Abstract
Non-alcoholic fatty liver disease (NAFLD) progresses from simple steatosis to steatohepatitis (NASH), which may then progress to the development of cirrhosis and hepatocarcinoma. NASH is characterized by both steatosis and inflammation. Control of inflammation in NASH is a key step for the prevention of disease progression to severe sequalae. Intestinal dysbiosis has been recognized to be an important causal factor in the pathogenesis of NASH, involving both the accumulation of lipids and aggravation of inflammation. The effects of gut dysbiosis are mediated by adverse shifts of various intestinal commensal bacterial genera and their associated metabolites such as butyrate, tryptophan, and bile acids. In this review, we focus on the roles of tryptophan and its metabolites in NASH in association with intestinal dysbiosis and discuss possible therapeutic implications.
Keywords: Nonalcoholic steatohepatitis, intestinal microbiome, dysbiosis, inflammation, tryptophan
Introduction
Non-alcoholic fatty liver disease (NAFLD) occurs in about one third of the population worldwide. 1 NAFLD develops from simple steatosis to steatohepatitis (NASH), cirrhosis, and eventually to hepatocellular carcinoma. It is closely associated with metabolic syndrome including obesity, insulin resistance, hyperlipidemia, and hypertension.2,3 However, NAFLD could happen without metabolic syndrome. 4 Currently there are no FDA approved therapeutic agents for the treatment of the disease. 5 Further studies on the pathogenesis of NASH could provide opportunities to investigate effective therapeutic approaches. The key pathological characteristic of NASH is inflammation, which is a major mediator promoting the development of NASH and progression to cirrhosis and hepatocellular carcinoma. Many factors could be involved in the NASH inflammatory process, such as genetics, epigenetic, environmental agents, nutritional, and adverse shifts in the intestinal microbiota. 6
The intestinal microbiota contains trillions of microbes, which have known benefits to health that studies with certain bacterial genera have reported. 7 Dysregulation of the gut microbiota (gut dysbiosis) is associated with many chronic metabolic-inflammatory diseases including NAFLD. In this narrative review, the focus is on the effects of intestinal dysbiosis on metabolic pathways of tryptophan in the pathogenesis of NASH and possible therapeutic implications.
Gut Dysbiosis and Inflammation in NASH
The association between the intestinal microbiota and non-alcoholic steatohepatitis (NASH) has been extensively documented and modulation of the gut microbiota has been advanced as an approach for treating the disease. 8 The associated mechanism for a causal effect of intestinal dysbiosis has been linked with adverse shifts in gut microbial metabolites. In NASH, gut dysbiosis has been demonstrated by both animal experiments and clinical studies. 2
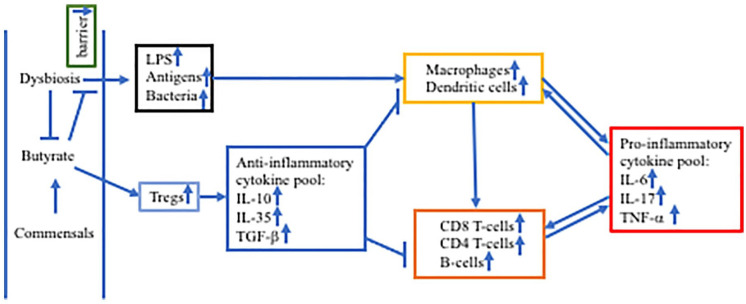
The analysis of gut microbiota in NAFLD showed that commensal bacteria had a decreased abundance in the gram-positive Firmicute phylum and opportunistic pathobionts were increased such as those from the gram-negative Proteobacterial phylum.2,3 There were different microbial signatures in different NAFLD stages. These gut microbiota signatures were identified at the family and genus levels. Overall, the gut dysbiosis in NASH promotes a proinflammatory environment, which results in increased gut permeability. Increased gut permeability can cause the translocation of endotoxin lipopolysaccharides (LPS), bacteria, and antigens into the portal circulation and thence into the liver, resulting in hepatic inflammation (Figure 1). 9
Figure 1.
Intestinal dysbiosis and aggravated inflammation in NASH.
Abbreviations: IL, interleukin; LPS, lipopolysaccharides; TGF-β, transforming growth factor-beta; TNF-β, tumor necrosis factor-alpha; Tregs, regulatory T-cells.
Reduced butyrate production in dysbiosis causes decreased intestinal barrier, leading to translocation of LPS, antigens, and bacteria to the liver which activate macrophages and dendritic cells to produce pro-inflammatory cytokines IL-6, IL-17, and TNF-α. Macrophages and Dendritic cells also present antigens to and activate CD8+ T-cells, CD4+ T-cells, and B-cells. Accumulated pro-inflammatory cytokines further stimulate both innate and adaptive immune cells, forming feed-forward regulation. Butyrate activates Tregs to secrete anti-inflammatory cytokines IL-10, IL-35, and TGF-β to reduce innate and adaptive immunities.
