Key Points
Question
Is the pathologic response of the index lymph node (ILN) concordant with the pathologic response of the total lymph node bed?
Findings
In this pathologic response analysis of resection specimens of 82 patients with stage III melanoma treated with neoadjuvant ipilimumab plus nivolumab, the pathologic response in the ILN was concordant with the response of the total lymph node bed in 99% of cases. In 96% of cases concordance was found when comparing the ILN response with every individual lymph node response.
Meaning
The findings of this study support an ILN response–directed treatment approach (ie, omission of extended lymph node dissection in patients with major pathologic response); ongoing investigation is warranted in melanoma and other tumor types.
Abstract
Importance
Neoadjuvant checkpoint inhibition in patients with high-risk stage III melanoma shows high pathologic response rates associated with a durable relapse-free survival. Whether a therapeutic lymph node dissection (TLND) can be safely omitted when a major pathologic response in the largest lymph node metastasis at baseline (index lymph node; ILN) is obtained is currently being investigated. A previous small pilot study (n = 12) showed that the response in the ILN may be representative of the pathologic response in the entire TLND specimen.
Objective
To assess the concordance of response between the ILN and the total lymph node bed in a larger clinical trial population.
Design, Setting, and Participants
Retrospective pathologic response analysis of a multicenter clinical trial population of patients from the randomized Study to Identify the Optimal Adjuvant Combination Scheme of Ipilimumab and Nivolumab in Melanoma Patients (OpACIN) and Optimal Neo-Adjuvant Combination Scheme of Ipilimumab and Nivolumab (OpACIN-neo) trials. Included patients were treated with 6 weeks neoadjuvant ipilimumab plus nivolumab. Patient inclusion into the trials was conducted from August 12, 2015, to October 24, 2016 (OpACIN), and November 24, 2016, and June 28, 2018 (OpACIN-neo). Data were analyzed from April 1, 2020, to August 31, 2021.
Main Outcomes and Measures
Concordance of the pathologic response between the ILN and the TLND tumor bed. The pathologic response of the ILN was retrospectively assessed according to the International Neoadjuvant Melanoma Consortium criteria and compared with the pathologic response of the entire TLND specimen.
Results
A total of 82 patients treated with neoadjuvant ipilimumab and nivolumab followed by TLND (48 [59%] were male; median age, 58.5 [range, 18-80] years) were included. The pathologic response in the ILN was concordant with the entire TLND specimen response in 81 of 82 patients (99%) and in 79 of 82 patients (96%) concordant when comparing the ILN response with the response in every individual lymph node. In the single patient with a discordant response, the ILN response (20% viable tumor, partial pathologic response) underestimated the entire TLND specimen response (5% viable, near-complete pathologic response). Two other patients each had 1 small nonindex node that contained 80% viable tumor (pathologic nonresponse) whereas all other lymph nodes (including the ILN) showed a partial pathologic response. In these 2 patients, the risk of regional relapse might potentially have been increased if TLND had been omitted.
Conclusions and Relevance
The results of this study suggest that the pathologic response of the ILN may be considered a reliable indicator of the entire TLND specimen response and may support the ILN response-directed omission of TLND in a prospective trial.
This pathologic response study assesses the concordance of the index lymph node pathologic response with the pathologic response in the entire therapeutic lymph node dissection specimen in patients enrolled in 2 clinical trials.
Introduction
Historically, the outcome of patients with stage III melanoma was poor with a high risk of relapse.1,2 Adjuvant therapy with monoclonal antibodies targeting cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) or programmed cell death protein 1 (PD-1) and adjuvant therapies targeting BRAF and MEK have improved the relapse-free survival (RFS). Still, 40% to 50% of patients with high-risk stage III melanoma relapse within 3 to 5 years.3,4,5 Results of early clinical trials show that neoadjuvant therapy with checkpoint inhibitors induces high pathologic response rates, especially with the combination of anti-PD-1 and anti-CTLA-4 antibodies, with subsequent durable RFS outcomes that may outperform adjuvant therapy.6,7,8
The Study to Identify the Optimal Adjuvant Combination Scheme of Ipilimumab and Nivolumab in Melanoma Patients (OpACIN) (NCT02437279) and Optimal Neo-Adjuvant Combination Scheme of Ipilimumab and Nivolumab (OpACIN-neo) (NCT02977052) trials showed that following neoadjuvant ipilimumab plus nivolumab, only 1 of 71 patients (2%) with a pathologic response (defined as ≤50% viable tumor) vs 15 of 23 with pathologic nonresponse (65%) (defined as ≥50% viable tumor) relapsed after a median follow-up of 36.7 and 24.6 months, respectively. This study, and a larger pooled analysis, indicates that pathologic response serves as surrogate outcome marker for RFS.9,10 In addition, long-term benefit was observed in patients with stage IV melanoma achieving a complete response on checkpoint inhibition, even after cessation of therapy.11,12,13,14 These observations raise the question of whether a therapeutic lymph node dissection (TLND) in patients with stage III melanoma achieving major pathologic responses to neoadjuvant immunotherapy has additional benefit. We hypothesize that TLND might be omitted in those patients without affecting RFS.
