This systematic review and meta-analysis aims to determine the pooled occurrence of cancers, in particular breast and prostate cancers, among those who were ever treated with spironolactone.
Key Points
Question
Is use of spironolactone associated with increased risk of cancer?
Findings
In this systematic review and meta-analysis of 7 observational studies, spironolactone use was not associated with statistically significant increased risks of breast or other solid organ cancers. It was also associated with a decreased risk of prostate cancer.
Meaning
Spironolactone use does not appear to be associated with a meaningful increase in risk of cancer occurrence.
Abstract
Importance
While originally approved for the management of heart failure, hypertension, and edema, spironolactone is commonly used off label in the management of acne, hidradenitis, androgenetic alopecia, and hirsutism. However, spironolactone carries an official warning from the US Food and Drug Administration regarding potential for tumorigenicity.
Objective
To determine the pooled occurrence of cancers, in particular breast and prostate cancers, among those who were ever treated with spironolactone.
Data Sources
PubMed, Cochrane Library, Embase, and Web of Science were searched from inception through June 11, 2021. The search was restricted to studies in the English language.
Study Selection
Included studies reported the occurrence of cancers in men and women 18 years and older who were exposed to spironolactone.
Data Extraction and Synthesis
Two independent reviewers (K.B. and H.H.) selected studies, extracted data, and appraised the risk of bias using the Newcastle-Ottawa Scale. Studies were synthesized using random effects meta-analysis.
Main Outcomes and Measures
Cancer occurrence, with a focus on breast and prostate cancers.
Results
Seven studies met eligibility criteria, with sample sizes ranging from 18 035 to 2.3 million and a total population of 4 528 332 individuals (mean age, 62.6-72.0 years; in the studies without stratification by sex, women accounted for 17.2%-54.4%). All studies were considered to be of low risk of bias. No statistically significant association was observed between spironolactone use and risk of breast cancer (risk ratio [RR], 1.04; 95% CI, 0.86-1.22; certainty of evidence very low). There was an association between spironolactone use and decreased risk of prostate cancer (RR, 0.79; 95% CI, 0.68-0.90; certainty of evidence very low). There was no statistically significant association between spironolactone use and risk of ovarian cancer (RR, 1.52; 95% CI, 0.84-2.20; certainty of evidence very low), bladder cancer (RR, 0.89; 95% CI, 0.71-1.07; certainty of evidence very low), kidney cancer (RR, 0.96; 95% CI, 0.85-1.07; certainty of evidence low), gastric cancer (RR, 1.02; 95% CI, 0.80-1.24; certainty of evidence low), or esophageal cancer (RR, 1.09; 95% CI, 0.91-1.27; certainty of evidence low).
Conclusions and Relevance
In this systematic review and meta-analysis, spironolactone use was not associated with a substantial increased risk of cancer and was associated with a decreased risk of prostate cancer. However, the certainty of the evidence was low and future studies are needed, including among diverse populations such as younger individuals and those with acne or hirsutism.
Introduction
Spironolactone, a synthetic 17-lactone steroid, is a potassium-sparing diuretic that also displays antagonistic effects on the androgen and progesterone receptors.1 It is approved by the US Food and Drug Administration (FDA) for the treatment of heart failure, edema and ascites, hypertension, and primary hyperaldosteronism. In addition, spironolactone is often used as an off-label treatment for acne, hidradenitis, androgenetic alopecia, and hirsutism owing to its antiandrogenic properties.2 Particularly for women with acne, spironolactone has shown promise as an effective option that can reduce reliance on oral antibiotics.3,4,5
Spironolactone is also frequently used as part of gender-affirming care, and it is included in the Endocrine Society guidelines as a part of hormone regimens for transgender women (male transitioning to female).6,7,8 For transgender women seeking feminine physical characteristics, antiandrogens such as spironolactone can reduce muscle hypertrophy and male body hair growth, induce skin softening, change body fat distribution, and cause testicular shrinkage.8 For transgender men, spironolactone can treat hormonal acne and androgenic hair loss.7
However, spironolactone carries an official FDA warning regarding possible tumorigenicity.9 This warning is largely based on animal studies using doses up to 150 times greater than human doses and found in the development of hepatic, testicular, and breast adenomas.10 Several observational studies have not confirmed such a risk when spironolactone is used in typical clinical practice.11,12,13,14 In addition, because prostate cancer is an androgen-sensitive tumor, it is possible that spironolactone use might be associated with reduced incidence of the development of prostate cancer.15 Given this official warning and the uncertainty regarding the potential association between spironolactone use and cancer, the purpose of this study was to conduct a systematic review and meta-analysis to synthesize the evidence with respect to the risk of cancer in the setting of spironolactone use.