LPS plays a key role in the pathogenesis of NASH by promoting inflammatory responses. Several studies have shown that LPS and TLR4 are increased in the liver of NASH patients.10 -12 LPS can bind to TLR4, which in turn activates MyD88/NF-kB cascade, leading to increased secretion of proinflammatory cytokines. TLR4 can also activate NF-kB through TRIF. 13 As TLR4 is expressed in many types of cells in the liver (eg, both parenchymal and non-parenchymal cell types), such as macrophages/dendritic cells, Kupffer cells, hepatocytes, and stellate cells, LPS can activate all these cells to secret proinflammatory cytokines.14,15
Both liver-resident macrophages (Kupffer cells) and recruited macrophages play a central role in the pathogenesis of NASH. 16 Carpino et al 11 showed that TLR4 activated MyD88 and NF-kB in macrophages. Over stimulation by LPS and translocated bacteria as well as other metabolites, macrophages differentiated into proinflammatory type M1 while anti-inflammatory type M2 were decreased. In a mouse model, macrophage infiltration into the liver was increased with increased expression of proinflammatory factors CCR2 and MCP-1. 17 Knockout of TLR4, or TLR9, or MyD88 reduced macrophage infiltration and CCR2 and MCP-1 expression, indicating these signaling molecules play critical roles in the pathogenesis of NASH. In addition, depletion of Kupffer cells ameliorated hepatic inflammation in the model, suggesting the involvement of Kupffer cells in the inflammation. Activation of Kupffer cells increased secretion of TNF-alpha, which accelerated hepatic inflammation. 18 In addition, cenicriviroc, a dual antagonist of CCR2/CCR5 reduced the accumulation of monocyte-derived macrophages. 19
Recently several studies have associated gut dysbiosis with changes of the important roles of T cells and B cells in NASH. Rai et al. 20 demonstrated the important adverse effects of intestinal dysbiosis (increased proteobacteria and decreased bacteroides) in increased homing and activation of CD4+ T-cells in NASH in a mouse model established by F11r knockout and consumption of a Western diet. Barrow et al 21 demonstrated that intrahepatic B-cells switched to a proinflammatory profile, with increased secretion of proinflammatory cytokines. The associated mechanism was linked to activation of the MyD88 pathway. Fecal Microbiota Transplantation from NAFLD patients to mice promoted the development of NASH with accumulation and activation of intrahepatic B cells. Hass et al 22 reported that CD8+ T cells were increased in the liver of NASH patients, which were collocated with inflammatory foci and hepatocyte (stress/damage) ballooning.
The mechanisms of the effects of gut dysbiosis on the pathogenesis could also be mediated by many metabolites from commensal bacterial biochemical actions, such as short-chain fatty acids, bile acids, and amino acids. 23 The effect of reduced bacterial production of short-chain fatty acids, particularly butyrate has been extensively studied. 24 Butyrate can activate regulatory T cells and thus has anti-inflammatory effect through inhibiting Th17 cells and cytotoxic T cells. 9 When butyrate levels are decreased in NASH, the anti-inflammatory mechanism through regulatory T cells is reduced, which facilitates the formation and progression of inflammation in NASH. In mouse models of NASH, oral administration of butyrate increased the intestinal barrier and reduced the pathological changes in the liver.25 -27 Interactions of bile acids with commensal intestinal bacteria have also been demonstrated to have important effects on lipid accumulation and inflammatory responses. Furthermore, altered tryptophan metabolism has been associated with hepatic inflammation in NASH, which is discussed below.
The Roles of Tryptophan and Its Metabolites in Inflammation
Tryptophan is an essential amino acid, which must be supplied from the diet. Most tryptophan can be absorbed by intestinal epithelial cells through solute carrier proteins. 28 It is metabolized in the cells or transported into the bloodstream and end organs for protein synthesis and metabolism. Most tryptophan is metabolized through Kynurenine (Kyn) pathway, which accounts for approximately 90% to 95%, whereas 1% to 2% tryptophan is converted into serotonin (5-HT) and 4% to 6% undergoes indole pathway.29,30 In extra-intestinal tissues, tryptophan is either used to produce proteins with various functions such as enzymes, neurotransmitters, and muscles or metabolized through Kyn and 5-HT pathways. 31 Tryptophan is also necessary for the production of other active molecules such as niacin (vitamin B3).
The resulting metabolites from tryptophan metabolism pathways could be pro-inflammatory and anti-inflammatory as well as immunomodulatory. These pathways need to be sophisticatedly regulated so that adequate levels of tryptophan and its metabolites are transported to the systemic circulation. Dysregulation of the tryptophan pathway has been associated with various chronic diseases such as cancer, depression, multiple sclerosis, inflammatory bowel diseases, and cardiovascular diseases.32,33 For example, increased quinolinic acid (QUIN) levels are associated with depression. 34 Intestinal inflammation can occur if there is a deficiency of tryptophan and/or key metabolites of tryptophan, which can also be caused by abnormalities in microbiome tryptophan metabolism.35,36 The products from Kyn pathway can also affect neurotransmitters; kynurenic acid (KYNA) inhibits glutamate receptor while QUIN activates it. 37 Kyn can protect the retina of the eye from UV. Kyn/tryptophan has been used to indicate the inflammatory status; although, it could be unreliable in some cases.35,38,39 Serotonin is an important regulator of appetite, sleep, mood, behavior, cognition, and pain and its metabolite melatonin controls sleep pattern, circadian rhythms of behavior, and physiology. 31 Unabsorbed tryptophan is metabolized by bacteria into indole and its derivatives, which can improve gut barrier and modulate immune responses through Aryl hydrocarbon receptor (AHR). 40 We will emphasize on the discussion on the tryptophan metabolism pathways in NASH in association with gut dysbiosis.