A reliable indicator of the pathologic response within the entire lymph node basin specimen is a prerequisite for potential omission of TLND in patients with MPR. The RECISTv1.1 radiologic response on computed tomography (CT) imaging after neoadjuvant immunotherapy underestimates the degree of pathologic response, possibly because residual viable tumor can be radiologically difficult to distinguish from regressed tumor bed and because intratumoral immune infiltration may mimic tumor progression (pseudoprogression).7,15 Another method is the pathologic assessment of a single (likely the largest) tumor-containing lymph node (the index lymph node [ILN]) as a representation of the entire lymph node bed. In breast cancer, the MARI (marking axillary lymph node with radioactive iodine seeds) procedure and selective removal of the nodes showed a low false-negative rate of residual viable tumor after neoadjuvant chemotherapy.16 However, neoadjuvant immunotherapy data on the representativeness of the pathologic response of the ILN for the total tumor bed response in breast cancer were lacking.
A previous trial17 demonstrated that the pathologic response in the ILN reliably indicated the response in the entire TLND specimen in a small pilot cohort (Magnetic Seed Localization for Melanoma [MeMaLoc] trial), of 12 patients with stage III melanoma treated with neoadjuvant ipilimumab plus nivolumab in the OpACIN-neo trial.17 Herein, we assess the concordance of the ILN pathologic response with the pathologic response in the entire TLND specimen in a larger retrospective analysis of patients enrolled in the OpACIN and OpACIN-neo trials.
Methods
Patients
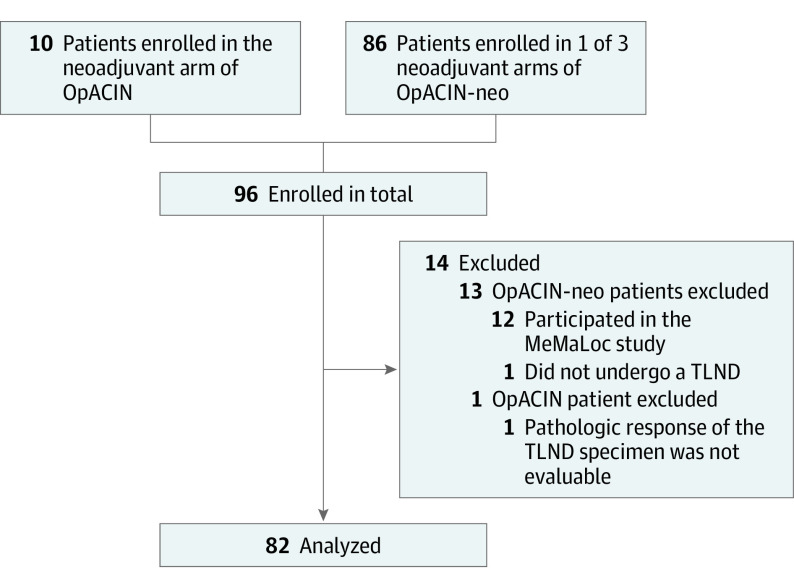
For this retrospective analysis, 82 of 96 patients with histologically confirmed resectable stage III melanoma and 1 or more macroscopic lymph node metastases were identified from the neoadjuvant arm of the OpACIN trial and the OpACIN-neo trial. The 14 patients who had not undergone a TLND, who had already participated in the MeMaLoc substudy17 of OpACIN-neo, or patients in whom the pathologic response was not evaluable were excluded (Figure 1). Patients were enrolled at The Netherlands Cancer Institute (NKI) (OpACIN: n = 9, OpACIN-neo: n = 33), Melanoma Institute Australia (MIA) (OpACIN-neo: n = 38), and Karolinska University Hospital (KS) (OpACIN-neo: n = 2). Patient inclusion into the trials was conducted from August 12, 2015, to October 24, 2016 (OpACIN), and November 24, 2016, and June 28, 2018 (OpACIN-neo). Data were analyzed from April 1, 2020, to August 31, 2021. Patients received 1 or 2 cycles of neoadjuvant ipilimumab plus nivolumab and underwent a TLND after 6 weeks. Patients in the OpACIN trial were treated with 2 cycles of ipilimumab, 3 mg/kg, plus nivolumab, 1 mg/kg, and patients in the OpACIN-neo trial received either 2 cycles ipilimumab, 3 mg/kg, plus nivolumab, 1 mg/kg, 2 cycles ipilimumab 1 mg/kg plus nivolumab 3 mg/kg, or 2 cycles of ipilimumab, 3 mg/kg, followed by 2 cycles of nivolumab, 3 mg/kg.6,7 Baseline imaging was performed using both whole-body positron emission tomography (PET)/CT and CT of the neck, thorax, abdomen, and pelvis. The latter was repeated in week 6. Both the OpACIN and OpACIN-neo studies were conducted in accordance with the protocol and Good Clinical Practice Guidelines as defined by the International Conference on Harmonization and the Declaration of Helsinki.18 All participating patients provided written informed consent before enrollment. The medical ethics committees of each participating center of both studies approved the study protocol. The approval of this analysis is covered by the study-specific medical ethics approval of both studies. This analysis is part of the extensive pathologic response assessment of the OpACIN and OpACIN-neo trials.
