays the secretion and synthesis of Ig and conversion of antibodies, resulting in a decline of immune function [14,15]. Serum IgA, IgG and IgM in the case group were significantly lower than those of the control group. Serum IgA, IgG, and IgM levels in children with rotavirus enteritis with mild dehydration were lower than those without dehydration, but higher than those with moderate dehydration. The results of the study showed that the humoral immunity of children with rotavirus enteritis was weakened with increasing severity of dehydration.
Studies have shown that rotavirus infection induces myocardial damage, and the pathogenesis of myocardial damage may be caused by the entry of rotavirus into the circulation through the gastrointestinal barrier, leading to damage to several organs [16]. Cardiomyocyte enzymes are widely distributed throughout the body, with highest levels in skeletal muscle and myocardium, and the cardiomyocyte membranes prevent extravasation of cardiomyocyte enzymes into the blood. When the myocardium is damaged, cardiac enzymes were released into the blood circulation, leading to elevated serum LDH, CK, and CK-MB levels in the case group [17]. CK-MB is rapidly elevated in the early stage of myocardial damage [18]. Bonkoungou et al. have shown that more than 50% of children with rotavirus enteritis have abnormally high expression of CK-MB, which may lead to sudden death [19]. In this study, LDH, α-HBDH, CK, and CK-MB in the case group were significantly higher than those of the control group. Serum LDH, α-HBDH, CK, and CK-MB in children with rotavirus enteritis with mild dehydration were higher than those without dehydration, but lower than those with moderate dehydration. The findings suggest that myocardial damage in children with rotavirus enteritis may be related to electrolyte disturbances and circulatory disturbances caused by dehydration.
In rotavirus infection, serum trace elements show a significant decrease, and the changes in trace elements are associated with complications, indicating a close relationship between trace elements and infectious and viral diseases. Zinc is directly involved in the synthesis of proteins and nucleic acids, humoral immunity, cellular immunity and energy metabolism. In the early stages of infectious diseases, leukocytes and phagocytes release leukocyte endogenous mediators (LEN) that directly contribute to copper and zinc metabolism. The decrease in serum zinc during the early stage of infection may be due to the action of LEN at metastatic sites on the surface of hepatocytes, which impedes zinc uptake by hepatocytes, and results in reduced dietary intake, leading to increased gastrointestinal loss and small intestinal malabsorption, which is aggravated by diarrhea. Agarwal et al. have found that children with rotavirus enteritis have low expression of serum zinc [20], and Sakai et al. [21] have pointed out that blood trace elements are closely related to severity and prognosis of enteritis through animal model. In this study, serum zinc, magnesium, and calcium in the case group were significantly lower than those in the control group. Serum zinc, magnesium and calcium in children with rotavirus enteritis with mild dehydration were lower than those without dehydration, but higher than those with moderate dehydration. The results of the study showed that children with rotavirus enteritis had lower levels of the zinc, magnesium and calcium. The levels of the trace elements zinc, enzymes and calcium were decreased further with the increased level of dehydration. The decrease in magnesium may be because [22,23]: children with rotavirus enteritis often have diarrhea, leading to malabsorption and insufficient magnesium intake and excretion with feces; loss of acid-base balance leading to intracellular translocation of magnesium ions; dehydration allows a secondary increase in aldosterone, reducing magnesium reabsorption by the distal tubule of the kidney, leading to increased excretion of magnesium. The decrease in calcium may be due to the opening of calcium ion channels that cause the inward flow of calcium into the cells, which is involved in the release of chemical mediators [24].
Epithelial cells are not only a physical barrier against infection, but also play an important role in the immune response and tissue remodeling after healing. Oral epithelial cells can recognize invading pathogens and stimulate epithelial cells to produce inflammatory cytokines by activating signaling pathways [25]. As pro-inflammatory cytokines, IL-6 and IL-8 are mainly produced by T cells and fibroblasts and play a key role in humoral and cellular immunity. IL-6 and IL-8 act together with TNF-α to increase vascular permeability, destroy intestinal mucosa, and ultimately promote the inflammatory response, which plays a vital role in the pathogenesis of rotavirus enteritis [26,27]. In this study, serum IL-6, IL-8, and TNF-α in the case group were significantly higher than those in the control group. Serum IL-6, IL-8, and TNF-α in children with rotavirus enteritis with mild dehydration were higher than those without dehydration, but lower than those with moderate dehydration. The results of the study showed that rotavirus enteritis produces an inflammatory response, and children with more severe dehydration exhibit a greater inflammatory response.