Indole pathway
Tryptophan is metabolized to indole by deamination by the bacterial enzyme tryptophanase. 41 Tryptophanase is produced by various commensal intestinal bacterial genera such as Prevotella, Bacteroides, Fusobacterium, Escherichia. Sasaki-Imamura et al 42 characterized the expression of the gene encoding tryptophanase tnaA in 22 Prevotella species and found 6 species expressed tryptophanase. Indole promoted biofilm formation, which protected indole-producing bacteria from invasion of other bacteria; exogenous tryptophan increased indole production and biofilm formation. 43
Intestinal dysbiosis associated with NAFLD has been demonstrated to cause decreased production of indole and its derivatives in both animal models and humans.44,45 Levels of the indole derivative, indole-3-acetate, was lower in germ-free mice than in conventionally raised mice. 44 In addition, a high-fat diet also resulted in lower levels of indo-3-acetate. 44 In humans, blood levels of indole were inversely correlated with body mass index. 45 The blood levels of indole in obese patients were significantly lower than that observed in lean patients and inversely correlated with hepatic lipid accumulation. 45
Indole and indole derivatives such as indoxyl-3-sulfate, indole-3-propionic acid, and indole-3-aldehyde have been shown to have anti-inflammatory effects. 46 Knudsen et al 47 showed that administration of low levels of indole (8-10 mg/kg) decreased liver inflammation in ob/ob mice, as indicated by decreased expression of cd68 and Itgax (Cd11c) in macrophages and Ccl2 and Cxcl2 in neutrophils and monocytes. Beaumont et al 48 showed that indole (20-50 mg/kg) reduced LPS-induced liver inflammation in both ob/ob and control mice through the inhibition of the NF-kB signaling pathway. Indole reduced both hepatic steatosis and inflammation through activation PFKFB3 and suppressed macrophage activities in PFKFB3-dependent manner.45,49 Shimada et al 50 demonstrated that germ-free mice had lower levels of indole and increased intestinal barrier permeability compared to specific-pathogen-free mice; and oral administration of indole protected against dextran sodium sulfate induced epithelial damage and colitis. Ji et al 51 demonstrated that indole-3-acetic acid reduced lipogenesis and inflammation as well as indicators of metabolic syndrome. Another study showed that administration of indole-3-propionic acid in high-fat diet fed rats reduced intestinal dysbiosis, decreased intestinal barrier permeability, decreased blood LPS levels and hepatic inflammatory cytokines. 52 Therefore, indole and its derivatives may be important mediators in the recovery of intestinal dysbiosis and hence in the pathogenesis of NASH and supplementation may provide beneficial therapeutic effects.
Serotonin pathway
Serotonin is synthesized from tryptophan by tryptophan hydroxylase 1 (TPH1) in peripheral non-nervous tissue and by TPH2 in the central and peripheral nervous system. 54 As 5-HT cannot pass through blood brain barrier, the two 5-HT systems are separated. 54 The small amount of 5-HT that is synthesized in brain plays a critical role for neurophysiology.
It has been demonstrated that 5-HT is increased in NASH both in patients and animal models. Wang et al 55 characterized blood levels of 5-HT in NASH patients and found that it was highly increased. Choi et al 56 showed that the hepatic steatosis induced by a high-fat diet was dependent on increased blood concentrations of 5-HT. Fluoxetine induced hepatic lipid accumulation through upregulation of TPH1 expression and subsequent increased blood concentration of 5-HT. 57
In a high-fat high-sucrose diet rat model, inhibition of TPH1 by LP533401 or dietary control of tryptophan reduced hepatic steatosis and expression of inflammatory factors Tnfa, Il-6, and Mcp-1 genes. 55 In BRL-3A cells, 5-HT increased expression of lipogenesis-related genes Fas, Cd36, and Plin2. Wang et al 55 also demonstrated that 5-HT bound to its receptor HTR2A activated PPAR-gamma2, progressing lipid accumulation and proinflammatory factor production. PPAR-gamma2 is known to be involved in the pathogenesis of NASH. 58 Intestinal-specific knockout of THP1 or liver-specific knockout of Htr2a decreased hepatic steatosis. Treatment of high fat diet mice with para-chlorophenylalanine to inhibit 5-HT synthesis or with sarpogrelate to inhibit HTR2A activity reduced hepatic lipid accumulation.47,50 Crane et al. 53 found another mechanism for protective effect of inhibition of THP1 on high-fat diet induced NAFLD. Inhibition of THP1 reduced blood levels of 5-HT, which increased brown adipose tissue sensitivity to noradrenaline and beta-adrenergic receptor, leading to increased thermogenesis and thus reduced lipid accumulation and inflammation.