Figure 1. Patient Flow Diagram.
MeMaLoc indicates Magnetic Seed Localization for Melanoma; OpACIN, Study to Identify the Optimal Adjuvant Combination Scheme of Ipilimumab and Nivolumab in Melanoma Patients; OpACIN-neo, Optimal Neo-Adjuvant Combination Scheme of Ipilimumab and Nivolumab; TLND, therapeutic lymph node dissection.
Pathologic Assessment
The histopathologic response assessment of the surgical resection specimens was performed locally by experienced pathologists of the NKI (B.v.d.W.), MIA (R.A.S., R.V.R., and/or A.J.C.) or Karolinska using hematoxylin-eosin (H&E)–stained slides. For the OpACIN-neo cases, scanned images of the H&E slides were also blinded and cross reviewed by the pathologists at NKI and MIA. As described previously, the interobserver reproducibility of the pathologic response assessment of both pathology teams was high.15
The histopathologic response assessment was performed according to the International Neoadjuvant Melanoma Consortium (INMC) criteria distinguishing 4 response categories based on the percentage of viable tumor: pathologic complete response (pCR; no viable tumor), near-pCR (1% to ≤10% residual viable tumor), pathologic partial response (pPR; >10% to ≤50% residual viable tumor), or no pathologic response (pNR; >50% residual viable tumor).19 The percentage of viable tumor was defined as the percentage of the area of the tumor bed (area of viable tumor and/or regressed tumor) occupied by viable tumor. The percentage of viable tumor was assessed for all lymph nodes with evidence of a tumor bed separately, and for the TLND specimen by dividing the total area of viable tumor by the total area of tumor bed. For this retrospective analysis, both pathology teams of NKI and MIA reanalyzed all cases to reaffirm the response assessment of all lymph nodes separately and to allocate the ILN.
Definitions of Index Lymph Node and Concordance
In a prospective setting, the ILN may be regarded as the largest lymph node at baseline imaging (CT and/or ultrasonographic imaging) and marked pretreatment.17 In this pathologic assessment study, the ILN was defined as the largest lymph node occupied by signs of viable or treated tumor (tumor bed) in the dissection specimen instead of the largest lymph node at baseline imaging. The pathologic response of the ILN and response of the TLND specimen were regarded as concordant if the pathologic response categories were in the same subcategory of pathologic response (pCR, near-pCR, pPR, or pNR) according to the INMC criteria.
Statistical Analysis
Continuous variables were described as median values with IQRs. No test was used for the comparison of variables, and statistical significance was not a factor in this analysis. Analysis was performed with Excel Professional Plus 2016 (Microsoft).
Results
A total of 82 posttreatment node field specimens were analyzed from 82 patients who were treated with neoadjuvant ipilimumab plus nivolumab followed by subsequent TLND in the OpACIN and OpACIN-neo studies (48 [59%] were male; 34 [41%] were female; median age, 58.5 [range, 18-80] years). Other baseline and demographic characteristics of all patients are shown in Table 1. Lymph node metastases were located in the axilla (51% [42 of 82]), neck (16% [13 of 82]), axilla and neck (4% [3 of 82]), groin (28% [23 of 82]), or epitrochlear fossa (1% [1 of 82]). The median time from the first immunotherapy cycle to TLND was 6.4 weeks (IQR, 6.0-6.9 weeks). A pathologic response was achieved in 60 of 82 patients (73%). Within median follow-up duration of 48.0 months (IQR, 44.3-50.2 months) in the OpACIN trial and 24.6 months (IQR, 21.6-27.6 months) in the OpACIN-neo trial, 14 patients relapsed. All patients with relapse in this cohort had a pathologic nonresponse.