In conclusion, children with rotavirus enteritis have low levels of trace elements and decreased immune function, and the body will produce an inflammatory response, which eventually leads to myocardial damage. Children with more severe dehydration exhibited lower trace elements level and humoral immune function, and severer myocardial damage and inflammatory response. Therefore, early detection of humoral immune, myocardial damage, trace elements and inflammatory factors levels can accurately assess the condition and provide supplementary reference for clinical treatment. The shortcomings of this study are as follows: (1) the sample size was relatively small; (2) humoral immunity, myocardial damage, trace elements and inflammatory factors in children with rotavirus enteritis at different time points were not explored; (3) the association between humoral immunity, myocardial damage, trace elements, and inflammatory factors was not discussed. Therefore, the study needs to be verified with larger samples and to analyze the correlation between changes in levels of humoral immunity, myocardial damage, trace elements and inflammatory factors performed in children at different time points.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure of conflict of interest
None.
References
- 1.Chen F, Knutson TP, Ciarlet M, Sturos M, Marthaler DG. Complete genome characterization of a rotavirus B (RVB) strain identified in Alpine goat kids with enteritis reveals inter-species transmission with RVB bovine strains. J Gen Virol. 2018;99:457–463. doi: 10.1099/jgv.0.001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soares-Weiser K, Bergman H, Henschke N, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2019;2019:CD008521. doi: 10.1002/14651858.CD008521.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Getachew HB, Dahl RM, Lopman BA, Parashar UD. Rotavirus vaccines and health care utilization for diarrhea in US children, 2001 to 2015. Pediatr Infect Dis J. 2018;37:943–948. doi: 10.1097/INF.0000000000001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Geng P, Liu Y, Wu J, Qiao H, Xie Y, Yin N, Chen L, Lin X, Liu Y, Yi S, Zhang G, Li H, Sun M. Rotavirus-encoded virus-like small RNA triggers autophagy by targeting IGF1R via the PI3K/Akt/mTOR pathway. Biochim Biophys Acta Mol Basis Dis. 2018;1864:60–68. doi: 10.1016/j.bbadis.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Sadiq A, Bostan N, Yinda KC, Naseem S, Sattar S. Rotavirus: genetics, pathogenesis and vaccine advances. Rev Med Virol. 2018;28:e2003. doi: 10.1002/rmv.2003. [DOI] [PubMed] [Google Scholar]
- 6.Tarris G, Belliot G, Callier P, Huet F, Martin L, de Rougemont A. Pathology of rotavirus-driven multiple organ failure in a 16-month-old boy. Pediatr Infect Dis J. 2019;38:e326–e328. doi: 10.1097/INF.0000000000002472. [DOI] [PubMed] [Google Scholar]
- 7.Ianiro G, Micolano R, Di Bartolo I, Scavia G, Monini M. Group A rotavirus surveillance before vaccine introduction in Italy, September 2014 to August 2017. Euro Surveill. 2019;24:1800418. doi: 10.2807/1560-7917.ES.2019.24.15.1800418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinnouho GM, Bernstein RM, Barffour MA, Arnold CD, Wessells KR, Ratsavong K, Bounheuang B, Kounnavong S, Hess SY. Impact of two forms of daily preventive zinc or therapeutic zinc supplementation for diarrhea on hair cortisol concentrations among rural laotian children: a randomized controlled trial. Nutrients. 2018;11:47. doi: 10.3390/nu11010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shekarian T, Sivado E, Jallas AC, Depil S, Kielbassa J, Janoueix-Lerosey I, Hutter G, Goutagny N, Bergeron C, Viari A, Valsesia-Wittmann S, Caux C, Marabelle A. Repurposing rotavirus vaccines for intratumoral immunotherapy can overcome resistance to immune checkpoint blockade. Sci Transl Med. 2019;11:eaat5025. doi: 10.1126/scitranslmed.aat5025. [DOI] [PubMed] [Google Scholar]
- 10.Mikounou Louya V, Nguekeng Tsague B, Ntoumi F, Vouvoungui C, Kobawila SC. High prevalence of norovirus and rotavirus co-infection in children with acute gastroenteritis hospitalised in Brazzaville, Republic of Congo. Trop Med Int Health. 2019;24:1427–1433. doi: 10.1111/tmi.13317. [DOI] [PubMed] [Google Scholar]