Methods
Eligibility Criteria
In this analysis, we included studies reporting on the risk of cancer development in women or men at least 18 years old who were exposed to spironolactone, regardless of the primary indication for spironolactone. We included any type of comparators (eg, not exposed to spironolactone or exposure to other diuretics such as furosemide). This systematic review of observational studies followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines checklist.16 The protocol was registered in PROSPERO (CRD42021262406).
Information Sources and Search Strategy
Searches were conducted in PubMed, Cochrane Library, Embase, and Web of Science from inception through June 11, 2021. The search used across all 4 databases was developed in conjunction with a medical reference librarian and was restricted to studies in the English language. The specific search syntax is included in the eAppendix in the Supplement.
In addition to this search strategy, to ensure potentially relevant articles were not excluded, PubMed was also searched using appropriate Medical Subject Heading terms, such as spironolactone, cancer, adenoma, and neoplasm (eAppendix in the Supplement). These terms were identified by reviewing the Medical Subject Heading terms associated with the most relevant articles identified by the initial search. A similar search was performed in Cochrane Library.
Study Selection
Two investigators (K.B. and H.H.) independently screened the titles and abstracts of all identified citations. The articles in full text were obtained for potentially relevant citation and reviewed for inclusion in the final analysis. Discrepancies were resolved by a senior author (J.S.B.).
Data Collection Process
Two investigators (K.B. and H.H.) independently extracted data from each study using a standard template, with discrepancies resolved by a senior author (J.S.B.). For each study that did not present risk ratios (RRs), we contacted corresponding authors by email to attempt to acquire primary data. However, we were unable to obtain primary data for these studies.
Data Items
The information collected on methods included aim of intervention, aim of study, study design, methods of recruitment of participants, inclusion and exclusion criteria for participation in the study, ethical approval, funding, and statistical methods. Participant information collected included general description, geographic location, health care setting, total number, age, gender, ethnicity, and any other sociodemographic data describing the study populations. Information on intervention collected included details of intervention, details of control, details of cointerventions in other groups, and delivery of intervention. Outcome information collected included number of malignant tumors and effect measures reported (RRs, odds ratios, or hazard ratios [HRs], and their 95% CIs).
Risk of Bias
Risk of bias was assessed using the Newcastle-Ottawa Scale.17,18,19 This scale uses a 9-star system evaluating 3 domains: (1) selection bias, (2) confounding bias, and (3) detection and outcome measurement bias.
In assessing selection bias for case-control studies, points are given if there is independent validation of the cases, consecutive or obviously representative series of cases with no potential for selection bias, use of community controls, and no history of disease (end point) in the controls. In cohort studies, the criteria included representativeness of the exposed cohort, if the nonexposed cohort is taken from the same community as the exposed cohort, if there is a secure record or structured interview for ascertainment of exposure, and if the outcome of interest was not present at the start of the study. In assessing comparability bias in case-control and cohort studies, points are given if the study controls for the most important risk factor (ie, age) and if the study controls for any other additional risk factor. In assessing exposure bias in case-control studies, points are given if there is secure record used to ascertain the exposure, same method of ascertainment for cases and controls, and equivalent nonresponse rate in control and exposure groups. In assessing outcome bias in cohort studies, criteria include independent blind assessment of outcome or record linkage, if follow-up was long enough for outcome to occur, and if there was complete follow-up or if patients lost is unlikely to introduce meaningful follow-up bias.
A score above 7 was considered low risk of bias.20,21 Two investigators (K.B. and H.H.) independently appraised each article, and discrepancies were resolved by a senior author (J.S.B.).
Effect Measures
Data were combined when statistically homogeneous and expressed as RRs with their associated 95% CIs. Calculated effect ratios were transformed into RRs using previously published formulas.22,23 When a formula required a baseline risk that was not reported in the study or could not be calculated, we used the methodology reported in previous meta-analyses of rates and obtained the average national baseline risk for the matching population in terms of years in which the study was conducted and age of participants.22,23 Standard errors were used to calculate the inverse variance weights. When not reported, standard errors were derived from 95% CIs or P values.24
Synthesis Methods
Studies were grouped by individual cancer types reported. When only 1 study reported a type of cancer or the reported data was not sufficient to be pooled, narrative synthesis was used to summarize associations. When more than 2 studies reported on the same type of cancer, analyses were performed using the inverse variance method with a random effects model in Stata, version 15 (StataCorp). Statistical heterogeneity was assessed by the inconsistency score (I2), with an I2 of 50% or greater considered moderate to considerable heterogeneity. Subgroup analyses were performed when substantial heterogeneity was observed and if there was at least 1 study per group for each type of cancer. We considered exploring clinical heterogeneity by the indication of spironolactone (any, hypertension, or heart failure) and by gender (female vs male), and methodological heterogeneity by the risk of confounding and immortal time bias (low vs high). Sensitivity analyses were performed to evaluate the robustness of findings by type of estimate used (converted RRs vs data reported).