Table 1. Baseline Characteristics of 82 Patients Enrolled in OpACIN and OpACIN-neo.
| Characteristic | No. (%)a |
|---|---|
| Study | |
| OpACIN | 9 (11) |
| OpACIN-neo | 73 (89) |
| Institute | |
| NKI | 42 (51) |
| MIA | 38 (46) |
| KS | 2 (2) |
| Age, median (range), y | 58.5 (18-80) |
| Sex | |
| Male | 48 (59) |
| Female | 34 (41) |
| Clinical tumor stage (AJCC 8th edition) | |
| IIIB | 51 (62) |
| IIIC | 31 (38) |
| Previous lymph node surgical procedure | |
| Previous sentinel node biopsy | 25 (30) |
| Previous lymph node dissection | 6 (8) |
| Location of affected lymph node | |
| Neck | 13 (16) |
| Axilla | 42 (51) |
| Axilla plus neck | 3 (4) |
| Groin | 23 (28) |
| Epitrochlear fossa | 1 (1) |
| No. of target lesions on CT | |
| 1 | 66 (80) |
| ≥2 | 16 (20) |
| Sum of diameter target lesions, median (IQR), mm | 24 (17.25-36.25) |
| Presurgical treatment regimen | |
| 2x I3N1 | 35 (43) |
| 2x I1N3 | 25 (30) |
| 2x I3 followed by 2x N3 | 22 (27) |
Abbreviations: AJCC, American Joint Committee on Cancer; IxNx, ipilimumab x mg/kg plus nivolumab x mg/kg; KS, Karolinska University Hospital; MIA, Melanoma Institute Australia; NKI, Netherlands Cancer Institute; OpACIN, Study to Identify the Optimal Adjuvant Combination Scheme of Ipilimumab and Nivolumab in Melanoma Patients; OpACIN-neo, Optimal Neo-Adjuvant Combination Scheme of Ipilimumab and Nivolumab.
Percentages may not total 100 due to rounding.
During surgery, a median of 19 lymph nodes (IQR, 12-31 nodes) were retrieved from the node field specimen (Table 2). Histopathologic assessment revealed that in 56 TLND specimens (68%), 1 lymph node was involved, and in 26 TLND specimens (32%), there was evidence of viable or regressed tumor in 2 or more nodes (Figure 2). The pathologic response in the ILN was concordant with the response of the entire TLND specimen in 81 of 82 patients (99%) according to the INMC pathologic response subgroup classification. In the single patient with a discordant response, both the TLND specimen and ILN specimen demonstrated pathologic response, but the degree of response in the entire TLND tumor bed (5% viable tumor, near pCR) was underestimated by the ILN response (20% viable tumor, partial response) (Figure 2 and Figure 3, A-D). In total, 34 patients (41%) achieved a pCR, 15 patients (18%) a near-pCR, and 11 patients (13%) a pPR in their ILN. In the entire TLND specimen, these were 34 (41%), 16 (20%), and 10 (12%) patients, respectively. There were 22 patients (27%) who did not achieve a pathologic response (pNR) in either their ILN or in the entire TLND specimen (Table 2).
Table 2. Overall Pathologic Results in 82 Patients Enrolled in OpACIN and OpACIN-neo.
| Pathologic result | Median (IQR) |
|---|---|
| Total node count per node field | |
| Median (IQR) | 19 (12-31) |
| Neck | 62 (41-76) |
| Axilla | 22 (14-29) |
| Axilla plus neck | 73 (52-81) |
| Groin | 9 (8-17) |
| Epitrochlear fossa | 1 (NA) |
| Node count with signs of viable or treated tumor | |
| Median (IQR) | 1 (1-2) |
| Neck | 1 (1-2) |
| Axilla | 1 (1-1) |
| Axilla plus neck | 20 (18-22) |
| Groin | 1 (1-2) |
| Epitrochlear fossa | 1 (NA) |
| Response ILN, No. (%)a | |
| pCR | 34 (41) |
| near-pCR | 15 (18) |
| pPR | 11 (13) |
| pNR | 22 (27) |
| Response total node field, No. (%)a | |
| pCR | 34 (41) |
| near-pCR | 16 (20) |
| pPR | 10 (12) |
| pNR | 22 (27) |
| ILN concordant with total node field, No. (%)a | |
| Yes | 81 (99) |
| No | 1 (1) |
| ILN concordant with every individual node response, No. (%)a | |
| Yes | 79 (96) |
| No | 3 (4) |
Abbreviations: ILN, index lymph node; NA, not applicable; near-pCR, near pathologic complete response; OpACIN, Study to Identify the Optimal Adjuvant Combination Scheme of Ipilimumab and Nivolumab in Melanoma Patients; OpACIN-neo, Optimal Neo-Adjuvant Combination Scheme of Ipilimumab and Nivolumab; pNR, pathologic nonresponse; pPR, pathologic partial response.
Percentages may not total 100 due to rounding.
Figure 2. Concordance of the Pathologic Response in ILN With Pathologic Response in Entire Lymph Node Bed.