Although 5-HT has pro-inflammatory effect, its metabolite melatonin has been demonstrated to have protective effect in NAFLD. Treatment of NAFLD patients with melatonin reduced blood levels of proinflammatory cytokines, improved fat deposit and decreased liver enzyme activities.59,60 The associated mechanisms have been studied in animal models. Melatonin was reported to inhibit LPS-induced SREBP-c, 61 HFD-stimulated p38 MAPK and JNK pathways, 62 microRNA34a-5p expression, 63 and NLRP3 inflammasome. 64
Kynurenine pathway
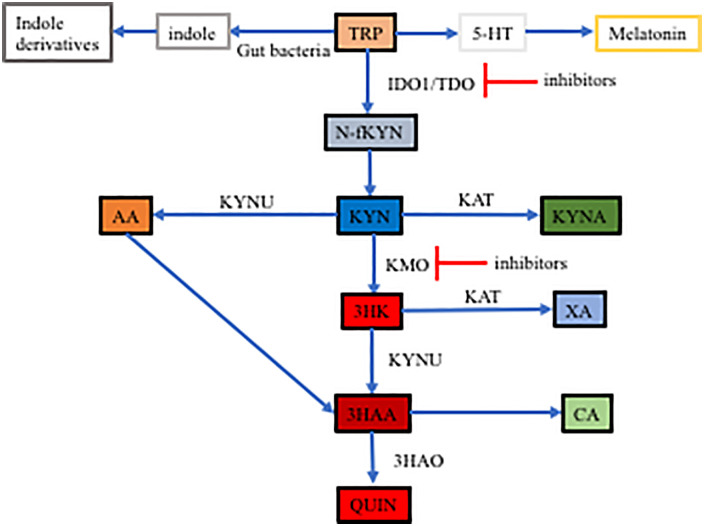
In Kyn pathway, tryptophan is metabolized to N-formyl-Kyn through tryptophan 2,3-dioxygenase (TDO) which is in the liver only and indoleamine-2,3-dioxygenase isoforms 1 and 2 (IDO1 and IDO2) (Figure 2). 65 IDO1 expresses extensively in the intestinal and extra-intestinal tissues but IDO2 is restricted to the liver, kidney, dendritic cells, and B cells. 66 IDO2 has much lower catabolizing activity than IDO1. 66 Unstable N-formyl-Kyn is quickly converted into Kyn by the enzyme formamidase. Kyn is a central molecule of the Kyn pathway and is further metabolized into 3 derivatives. The metabolites of Kyn could be proinflammatory such as QUIN or anti-inflammatory such as KYNA. 67 Kyn is catalyzed by Kyn 3-monooxygenase (KMO) into 3HK (3-hydroxykynurenine), which is converted into 3-HAA (3-hydroxy anthranilic acid) by kynureninase (KYNU) and further metabolized to QUIN. Kyn can also be converted into anthranilic acid (AA) by KYNU and AA is converted to 3-HAA. In these 2 metabolizing routes, the main metabolites are proinflammatory. Kyn can be converted by Kyn aminotransferase (KAT) into KYNA.
Figure 2.
Tryptophan metabolism pathway in the intestines.
Kynurenine is catalyzed by various enzyme to metabolites in the intestines in 3 pathways.
Abbreviations: AA, anthranilic acid; IDO1, tryptophan 2,3-dioxygenase 1; KAT, Kyn aminotransferase; KMO, Kyn 3-monooxygenase KMO; Kyn, kynurenine; KYNA, kynurenic acid; KYNU, kynureninase; QUIN, quinolinic acid; TRP, tryptophan; 3-HAA, 3-hydroxy anthranilic acid; 3HAO, 3-hydroxyanthranilate 3.4-dioxygenase; 3HK, 3-hydroxykynurenine.
Changes in the Kyn pathway have been associated with inflammation. 68 When QUIN production increases and KYNA production decreases, this is indicative of proinflammatory status. 68 It was reported that intrastriatal injections of QUIN caused increased inflammatory cytokines IL-6 and TNF-alpha, while KYNA had a protective effect. 69 KYNA could block QUIN-induced neurodegeneration. 70 KMO, an enzyme that converts Kyn into the QUIN precursor, was increased in various inflammatory diseases and cancer. 70 Inhibition of KMO, which decreases QUIN and increases KYNA can reduce inflammatory factors and thus protect from inflammatory diseases. 70 In acute pancreatitis, KMO was increased and correlated with the severity of the disease; inhibition of KMO resulted in improvement of inflammation.71,72 KMO has also been found to be increased in cancers and associated with increased proliferation, invasion and migration of cancer cells.73,74 It is plausible that this effect may be caused by the activation of proinflammatory signaling pathways.
The Kyn pathway could play an important role in NASH development, given that LPS is increased in the intestines in NASH and progresses inflammation. Both LPS and inflammatory cytokines can activate IDO1. Liu et al 75 and Fujigaki et al 76 also demonstrated that IL-1beta and IFN-gamma induced IDO1. LPS has also been demonstrated to increase KMO and thus alter the Kyn pathway to facilitate QUIN production. 77 Laurans et al.78,79 showed that IDO1 was increased in obesity, which has been posited as causal for shifts in tryptophan metabolism from indole and IL-22 production to Kyn. Knockout or inhibition of IDO1 improved intestinal barrier and decreased endotoxinemia. 79 In contrast, in an IDO1 knockout, high-fat diet induced NAFLD murine model, liver inflammation was markedly increased with infiltration of macrophages and T lymphocytes as well as increased pro-inflammatory cytokines IL-1beta, IL-6, TNF-alpha. 80 This suggests that normal IDO1 activity is necessary as Kyn is not only converted into pro-inflammatory metabolites but anti-inflammatory metabolites.
KYNA has been shown to be beneficial in a NAFLD model; it decreases lipogenic gene expression and lipid accumulation through activation of AMP-activated protein Kinase. 81 Counter intuitively, a study showed that chemotherapy-induced intestinal toxicity in mouse models including irinotecan-induced rat diarrhea, vincristine-induced rat ileus, and DDS-induced colitis caused dramatically increased Kyn and KYNA. 82 The study also showed that the Kyn/TRP (tryptophan) ratio was increased, indicating IDO1 was increased in these models. In these models, IL-6 was also increased. The associated mechanisms were studied in cell culture systems. In cell culture, Kyn and KYNA promoted wound healing. IL-6 also increased Kyn and KYNA with wound healing, which was inhibited by IDO1 inhibitor 1 methyl-tryptophan. The mechanism has been associated with activation of AHR. Kyn and KYNA activated AHR, which increased IL-6 production and IL-6 activated IDO1 to form a loop. However, Kyn and KYNA were not sufficient to initiate the feed-forward loop. IL-6 at concentrations of 2.5 ng/ml was sufficient to initiate the loop. These studies indicate the complexity of the Kyn pathway in the pathogenesis of NASH.
