Abstract
This is a focused review looking at the pharmacological support in cardiogenic shock. There are a plethora of data evaluating vasopressors and inotropes in septic shock, but the data are limited for cardiogenic shock. This review article describes in detail the pathophysiology of cardiogenic shock, the mechanism of action of different vasopressors and inotropes emphasizing their indications and potential side effects. This review article incorporates the currently used specific risk-prediction models in cardiogenic shock as well as integrates data from many trials on the use of vasopressors and inotropes. Lastly, this review seeks to discuss the future direction for vasoactive medications in cardiogenic shock.
Keywords: Cardiogenic shock, acute myocardial infarction, vasopressors, inotropes, mechanical circulatory support
Introduction
Cardiogenic shock is defined as inadequate tissue hypoperfusion caused due to a primary cardiac dysfunction.1,2 Delayed recognition and treatment of cardiogenic shock leads to rapid deterioration in clinical status with nearly 50% in-hospital mortality in the contemporary era despite advances in medical therapies, specifically prompt mechanical revascularization with thrombolysis, percutaneous coronary intervention, and coronary artery bypass grafting.2-4 The overall incidence of acute myocardial infarction (AMI) with cardiogenic shock is 5% to 10%, with higher rates in ST-segment-elevation MI as compared to non-ST-segment-elevation MI. 5 The overall incidence of cardiogenic shock in the past 2 decades have remained unchanged. cardiogenic shock is still the commonest cause of mortality in patients admitted to the hospital with AMI. 4 Studies by Fang et al 6 and Helgestad et al 7 have shown a decrease in the incidence of AMI-cardiogenic shock, whereas the studies by Babaev 8 and Kolte 9 have reported increased to unchanged incidence of AMI-cardiogenic shock in the past 2 to 3 decades.10,11 Though the in-hospital mortality has improved in recent times, 12 the long-term mortality remains elevated at nearly 50%.13,14
Definition
The clinical criteria for the diagnosis of cardiogenic shock based on the SHOCK (Should We Emergently Revascularize Occluded Coronaries For Cardiogenic Shock) and the IABP-SHOCK II (Intra-aortic Balloon Pump in Cardiogenic Shock II) trials were defined by systolic blood pressure (SBP) <90 mm Hg for >30 minutes, use of mechanical or pharmacologic support to maintain SBP >90 mm Hg, urine output <30 mL/hour, cardiac index (CI) <2.2 L/minute/m2, pulmonary capillary wedge pressure (PCWP) >15 mm Hg and lactate >2 mmol/L.2,4 The 2016 European Society of Cardiology-Heart Failure guidelines include clinical criteria along with hemodynamic criteria in the definition of cardiogenic shock: SBP <90 mm Hg despite appropriate fluid resuscitation with clinical and laboratory evidence of end organ damage.15-17 Clinical criteria were defined as cold extremities, oliguria, altered mental status change, and narrow pulse pressure, and laboratory abnormalities included metabolic acidosis, elevated serum lactate and elevated creatinine.4,18
Etiology
The commonest etiology of cardiogenic shock is left ventricular (LV) failure in the setting of AMI. Cardiogenic shock is seen more commonly with anterior AMI 19 when compared to inferior AMI.3,20 Acute complications of AMI such as ventricular septal rupture, 21 LV free wall rupture, acute mitral regurgitation, and cardiac tamponade account for the other ischemic etiologies of cardiogenic shock. 3 Other non-ischemic etiologies 22 include valvular regurgitation and stenosis, aortic dissection, primary LV dysfunction like acute decompensated heart failure, post cardiotomy syndromes, 23 takotsubo cardiomyopathy,24,25 myocarditis, infiltrative disorders 26 (like sarcoidosis, amyloidosis, hemochromatosis), acute tachyarrhythmia and bradyarrhythmia, 27 infective endocarditis, pericarditis and constrictive pericarditis. 28 Rare causes include severe hypertrophic obstructive cardiomyopathy, peri, and post-partum cardiomyopathy. 29 Pharmacological agents like beta blockers when used in early AMI-cardiogenic shock were associated with higher incidence of cardiogenic shock in the COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction) trial. 30 Overdose with flecainide, 31 calcium channel blockers 32 and digoxin have been implicated in cardiogenic shock in some case reports.33,34
Staging of Cardiogenic Shock
Patients with cardiogenic shock have a varying continuum of presentation ranging from isolated myocardial dysfunction to multiorgan dysfunction with severe hemodynamic compromise to cardiac arrest. 35 The prognosis of cardiogenic shock is also variable in patients with different etiologies, severity, and comorbid condition.35,36 The Society for Angiography and Cardiovascular Interventions (SCAI) has recently proposed a classification system for cardiogenic shock encompassing clinical, biochemical, and hemodynamic parameters to guide treatment and classify outcomes. The 5 stages of cardiogenic shock are (Figure 1):
Figure 1.
The society for angiography and cardiovascular interventions (SCAI) staging of cardiogenic shock.
Adapted with permission from Baran et al. 35
Abbreviations: CPR, cardiopulmonary resuscitation; CVP, central venous pressure; ECMO, extra corporeal membrane oxygenation; JVP, jugular venous pulsations; LFTs, liver function test; MAP, mean arterial blood pressure; PA Sat, pulmonary artery saturation; PCWP, pulmonary capillary wedge pressure; PEA, pulseless electrical activity; SBP, systolic blood pressure.
Stage A: Patients “at risk” of developing cardiogenic shock and include patients with an acute MI, previous or subacute MI and decompensated heart failure. Cardiovascular examination is normal with adequate perfusion status. Renal function and lactate are also within normal limits. Hemodynamic parameters reflecting normotension, CI >2.5 L/minute/m2, central venous pressure <10 mm Hg and pulmonary artery saturation >65%.
Stage B: Beginning of cardiogenic shock/compensated cardiogenic shock; patient with clinical evidence of tachycardia and hypotension but without hypoperfusion. Cardiovascular examination with elevated jugular venous pressure, rales in the lung fields but with normal perfusion. Evidence of elevated BNP and mild renal impairment on labs. Hemodynamics with SBP <90 mm Hg or mean arterial pressure (MAP) <60 or >30 mm Hg drop from baseline. Pulse >100 bpm, CI > 2.2 L/minute/m2, pulmonary artery saturation >65%.
Stage C: Classic cardiogenic shock; Patient with hypoperfusion, presenting as relative hypoperfusion requiring pharmacological or mechanical support beyond volume resuscitation to restore perfusion. Physical examination indicative of multiorgan dysfunction and hypoperfusion—volume overload, respiratory distress with extensive rales (Killip class 3/4) requiring positive pressure or mechanical ventilation, cold clammy extremities, alteration of mental status, urine output <30 mL/hour. Biochemical markers with elevated lactate, doubling of creatinine or >50% drop in eGFR and elevated BNP. Hemodynamics with SBP <90 mm Hg, MAP <60 mm Hg or >30 mm Hg drop from baseline and with drugs/devices to maintain SBP above these targets. CI < 2.2 L/minute/m2, PCWP > 15 mm Hg, pulmonary artery pulsatility index 37 <1.85 and cardiac power output ⩽0.6 W.
Stage D: Deteriorating/doom; Patient with clinical and hemodynamic deterioration despite intensive initial resuscitation for >30 minutes with failure to respond, requiring further escalation of therapy. Clinical exam with any stage C features. Labs indicating deterioration from stage C. Hemodynamics with parameters in stage C and requiring multiple vasopressors or addition of mechanical circulatory support devices to maintain perfusion.
