Key Points
Question
Is there an infectious etiology associated with widespread hyperkeratotic lesions, clinical decline, and fatality in solid organ transplant recipients?
Findings
In this case series study, human polyomavirus 9 was identified in 3 patients’ lesional skin using high-throughput illumina sequencing. Human polyomavirus 9 was also present in blood, oral swabs, ocular swabs, urine samples, and lung samples (from autopsy).
Meaning
Human polyomavirus 9 was associated with a widespread cutaneous eruption, and potentially associated with cutaneous and systemic disease including pulmonary failure and death in solid organ transplant recipients.
This case series describes previously undescribed hyperkeratotic lesions, clinical decline, and fatality associated with human polyomavirus 9 in solid organ transplant recipients in a single university medical center.
Abstract
Importance
We describe the first report to our knowledge of cutaneous and systemic pathogenicity of human polyomavirus 9 in solid organ transplant recipients.
Objective
Three solid organ transplant recipients developed a widespread, progressive, violaceous, and hyperkeratotic skin eruption. All died from pulmonary and multiorgan failure around 1 year from onset of the rash. Routine clinical diagnostic testing could not identify any causative agent; therefore, samples and autopsies were investigated for novel pathogens using high-throughput sequencing.
Design, Setting, and Participants
This case series, including 3 solid organ transplant recipients who developed characteristic pink, violaceous, or brown hyperkeratotic papules and plaques throughout the body, was conducted at the Columbia University Medical Center. Lesional skin biopsies were collected from all 3 patients and subjected to high-throughput illumina sequencing for identification of microbial pathogens. Human polyomavirus 9 was identified in lesional skin biopsies. We subsequently collected ocular swabs, oral swabs, urine samples, and blood samples from patients, and organ tissues at autopsy in 1 patient. We investigated these samples for the presence of human polyomavirus 9 using in situ hybridization and quantitative polymerase chain reaction (PCR) assays.
Main Outcomes and Measures
A description of the clinical and pathologic findings of 3 patients.
Results
This case series study found that human polyomavirus 9 was detected in the skin biopsies of all 3 patients by a capture-based high-throughput sequencing method platform (VirCapSeq-VERT). Human polyomavirus 9 was also detected in blood, oral, ocular swabs, and urine by real-time polymerase chain reaction (PCR) assay. In situ hybridization and quantitative PCR assays were performed on the skin biopsies from 3 patients and lung autopsy of 1 patient, which showed the presence of human polyomavirus 9 messenger RNA transcripts, indicating active viral replication and pathogenesis in the skin and lungs.
Conclusions and Relevance
Human polyomavirus 9 was associated with the widespread cutaneous eruption. All 3 patients had progression of cutaneous disease, accompanied by clinical deterioration, pulmonary failure, and death. One patient underwent autopsy and human polyomavirus 9 was identified in the lungs and paratracheal soft tissue. These findings suggest that human polyomavirus 9 may be associated with cutaneous and possibly pulmonary infection and death in solid organ transplant recipients.
Introduction
Human polyomavirus 9 (HPyV9) was first isolated from urine and serum of a kidney transplant recipient.1 Human polyomavirus 9 viremia is reported in approximately 20% of solid organ transplant recipients (SOTRs) but never in healthy individuals.2,3 Antibodies against HPyV9 are reported in 17% to 29% of healthy blood donors and in SOTRs immediately after transplantation.2,4 However, increasing numbers of SOTRs become seropositive years subsequent to transplantation, indicating risk of HPyV9 infection in SOTRs. Despite the significant prevalence, HPyV9 has not been implicated in the pathogenesis of human disease.
We describe 3 SOTRs who developed painful, progressive, hyperkeratotic skin lesions and died within 14 months after onset of disease with pulmonary failure. Patients had high viral loads of HPyV9 in lesional skin, serum, and lung autopsy tissues. We report that HPyV9 was associated with cutaneous infection and possibly with pulmonary infection and death in SOTRs.