Reporting Bias Assessment and Certainty Assessment
A funnel plot and an Egger regression asymmetry test was planned if more than 6 studies reported data on the same type of cancer to assess publication bias and small-study effects in the meta-analysis.25,26 However, none of the reported cancers met the criteria. The strength of the evidence was assessed using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach.
Results
Study Selection
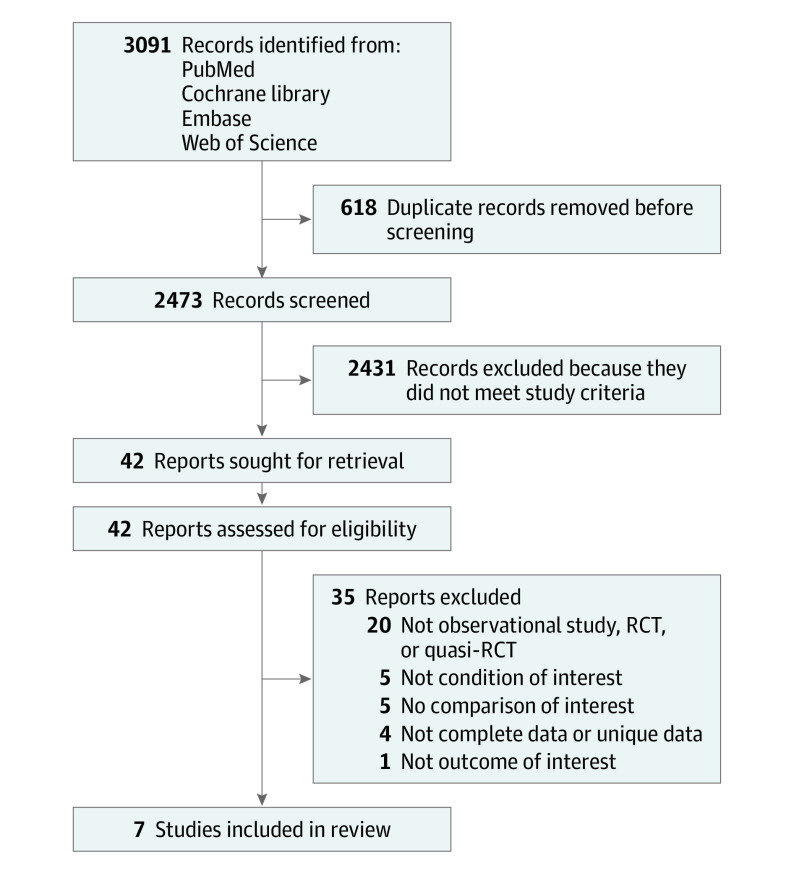
A PRISMA flow diagram summarizes the study selection process (Figure 1). The initial searches identified 3091 records, from which 42 full-text articles were assessed. A total of 7 observational studies were included in the review.
Figure 1. PRISMA Flow Diagram.
RCT indicates randomized clinical trial.
Study Characteristics
A total population of 4 528 332 individuals was included across all of the studies identified (Table). The observational studies included in the quantitative analysis investigated prostate (n = 4), breast (n = 3), ovarian (n = 2), bladder (n = 3), kidney (n = 2), gastric (n = 2), and esophageal (n = 2) cancers. The sample size of included studies ranged from 18 035 to 2.3 million. Studies were conducted using data from 6 continents (Europe [n = 5], North America [n = 2], South America [n = 1], Africa [n = 1], Asia [n = 2], and Australia [n = 1]). Four studies provided stratified analyses by sex, and 3 studies did not conduct analyses stratified by sex. In the studies without stratification by sex, the lowest percentage of women was 17.2% and the highest percentage of women was 54.4%. The mean age of individuals included in the studies ranged from 62.6 to 72.0 years.