Stage E: Extremis; Patient with circulatory collapse in refractory cardiac arrest with ongoing cardiopulmonary resuscitation 38 and/or supported by extra corporeal membrane oxygenation 39 and multiple simultaneous interventions. Physical exam with pulselessness, cardiac collapse, mechanical ventilation or use of defibrillator. Biochemical abnormalities with pH <7.2, lactate ⩾ 5, critically deteriorating biomarkers. Hemodynamics indicating no SBP without resuscitation, pulseless electrical activity or refractory ventricular tachycardia ventricular fibrillation and hypotension without mechanical support. 40
Use of Vasoactive Medications in Various CS Stages
In the staging of CS by SCAI, vasoactive medications have been treated as a binary factor, but cumulative totals have not been considered.35,41 The outcomes of use of vasopressors and inotropes in different stages of CS has not been studied in randomized control trials. Several observational studies performed to validate the SCAI CS staging and to predict the mortality in various stages have demonstrated the use of vasoactive medications in different CS stages and the mortality outcomes. A study by Thayer et al, 42 showed that, out of the 1414 study participants in various stages of CS, the number and the requirements of vasoactive medications steadily increased with deteriorating SCAI CS stages (100% use of 2+ vasoactive medications in SCAI Stage E vs 57.8% use of 2+ vasoactive medications in SCAI Stage D CS). Another study by Jentzer et al 43 with a study population of 10,004 patients demonstrated increasing number of vasoactive agents use and earlier initiation of vasoactive agents in Stage D and E compared to earlier SCAI stages of shock (0.4 ± 0.6 and 34% in Stage D vs 1.4 ± 1.0 and 85% in Stage E). Higher stages of CS had aggressive use of vasoactive medications and higher mortality compared to lower stages. These studies, evaluate vasopressors and inotropes as a relative number of each medication. Prior work from the septic shock literature has shown that cumulative vasoactive medication measurements have significant mortality prediction41,44,45. However, the influence of cumulative vasoactive medication measurements and the influence of these medications on the SCAI Shock staging remains to be studied.
Pathophysiology
The pathophysiology of cardiogenic shock is complex and is characterized by profound depression of myocardial contractility resulting in a downward spiral in which progressive myocardial dysfunction leads to decreased stroke volume, cardiac output (CO) and low MAP, which reduces the myocardial perfusion. 46 This constitutes a vicious cycle which exacerbates ischemia, and further depress the myocardial function, stroke volume, and systemic perfusion.1,2 In cardiogenic shock, sympathetic stimulation occurs as a compensatory mechanism that increases CO by increasing the heart rate (HR) and contractility, at the cost of increased myocardial oxygen demand. 46 Compensatory peripheral vasoconstriction increases MAP at the cost of increased myocardial afterload and cause further worsening of myocardial function. The reduction in CI leads to severe tissue hypoperfusion, can worsen the ischemia and may finally result in refractory shock and subsequent death.46,47 As shock progresses, there is prolonged cellular hypoxia leading to depletion of adenosine triphosphate and intracellular energy reserves. This causes the active energy-dependent ion transport pumps to fail, eventually causing the myocardial cells to swell by osmosis due to the build-up of intracellular sodium, calcium, and hydrogen. These changes in membrane potential can lead to apoptosis of the cells through the activation of caspases and eventual cell death. 48 Alternatively, cardiac injury triggers systemic inflammation which can induce pathological vasodilation. Inducible nitric oxide synthase after exposure to inflammatory mediators produce high nitric oxide levels, along with its derivative, peroxynitrite which are cardiotoxic. 49 Systemic vasodilation is also caused by other inflammatory mediators like interleukins and tumor necrosis factor and have been associated with mortality in cardiogenic shock.1,2 Vasopressors and inotropes are a group of drugs that create vasoconstriction or increase cardiac contractility in patients with cardiogenic shock. While vasopressors increase vasoconstriction leading to increased systemic vascular resistance (SVR), inotropes increase cardiac contractility and improve CO. These drugs work synergistically in maintaining MAP and organ perfusion summarized as MAP = CO × SVR 46 (Figure 2).
Figure 2.
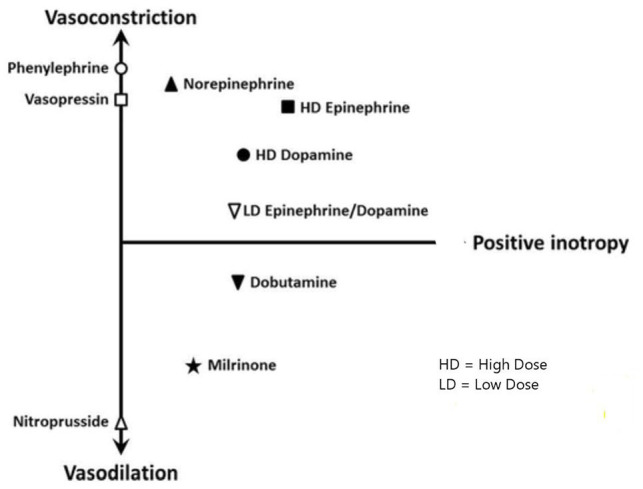
Vascular response to vasopressors and inotropic medications.
Adapted with permission from Jentzer et al. (2015). 49
Abbreviations: HD, high dose; LD, low dose.
Right ventricular (RV) failure occurs in the setting of diminished myocardial systolic and diastolic pressures. This leads to inadequate forward flow in the RV which contributes to decreased perfusion and increased venous pressures. 13 Due to the low-pressure circuit and smaller myocardial mass, the RV is prone to early dilatation as compared to the LV further compromising venous return and LV preload. Additionally, bowing of the interventricular septum into the LV further diminishing filling and stroke volume exacerbating the perfusion deficits. 50 A full discussion of the RV pathology is beyond the scope of the current review and we direct the readers to excellent prior reviews.14,46,47,50
Management of Cardiogenic Shock
The incidence and mortality of cardiogenic shock has remained unchanged in the last two decades at greater than 50% despite interventional and therapeutic advances.4,51 Although early revascularization (percutaneous coronary intervention/coronary artery bypass grafting) remains the only treatment modality with proven mortality benefits in the long term,3,52,53 cardiogenic shock is still the leading cause of death in hospitalized patients with AMI-cardiogenic shock.4,54 Diversity of the etiopathology, wide clinical spectrum of cardiogenic shock has led to the incongruity in standardizing diagnosis and variations in management strategies. 55 Hence a multidisciplinary shock team approach was conceptualized55-57 which has shown mortality benefit in cardiogenic shock. The multidisciplinary team approach involves rapid identification of shock state depending on the SHOCK, IABP SHOCK II or European Society of Cardiology 2015 definitions, activation of the multidisciplinary shock team involving interventional cardiologist, cardiothoracic surgeons, heart failure specialists and intensivists.58,59 This is followed by early invasive hemodynamic monitoring with optimizing medical therapy, early mechanical circulatory device support of the left or right ventricle and cardiac recovery. 56 Although the isolated use of mechanical circulatory support devices for cardiogenic shock was not associated with improved mortality outcomes, early use in the shock team approach was associated with improved mortality benefit.56,57 Initial medical management of cardiogenic shock along with invasive hemodynamic management to achieve adequate tissue perfusion, 60 maintenance of euvolemia and prevention of multiorgan dysfunction is recommended.