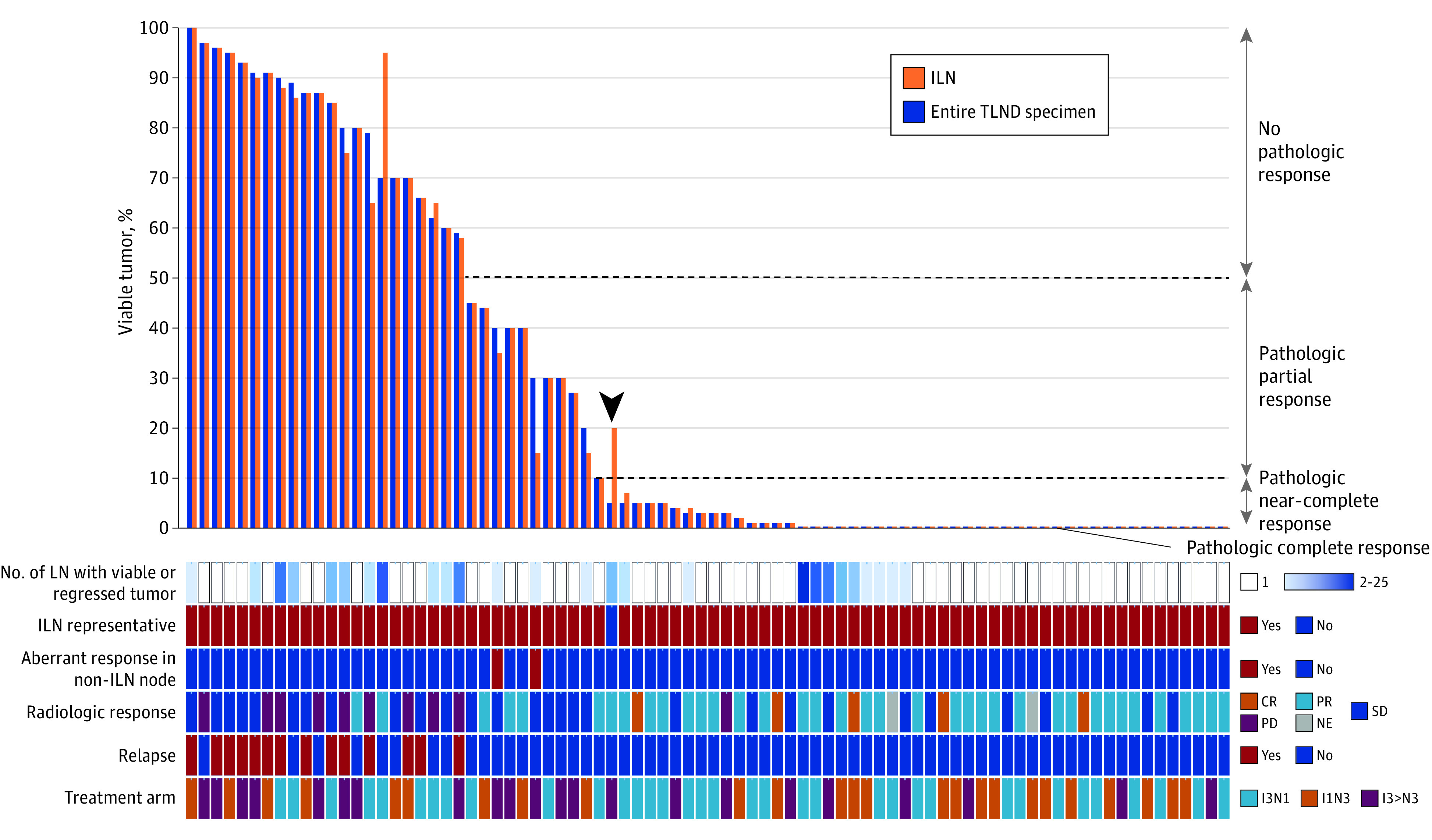
Waterfall plot of concordance of the pathologic response in the ILN with the pathologic response of the entire lymph node bed in each patient (column). The arrowhead denotes the 1 patient with discordant responses. CR indicates complete response; ILN, index lymph node; IxNx, ipilimumab x mg/kg plus nivolumab x mg/kg; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease; TLND, therapeutic lymph node dissection.