Case Reports
Patient 1 was a woman in her 60s with 3 kidney transplants, who developed a full body pruritic eruption 13 years after initial transplant. Patient 2 was a man in his 60s with 2 lung transplants who developed hyperpigmented and hyperkeratotic lesions on the elbows and ankles 9 years after initial transplant. Patient 3 was a man in his 70s with bilateral lung transplant who developed hyperpigmented, hyperkeratotic skin lesions on the face 7 years after transplant. All patients initially had acral lesions that generalized to the proximal extremities and trunk (Figure 1, A and B; eFigure 1 in the Supplement). Lesions were hyperkeratotic pink, brown, and violaceous papules and plaques with hyperkeratotic spines. As the eruption progressed, they experienced clinical decline including weakness, anorexia, and dyspnea. No infectious agent was found using routine diagnostic assays in any patient (Table).
Figure 1. Clinical and Histopathologic Images of Patient 3.
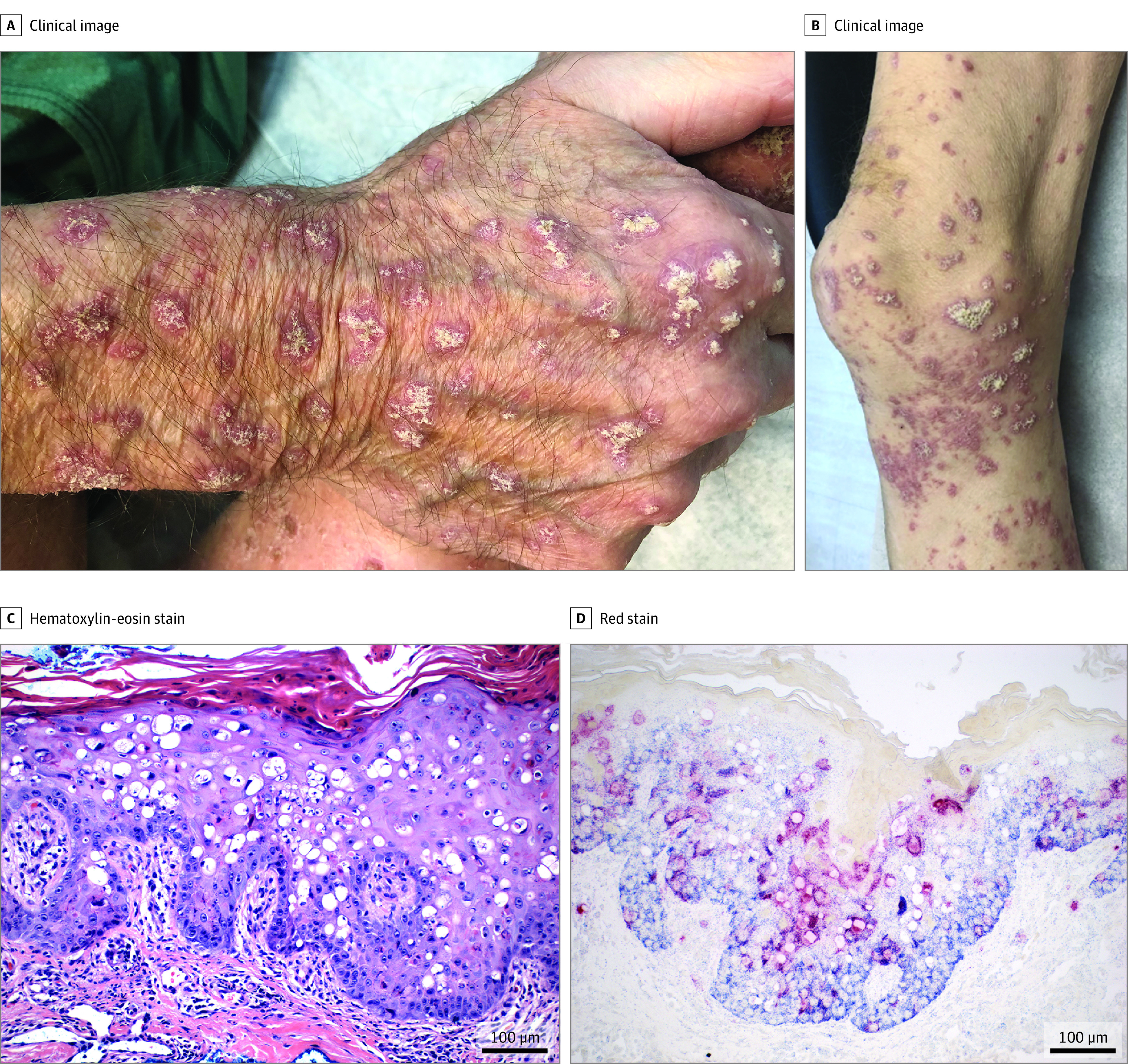
Patient 3 initially presented with thin pink plaques on the face that subsequently formed plaques with spicules. He developed painful keratotic papules and plaques on the distal extremities (A, B). C, Hematoxylin-eosin staining from patient 3 demonstrates hyperkeratosis, acanthosis, and patchy lymphocytic infiltrates in the superficial dermis, with scattered necrotic, dyskeratotic and vacuolated keratinocytes. D, In-situ hybridization demonstrates human polyomavirus 9 in the cytoplasm and nuclei of keratinocytes (red staining).