Table. Summary of Included Studies.
| Source | Study design | Sample size | Mean age, y | Women, % | Men, % | Database | Key eligibility criteria | Key exclusion criteria | Duration of follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Chuang et al,29 2017 (Taiwan) | Retrospective case-control study | 32 167 | 70.9 | 23.9 | 76.1 | National Health Insurance Research Database | Age >20 y, hypertension, with or without a new diagnosis of urinary tract cancer | Incomplete information on age or sex | 2005 to 2011 |
| Mackenzie et al,13 2017 (United Kingdom) | Retrospective matched cohort study | 222 225 | NA | 54.4 | 45.6 | Clinical Practice Research Datalink | Individuals with ≥2 prescriptions for spironolactone matched with controls on age and medical history | History of cancer or ascites | 1986 to 2013, practice’s last follow-up date or patient’s last contact |
| Biggar et al,11 2013 (Denmark) | Retrospective cohort study | 2.3 million | 62.6 | 100 | 0 | Danish Register of Medicinal Products Statistics | Age >20 y | History of prior cancer | 1995 to 2010, to the first cancer diagnosis, to death, or to emigration or disappearance |
| Sabatier et al,28 2019 (worldwide) | Retrospective case-control study | 1 748 950 | 67.0 | 100 | 0 | VigiBase (World Health Organization) | Age >50 y | Taking drugs that are possible risk factors for cancer, putative reports, and non–health professional reports | 1981 to 2017 |
| Busby et al,27 2017 (Scotland) | Retrospective case-control study | 18 035 | 69.2 | 17.2 | 82.8 | Primary Care Clinical Information Unit Research | First-time esophageal or gastric cancer with up to 5 controls matched on age, gender, diagnosis year, and general practice | Preexisting cancer diagnosis and patients with <3 y of exposure | 1/1/1993 to 4/30/2011 |
| Beckmann et al,30 2019 (Sweden) | Retrospective case-control study | 188 393 | NA | 0 | 100 | Prostate Cancer Database Sweden | Men with prostate cancer matched to controls by birth year and county | None | 2014 to 2016 |
| Hiebert et al,31 2020 (Canada) | Retrospective cohort study | 18 562 | 72.0 | 0 | 100 | Clinical and administrative databases from the Manitoba Centre for Health Policy | Age >18 y, new diagnosis of heart failure, resident of Manitoba between 1/1/2007 and 3/31/2007 | New cancer diagnosis in the 5-y period preceding heart failure diagnosis or those with metastatic solid tumor cancer | 1/1/2007 to 3/31/2007 |
Abbreviation: NA, not available.
Common inclusion criteria for studies included in the quantitative analysis were age of at least 18 years with or without spironolactone exposure. Some studies included individuals with specific medical history, such as hypertension or urinary tract cancers. Common exclusion criteria included history of cancer (Table).
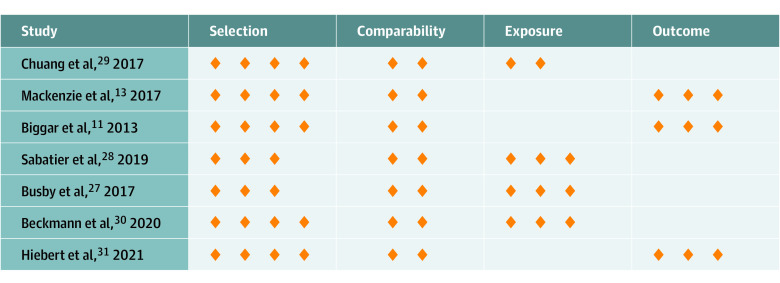
Risk of Bias
Of the 7 included studies, all were of low risk of bias (total score above 7 of ≥9 on the Newcastle-Ottawa Scale). The mean score on the Newcastle-Ottawa Scale was 8.43 of 9 stars (Figure 2). Five of 7 studies received all 4 stars for selection criteria. In the study by Busby et al,27 the selection was given 3 stars owing to the authors’ stated difficulty of ascertaining a gastroesophageal junction tumor. Similarly, the study by Sabatier et al28 received 3 stars because the noncases in the study were not derived from the same population as the cases, with statistically significant differences in several characteristics across the 2 groups. All studies met both criteria in the comparability category, with the exception of Sabatier et al,28 which did not control for the most important risk factor (ie, age). Three of 4 case-control studies received all 3 stars for exposure criteria. Chuang et al29 used different databases and was downgraded owing to inability to ascertain nonresponse rate in each group. All 3 cohort studies received all 3 stars for outcome criteria.
Figure 2. Risk of Bias Summary.
Risk of bias was assessed using the Newcastle-Ottawa Scale. The orange diamonds presented here represent the stars used in this scoring system.