- 11.Debellut F, Clark A, Pecenka C, Tate J, Baral R, Sanderson C, Parashar U, Kallen L, Atherly D. Re-evaluating the potential impact and cost-effectiveness of rotavirus vaccination in 73 Gavi countries: a modelling study. Lancet Glob Health. 2019;7:e1664–e1674. doi: 10.1016/S2214-109X(19)30439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pye R, Patchett A, McLennan E, Thomson R, Carver S, Fox S, Pemberton D, Kreiss A, Baz Morelli A, Silva A, Pearse MJ, Corcoran LM, Belov K, Hogg CJ, Woods GM, Lyons AB. Immunization strategies producing a humoral IgG immune response against devil facial tumor disease in the majority of Tasmanian devils destined for wild release. Front Immunol. 2018;9:259. doi: 10.3389/fimmu.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cachat J, Deffert C, Alessandrini M, Roux-Lombard P, Le Gouellec A, Stasia MJ, Hugues S, Krause KH. Altered humoral immune responses and IgG subtypes in NOX2-deficient mice and patients: a key role for NOX2 in antigen-presenting cells. Front Immunol. 2018;9:1555. doi: 10.3389/fimmu.2018.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keskin Yildirim Z, Buyukavci M. Assessment of humoral immunity to hepatitis b, measles, rubella, and mumps in children after chemotherapy. J Pediatr Hematol Oncol. 2018;40:e99–e102. doi: 10.1097/MPH.0000000000001072. [DOI] [PubMed] [Google Scholar]
- 15.Lee SA, Jang SH, Kim BH, Shibata T, Yoo J, Jung Y, Kawabata SI, Lee BL. Insecticidal activity of the metalloprotease AprA occurs through suppression of host cellular and humoral immunity. Dev Comp Immunol. 2018;81:116–126. doi: 10.1016/j.dci.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Kumar L, Puliyel J. New pentavalent rotavirus vaccine shows little efficacy against diarrhea. Vaccine. 2019;37:3945. doi: 10.1016/j.vaccine.2018.05.105. [DOI] [PubMed] [Google Scholar]
- 17.Selimoglu SM, Kasap M, Akpinar G, Karadenizli A, Wis AM, Gormus U. Improved production of highly active and pure human creatine kinase MB. J Mol Microbiol Biotechnol. 2018;28:28–36. doi: 10.1159/000486716. [DOI] [PubMed] [Google Scholar]
- 18.Jo MS, Lee J, Kim SY, Kwon HJ, Lee HK, Park DJ, Kim Y. Comparison between creatine kinase MB, heart-type fatty acid-binding protein, and cardiac troponin T for detecting myocardial ischemic injury after cardiac surgery. Clin Chim Acta. 2019;488:174–178. doi: 10.1016/j.cca.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Bonkoungou IJO, Ouédraogo N, Tamini L, Teguera RK, Yaméogo P, Drabo MK, Medah I, Barro N, Sharma S, Svensson L, Nordgren J. Rotavirus and norovirus in children with severe diarrhea in Burkina Faso before rotavirus vaccine introduction. J Med Virol. 2018;90:1453–1460. doi: 10.1002/jmv.25213. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal A, Gupta NK, Upadhyay A, Soni RK, Shah D, Jaiswal V. Serum zinc levels as a predictor of severity of acute diarrhea. Indian J Pediatr. 2018;85:179–183. doi: 10.1007/s12098-017-2493-z. [DOI] [PubMed] [Google Scholar]
- 21.Black R, Fontaine O, Lamberti L, Bhan M, Huicho L, El Arifeen S, Masanja H, Walker CF, Mengestu TK, Pearson L, Young M, Orobaton N, Chu Y, Jackson B, Bateman M, Walker N, Merson M. Drivers of the reduction in childhood diarrhea mortality 1980-2015 and interventions to eliminate preventable diarrhea deaths by 2030. J Glob Health. 2019;9:020801. doi: 10.7189/jogh.09.020801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trapani V, Petito V, Di Agostini A, Arduini D, Hamersma W, Pietropaolo G, Luongo F, Arena V, Stigliano E, Lopetuso LR, Gasbarrini A, Wolf FI, Scaldaferri F. Dietary Magnesium Alleviates Experimental Murine Colitis Through Upregulation of the Transient Receptor Potential Melastatin 6 Channel. Inflamm Bowel Dis. 2018;24:2198–2210. doi: 10.1093/ibd/izy186. [DOI] [PubMed] [Google Scholar]
- 23.Lewis TV, Neely S, Turman MA. Efficacy and tolerability of magnesium plus protein for managing hypomagnesemia in pediatric kidney transplant patients. Pediatr Transplant. 2018;22:e13170. doi: 10.1111/petr.13170. [DOI] [PubMed] [Google Scholar]
- 24.Salgado EN, Garcia Rodriguez B, Narayanaswamy N, Krishnan Y, Harrison SC. Visualization of calcium ion loss from rotavirus during cell entry. J Virol. 2018;92:e01327-18. doi: 10.1128/JVI.01327-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KY, Moon CH, Choi SH. Type I interferon and proinflammatory cytokine levels in cerebrospinal fluid of newborns with rotavirus-associated leukoencephalopathy. Brain Dev. 2018;40:211–217. doi: 10.1016/j.braindev.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Paparo L, Tripodi L, Bruno C, Pisapia L, Damiano C, Pastore L, Berni Canani R. Protective action of Bacillus clausii probiotic strains in an in vitro model of rotavirus infection. Sci Rep. 2020;10:12636. doi: 10.1038/s41598-020-69533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakim MS, Ding S, Chen S, Yin Y, Su J, van der Woude CJ, Fuhler GM, Peppelenbosch MP, Pan Q, Wang W. TNF-α exerts potent anti-rotavirus effects via the activation of classical NF-κB pathway. Virus Res. 2018;253:28–37. doi: 10.1016/j.virusres.2018.05.022. [DOI] [PubMed] [Google Scholar]