Vasoactive Medications in Cardiogenic Shock
Vasopressors are used in almost 90% of cardiogenic shock patients and form a class IIc and level C evidence of United States and European Society of Cardiology guidelines.1,61,62 To achieve increased cardiac performance both RV and LV function need augmentation. 50 This involves optimizing preload and decreasing cardiac afterload while achieving optimal contractility for the diseased ventricle. 48 Inotropic agents are indicated in patients with tissue hypoperfusion despite adequate volume resuscitation. 63 Cardiogenic shock is also associated with decreased vascular resistance due to various proinflammatory pathways which contributes to the hypotension.63-65 Vasopressors are used to maintain MAP and tissue perfusion pressures with refractory hypotension. Treatment of cardiogenic shock using pharmacologic agents must optimally balance achieving the best tissue perfusion without significantly increasing cardiac work.1,62,63 Hence the pharmacologic therapy should be used for the shortest duration and with the lowest possible dose. 64
Inotropes
Inotropes are a group of medicines that alter the cardiac contractility of the heart muscle and increase the force of myocardial contraction. Inotropes can be broadly divided into 3 groups: adrenergic agonists, phosphodiesterase III inhibitors, and calcium sensitizers.62,66 The adrenergic agonists exert their positive inotropic effects by acting on beta-adrenergic receptors. They increase the HR, stroke volume and the CO. 67 The adrenergic agonists, dobutamine, dopamine, norepinephrine, and epinephrine, can be classified further based on their effects on systemic vascular resistance (inopressors or inodilators). Milrinone and dobutamine are the only 2 inodilators approved in the United States. 66
Dopamine exerts its effects on cardiovascular system via 4 receptors: dopaminergic type 1 and 2 and adrenergic alpha 1 and beta 1 receptors. At lower doses (<2.5 μg/kg/minute), it causes vasodilation of coronary, renal and splanchnic vessels. At intermediate doses of 3 to 5 μg/kg/minute, it causes significant inotropic and chronotropic effects via beta-1 receptors of cardiomyocytes. At doses (>5 μg/kg/minute), it causes potent vasoconstriction via alpha-1 adrenergic receptors of the vessels. This could lead to severe hypertension and tachyarrhythmia at these high doses. Dopamine and epinephrine have strong beta-adrenergic effect and cause increased HR, stroke volume and CO. The effect of dopamine is maximum in the dose range of 5 to 10 μg/kg/minute, and higher degree of vasoconstriction at doses more than 10 μg/kg/minute. 67 The half -life of dopamine is less than 2 minutes and dose adjustments are not necessary in renal failure. 68
Dobutamine is a β adrenergic agonist with strong β1 adrenergic activity and weak β2 adrenergic and α1 adrenergic activity. The half -life of dobutamine is 2 minutes and dose adjustments are not necessary in renal failure. 69 It achieves steady state effects in minutes as it is rapidly cleared in the blood. It also acts on peripheral vasculature through vascular alpha-1 and beta-2 receptors. At low doses (<5 μg/kg/minute), it increases CO and lowers afterload by exerting vasodilatory action on peripheral vessels. At doses (>5 μg/kg/minute), dobutamine causes vasoconstriction via its agonistic action on alpha-1 receptors. When dobutamine is given more than 10 μg/kg/minute, it can worsen tachycardia in patients without additional CO increase. 67
Milrinone is a phosphodiesterase III inhibitor. Phosphodiesterase III enzyme is responsible for the degradation of cyclic adenosine monophosphate. Inhibition of this enzyme leads to increased cyclic adenosine monophosphate which increases the phosphorylation of calcium influx channels. This results in increased calcium concentration within the cells promoting actin-myosin cross bridging leading to increased myocardial contractility. It also causes peripheral vasodilation through its action on the vascular bed by inhibiting myosin light chain activation in the vascular smooth muscles. 70 The half -life of milrinone is 2.3 to 2.4 hours in heart failure as well as renal impairment. Milrinone should be initiated at doses less than 0.0625 to 0.125 mcg/kg/minute if creatinine clearance is 10 to 50 mL/minute. 70 Milrinone doses >0.5 μg/kg/minute can lead to hypotension. 49
Levosimendan is a calcium sensitizer that increases cardiac contractility by increasing the sensitivity of troponin C to intracellular calcium in the cardiomyocyte. Both milrinone and levosimendan are preferred over beta adrenergic inotropes in patients receiving beta blockers as their action does not involve the beta-adrenergic pathway. 66 Along with its positive inotropic effect, levosimendan causes peripheral vasodilation through the opening of ATP-sensitive potassium channel on the vasculature smooth muscle cells. The half -life of levosimendan is 1 hour and of its active metabolite is 70 to 80 hours. Renal impairment prolongs its half-life. Due to the increased half-life, the drug is found to persist even after 24hrs of cessation of the drug and this is used clinically by giving intermittent pulse doses of the drug. 71 It is not available in the United States. 71 Milrinone and levosimendan have longer renal clearance and half-life causing delayed steady state effects. 70
Vasopressors
Vasopressors act via multiple receptors to increase intracellular calcium in the vascular myocyte, causing peripheral vasoconstriction, increased systemic vascular resistance, and thus the MAP. The use of catecholamines is considered a cornerstone in the treatment of cardiogenic shock. Vasopressor agents are used in up to 90% of patients in cardiogenic shock. 62 They are a class IIb/c and class IIb/b recommendation of the European Society of Cardiology guidelines in the management of cardiogenic shock. 61
The commonly used catecholamine vasopressors are norepinephrine, epinephrine, dopamine, and phenylephrine. Vasopressors act through multiple receptors to augment cytosolic calcium availability in vascular myocytes causing vasoconstriction which increases systemic vascular resistance and MAP. 49 They all activate the α1 adrenergic receptors to increase MAP.
Randomized trials comparing inotropes and vasopressors have been difficult to perform and clinical evidence is scarce. 66 Current recommendations on the use of vasopressors are through meta-analysis, expert opinion, and review articles.