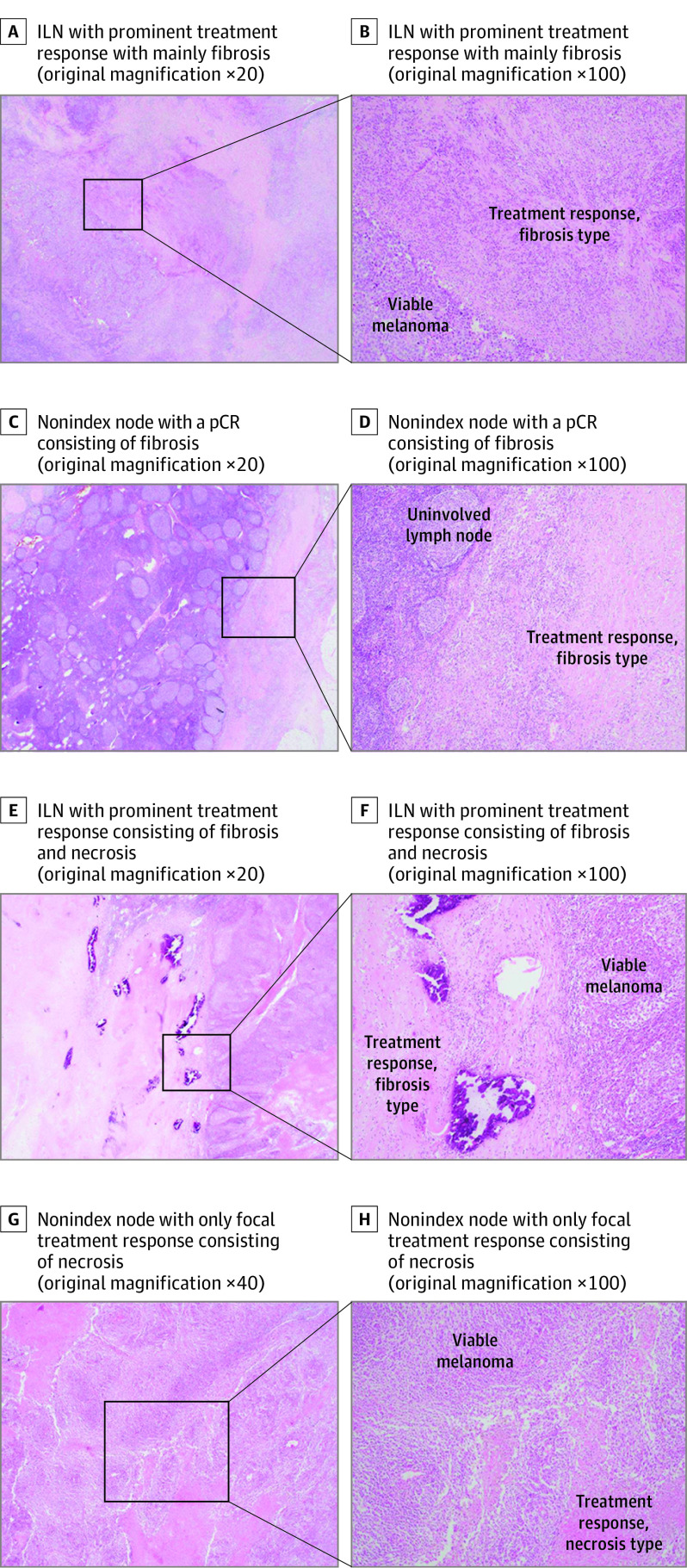
Figure 3. Discordant Pathologic Response in the Index Lymph Node (ILN) vs Nonindex Node After Neoadjuvant Immunotherapy for Stage III Melanoma.
A-D, Hematoxylin-eosin (H&E)–stained images of the patient with discordant responses in the ILN (20% viable tumor; pathologic partial response [pPR]) vs total lymph node tumor bed (5% viable tumor; near pathologic complete response [near-pCR]). A and B, ILN with prominent treatment response with mainly fibrosis. C and D, Nonindex node with a pCR consisting of fibrosis. E-H, H&E images of a patient with 1 small node with aberrant response (80% viable tumor; pathologic nonresponse [pNR]) whereas the ILN and total lymph node tumor bed showed a pPR. E-F, ILN with prominent treatment response consisting of fibrosis and necrosis. G-H, Nonindex node with only focal treatment response consisting of necrosis.
For patients with 2 or more lymph nodes with evidence of viable or regressed tumor, we searched for any nonindex nodes with notably less tumor regression or treatment effect compared with other involved lymph nodes, which may indicate a clone that was less responsive tumor to checkpoint inhibition. Two patients each had 1 small nonindex node with 80% viable tumor while their ILN showed a pPR. In both cases, no other nodes were involved. Taken together, the response of the entire tumor bed remained a pPR (Figure 2 and Figure 3, E-H). The 2 patients have not relapsed to date (follow-up ≥3 years), although the risk of relapse might potentially have been augmented if TLND would have been omitted. Thus, when comparing the pathologic response of the ILN with every individual lymph node response, the ILN was concordant in 79 of 82 cases (96%) compared with a concordance rate of 81 of 82 cases (99%) when the entire TLND specimen response was considered. Baseline tumor features (tumor stage, ulceration, mutation status, and PD-L1 status), baseline lymph node features (number of RECISTv1.1 target lesions or PET/CT positive nodes), or steroid administration due to toxic effects before surgical procedure did not indicate the discordance in pathologic responses in these patients.