Cancer Risk
Breast Cancer
Three studies provided data for this analysis,11,13,28 and there was no statistically significant association between spironolactone use and risk of breast cancer occurrence (RR, 1.04; 95% CI, 0.86-1.22; I2 = 85%). Certainty of evidence was very low and downgraded because of indirectness owing to the conversion of the effect estimates and differences in population; it was upgraded owing to addressing of residual confounding (Figure 3 and the eFigure in the Supplement). In sensitivity analyses, including only the reported (ie, published) data, spironolactone use was associated with decreased odds of breast cancer for 1 study reporting odds ratios (odds ratio, 0.75; 95% CI, 0.58-0.97).28 This finding contrasted with studies reporting incidence rate ratios (IRRs),11,13 in which spironolactone use was associated with an increased risk of breast cancer (IRR, 1.15; 95% CI, 1.05-1.26; eTable 1 in the Supplement).11,13,27,28,29,30,31
Figure 3. Summary of Evidence.
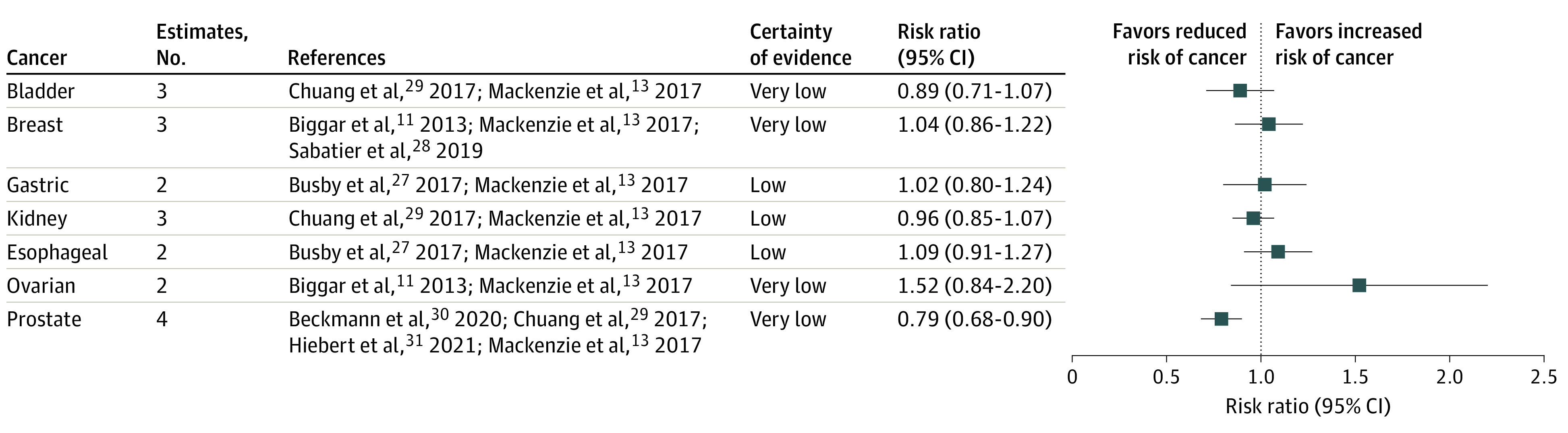
For associations between spironolactone use and risk of bladder cancer occurrence, certainty of evidence was very low and downgraded because of indirectness owing to the conversion of the effect estimates and inconsistency owing to differences in population; it was upgraded owing to addressing residual confounding. For risk of breast cancer, certainty of evidence was very low and downgraded because of indirectness owing to the conversion of the effect estimates and differences in population; it was upgraded owing to addressing residual confounding. For risk of gastric cancer, certainty of evidence was low and downgraded because of indirectness owing to the conversion of the effect estimates; it was upgraded owing to addressing residual confounding. For risk of kidney cancer, certainty of evidence was low and downgraded because of indirectness owing to the conversion of the effect estimates; it was upgraded owing to addressing residual confounding. For risk of esophageal cancer, certainty of evidence was low and downgraded because of indirectness owing to the conversion of the effect estimates; it was upgraded owing to addressing residual confounding. For risk of ovarian cancer, certainty of evidence was very low and downgraded because of indirectness owing to the conversion of the effect estimates and imprecision in the estimate; it was upgraded owing to addressing residual confounding. For risk of prostate cancer, certainty of evidence was very low and downgraded because of indirectness owing to the conversion of the effect estimates and inconsistency owing to differences in population; it was upgraded owing to addressing residual confounding.
Prostate Cancer
Four studies provided data for this analysis13,29,30,31 and demonstrated an association between spironolactone use and decreased risk of prostate cancer occurrence (RR, 0.79; 95% CI, 0.68-0.90; I2 = 74%). Certainty of evidence was very low and downgraded because of indirectness owing to the conversion of the effect estimates and inconsistency owing to differences in population; it was upgraded owing to addressing residual confounding (Figure 3 and the eFigure in the Supplement). When exploring the differences in the studied population, the risk of prostate cancer was similar in studies of heart failure (RR, 0.63; 95% CI, 0.42-0.95), hypertension (RR, 0.88; 95% CI, 0.83-0.95), and for any indication (RR, 0.76; 95% CI, 0.71-0.81) (eTable 2 in the Supplement).