Plausible Therapeutic Implications
As intestinal dysbiosis is causal for NASH, modulation of the intestinal microbiota has been proposed for the treatment of NASH. Multiple approaches could be implemented to improve the intestinal microbiota such as administration of probiotics, prebiotics, and synbiotics. Recently, a synbiotic has been proposed to treat NASH, which was formulated with Bifidobacteria sp and Fecalibacterium prausnitzii with the inclusion of dietary fibers with expectation of high production of butyrate, which reduces intestinal inflammation. 83 The reduced inflammation could affect tryptophan metabolism as described above to decrease production of proinflammatory tryptophan metabolites. All approaches to improve gut microbiota may correct an adversely shifted tryptophan metabolism in NASH; an area that has not been well elucidated, and as such further studies are warranted.
Modulation of intestinal commensal bacterial metabolites could be effective approaches for the treatment of NASH. Among them the adjustment of tryptophan pathway in NASH has not been well studied. There are several potential approaches to modulate tryptophan pathway, which may ameliorate NASH including supplementation of tryptophan, indole and indole derivatives, inhibiting metabolism of tryptophan to 5-HT, and altering the Kyn pathway.
Tryptophan has been supplemented into animal models of NAFLD but outcomes have been controversial. A study reported that supplementation of tryptophan to fructose fed mice ameliorated NAFLD indicated by decreased fat accumulation and liver/body weight through increasing gut barrier and modulation of serotonergic pathway. 84 However, another study revealed that supplementation of tryptophan increased steatosis in high-fat high-fructose fed mice but not in normal chow fed mice. 85 It was associated with increased production of 5-HT and activation of pro-inflammatory mTOR pathway. These controversial outcomes may reflect the different conditions in which tryptophan is skewed to produce different metabolites—proinflammatory or anti-inflammatory. Therefore, the supplementation of tryptophan for the treatment of NASH may need to be combined with the modulation of the key enzymes in tryptophan metabolism pathway.
In animal models, indole and indole derivatives are effective in reducing inflammation in NASH.47,48,51,52 This provides a potential for indole and indole derivatives to be used in the treatment of NASH. A recent study correlated the anti-inflammatory effects of garvage administration of indole-3-acetic acid in an animal model with an increase of indole-3-acetic acid in obese NAFLD patients after sleeve gastrectomy, which reduced hepatic inflammation. 86 In a mouse model, administration of indole-3-acetic acid led to activation and differentiation of hepatic M2 macrophages, thus reducing the ratio of M1/M2, which was confirmed in cell cultures of macrophages and hepatocytes. Sleeve gastrectomy is known to reduce steatohepatitis. 87 Demonstration of the anti-inflammatory effect of indole-3-acetic acid in an animal model highly suggests the use of indole derivatives in NASH patients, which warrants confirmation from a clinical trial. 71 Indeed, the natural indole derivative indole-3-carbinol, which has anti-inflammatory and chemo-preventive effects, has been used in phase I clinical trial for a safety, tolerability, and pharmacokinetics study. 88 In oral administration doses from 400 to 1200 mg, indole-3-carbinol is well tolerated. Pharmacokinetics study reported that indole-3-carbinol was rapidly metabolized into 3,3′-diindolylmethane (DIM) as only DIM was detected but no indole-3-carbinol in the blood samples. These results could indicate indole-3-acetic acid may also be safe for clinical trials.
Modulation of Kyn pathway could lead to increased anti-inflammatory metabolites and decreased pro-inflammatory metabolites. KMO inhibition is a practical option, which can increase KYNA and reduce QUIN. KMO has been extensively studied in neurological disorders and many KMO inhibitors have developed. The proinflammatory role of KMO in neurological chronic diseases have been well demonstrated. For example, KMO knockout in Huntington’s mouse model resulted in decreased toxic 3-HK and increased protective KYNA. 89 The blood levels of the proinflammatory cytokines were also decreased. A KMO inhibitor used in the Huntington’s model reduced 3HK and QUIN formation. 90 Furthermore, a brain permeable KMO inhibitor has been developed with potent effects, which is proposed to be used in a wide range of inflammatory neurological diseases. 91 The KMO inhibitors could also be tested for NASH and experience from the use of KMO inhibitors in neurological diseases may be helpful for NASH.
Overall, the disturbance of the tryptophan pathway could be an important part of gut dysbiosis-caused NASH and modulation of the pathway could have promising therapeutic implications in NASH. Several approaches could be adopted. However, it has been much less studied compared with that in neurological diseases. Future studies could clarify: (1) the roles of the specific bacterial species and enzymes produced from gut microbiota in the indole pathway, (2) the supplementation of individual metabolites from the tryptophan pathway and associated mechanisms involved in the pathogenesis of NASH, particularly recently identified NASH inflammatory T and B cells, (3) the effects of tryptophan supplementation in NASH in various conditions with different changes of tryptophan metabolism pathways as well as combination use of tryptophan with its metabolism pathway modulators, and (4) the interactions of metabolites/enzymes from tryptophan pathway with other bacterial metabolites and combination use such as butyrate and indole or butyrate and melatonin.