Discussion
Neoadjuvant checkpoint inhibition is being investigated in patients with high-risk stage III melanoma and reports show high pathologic response rates associated with long-term RFS.9,10 These results together with the durable complete responses on checkpoint inhibition in stage IV melanoma and the substantial morbidity of more extensive surgery20,21,22 may raise questions about the role of TLND in treatment in patients with stage III melanoma who achieve a major pathologic response to neoadjuvant immunotherapy. However, whether patients who may not require TLND can be reliably identified has been questioned as a as a reliable indicator of pathologic response in the melanoma-involved lymph node bed was lacking.
This is to our knowledge the first larger study that confirms the results of the earlier MeMaLoc pilot trial of 12 patients, which found the pathologic response of the ILN to neoadjuvant immunotherapy to be representative for the pathologic response of the total node field.17 With 99% concordance (96% when considering all individual node responses), the results of this study suggest that the ILN response may be regarded as a reliable indicator for the pathologic response of the entire TLND specimen, particularly as in the single discordant case (according to the INMC criteria) the ILN response underestimated the extent of the TLND specimen pathologic response. Comparable approaches have been investigated with chemotherapy and targeted therapy in breast cancer, in which the removal of a preoperatively labeled or clipped tumor-positive axillary node was a potentially feasible method for tailoring further treatment after neoadjuvant systemic therapy.23,24,25 However, these data are based on neoadjuvant chemotherapy trials, and comparable data from neoadjuvant immunotherapy trials are lacking to date. This study may have implications across oncology in an era of neoadjuvant immunotherapy and may encourage the assessment of the ILN pathologic response in other tumor types (eg, bladder, lung).
Ideally, noninvasive methods such as radiologic imaging should be able to estimate pathologic responses after neoadjuvant therapy. However, in previous studies in stage III melanoma, RECISTv1.1 radiologic response to neoadjuvant checkpoint inhibition underestimated the pathologic response rates.6,7,8 Similarly, in breast cancer, MRI and PET/CT imaging showed only modest results for response estimation after neoadjuvant chemotherapy with a pooled sensitivity of 0.88 and 0.77, and specificity of 0.69 and 0.78, respectively.26 Inconsistent outcomes were seen with minimally invasive image-guided biopsy methods in breast cancer, probably partly due to tumor heterogeneity and the variety in biopsy methods.27 In advanced melanoma, an increased immune-related pathologic response score in H&E-stained biopsies while receiving treatment was associated with objective response and improved overall survival,28 but further evidence on the representativeness for the total tumor bed response after neoadjuvant immunotherapy in stage III melanoma is warranted. Extensive improvements in radiomics and baseline or during treatment biomarkers in addition to less invasive approaches may help generate alternatives.
Given the concordance of pathologic response in the ILN and TLND specimens in this study, the added value of TLND in patients achieving an ILN pathologic response and to what degree of response (complete, near-complete, or partial response) the TLND could be safely omitted warrants further investigation.
The pooled analysis of the INMC including 6 neoadjuvant trials reported an association between pathologic response (≤50% residual viable tumor) and relapse-free survival with neoadjuvant immunotherapy.10 However, the patients (all but one) that were in this pooled analysis had undergone TLND irrespective of their pathologic response after neoadjuvant immunotherapy. The omission of TLND in patients who achieve a pCR or near-pCR (≤10% residual viable tumor) in their ILN is currently being prospectively evaluated in the PRADO (personalized response-directed surgery and adjuvant therapy after neoadjuvant combination of ipilimumab and nivolumab) trial.29
The association between any degree of pathologic response and relapse-free survival may be specific to systemic immunotherapy and may be consistent across other tumor types. Unlike chemotherapy and targeted therapies, immune checkpoint inhibitors provide ongoing and durable efficacy, even after cessation of treatment,11,12,13 thus limited residual viable disease after short-term neoadjuvant checkpoint inhibition might not have negative consequences in RFS.
In addition, heterogeneity of pathologic responses between different nodes could be of particular importance in a small subset of patients. This study reported 2 patients with only limited tumor regression in a single nonindex node while the remaining tumor bed showed a pathologic partial response, possibly indicating the presence of a less responsive tumor clone in some nodes to checkpoint inhibition, which might increase the risk of relapse when TLND is omitted.