Norepinephrine is the most frequently used vasopressor for the treatment of cardiogenic shock.2,72 Norepinephrine increases MAP without significant effect on the HR due its weak β adrenergic activity. Norepinephrine also increases CI without significant increase in myocardial oxygen demand due to its selective effect of β1 adrenergic receptor stimulation. 62 Norepinephrine has similar vasopressor potency as epinephrine and phenylephrine and vasopressor effect greater than dopamine. 73 The half -life of norepinephrine is 2 to 3 minutes and dose adjustments are not necessary in renal failure. 73 It is usually infused at a rate of 0.01 to 0.3 μg/kg/minute and can be titrated up to 1 μg/kg/minute to achieve the targeted blood pressure. Side effects include hypertension, peripheral vasoconstriction leading to ischemia and arrhythmia, although norepinephrine is less proarrhythmogenic compared to epinephrine and dopamine.74,75
Epinephrine is the second line vasopressor and inotrope which had both α adrenergic and β adrenergic activity. Epinephrine has stronger β adrenergic 1,2 > α adrenergic receptor activity. At low doses epinephrine increases CO due to positive inotropic and chronotropic effect through the β1 adrenergic receptors. The vasoconstriction induced by α adrenergic receptor activation is compensated by vasodilation through β2 adrenergic receptor induced vasodilation. This results in decrease of SVR and variable effects on MAP. But at higher doses due to predominant α adrenergic activity, epinephrine increase MAP by increasing HR, SVR and contractility. 76 The half -life of epinephrine is <5 minutes and dose adjustments are not necessary in renal failure. 62 The dose of epinephrine use in cardiogenic shock ranges from 0.01 to 0.3 ug/kg/minute. Due to its effects on SVR, epinephrine increases right ventricular pressure as well as pulmonary artery pressure. 76 Myocardial oxygen demand is increased due to its effect on heart rate. 77 Other side effects include arrhythmias, splanchnic vasoconstriction which is more pronounced when compared to norepinephrine and dopamine. 78 Epinephrine use is also associated with higher lactate and glucose levels. 79
Vasopressin acts through the vasopressin 1 receptors to cause vasoconstriction and raises MAP. It does not have any inotropic effects and causes reduction in HR and CO. 48 Vasopressin is used as a second line vasopressor in septic shock but evidence for its use in cardiogenic shock is lacking. There have been no randomized control trials on vasopressin use in cardiogenic shock. 79 The half -life of vasopressin is 10 to 20 minutes and dose adjustments are not necessary in renal failure. 80 The dose recommended ranges from 0.01 to 0.04 units/minute. Vasopressin was found to be helpful to wean off or in dose reduction of other catecholamines in septic shock. 81 Vasopressin use in vasoplegia syndrome following cardiac surgery and post-cardiotomy syndrome was associated with lower incidence of complications.82,83 Vasopressin can be used as an additional agent in vasodilatory shock in addition to norepinephrine in patients requiring high norepinephrine doses and in patients with refractory vasodilatory shock in AMI to improve MAP.26,84 There is a growing body of interest regarding the use of vasopressin in right ventricular failure and shock due to its selective action on pulmonary vascular bed. Vasopressin does not cause pulmonary vasoconstriction unlike epinephrine and dopamine. 62 Vasopressin is associated with lower risk of arrhythmias when compared to other catecholamines. 85 Vasopressin is more expensive when compared to other catecholamines and its escalation and dose titration are not well studied. Higher doses (>0.04 units/minute) of vasopressin is associated with systemic vasoconstriction and skin necrosis. Other side effects include hyponatremia and diabetes insipidus. 86
Phenylephrine is a pure α1 adrenergic agonist that increases MAP by increasing SVR which leads to decrease in cardiac contractility and reflex bradycardia. 77 Due to its adverse effects and paucity of adequately powered trials phenylephrine is not recommended in the treatment of cardiogenic shock. 63
Quantification of Inotropes and Vasopressors
Though vasopressors and inotropes are ubiquitously used in cardiogenic shock, prior literature in cardiogenic shock does not quantify vasoactive medications holistically. Prior work in non-cardiogenic shock states, such as septic shock, has shown cumulative vasoactive indices to predict mortality with higher accuracy that traditional risk scores. The Modified Cardiovascular Sequential Organ Failure Assessment score (Modified SOFA) by Yadav et al 45 demonstrated the importance of incorporating vasoactive medication indices into the existing cardiovascular component of the SOFA score. Modified SOFA score incorporates shock index as a substitute for MAP, serum lactate, and all vasoactive agents used in clinical practice. The vasopressors included were epinephrine, norepinephrine, vasopressin, dopamine, or phenylephrine, and the inotropes included were dobutamine and milrinone. The use of one vasoactive agent was assigned 2 points. Use of 2 or more vasoactive agents simultaneously was assigned 3 points. The use of higher doses of dopamine (>5 μg/kg/minute), epinephrine (>0.05 μg/kg/minute), or norepinephrine (>0.15 μg/kg/minute) at any time during the first 24 hours of ICU stay was assigned 4 points.
Our group has previously developed a risk prognostication system using a quantitative vasoactive medication scoring system, that was noted to be superior to the APACHE III and SOFA scores in outcomes prediction in septic shock.87,88 The following scoring systems (a) norepinephrine equivalents, 89 (b) vasoactive inotropic score,90,91 and (c) cumulative vasopressor index 92 were used to quantify the overall peak vasoactive medication requirements. A similar body of work is urgently needed in patients with cardiogenic shock to understand the correlation of vasoactive medication requirements with escalation to mechanical circulatory support, short-term mortality, development of organ failure and recovery of ventricular function.
The need for high-dose vasopressors reflects a potentially fatal underlying condition with a high risk of complication. Refractory shock is defined by persistent hypotension despite a high dose of vasopressor therapy (>0.5 μg/kg/minute norepinephrine equivalents).57,92 Patients requiring a high dose to maintain MAP can be considered to have refractory shock and are at high risk of death. 92 High doses of vasopressors are associated with adverse effects like arrhythmias, myocardial infarctions, digital ischemia, and acute kidney injury.26,41
Trials on Inotropes and Vasopressors Use
Randomized control and clinical trials on the use of vasopressors and inotropes in cardiogenic shock have been limited, and the recommendations for its use are largely from meta-analyses and expert opinions. A large Cochrane review did not demonstrate any superiority of individual inotropes and vasopressors in the treatment of cardiogenic shock. 93 The SOAP II (Sepsis Occurrence in Acutely ill Patients) trial was the largest clinical trial evaluating the use of vasopressors in shock. In an all-comer shock population, dopamine and norepinephrine had comparable outcomes. 75 In a sub-group analyses, use of dopamine was associated with higher mortality in patients with cardiogenic shock.1,75 Given the sub-group analyses of cardiogenic shock from the larger cohort of circulatory shock, the lack of differentiation on the etiology of cardiogenic shock, and the broad definitions used to define cardiogenic shock, the results of this trial need further validation in dedicated randomized trials. Dopamine was associated with higher rates of arrhythmia and gastrointestinal reaction.94,95 Dopamine is not indicated routinely in patients with cardiogenic shock. Similarly, a comparison of dobutamine to milrinone in an all-comer cardiogenic shock population, which was largely post-cardiotomy, did not demonstrate clear superiority of either agent. 96 In AMI patients with pre-shock (i.e. normotensive with signs of congestion) and acute heart failure, milrinone was associated with improved outcomes compared to placebo 96 In recent times, levosimendan has been keenly studied outside the United States as a potential therapy for cardiogenic shock. 97 A 6-hour infusion of levosimendan (0.1-0.2 μg/kg/minute) did not increase hypotension or ischemia significantly, alluding to the safety profile of this medication. Many studies98-100 have previously reported the favorable short-term effects of levosimendan treatment in a small series of patients with cardiogenic shock. In patients with ST-segment-elevation AMI complicated by cardiogenic shock, levosimendan increased CI, CO, and left ventricular ejection fraction at short-term significantly more than dobutamine, 101 however it did not demonstrate any long-term outcome improvements. 98
Levy et al 73 conducted a contemporary randomized trial comparing the effects and safety of norepinephrine and epinephrine in AMI-cardiogenic shock. The primary end points were similar in both groups. But the incidence of refractory shock was higher in the epinephrine group (10 of 27 [37%] vs norepinephrine 2 of 30 [7%]; P = .008). This led to the early termination of the study. They also showed that epinephrine was associated with higher heart rate, higher double product (indicating higher myocardial oxygen demands) and lactate levels when compared to norepinephrine. 73