Table. Additional Case Information.
| Variable | Patient | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Transplant type | Kidney | Lung | Lung |
| Transplant dates | December 2006 | March 2009 left lung | March 2013 right and left lungs |
| October 2013 | June 2014 right lung | ||
| November 2018 | |||
| Indication for transplantation | FSG | Interstitial lung disease | COPD |
| Emphysema | |||
| Medical comorbidities | Hypertension | Interstitial pulmonary fibrosis | Chronic kidney disease |
| COPD | Hypertension | Hypothyroidism | |
| FSG | Coronary artery disease | ||
| Associated symptoms | Dyspnea | Weight loss | Diarrhea |
| Weakness | Poor oral intake | Weight loss | |
| Malaise | Deconditioning | Dyspnea | |
| Anorexia | Confusion | Anorexia | |
| Dyspnea | Loss of taste | ||
| Reduced vision | |||
| Other infectious workup results | Negative serum CMV on PCR until critically ill, then low levels of reactivation | Negative serum CMV, VZV, HSV, EBV | Negative serum CMV, VZV, HSV, adenovirus, HHV6, parvovirus B19 |
| Negative serum BKV, JCV, VZV, HHV6, EBV, adenovirus, parvovirus B19 | Negative respiratory viral panel | Negative SARS-CoV-2 on PCR | |
| Negative lesional IHC for HPV | Negative lesional IHC for HPV | Negative lesional IHC for HPV | |
| Negative blood cultures | Negative blood cultures | Negative blood cultures | |
| Negative urine cultures | Negative urine cultures | Negative GI PCR panel | |
| Negative ascites fluid culture | Negative bronchioalveolar lavage cultures | Negative bronchioalveolar lavage cultures | |
| Negative respiratory viral panel | |||
| Anti-rejection medication | Mycophenolic acid | Mycophenolic acid | Mycophenolate mofetil |
| Tacrolimus | Tacrolimus | Tacrolimus | |
| Prednisone | Prednisone | Prednisone | |
| Attempted treatments for cutaneous disease | Acitretin-progression of disease; discontinuation of mycophenolic acid, and tacrolimus-progression of disease | NA | Reduction of immunosuppression-progression of disease; leflunomide-progression of disease; intravenous cidofovir-improvement in skin disease; acitretin-improvement in skin lesions |
| Cutaneous eruption onset | July 2019 | July 2018 | November 2019 |
| Death | November 2019 | February 2019 | February 2021 |
Abbreviations: BKV, BK polyomavirus; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; EBV, Epstein-Barr virus; FSG, focal segmental glomerulonephritis; GI, gastrointestinal; HHV6, human herpesvirus 6; HPV, human papillomavirus; HSV, herpes simplex virus; IHC, immunohistochemistry; JCV, JC polyomavirus; PCR, polymerase chain reaction; VZV, varicella zoster virus.
Patient 1 died 4 months after onset of disease, and the day after HPyV9 was detected in skin and serum using high-throughput sequencing (HTS) (eTable 1 in the Supplement).5,6 Given the similarities to patient 1, banked lesional skin biopsies of patient 2 were subjected to HTS and HPyV9 was detected. In patient 3, skin, blood, urine, ocular, and oral swab results were positive for HPyV9 (eTable 1 in the Supplement). Reduction in immunosuppression, systemic leflunomide, intravenous cidofovir, and acitretin led to transient improvements in patient 3, although not accompanied by a decrease in the viral load. His cutaneous lesions progressed, and the viral load increased in his blood. Lesions evolved during treatment to resemble generalized keratoacanthomas (eFigure 1G in the Supplement). He developed increasing dyspnea 1 year after disease onset, was hospitalized for hemoptysis, placed on comfort care, and died. Autopsy findings revealed the cause of death to be diffuse alveolar hemorrhage. Study was approved by the institutional review board at the Columbia University Medical Center and participants were recruited with written informed consent.