Footnotes
Author Contributions: JC and LV developed the concept of this work. Writing-original draft preparation was done by JC and LV. Writing - review and editing was done by LV, JDH and SH.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jiezhong Chen  https://orcid.org/0000-0003-1977-6143
https://orcid.org/0000-0003-1977-6143
References
- 1. Wong VW, Chitturi S, Wong GL, Yu J, Chan HL, Farrell GC. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol Hepatol. 2016;1:56-67. [DOI] [PubMed] [Google Scholar]
- 2. Aron-Wisnewsky J, Vigliotti C, Witjes J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279-297. [DOI] [PubMed] [Google Scholar]
- 3. Hrncir T, Hrncirova L, Kverka M, et al. Gut microbiota and NAFLD: pathogenetic mechanisms, microbiota signatures, and therapeutic interventions. Microorganisms. 2021;9:957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh SP, Anirvan P, Reddy KR, et al. Non-alcoholic fatty liver disease: not time for an obituary just yet! J Hepatol. 2021;74:972-974. [DOI] [PubMed] [Google Scholar]
- 5. Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17:484-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juanola O, Martínez-López S, Francés R, Gómez-Hurtado I. Non-alcoholic fatty liver disease: metabolic, genetic, epigenetic and environmental risk factors. Int J Environ Res Public Health. 2021;18:5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marras L, Caputo M, Bisicchia S, et al. The role of bifidobacteria in predictive and preventive medicine: a focus on eczema and hypercholesterolemia. Microorganisms. 2021;9:836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leung DH, Yimlamai D. The intestinal microbiome and paediatric liver disease. Lancet Gastroenterol Hepatol. 2017;2:446-455. [DOI] [PubMed] [Google Scholar]
- 9. Krautkramer KA, Fan J, Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2021;19:77-94. [DOI] [PubMed] [Google Scholar]
- 10. Nguyen-Lefebvre AT, Horuzsko A. Kupffer cell metabolism and function. J Enzymol Metab. 2015;1:101. [PMC free article] [PubMed] [Google Scholar]
- 11. Carpino G, Del Ben M, Pastori D, et al. Increased liver localization of lipopolysaccharides in human and experimental NAFLD. Hepatology. 2020;72:470-485. [DOI] [PubMed] [Google Scholar]
- 12. Sharifnia T, Antoun J, Verriere TG, et al. Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol. 2015;309:G270-G278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int. 2010;4:659-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li L, Chen L, Hu L, et al. Nuclear factor high-mobility group box1 mediating the activation of toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology. 2011;54:1620-1630. [DOI] [PubMed] [Google Scholar]
- 15. Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kazankov K, Jørgensen SMD, Thomsen KL, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145-159. [DOI] [PubMed] [Google Scholar]
- 17. Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310-G1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem. 2012;287:40161-40172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krenkel O, Puengel T, Govaere O, et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018;67:1270-1283. [DOI] [PubMed] [Google Scholar]
- 20. Rai RP, Liu Y, Iyer SS, et al. Blocking integrin α(4)β(7)-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J Hepatol. 2020;73:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barrow F, Khan S, Fredrickson G, et al. Microbiota-Driven activation of intrahepatic B cells aggravates NASH through innate and adaptive signaling. Hepatology. 2021;74:704-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haas JT, Vonghia L, Mogilenko DA, et al. Author correction: transcriptional network analysis implicates altered hepatic immune function in NASH development and resolution. Nat Metab. 2019;1:744-614. [DOI] [PubMed] [Google Scholar]
- 23. Chen J, Vitetta L. Gut Microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int J Mol Sci. 2020;21:5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campos-Perez W, Martinez-Lopez E. Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866:158900. [DOI] [PubMed] [Google Scholar]
- 25. Ye J, Lv L, Wu W, et al. Butyrate protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels. Front Microbiol. 2018;9:1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng M, Qu F, Chen L, et al. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J Endocrinol. 2020;245:425-437. [DOI] [PubMed] [Google Scholar]
- 27. Jin CJ, Sellmann C, Engstler AJ, Ziegenhardt D, Bergheim I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br J Nutr. 2015;114:1745-1755. [DOI] [PubMed] [Google Scholar]
- 28. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716-724. [DOI] [PubMed] [Google Scholar]
- 29. Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wyatt M, Greathouse KL. Targeting dietary and microbial tryptophan-indole metabolism as therapeutic approaches to colon cancer. Nutrients. 2021;13:1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-Tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res. 2009;2:45-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joisten N, Ruas JL, Braidy N, Guillemin GJ, Zimmer P. The kynurenine pathway in chronic diseases: a compensatory mechanism or a driving force? Trends Mol Med. 2021;27:946-954. [DOI] [PubMed] [Google Scholar]
- 33. Sofia MA, Ciorba MA, Meckel K, et al. Tryptophan metabolism through the kynurenine pathway is associated with endoscopic inflammation in ulcerative colitis. Inflamm Bowel Dis. 2018;24:1471-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogyu K, Kubo K, Noda Y, et al. Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:16-25. [DOI] [PubMed] [Google Scholar]