Tarvasmaki et al, 72 in a sub-study of the CardShock study, attempted to evaluate the real-life use and outcomes of vasopressors and inotropes in cardiogenic shock. Of the 219 patients, Vasopressors and/or inotropes were used in 94% of the study population. Norepinephrine was used in 75% and epinephrine in 21% of patients. Epinephrine was independently associated with increased mortality with odds ratio of 5.2(95 % confidence interval 1.88, 14.7, P = .002). Norepinephrine was also associated with worsening renal and cardiac markers at the first few days. Dobutamine (49%) and levosimendan (24%) was the commonly used inotrope in the study. 72
In a meta-analysis, Karami et al 102 investigated current evidence on outcomes on inotropes and vasopressors in patients with AMI related CS. They found that treatment with noradrenaline, adrenaline, levosimendan, dobutamine, and dopamine were not associated with a difference in short term or long-term mortality. The overall quality of evidence was graded low. They also found a positive trend toward better outcome with levosimendan, compared with control. Overall, they found insufficient evidence that vasopressors and inotropes routinely are associated with reduced mortality in patients with AMI-CS and hence emphasized on the need for proper randomized trials. Uhlig et al 103 assessed the efficacy and safety of cardiac care with positive inotropic agents and vasodilators in CS or low cardiac output syndrome due to AMI, heart failure or after cardiac surgery. The study compared the efficacy of levosimendan versus dobutamine, enoximone or placebo; enoximone versus dobutamine, piroximone or epinephrine-nitroglycerine; epinephrine versus norepinephrine or norepinephrine-dobutamine; dopexamine versus dopamine; milrinone versus dobutamine and dopamine-milrinone versus dopamine-dobutamine. With a low to very low quality of evidence, all these comparisons showed uncertainty on the effect of inotropic/vasodilatory drugs on all-cause mortality. Leopold et al 104 evaluated the association between epinephrine use and short-term mortality in all-cause CS participants. A positive correlation was found between percentage of epinephrine use and short-term mortality. They found that in the hemodynamic management of CS patients, epinephrine was associated with threefold increase of risk of death. 104
The American Heart Association guidelines 1 for the management of CS mentions that norepinephrine may be the vasopressor of choice in many patients with CS as it is associated with fewer arrhythmias. 1 However, the optimal first-line vasoactive medication in CS remains unclear and no specific drug is mentioned. Due to the heterogeneity in the etiology and inciting factors for CS, these guidelines recommend different vasoactive medications for each presentation of CS. The European Society of Cardiology 2017 Guidelines 61 for the management of AMI-CS patients with ST-elevation recommend norepinephrine as a vasoconstrictor of choice when BP is low and tissue perfusion pressure is insufficient (class IIb) based on a study (De Backer et al ) 75 showing lower rate of arrhythmia and lower mortality compared to dopamine subgroup. To improve cardiac contractility (class IIb), inotropes like dobutamine may be given simultaneously to norepinephrine. 61
The range of studies on the use of all these drugs in CS discussed in (Table 1) clearly shows much uncertainty surrounding the subject. Nevertheless, inotropes and vasopressors continue to be an essential component in the management of CS by maintaining MAP of 65 to 70 mm Hg and thereby preventing tissue hypoperfusion and the resulting organ dysfunction. 103 As demonstrated by Pei et al, 106 currently, the use of these drugs varies between physicians and the conditions in different centers. Therefore, they suggest the rationalization and clinical judgment in the use of these drugs for management of shock needs to be improved and the treatments must be standardized. 106 Lastly, the introduction of the SCAI stages of CS need to be retrospectively applied to the older trials, to understand the stage of CS at which these medications were initiated, which may explain the heterogeneity in the data.
Table 1.
Trials of inotropes and vasopressors in shock.
| Study | Country | N | Comparator | Outcomes | Mortality |
|---|---|---|---|---|---|
| SOAP, 2002 | European Union | 3147 | Dopamine vs other catecholamines | Increased intensive care mortality rates with dopamine | No difference |
| SOAP II, 2010 | Belgium | 1679 | Dopamine vs norepinephrine | Dopamine had higher incidence of arrhythmias compared to norepinephrine | No difference |
| Samimi-Fard et al 98 | Spain | 22 | Levosimendan vs dobutamine | Levosimendan did not improve long term survival | No difference |
| Levy et al 73 | France | 57 | Epinephrine vs norepinephrine | Higher incidence of refractory cardiogenic shock with epinephrine | No difference |
| Lewis et al 96 | USA | 100 | Milrinone vs dobutamine | Milrinone was a safe alternative as an initial inotrope in cardiogenic shock | No difference |
| Tarvasmaki et al 72 | Finland | 219 | Vasopressors and inotropes | Norepinephrine with either dobutamine or levosimendan were prognostically similar | Increased with epinephrine |
| Hajjar et al 83 | Brazil | 330 | Vasopressin vs norepinephrine | Vasopressin can be used as a first line vasopressor agent in postcardiac surgery | No difference |
| RUSSLAN, 2002 | Russia, Latvia | 504 | Levosimendan vs placebo | Sixhours of levosimendan did not increase hypotension or ischemia significantly | Lower with levosimendan |
| REVIVE-II, 2006 | USA | 600 | Levosimendan vs placebo | Reduction in BNP and duration of hospitalization with levosimendan. | Higher with levosimendan |
| SURVIVE, 2006 | USA | 1327 | Levosimendan vs dobutamine | No difference with long outcomes | Lower with levosimendan |
| LEAF, 2014 | Norway | 61 | Levosimendan vs placebo | Levosimendan improved contractility in post ischemic myocardium | No difference |
| Annane et al (2007) 105 | France | 330 | Norepinephrine and dobutamine vs epinephrine | No difference in efficacy or safety | No difference |
| Russell et al 81 | Canada | 778 | Vasopressin vs norepinephrine | Vasopressin did not reduce mortality in septic shock compared to norepinephrine | No difference |
Suggested Approach
As acknowledged in the review of existing clinical trials, there remains significant heterogeneity in the literature due to differing inclusion criteria, differences in severity of illness (SCAI staging), varying etiology (AMI vs non-AMI), and lack of adequate controlling for confounding, specifically concomitant cardiac arrest. In light of these limitations, our proposed algorithm needs to be individualized to patients. The majority of the data recommends norepinephrine as the primary vasoactive medication of choice, with the subsequent addition of vasopressin or epinephrine to achieve additional vasopressor with or without inotropic support. Further escalation to additional inotropic support such as higher dose epinephrine versus initiation of dobutamine and milrinone need to bee cautiously titrated against the complications of tachyarrhythmias and peripheral vasodilation. In patients with AMI etiology of CS, vasopressors might be preferred to inotropes to prevent worsening of myocardial ischemia, whereas in acute heart failure with CS, inotropes might be indicated to assist in decongestion. Often when a second or third vasoactive medication is required, we encourage the use of invasive hemodynamic measurement to understand the impact of cardiogenic versus distributive shock to appropriately titrate further medications. This will additionally assist with assessing biventricular function and providing baseline information for escalation to mechanical circulatory support as indicated.
Conclusions
Although vasopressors and inotropes are used as a mainstay of pharmacologic management of cardiogenic shock, studies on these agents are scarce. The review highlights the pharmacologic management of cardiogenic shock focusing on vasoactive medications. Vasopressors and inotropes are reasonable options for initial medical management of cardiogenic shock to optimize volume status and maintain tissue perfusion. But safety concerns and risk profile indicate that the use of these medications should be for the shortest duration of time with the lowest doses. An individualized patient approach should be adapted to choose the vasoactive agents based on the hemodynamic and clinical profile. Pharmacologic management with early use of mechanical circulatory devices in a shock team approach has demonstrated improved outcomes in cardiogenic shock, but randomized control trials are needed to study the detailed incorporation of vasoactive and inotropic agents in the setting of cardiogenic shock teams and protocols.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: Review design, literature review: AS, GG, SV.
Data management, data analysis, drafting of manuscript: AS, GG, SV.
Access to data: AS, GG, LS, DG, WJN, WAJ, SV.
Manuscript revision, intellectual revisions, mentorship: LS, DG, WJN, WAJ, SV.
Final approval: AS, GG, LS, DG, WJN, WAJ, SV.
Disclosures: All authors report no relevant disclosures related to the contents of this manuscript.