Other Cancers
There was no statistically significant association between spironolactone use and risk of ovarian, bladder, kidney, gastric, or esophageal cancers. Across 2 studies assessing risk of ovarian cancer (RR, 1.52; 95% CI, 0.84-2.20; I2 = 86%),11,13 certainty of evidence was very low and downgraded because of indirectness owing to the conversion of the effect estimates and imprecision in the estimate; it was upgraded owing to addressing residual confounding. Across 2 studies and 3 estimates assessing risk of bladder cancer (RR, 0.89; 95% CI, 0.71-1.07; I2 = 85%),13,29 certainty of evidence was very low and downgraded because of indirectness owing to the conversion of the effect estimates and inconsistency owing to differences in population; it was upgraded owing to addressing residual confounding. Across 2 studies and 3 estimates assessing risk of kidney cancer (RR, 0.96; 95% CI, 0.85-1.07; I2 = 30%),13,29 certainty of evidence was low and downgraded because of indirectness owing to the conversion of the effect estimates; it was upgraded owing to addressing residual confounding. Across 2 studies assessing risk of gastric cancer (RR, 1.02; 95% CI, 0.80-1.24; I2 = 0%),13,27 certainty of evidence was low and downgraded because of indirectness owing to the conversion of the effect estimates; it was upgraded owing to addressing residual confounding. Across 2 studies assessing risk of esophageal cancer (RR, 1.09; 95% CI, 0.91-1.27; I2 = 0%),13,27 certainty of evidence was low and downgraded because of indirectness owing to the conversion of the effect estimates; it was upgraded owing to addressing residual confounding. In subgroup analyses, there were differences in the risk of bladder cancer between women (RR, 0.81; 95% CI, 0.73-0.91) and men (RR, 1.03; 95% CI, 0.95-1.11) (Figure 3 and the eFigure in the Supplement).
Narrative Synthesis
In a retrospective matched cohort study, there was no statistically significant association between spironolactone use and thyroid cancer (HR, 0.67; 95% CI, 0.23-2.00).13 Mackenzie et al13 found no association between spironolactone use and endometrial cancer (HR, 0.90; 95% CI, 0.63-1.30), and Biggar et al11 reported no statistically significant association between spironolactone use and cervical cancer (IRR, 1.05; 95% CI, 0.63-1.75). In addition, Mackenzie et al13 reported no statistically significant association between spironolactone use and colorectal cancer (HR, 0.91; 95% CI, 0.80-1.04), pancreatic cancer (HR, 1.22; 95% CI, 0.93-1.60), pharyngeal cancer (HR, 0.76; 95% CI, 0.32-1.82), or myelomonoblastic/myelomonocytic leukemia (HR, 1.44; 95% CI, 0.84-2.47).
Discussion
This systematic review and meta-analysis evaluated the potential association between spironolactone use and risk of cancer. In the primary analysis, we found no statistically significant associations between spironolactone use and breast, ovarian, bladder, kidney, gastric, or esophageal cancers. In addition, spironolactone use was associated with a decreased risk of prostate cancer. In light of the FDA-mandated warning cautioning that “unnecessary use of this drug should be avoided,”9 these data are reassuring that treatment with spironolactone is unlikely to be associated with a meaningful increased risk of cancer when prescribed at typical clinical doses.
For patients with acne, spironolactone represents an important alternative to oral antibiotics.4 While there have been concerns about use of spironolactone and the development of breast cancer, the results of this meta-analysis are reassuring. In addition, prior studies have suggested that use of oral antibiotics may be associated with an increased risk of breast and color cancers.32,33,34 Taken together, these findings suggest that spironolactone may have lower malignant potential compared with oral antibiotics for women with acne.