- 35. Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Idzko M, Pitchford S, Page C. Role of platelets in allergic airway inflammation. J Allergy Clin Immunol. 2015;135:1416-1423. [DOI] [PubMed] [Google Scholar]
- 38. Badawy AA, Guillemin G. The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2,3-dioxygenase: time for appraisal. Int J Tryptophan Res. 2019;12:1178646919868978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen Y, Xie Z, Xiao C, et al. Peripheral kynurenine/tryptophan ratio is not a reliable marker of systemic indoleamine 2,3-dioxygenase: a lesson drawn from patients on hemodialysis. Oncotarget. 2017;8:25261-25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Modoux M, Rolhion N, Mani S, Sokol H. Tryptophan metabolism as a pharmacological target. Trends Pharmacol Sci. 2021;42:60-73. [DOI] [PubMed] [Google Scholar]
- 41. Jin UH, Lee SO, Sridharan G, et al. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 2014;85:777-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sasaki-Imamura T, Yoshida Y, Suwabe K, Yoshimura F, Kato H. Molecular basis of indole production catalyzed by tryptophanase in the genus Prevotella. FEMS Microbiol Lett. 2011;322:51-59. [DOI] [PubMed] [Google Scholar]
- 43. Sasaki-Imamura T, Yano A, Yoshida Y. Production of indole from L-tryptophan and effects of these compounds on biofilm formation by Fusobacterium nucleatum ATCC 25586. Appl Environ Microbiol. 2010;76:4260-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krishnan S, Ding Y, Saeidi N, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23:1099-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ma L, Li H, Hu J, et al. Indole alleviates diet-induced hepatic steatosis and inflammation in a manner involving myeloid cell 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3. Hepatology. 2020;72:1191-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rothhammer V, Mascanfroni ID, Bunse L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Knudsen C, Neyrinck AM, Leyrolle Q , et al. Hepatoprotective effects of indole, a Gut microbial metabolite, in leptin-deficient obese mice. J Nutr. 2021;151:1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beaumont M, Neyrinck AM, Olivares M, et al. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J. 2018;32:fj201800544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dai X, Hou H, Zhang W, et al. Microbial metabolites: critical regulators in NAFLD. Front Microbiol. 2020;11:567654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shimada Y, Kinoshita M, Harada K, et al. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One. 2013;8:e80604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ji Y, Gao Y, Chen H, Yin Y, Zhang W. Indole-3-Acetic acid alleviates nonalcoholic fatty liver disease in mice via attenuation of hepatic lipogenesis, and oxidative and inflammatory stress. Nutrients. 2019;11:2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao ZH, Xin FZ, Xue Y, et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp Mol Med. 2019;51:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Crane JD, Palanivel R, Mottillo EP, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunctionby promoting brown adiposetissue thermogenesis. Nat Med. 2015;21:166-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. [DOI] [PubMed] [Google Scholar]
- 55. Wang L, Fan X, Han J, et al. Gut-Derived serotonin contributes to the progression of non-alcoholic steatohepatitis via the liver HTR2A/PPARγ2 pathway. Front Pharmacol. 2020;11:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Choi W, Namkung J, Hwang I, et al. Publisher correction: serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat Commun. 2019;10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ayyash A, Holloway AC. Fluoxetine-induced hepatic lipid accumulation is linked to elevated serotonin production. Can J Physiol Pharmacol. 2021;99:983-988. [DOI] [PubMed] [Google Scholar]
- 58. Morán-Salvador E, López-Parra M, García-Alonso V, et al. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011;25:2538-2550. [DOI] [PubMed] [Google Scholar]
- 59. Celinski K, Konturek PC, Slomka M, et al. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease-14 months follow up. J Physiol Pharmacol. 2014;65:75-82. [PubMed] [Google Scholar]
- 60. Bahrami M, Cheraghpour M, Jafarirad S, et al. The effect of melatonin on treatment of patients with non-alcoholic fatty liver disease: a randomized double blind clinical trial. Complement Ther Med. 2020;52:102452. [DOI] [PubMed] [Google Scholar]
- 61. Chen X, Zhang C, Zhao M, et al. Melatonin alleviates lipopolysaccharide-induced hepatic SREBP-1c activation and lipid accumulation in mice. J Pineal Res. 2011;51:416-425. [DOI] [PubMed] [Google Scholar]
- 62. Sun H, Wang X, Chen J, et al. Melatonin improves non-alcoholic fatty liver disease via MAPK-JNK/P38 signaling in high-fat-diet-induced obese mice. Lipids Health Dis. 2016;15:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stacchiotti A, Grossi I, García-Gómez R, et al. Melatonin effects on non-alcoholic fatty liver disease are related to MicroRNA-34a-5p/Sirt1 axis and autophagy. Cells. 2019;8:1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yu Y, Chen D, Zhao Y, Zhu J, Dong X. Melatonin ameliorates hepatic steatosis by inhibiting NLRP3 inflammasome in db/db mice. Int J Immunopathol Pharmacol. 2021;35:20587384211036819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ito H, Hoshi M, Ohtaki H, et al. Ability of IDO to attenuate liver injury in alpha-galactosylceramide-induced hepatitis model. J Immunol. 2010;185:4554-4560. [DOI] [PubMed] [Google Scholar]