ORCID iDs: Gayathri Gurumurthy  https://orcid.org/0000-0003-2481-7149
https://orcid.org/0000-0003-2481-7149
Lakshmi Sridharan  https://orcid.org/0000-0002-4855-4892
https://orcid.org/0000-0002-4855-4892
Saraschandra Vallabhajosyula  https://orcid.org/0000-0002-1631-8238
https://orcid.org/0000-0002-1631-8238
References
- 1. Van Diepen S, Katz JN, Albert NM, et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Lippincott Williams and Wilkins; 2017:e232-e268. [DOI] [PubMed] [Google Scholar]
- 2. Katz JN, Stebbins AL, Alexander JH, et al. Predictors of 30-day mortality in patients with refractory cardiogenic shock following acute myocardial infarction despite a patent infarct artery. Am Heart J. 2009;158:680-687. [DOI] [PubMed] [Google Scholar]
- 3. Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction–etiologies, management and outcome: a report from the SHOCK trial registry. SHould we emergently revascularize occluded coronaries for cardiogenic shocK? J Am Coll Cardiol. 2000;36:1063-1070. [DOI] [PubMed] [Google Scholar]
- 4. Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction a population-based perspective. Circulation. 2009;119:1211-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thiele H, Zeymer U, Neumann F-J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287-1296. [DOI] [PubMed] [Google Scholar]
- 6. Fang J, Mensah GA, Alderman MH, Croft JB. Trends in acute myocardial infarction complicated by cardiogenic shock, 1979-2003, United States. Am Heart J. 2006;152:1035-1041. [DOI] [PubMed] [Google Scholar]
- 7. Helgestad OKL, Josiassen J, Hassager C, et al. Temporal trends in incidence and patient characteristics in cardiogenic shock following acute myocardial infarction from 2010 to 2017: a Danish cohort study. Eur J Heart Fail. 2019;21:1370-1378. [DOI] [PubMed] [Google Scholar]
- 8. Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294:448-454. [DOI] [PubMed] [Google Scholar]
- 9. Kolte D, Khera S, Aronow WS, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Subramaniam AV, Barsness GW, Vallabhajosyula S, Vallabhajosyula S. Complications of temporary percutaneous mechanical circulatory support for cardiogenic shock: an appraisal of contemporary literature. Cardiol Ther. 2019;8:211-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vallabhajosyula S, O’Horo JC, Antharam P, et al. Concomitant intra-aortic balloon Pump use in cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation. Circ Cardiovasc Interv. 2018;11:e006930. [DOI] [PubMed] [Google Scholar]
- 12. Vallabhajosyula S, Dunlay SM, Barsness GW, Rihal CS, Holmes DR, Jr, Prasad A. Hospital-Level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol. 2019;124:491-498. [DOI] [PubMed] [Google Scholar]
- 13. Vallabhajosyula S, Payne SR, Jentzer JC, et al. Long-Term outcomes of acute myocardial infarction with concomitant cardiogenic shock and cardiac arrest. Am J Cardiol. 2020;133:15-22. [DOI] [PubMed] [Google Scholar]
- 14. Jentzer JC, Ahmed AM, Vallabhajosyula S, et al. Shock in the cardiac intensive care unit: changes in epidemiology and prognosis over time. Am Heart J. 2021;232:94-104. [DOI] [PubMed] [Google Scholar]
- 15. Ponikowski P, Voors AA, Anker SD, et al. [2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure]. Kardiol Pol. 2016;74:1037-1147. Wytyczne ESC dotyczace diagnostyki i leczenia ostrej i przewleklej niewydolnosci serca w 2016 roku. [DOI] [PubMed] [Google Scholar]
- 16. Vallabhajosyula S, Barsness GW, Vallabhajosyula S. Multidisciplinary teams for cardiogenic shock. Aging. 2019;11:4774-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vallabhajosyula S, Dunlay SM, Prasad A, et al. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2019;73:1781-1791. [DOI] [PubMed] [Google Scholar]
- 18. Vallabhajosyula S, Ya’Qoub L, Singh M, et al. Response by Vallabhajosyula to letter regarding article, “Sex disparities in the management and outcomes of cardiogenic shock complicating acute myocardial infarction in the young”. Circ Heart Fail. 2021;14:e007154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vallabhajosyula S, Dunlay SM, Prasad A, et al. Cardiogenic shock and cardiac arrest complicating ST-segment elevation myocardial infarction in the United States, 2000-2017. Resuscitation. 2020;155:55-64. [DOI] [PubMed] [Google Scholar]
- 20. Vallabhajosyula S, Kumar V, Vallabhajosyula S, et al. Acute myocardial infarction-cardiogenic shock in patients with prior coronary artery bypass grafting: a 16-year national cohort analysis of temporal trends, management and outcomes. Int J Cardiol. 2020;310:9-15. [DOI] [PubMed] [Google Scholar]
- 21. Vallabhajosyula S, Prasad A, Gulati R, Barsness GW. Contemporary prevalence, trends, and outcomes of coronary chronic total occlusions in acute myocardial infarction with cardiogenic shock. Int J Cardiol Heart Vasc. 2019;24:100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alpert JS, Becker RC. Cardiogenic shock: elements of etiology, diagnosis, and therapy. Clin Cardiol. 1993;16:182-190. [DOI] [PubMed] [Google Scholar]
- 23. Charlesworth M, Venkateswaran R, Barker JM, Feddy L. Postcardiotomy VA-ECMO for Refractory Cardiogenic Shock. BioMed Central Ltd; 2017:116-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stiermaier T, Eitel C, Desch S, et al. Incidence, determinants and prognostic relevance of cardiogenic shock in patients with Takotsubo cardiomyopathy. Eur Heart J Acute Cardiovasc Care. 2016;5:489-496. [DOI] [PubMed] [Google Scholar]
- 25. Vallabhajosyula S, Dunlay SM, Murphree DH, Jr., et al. Cardiogenic shock in Takotsubo cardiomyopathy versus acute myocardial infarction: an 8-year national perspective on clinical characteristics, management, and outcomes. JACC Heart Fail. 2019;7:469-476. [DOI] [PubMed] [Google Scholar]
- 26. Mody KP, Takayama H, Landes E, et al. Acute mechanical circulatory support for fulminant myocarditis complicated by cardiogenic shock. J Cardiovasc Transl Res. 2014;7:156-164. [DOI] [PubMed] [Google Scholar]
- 27. Vallabhajosyula S, Patlolla SH, Verghese D, et al. Burden of arrhythmias in acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol. 2020;125:1774-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schaikewitz MF, Nnaoma CB, Meredith RD, et al. Acute myocardial infarction with cardiogenic shock due to pericardial constriction and multivessel coronary obstruction. JACC: Case Rep. 2020;2:1708-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cooper HA, Panza JA. Cardiogenic Shock. Elsevier; 2013:567-580. [DOI] [PubMed] [Google Scholar]
- 30. Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial.. Lancet. 2005;366:1622-1632. [DOI] [PubMed] [Google Scholar]
- 31. Timperley J, Mitchell AR, Brown PD, West NE. Flecainide overdose – support using an intra-aortic balloon pump. BMC Emerg Med. 2005;5:1-3. doi: 10.1186/1471-227x-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nanda U, Ashish A, Why HJ. Modified release verapamil induced cardiogenic shock. Emerg Med J. 2005;22:832-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vallabhajosyula S, Dunlay SM, Barsness GW, et al. Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction-related cardiogenic shock. PLoS One. 2019;14:e0222894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vallabhajosyula S, Prasad A, Sandhu GS, et al. Mechanical circulatory support-assisted early percutaneous coronary intervention in acute myocardial infarction with cardiogenic shock: 10-year national temporal trends, predictors and outcomes. EuroIntervention. 2021;16:e1254-e1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Societ. Catheterization and Cardiovascular Interventions. 2019;94:29-37. [DOI] [PubMed] [Google Scholar]