Ovarian cancer is a hormone-sensitive cancer that could be postulated to be increased with spironolactone use. Although Biggar et al11 found an increased risk of ovarian cancer among women treated with spironolactone, particularly within the first year of treatment, the results of this meta-analysis do not support a clear association between spironolactone use and ovarian cancer. Notably, Biggar et al found a similarly increased risk of ovarian cancer within the first year of use with furosemide and noted that use of spironolactone was found to be more common in the months just prior to formal diagnosis of ovarian cancer. Because women with ovarian cancer often present with abdominal swelling, Biggar et al suggest that symptomatic management of abdominal swelling with spironolactone prior to the formal diagnosis of ovarian cancer could explain this finding.11,35
There was a decreased risk of prostate cancer observed among men treated with spironolactone. This finding is consistent with the hypothesis that the antiandrogenic properties of spironolactone could be protective against prostate cancer.1 Because prostate cancer is one of the most common cancers in men, this factor may be a reason to consider spironolactone over other diuretics in the management of heart failure and edema in men.36 Spironolactone may also be a potential consideration for future study with respect to primary prevention and treatment of prostate cancer.
Strengths and Limitations
This systematic review and meta-analysis has some strengths, including the large sample sizes of the included studies. Furthermore, these studies include long follow-up periods, typically between 5 and 20 years. The use of comprehensive health care registries, such as the Clinical Practice Research Datalink and the Danish Register of Medicinal Products Statistics, allowed for rigorous adjustment for potential confounders and accurate outcome identification in the setting of nationalized health care systems.
However, this systematic review and meta-analysis has potential limitations. Despite the large sample size, for rarer outcomes such as ovarian cancer, the confidence intervals for the estimates are wide and we cannot entirely exclude the potential for a meaningful increase in cancer risk. The included studies are largely in European individuals (via Clinical Practice Research Datalink, Danish Register of Medicinal Products Statistics, and VigiBase) and may not generalize to other populations.28 In addition, many of the studies predominantly included older individuals and may not generalize to younger populations, such as those treated with spironolactone for acne. We were not able to examine whether there are dose-dependent associations between spironolactone and the outcomes of interest. In addition, although we requested primary data from all study authors, for some studies raw data were not available and calculated effect ratios were transformed into RRs, which resulted in downgrading the certainty of evidence owing to indirectness. In sensitivity analyses, there were some differences in the effect estimates depending on which studies were included, particularly for breast cancer, and these results should be interpreted with caution. The search was also limited to studies in the English language and may have missed potentially relevant studies in the non-English literature.
Conclusions
This systematic review and meta-analysis provides reassuring data that use of spironolactone, an important treatment for patients with acne, hidradenitis, androgenetic alopecia, and hirsutism, is unlikely to be associated with a substantial increased risk of cancer. However, the certainty of the evidence was low. Future studies are needed, particularly among diverse populations such as younger individuals and those with acne or hirsutism.
eAppendix. Search Strategy
eFigure. Forest Plot Including All Individual Studies
eTable 1. Sensitivity Analyses
eTable 2. Subgroup Analysis to Explore Heterogeneity (only analyses with I2>50%)
References
- 1.Lainscak M, Pelliccia F, Rosano G, et al. Safety profile of mineralocorticoid receptor antagonists: spironolactone and eplerenone. Int J Cardiol. 2015;200:25-29. doi: 10.1016/j.ijcard.2015.05.127 [DOI] [PubMed] [Google Scholar]
- 2.Searle TN, Al-Niaimi F, Ali FR. Spironolactone in dermatology: uses in acne and beyond. Clin Exp Dermatol. 2020;45(8):986-993. doi: 10.1111/ced.14340 [DOI] [PubMed] [Google Scholar]
- 3.Barbieri JS, Bhate K, Hartnett KP, Fleming-Dutra KE, Margolis DJ. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155(3):290-297. doi: 10.1001/jamadermatol.2018.4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbieri JS, Spaccarelli N, Margolis DJ, James WD. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80(2):538-549. doi: 10.1016/j.jaad.2018.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg V, Choi JK, James WD, Barbieri JS. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84(5):1348-1355. doi: 10.1016/j.jaad.2020.12.071 [DOI] [PubMed] [Google Scholar]
- 6.Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. ; Endocrine Society . Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(9):3132-3154. doi: 10.1210/jc.2009-0345 [DOI] [PubMed] [Google Scholar]
- 7.Ragmanauskaite L, Kahn B, Ly B, Yeung H. Acne and the Lesbian, Gay, Bisexual, or Transgender Teenager. Dermatol Clin. 2020;38(2):219-226. doi: 10.1016/j.det.2019.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomlins L. Prescribing for transgender patients. Aust Prescr. 2019;42(1):10-13. doi: 10.18773/austprescr.2019.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldactone. Package insert. Pfizer; 2008.