Results
Skin Pathology
Skin tissues from all patients demonstrated hyperkeratosis, acanthosis, prominent dyskeratosis, scattered necrotic and vacuolated keratinocytes in the epidermis. A patchy lymphocytic infiltrate was present in the superficial dermis (Figure 1C; eFigure 2 in the Supplement).
High-Throughput Sequencing and qPCR Analysis
Findings on HTS revealed HPyV9 viral reads in the skin of all patients.5 The samples were negative for all other viral and bacterial pathogens on HTS. Bacterial reads were skin-flora only. We developed a quantitative PCR (qPCR) assay targeting the VP1 gene of HPyV9. Presence of HPyV9 mRNA transcripts in DNase-treated RNA from skin tissues indicated active HPyV9 replication.7 Other samples were positive for HPyV9 DNA but lacked mRNA transcripts in DNase-treated RNA (eTable 1 and eFigure 2F in the Supplement). Two perilesional biopsies in unaffected skin from patient 3 were negative for HPyV9 (eTable 1 in the Supplement). Serum, oral and ocular swabs, and multiple organ tissue samples collected during patient 3′s autopsy were tested with HPyV9 qPCR (eTable 1 in the Supplement). Lung autopsy revealed HPyV9 DNA (3.91E+05 copies/reaction) and HPyV9 mRNA transcripts (1.44E+02 copies/reaction), suggesting viral replication in the lungs. Paratracheal tissue was positive for HPyV9 DNA but other autopsy tissues were negative. The increased viral loads and presence of mRNA in DNase-treated RNA from serum, ocular and oral swabs collected at autopsy suggested systemic infection and pathogenicity (eTable 1 in the Supplement). Extended methods, results for HTS and qPCR, genomic sequencing, mutational and phylogenic analyses are described in the eMethods section and shown in eFigure 3 and eTable 2 in in the Supplement.
In-Situ Hybridization (ISH)
Skin biopsies from patients and controls were subjected to ISH with HPyV9 VP1 gene-specific (Red Cy3 probe), and human GAPDH probes (Blue Vy5.5 probe) (eAppendix in the Supplement). Findings on ISH revealed HPyV9 mRNA transcripts in the cytoplasm and nuclei of keratinocytes in all layers of the lesional epidermis (Figure 1D; eFigure 2 in the Supplement). Extended ISH methods and results are are described in the eMethods and shown in eFigure 4 in the Supplement.
Autopsy
In a complete autopsy, both lungs were grossly congested and consolidated. Microscopic findings demonstrated extensive recent and organized diffuse alveolar hemorrhage with hemosiderin deposits (Figure 2A; eFigure 5A in the Supplement), but without evidence of capillaritis. A nonspecific interstitial fibrosis and numerous dense fibroblastic foci were found in lungs (eFigure 5D in the Supplement). Findings for HPyV9 on ISH were positive in lung (alveolar epithelium, macrophages, interstitial cells) (Figure 2) and focal paratracheal chronic inflammation. Extended results for autopsy are described in the eMethods and shown in eFigure 5 in the Supplement.
Figure 2. Lung Autopsy Findings in Patient 3.