- 36. Vallabhajosyula S, Arora S, Lahewala S, et al. Temporary mechanical circulatory support for refractory cardiogenic shock before left ventricular assist device surgery. J Am Heart Assoc. 2018;7:e010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vallabhajosyula S, Shankar A, Patlolla SH, et al. Pulmonary artery catheter use in acute myocardial infarction-cardiogenic shock. ESC Heart Fail. 2020;7:1234-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vallabhajosyula S, Prasad A, Dunlay SM, et al. Utilization of palliative care for cardiogenic shock complicating acute myocardial infarction: a 15-year national perspective on trends, disparities, predictors, and outcomes. J Am Heart Assoc. 2019;8:e011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vallabhajosyula S, Vallabhajosyula S, Vaidya VR, et al. Venoarterial extracorporeal membrane oxygenation support for ventricular tachycardia ablation: a systematic review. ASAIO J. 2020;66:980-985. [DOI] [PubMed] [Google Scholar]
- 40. Vallabhajosyula S, Arora S, Sakhuja A, et al. Trends, predictors, and outcomes of temporary mechanical circulatory support for postcardiac surgery cardiogenic shock. Am J Cardiol. 2019;123:489-497. [DOI] [PubMed] [Google Scholar]
- 41. Vallabhajosyula S, Jentzer JC, Kotecha AA, et al. Development and performance of a novel vasopressor-driven mortality prediction model in septic shock. Ann Intensive Care. 2018;8:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thayer KL, Zweck E, Ayouty M, et al. Invasive hemodynamic assessment and classification of In-hospital mortality risk among patients with cardiogenic shock. Circ Heart Fail. 2020;13:e007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jentzer JC, van Diepen S, Barsness GW, et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74:2117-2128. [DOI] [PubMed] [Google Scholar]
- 44. Jentzer JC, Vallabhajosyula S, Khanna AK, Chawla LS, Busse LW, Kashani KB. Management of refractory vasodilatory shock. Chest. 2018;154:416-426. [DOI] [PubMed] [Google Scholar]
- 45. Yadav H, Harrison AM, Hanson AC, Gajic O, Kor DJ, Cartin-Ceba R. Improving the accuracy of cardiovascular component of the sequential organ failure assessment score. Crit Care Med. 2015;43:1449-1457. [DOI] [PubMed] [Google Scholar]
- 46. Hollenberg SM. Cardiogenic shock. Crit Care Clin. 2001;17:391-410. [DOI] [PubMed] [Google Scholar]
- 47. Vallabhajosyula S, Ya’Qoub L, Kumar V, et al. Contemporary national outcomes of acute myocardial infarction-cardiogenic shock in patients with prior chronic kidney disease and end-stage renal disease. J Clin Med. 2020;9:3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jentzer JC, Hollenberg SM. Vasopressor and inotrope therapy in cardiac critical care. J Intensive Care Med. 2021;36:843-856. [DOI] [PubMed] [Google Scholar]
- 49. Jentzer JC, Coons JC, Link CB, Schmidhofer M. Pharmacotherapy Update on the Use of Vasopressors and Inotropes in the Intensive Care Unit. SAGE Publications Ltd; 2015:249-260. [DOI] [PubMed] [Google Scholar]
- 50. de Asua I, Rosenberg A. On the Right Side of the Heart: Medical and Mechanical Support of the Failing Right Ventricle. SAGE Publications Inc; 2017:113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. New Engl J Med. 1999;341:625-634. [DOI] [PubMed] [Google Scholar]
- 52. Hoedw-Tszuds T. Management of cardiogenic shock complicating myocardial infarction: an update 2019. ESC Heart Fail. 2019;40:2671-2683. [DOI] [PubMed] [Google Scholar]
- 53. Wayangankar SA, Bangalore S, McCoy LA, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the cathpci registry. JACC Cardiovasc Interv. 2016;9:341-351. [DOI] [PubMed] [Google Scholar]
- 54. Jeger RV, Radovanovic D, Hunziker PR, et al. Ten-year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med. 2008;149:618-626. [DOI] [PubMed] [Google Scholar]
- 55. Lee F, Hutson JH, Boodhwani M, et al. Multidisciplinary code shock team in cardiogenic shock: a Canadian centre experience. CJC Open. 2020;2:249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tehrani BN, Truesdell AG, Sherwood MW, et al. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol. 2019;73:1659-1669. [DOI] [PubMed] [Google Scholar]
- 57. Taleb I, Koliopoulou AG, Tandar A, et al. Shock Team Approach in Refractory Cardiogenic Shock Requiring Short-Term Mechanical Circulatory Support: A Proof of Concept. Lippincott Williams and Wilkins; 2019:98-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vallabhajosyula S, Dunlay SM, Barsness GW, et al. Sex disparities in the use and outcomes of temporary mechanical circulatory support for acute myocardial infarction-cardiogenic shock. CJC Open. 2020;2:462-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vallabhajosyula S, Vallabhajosyula S, Dunlay SM, et al. Sex and gender disparities in the management and outcomes of acute myocardial infarction-cardiogenic shock in older adults. Mayo Clin Proc. 2020;95:1916-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40:2671-2683. [DOI] [PubMed] [Google Scholar]
- 61. Ibanez B, James S, Agewall S. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed). 2017;70(12):1082. [DOI] [PubMed] [Google Scholar]
- 62. Levy B, Klein T, Kimmoun A. Vasopressor use in cardiogenic shock. Curr Opin Crit Care. 2020;26:411-416. [DOI] [PubMed] [Google Scholar]
- 63. Levy B, Buzon J, Kimmoun A. Inotropes and Vasopressors Use in Cardiogenic Shock: When, Which and How Much? Lippincott Williams and Wilkins; 2019:384-390. [DOI] [PubMed] [Google Scholar]
- 64. Vallabhajosyula S, Dunlay SM, Bell MR, et al. Epidemiological trends in the timing of In-hospital death in acute myocardial infarction-cardiogenic shock in the United States. J Clin Med. 2020;9:E2094. doi: 10.3390/jcm9072094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vallabhajosyula S, Subramaniam AV, Murphree DH, Jr, et al. Complications from percutaneous-left ventricular assist devices versus intra-aortic balloon pump in acute myocardial infarction-cardiogenic shock. PLoS One. 2020;15:e0238046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Polyzogopoulou E, Arfaras-Melainis A, Bistola V, Parissis J. Inotropic agents in cardiogenic shock. Curr Opin Crit Care. 2020;26:403-410. [DOI] [PubMed] [Google Scholar]
- 67. Bistola V, Arfaras-Melainis A, Polyzogopoulou E, Ikonomidis I, Parissis J. Inotropes in acute heart failure: from guidelines to practical use: therapeutic options and clinical practice. Card Fail Rev. 2019;5:133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Le Corre P, Malledant Y, Tanguy M, Le Verge R. Steady-state pharmacokinetics of dopamine in adult patients. Crit Care Med. 1993;21:1652-1657. [DOI] [PubMed] [Google Scholar]
- 69. Ruffolo RR., Jr. The pharmacology of dobutamine. Am J Med Sci. 1987;294:244-248. [DOI] [PubMed] [Google Scholar]
- 70. Chong LYZ, Satya K, Kim B, Berkowitz R. Milrinone dosing and a culture of caution in clinical practice. Cardiol Rev. 2018;26:35-42. [DOI] [PubMed] [Google Scholar]