- 66. Merlo LMF, DuHadaway JB, Montgomery JD, et al. Differential roles of IDO1 and IDO2 in T and B cell inflammatory immune responses. Front Immunol. 2020;11:1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Baumgartner R, Forteza MJ, Ketelhuth DFJ. The interplay between cytokines and the kynurenine pathway in inflammation and atherosclerosis. Cytokine. 2019;122:154148. [DOI] [PubMed] [Google Scholar]
- 68. Baumgartner R, Berg M, Matic L, et al. Evidence that a deviation in the kynurenine pathway aggravates atherosclerotic disease in humans. J Intern Med. 2021;289:53-68. [DOI] [PubMed] [Google Scholar]
- 69. Ferreira FS, Schmitz F, Marques EP, Siebert C, Wyse ATS. Intrastriatal quinolinic acid administration impairs redox homeostasis and induces inflammatory changes: prevention by kynurenic acid. Neurotox Res. 2020;38:50-58. [DOI] [PubMed] [Google Scholar]
- 70. Zwilling D, Huang SY, Sathyasaikumar KV, et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145:863-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mole DJ, Webster SP, Uings I, et al. Kynurenine-3-monooxygenase inhibition prevents multiple organ failure in rodent models of acute pancreatitis. Nat Med. 2016;22:202-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Skouras C, Zheng X, Binnie M, et al. Increased levels of 3-hydroxykynurenine parallel disease severity in human acute pancreatitis. Sci Rep. 2016;6:33951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jin H, Zhang Y, You H, et al. Prognostic significance of kynurenine 3-monooxygenase and effects on proliferation, migration, and invasion of human hepatocellular carcinoma. Sci Rep. 2015;5:10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Heng B, Bilgin AA, Lovejoy DB, et al. Differential kynurenine pathway metabolism in highly metastatic aggressive breast cancer subtypes: beyond IDO1-induced immunosuppression. Breast Cancer Res. 2020;22:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu JJ, Raynal S, Bailbé D, et al. Expression of the kynurenine pathway enzymes in the pancreatic islet cells. Activation by cytokines and glucolipotoxicity. Biochim Biophys Acta. 2015;1852:980-991. [DOI] [PubMed] [Google Scholar]
- 76. Fujigaki S, Saito K, Sekikawa K, et al. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-γ-independent mechanism. Eur J Immunol. 2001;31:2313-2318. [DOI] [PubMed] [Google Scholar]
- 77. Molteni R, Macchi F, Zecchillo C, et al. Modulation of the inflammatory response in rats chronically treated with the antidepressant agomelatine. Eur Neuropsychopharmacol. 2013;23:1645-1655. [DOI] [PubMed] [Google Scholar]
- 78. Cussotto S, Delgado I, Anesi A, et al. Tryptophan metabolic pathways are altered in obesity and are associated with systemic inflammation. Front Immunol. 2020;11:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Laurans L, Venteclef N, Haddad Y, et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med. 2018;24:1113-1120. [DOI] [PubMed] [Google Scholar]
- 80. Nagano J, Shimizu M, Hara T, et al. Effects of indoleamine 2,3-dioxygenase deficiency on high-fat diet-induced hepatic inflammation. PLoS One. 2013;8:e73404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pyun DH, Kim TJ, Kim MJ, et al. Endogenous metabolite, kynurenic acid, attenuates nonalcoholic fatty liver disease via AMPK/autophagy- and AMPK/ORP150-mediated signaling. J Cell Physiol. 2021;236:4902-4912. [DOI] [PubMed] [Google Scholar]
- 82. Wang D, Li D, Zhang Y, et al. Functional metabolomics reveal the role of AHR/GPR35 mediated kynurenic acid gradient sensing in chemotherapy-induced intestinal damage. Acta Pharm Sin B. 2021;11:763-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ralli T, Neupane YR, Saifi Z, Kohli K. Gut microbiota as an emerging therapeutic avenue for the treatment of non-alcoholic fatty liver disease. Curr Pharm Des. 2021;27:4677-4685. [DOI] [PubMed] [Google Scholar]
- 84. Ritze Y, Bárdos G, Hubert A, Böhle M, Bischoff SC. Effect of tryptophan supplementation on diet-induced non-alcoholic fatty liver disease in mice. Br J Nutr. 2014;112:1-7. [DOI] [PubMed] [Google Scholar]
- 85. Osawa Y, Kanamori H, Seki E, et al. L-tryptophan-mediated enhancement of susceptibility to nonalcoholic fatty liver disease is dependent on the mammalian target of rapamycin. J Biol Chem. 2011;286:34800-34808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang Y, Wang G, Bai J, et al. Role of indole-3-acetic acid in NAFLD amelioration after sleeve gastrectomy. Obes Surg. 2021;31:3040-3052. [DOI] [PubMed] [Google Scholar]
- 87. de Brito E, Silva MB, Tustumi F, de Miranda Neto AA, Dantas ACB, Santo MA, Cecconello I. Gastric bypass compared with sleeve gastrectomy for nonalcoholic fatty liver disease: a systematic review and meta-analysis. Obes Surg. 2021;31:2762-2772. [DOI] [PubMed] [Google Scholar]
- 88. Reed GA, Arneson DW, Putnam WC, et al. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477-2481. [DOI] [PubMed] [Google Scholar]
- 89. Bondulich MK, Fan Y, Song Y, Giorgini F, Bates GP. Ablation of kynurenine 3-monooxygenase rescues plasma inflammatory cytokine levels in the R6/2 mouse model of Huntington’s disease. Sci Rep. 2021;11:5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Beaumont V, Mrzljak L, Dijkman U, et al. The novel KMO inhibitor CHDI-340246 leads to a restoration of electrophysiological alterations in mouse models of Huntington’s disease. Exp Neurol. 2016;282:99-118. [DOI] [PubMed] [Google Scholar]
- 91. Kimura H, Suda H, Kassai M, et al. N-(6-phenylpyridazin-3-yl)benzenesulfonamides as highly potent, brain-permeable, and orally active kynurenine monooxygenase inhibitors. Bioorg Med Chem Lett. 2021;33:127753. [DOI] [PubMed] [Google Scholar]