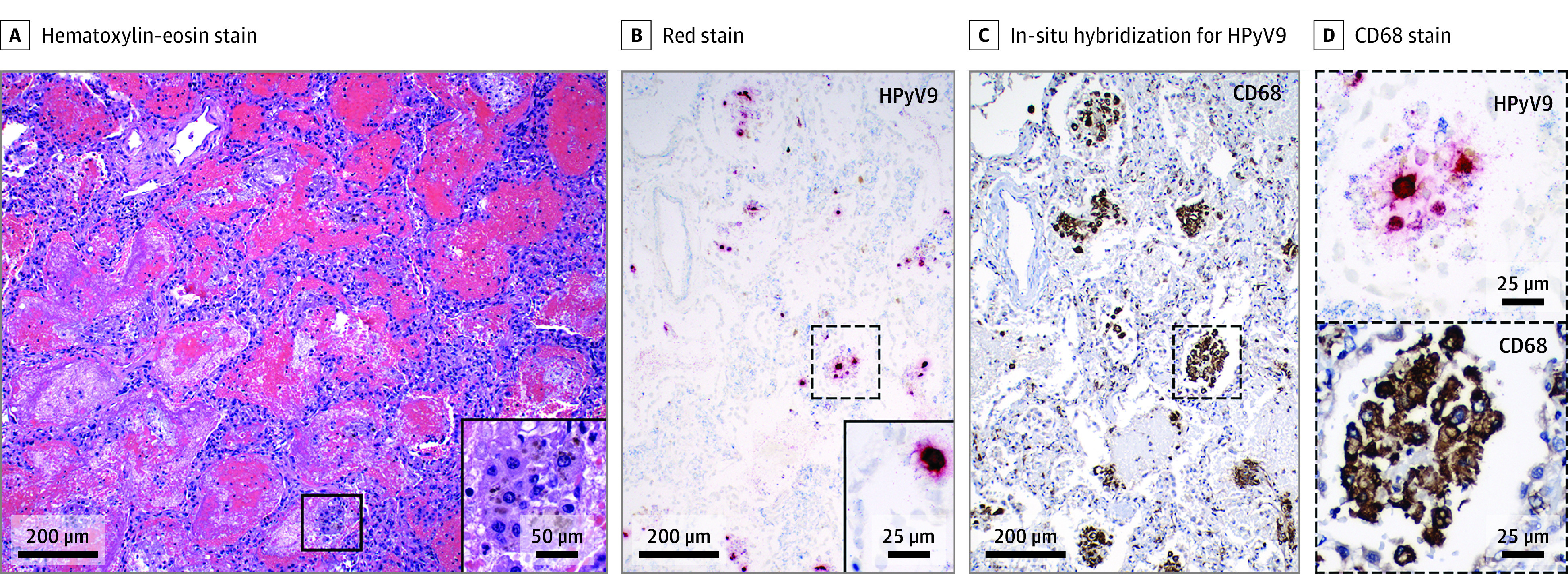
A, Acute bilateral diffuse alveolar hemorrhage with hemosiderin-laden macrophages (inset). B, Human polyomavirus 9 (red staining) is present in lung by in-situ hybridization (dashed box enlarged in D), seen in macrophages in alveolar space (C, dashed box enlarged in D) and alveolar epithelial cells (inset, panel B).
Discussion
We report 3 SOTRs who developed progressive, severe, hyperkeratotic skin lesions associated with HPyV9 infection and died with systemic disease and pulmonary failure. The histopathologic findings resembled previously described HPyV6 and HPyV7 skin eruptions, with dyskeratotic cells throughout the epidermis with a peacock plumage pattern of eosinophilic inclusions in the stratum corneum.8,9,10 The findings of acanthosis, hyperkeratosis, dyskeratosis, necrotic and vacuolated keratinocytes on these patients’ biopsies prompted further investigation for human polyomavirus infection.
Lesional skin biopsies were notable for high levels of HPyV9. The presence of viral mRNA transcripts in HTS, qPCR, and ISH suggest active replication of HPyV9 in skin biopsy samples and suggest HPyV9 was associated with the dermatoses in all 3 patients.
Autopsy of patient 3 revealed that the cause of death was diffuse alveolar hemorrhage (DAH). Numerous fibroblastic foci were also present, which represent organizing acute lung injury. These features would have severely impaired pulmonary function. There was no evidence of acute rejection in the transplanted lungs. Presence of HPyV9 mRNA and HPyV9 DNA in lung alveolar epithelium, macrophages, and interstitial cells indicated active viral replication in lung tissues. Although the underlying etiology for DAH includes connective tissue disease (CTD), medications, coagulopathy, or infection, patient 3 had no clinical evidence of these disorders. The only infectious agent found on autopsy was HPyV9. Given the high viral load of HPyV9 in the skin, accompanied by viremia and HPyV9 mRNA in lung tissue, we propose that HPyV9 infection was associated with patient 3′s death. Patients 1 and 2 did not undergo autopsy; however, both patients also had pulmonary failure of undiagnosed etiology and fatal outcome.