- 71. Bouchez S, Fedele F, Giannakoulas G, et al. Levosimendan in acute and advanced heart failure: an expert perspective on posology and therapeutic application. Cardiovasc Drugs Ther. 2018;32:617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tarvasmäki T, Lassus J, Varpula M, et al. Current real-life use of vasopressors and inotropes in cardiogenic shock - adrenaline use is associated with excess organ injury and mortality. Crit Care. 2016;20:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Levy B, Clere-Jehl R, Legras A, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72:173-182. [DOI] [PubMed] [Google Scholar]
- 74. Myburgh JA, Higgins A, Jovanovska A, Lipman J, Ramakrishnan N, Santamaria J. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med. 2008;34:2226-2234. [DOI] [PubMed] [Google Scholar]
- 75. De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. New Engl J Med. 2010;362:779-789. [DOI] [PubMed] [Google Scholar]
- 76. Nativi-Nicolau J, Selzman CH, Fang JC, Stehlik J. Pharmacologic Therapies for Acute Cardiogenic Shock. Lippincott Williams and Wilkins; 2014:250-257. [DOI] [PubMed] [Google Scholar]
- 77. Tewelde SZ, Liu SS, Winters ME. Cardiogenic shock. Cardiol Clin. 2018;36:53-61. [DOI] [PubMed] [Google Scholar]
- 78. De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. 2003;31:1659-1667. [DOI] [PubMed] [Google Scholar]
- 79. Amado J, Gago P, Santos W, Mimoso J, de Jesus I. Choque cardiogénico – fármacos inotrópicos e vasopressores. Rev Port Cardiol. 2016;35:681-695. [DOI] [PubMed] [Google Scholar]
- 80. Pelletier JS, Dicken B, Bigam D, Cheung PY. Cardiac effects of vasopressin. J Cardiovasc Pharmacol. 2014;64:100-107. [DOI] [PubMed] [Google Scholar]
- 81. Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. New Engl J Med. 2008;358:877-887. [DOI] [PubMed] [Google Scholar]
- 82. Dünser MW, Bouvet O, Knotzer H, et al. Vasopressin in cardiac surgery: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2018;32:2225-2232. [DOI] [PubMed] [Google Scholar]
- 83. Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, et al. Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgery: the VANCS randomized controlled trial. Anesthesiology. 2017;126:758-793. [DOI] [PubMed] [Google Scholar]
- 84. Jolly S, Newton G, Horlick E, et al. Effect of vasopressin on hemodynamics in patients with refractory cardiogenic shock complicating acute myocardial infarction. Am J Cardiol. 2005;96:1617-1620. [DOI] [PubMed] [Google Scholar]
- 85. McIntyre WF, Um KJ, Alhazzani W, et al. Association of Vasopressin Plus Catecholamine Vasopressors Vs Catecholamines Alone With Atrial Fibrillation in Patients With Distributive Shock a Systematic Review and meta-Analysis. American Medical Association; 2018:1889-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Malay MB, Ashton JL, Dahl K, et al. Heterogeneity of the vasoconstrictor effect of vasopressin in septic shock. Crit Care Med. 2004;32:1327-1331. [DOI] [PubMed] [Google Scholar]
- 87. Vallabhajosyula S, Kumar M, Pandompatam G, et al. Prognostic impact of isolated right ventricular dysfunction in sepsis and septic shock: an 8-year historical cohort study. Ann Intensive Care. 2017;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vallabhajosyula S, Sakhuja A, Geske JB, et al. Role of admission Troponin-T and serial Troponin-T testing in predicting outcomes in severe sepsis and septic shock. J Am Heart Assoc. 2017;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Khanna A, English SW, Wang XS, et al. Angiotensin II for the treatment of vasodilatory shock. New Engl J Med. 2017;377:419-430. [DOI] [PubMed] [Google Scholar]
- 90. Nguyen HV, Havalad V, Aponte-Patel L, et al. Temporary biventricular pacing decreases the vasoactive-inotropic score after cardiac surgery: a substudy of a randomized clinical trial. J Thorac Cardiovasc Surg. 2012;146:296-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gaies MG, Jeffries HE, Niebler RA, et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the pediatric cardiac critical care consortium and virtual PICU system registries. Pediatr Crit Care Med. 2014;15:529-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Reyentovich A, Barghash MH, Hochman JS. Management of refractory cardiogenic shock. Nat Rev Cardiol. 2016;13:481-492. [DOI] [PubMed] [Google Scholar]
- 93. Unverzagt S, Wachsmuth L, Hirsch K, et al. Inotropic agents and vasodilator strategies for acute myocardial infarction complicated by cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2014;1:CD009669. [DOI] [PubMed] [Google Scholar]
- 94. De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med. 2012;40:725-730. [DOI] [PubMed] [Google Scholar]
- 95. Rui Q, Jiang Y, Chen M, Zhang N, Yang H, Zhou Y. Dopamine Versus Norepinephrine in the Treatment of Cardiogenic Shock. Lippincott Williams and Wilkins; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lewis TC, Aberle C, Altshuler D, Piper GL, Papadopoulos J. Comparative Effectiveness and Safety Between Milrinone or Dobutamine as Initial Inotrope Therapy in Cardiogenic Shock. SAGE Publications Ltd; 2019:130-138. [DOI] [PubMed] [Google Scholar]
- 97. Moiseyev V. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J. 2002;23:1422-1432. [DOI] [PubMed] [Google Scholar]
- 98. Samimi-Fard S, García-González MJ, Domínguez-Rodríguez A, Abreu-González P. Effects of levosimendan versus dobutamine on long-term survival of patients with cardiogenic shock after primary coronary angioplasty. Int J Cardiol. 2008;127:284-287. [DOI] [PubMed] [Google Scholar]
- 99. Lehmann A, Kiessling AH, Isgro F, Zeitler C, Thaler E, Boldt J. Levosimendan in patients with acute myocardial ischaemia undergoing emergency surgical revascularization. Eur J Anaesthesiol. 2008;25:224-229. [DOI] [PubMed] [Google Scholar]
- 100. García-González MJ, Domínguez-Rodríguez A, Ferrer-Hita JJ. Utility of levosimendan, a new calcium sensitizing agent, in the treatment of cardiogenic shock due to myocardial stunning in patients with ST-elevation myocardial infarction: a series of cases. J Clin Pharmacol. 2005;45:704-708. [DOI] [PubMed] [Google Scholar]
- 101. Husebye T, Eritsland J, Müller C, et al. Levosimendan in acute heart failure following primary percutaneous coronary intervention-treated acute ST-elevation myocardial infarction. Results from the LEAF trial: a randomized, placebo-controlled study. Eur J Heart Fail. 2013;15:565-572. [DOI] [PubMed] [Google Scholar]
- 102. Karami M, Hemradj VV, Ouweneel DM, et al. Vasopressors and inotropes in acute myocardial infarction related cardiogenic shock: a systematic review and meta-analysis. J Clin Med. 2020;9:E2051. doi: 10.3390/jcm9072051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Uhlig K, Efremov L, Tongers J, et al. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2020;11:CD009669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Léopold V, Gayat E, Pirracchio R, et al. Epinephrine and short-term survival in cardiogenic shock: an individual data meta-analysis of 2583 patients. Intensive Care Med. 2018;44:847-856. [DOI] [PubMed] [Google Scholar]
- 105. Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet. 2007;370:676-684. [DOI] [PubMed] [Google Scholar]
- 106. Pei XB, Ma PL, Li JG, et al. Extensive variability in vasoactive agent therapy: a nationwide survey in Chinese intensive care units. Chin Med J (Engl). 2015;128:1014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]