- 10.Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-73.e33. doi: 10.1016/j.jaad.2015.12.037 [DOI] [PubMed] [Google Scholar]
- 11.Biggar RJ, Andersen EW, Wohlfahrt J, Melbye M. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37(6):870-875. doi: 10.1016/j.canep.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 12.Mackenzie IS, Macdonald TM, Thompson A, Morant S, Wei L. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447. doi: 10.1136/bmj.e4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie IS, Morant SV, Wei L, Thompson AM, MacDonald TM. Spironolactone use and risk of incident cancers: a retrospective, matched cohort study. Br J Clin Pharmacol. 2017;83(3):653-663. doi: 10.1111/bcp.13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year followup study. J Cutan Med Surg. 2002;6(6):541-545. doi: 10.1177/120347540200600604 [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg SL, Koupparis A, Robinson ME. Differing levels of testosterone and the prostate: a physiological interplay. Nat Rev Urol. 2011;8(7):365-377. doi: 10.1038/nrurol.2011.79 [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(n71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA. 2015;314(22):2373-2383. doi: 10.1001/jama.2015.15845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotenstein LS, Torre M, Ramos MA, et al. Prevalence of burnout among physicians: a systematic review. JAMA. 2018;320(11):1131-1150. doi: 10.1001/jama.2018.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute . Accessed December 30, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 20.Cui CJ, Wang GJ, Yang S, Huang SK, Qiao R, Cui W. Tissue Factor-bearing MPs and the risk of venous thrombosis in cancer patients: a meta-analysis. Sci Rep. 2018;8(1):1675. doi: 10.1038/s41598-018-19889-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 22.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. 2014;348:f7450. doi: 10.1136/bmj.f7450 [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Yu KF. What’s the relative risk? a method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690-1691. doi: 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 24.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drucker AM, Fleming P, Chan AW. Research techniques made simple: assessing risk of bias in systematic reviews. J Invest Dermatol. 2016;136(11):e109-e114. doi: 10.1016/j.jid.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 26.Page MJ, Higgins JPT, Sterne JAC. Assessing risk of bias due to missing results in a synthesis. In: Higgins J, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2021. [Google Scholar]
- 27.Busby J, Murchie P, Murray L, et al. The effect of medications which cause inflammation of the gastro-oesophageal tract on cancer risk: a nested case-control study of routine Scottish data. Int J Cancer. 2017;140(8):1828-1835. doi: 10.1002/ijc.30612 [DOI] [PubMed] [Google Scholar]
- 28.Sabatier P, Amar J, Montastruc F, et al. Breast cancer and spironolactone: an observational postmarketing study. Eur J Clin Pharmacol. 2019;75(11):1593-1598. doi: 10.1007/s00228-019-02740-y [DOI] [PubMed] [Google Scholar]
- 29.Chuang YW, Yu MC, Huang ST, et al. Spironolactone and the risk of urinary tract cancer in patients with hypertension: a nationwide population-based retrospective case-control study. J Hypertens. 2017;35(1):170-177. doi: 10.1097/HJH.0000000000001130 [DOI] [PubMed] [Google Scholar]
- 30.Beckmann K, Garmo H, Lindahl B, et al. Spironolactone use is associated with lower prostate cancer risk: a population-wide case-control study. Prostate Cancer Prostatic Dis. 2020;23(3):527-533. doi: 10.1038/s41391-020-0220-8 [DOI] [PubMed] [Google Scholar]
- 31.Hiebert BM, Janzen BW, Sanjanwala RM, Ong AD, Feldman RD, Kim JO. Impact of spironolactone exposure on prostate cancer incidence amongst men with heart failure: a pharmacoepidemiological study. Br J Clin Pharmacol. 2021;87(4):1801-1813. doi: 10.1111/bcp.14568 [DOI] [PubMed] [Google Scholar]
- 32.Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67(4):672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman GD, Oestreicher N, Chan J, Quesenberry CP Jr, Udaltsova N, Habel LA. Antibiotics and risk of breast cancer: up to 9 years of follow-up of 2.1 million women. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2102-2106. doi: 10.1158/1055-9965.EPI-06-0401 [DOI] [PubMed] [Google Scholar]
- 34.Velicer CM, Heckbert SR, Lampe JW, Potter JD, Robertson CA, Taplin SH. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291(7):827-835. doi: 10.1001/jama.291.7.827 [DOI] [PubMed] [Google Scholar]
- 35.Bankhead CR, Kehoe ST, Austoker J. Symptoms associated with diagnosis of ovarian cancer: a systematic review. BJOG. 2005;112(7):857-865. doi: 10.1111/j.1471-0528.2005.00572.x [DOI] [PubMed] [Google Scholar]
- 36.Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524-548. doi: 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Search Strategy
eFigure. Forest Plot Including All Individual Studies
eTable 1. Sensitivity Analyses
eTable 2. Subgroup Analysis to Explore Heterogeneity (only analyses with I2>50%)