Limitations
We acknowledge that sample size is limited in our study, but we report a serious illness in SOTR. We have recently started caring for a fourth patient with similar cutaneous presentation, presenting after transplantation, and HPyV9 has been detected in skin and blood samples. Given the number of cases we have identified at 1 institution, we believe that there are other undiagnosed patients. No commercial diagnostic tests or treatment for HPyV9 infection are available.11 We developed an HPyV9 qPCR assay for rapid diagnosis, which can help with early diagnosis.12
Conclusions
Physicians should consider HPyV9 infection when a SOTR develops widespread hyperkeratotic lesions, starting with an acral predominance, with no clear alternative diagnosis. Patients may appear stable initially but should be closely monitored for clinical decline. Our study should lead to further research efforts focusing on rapid and early detection of HPyV9 in immunocompromised patients, better understanding of the extent of clinical disease, and finding targeted therapy for this devastating disease.
eMethods
eTable 1. qPCR analysis of RNA and DNA extracts
eTable 2. Full genome mutational analyses
eFigure 1. Additional Clinical Photographs
eFigure 2. Skin histopathology and ISH of patients
eFigure 3. Genomic organization of HPyV9
eFigure 4. Control experiments on skin biopsies
eFigure 5. Lung autopsy findings in Patient 3
eReferences
References
- 1.Scuda N, Hofmann J, Calvignac-Spencer S, et al. A novel human polyomavirus closely related to the african green monkey-derived lymphotropic polyomavirus. J Virol. 2011;85(9):4586-4590. doi: 10.1128/JVI.02602-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Meijden E, Wunderink HF, van der Blij-de Brouwer CS, et al. Human polyomavirus 9 infection in kidney transplant patients. Emerg Infect Dis. 2014;20(6):991-999. doi: 10.3201/eid2006.140055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fajfr M, Pliskova L, Kutova R, et al. Human polyomavirus 9 in immunocompromised patients in the University Hospital in Hradec Kralove, Czech Republic. J Med Virol. 2017;89(12):2230-2234. doi: 10.1002/jmv.24892 [DOI] [PubMed] [Google Scholar]
- 4.Gossai A, Waterboer T, Nelson HH, et al. Seroepidemiology of human polyomaviruses in a US population. Am J Epidemiol. 2016;183(1):61-69. doi: 10.1093/aje/kwv155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briese T, Kapoor A, Mishra N, et al. Virome capture sequencing enables sensitive viral diagnosis and comprehensive virome analysis. mBio. 2015;6(5):e01491-e15. doi: 10.1128/mBio.01491-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allicock OM, Guo C, Uhlemann AC, et al. BacCapSeq: a Platform for Diagnosis and Characterization of Bacterial Infections. mBio. 2018;9(5):e02007-18. doi: 10.1128/mBio.02007-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Carmichael GG. RNA processing in the polyoma virus life cycle. Front Biosci (Landmark Ed). 2009;14:4968-4977. doi: 10.2741/3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canavan TN, Baddley JW, Pavlidakey P, Tallaj JA, Elewski BE. Human polyomavirus-7-associated eruption successfully treated with acitretin. Am J Transplant. 2018;18(5):1278-1284. doi: 10.1111/ajt.14634 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen KD, Lee EE, Yue Y, et al. Human polyomavirus 6 and 7 are associated with pruritic and dyskeratotic dermatoses. J Am Acad Dermatol. 2017;76(5):932-940.e3. doi: 10.1016/j.jaad.2016.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho J, Jedrych JJ, Feng H, et al. Human polyomavirus 7-associated pruritic rash and viremia in transplant recipients. J Infect Dis. 2015;211(10):1560-1565. doi: 10.1093/infdis/jiu524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rijn AL, Wunderink HF, de Brouwer CS, van der Meijden E, Rotmans JI, Feltkamp MCW. Impact of HPyV9 and TSPyV coinfection on the development of BK polyomavirus viremia and associated nephropathy after kidney transplantation. J Med Virol. 2019;91(6):1142-1147. doi: 10.1002/jmv.25397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch HH, Randhawa PS; AST Infectious Diseases Community of Practice . BK polyomavirus in solid organ transplantation-guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13528. doi: 10.1111/ctr.13528 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. qPCR analysis of RNA and DNA extracts
eTable 2. Full genome mutational analyses
eFigure 1. Additional Clinical Photographs
eFigure 2. Skin histopathology and ISH of patients
eFigure 3. Genomic organization of HPyV9
eFigure 4. Control experiments on skin biopsies
eFigure 5. Lung autopsy findings in Patient 3
eReferences