Because the treatment period of 6 weeks was relatively short and most resection specimens in current analysis showed no substantial variety in pathologic responses between all lymph nodes, we considered that this retrospective assessment was unlikely to have affected the outcome. Prospective data on the use of the ILN for pathologic response assessment and on RFS outcomes following ILN response-directed tailoring of treatment are expected to be available from the PRADO extension cohort of OpACIN-neo in 2022. In PRADO, TLND was omitted in patients with high-risk stage III melanoma who achieved a complete or near-complete pathologic response (≤10% residual viable tumor) after neoadjuvant ipilimumab and nivolumab in their pretreatment marked ILN.
Limitations
This study has limitations, including its retrospective approach, using the largest lymph node in the resection specimen instead of the largest lymph node marked pretreatment as ILN (as was done in a small pilot study28 and the prospective PRADO trial)17 for assessment of concordance. Theoretically, the largest node in the resection specimen might not have been the largest node on imaging owing to lymphoid hyperplasia in response to checkpoint inhibition or heterogeneity in pathologic response between individual nodes. Because the treatment period of 6 weeks was relatively short and most resection specimens in current analysis showed no substantial variety in pathologic responses between all lymph nodes, we considered that this retrospective assessment was unlikely to have affected the outcome.
Conclusions
Results of this pathologic assessment study of the value of the ILN response suggest that the pathologic ILN response reliably estimates the pathologic response in the remaining TLND tumor bed. These data suggest the utility of the ILN response-directed approach and warrant ongoing investigation of the concept.
References
- 1.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199-6206. doi: 10.1200/JCO.2009.23.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gershenwald JE, Scolyer RA. Melanoma staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Ann Surg Oncol. 2018;25(8):2105-2110. doi: 10.1245/s10434-018-6513-7 [DOI] [PubMed] [Google Scholar]
- 3.Dummer R, Brase JC, Garrett J, et al. Adjuvant dabrafenib plus trametinib versus placebo in patients with resected, BRAFV600-mutant, stage III melanoma (COMBI-AD): exploratory biomarker analyses from a randomised, phase 3 trial. Lancet Oncol. 2020;21(3):358-372. doi: 10.1016/S1470-2045(20)30062-0 [DOI] [PubMed] [Google Scholar]
- 4.Eggermont AMM, Blank CU, Mandalà M, et al. ; EORTC Melanoma Group . Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(5):643-654. doi: 10.1016/S1470-2045(21)00065-6 [DOI] [PubMed] [Google Scholar]
- 5.Ascierto PA, Del Vecchio M, Mandalá M, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(11):1465-1477. doi: 10.1016/S1470-2045(20)30494-0 [DOI] [PubMed] [Google Scholar]
- 6.Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655-1661. doi: 10.1038/s41591-018-0198-0 [DOI] [PubMed] [Google Scholar]
- 7.Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948-960. doi: 10.1016/S1470-2045(19)30151-2 [DOI] [PubMed] [Google Scholar]
- 8.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649-1654. doi: 10.1038/s41591-018-0197-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozeman EA, Hoefsmit EP, Reijers ILM, et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat Med. 2021;27(2):256-263. doi: 10.1038/s41591-020-01211-7 [DOI] [PubMed] [Google Scholar]
- 10.Menzies AM, Amaria RN, Rozeman EA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med. 2021;27(2):301-309. doi: 10.1038/s41591-020-01188-3 [DOI] [PubMed] [Google Scholar]
- 11.Jansen YJL, Rozeman EA, Mason R, et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol. 2019;30(7):1154-1161. doi: 10.1093/annonc/mdz110 [DOI] [PubMed] [Google Scholar]
- 12.Long GV, Schachter J, Ribas A, et al. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in KEYNOTE-006. J Clin Oncol. 2018;36(suppl 15):9503. doi: 10.1200/JCO.2018.36.15_suppl.9503 [DOI] [Google Scholar]
- 13.Robert C. 5-Year characterization of complete responses in patients with advanced melanoma who received nivolumab plus ipilimumab or nivolumab alone. Ann Oncol. 2020;31(4):s734-s735. doi: 10.1016/j.annonc.2020.08.1206 [DOI] [Google Scholar]
- 14.Tan AC, Emmett L, Lo S, et al. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann Oncol. 2018;29(10):2115-2120. doi: 10.1093/annonc/mdy330 [DOI] [PubMed] [Google Scholar]
- 15.Rawson RV, Adhikari C, Bierman C, et al. Pathological response and tumour bed histopathological features correlate with survival following neoadjuvant immunotherapy in stage III melanoma. Ann Oncol. 2021;32(6):766-777. doi: 10.1016/j.annonc.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 16.Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378-382. doi: 10.1097/SLA.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 17.Schermers B, Franke V, Rozeman EA, et al. Surgical removal of the index node marked using magnetic seed localization to assess response to neoadjuvant immunotherapy in patients with stage III melanoma. Br J Surg. 2019;106(5):519-522. doi: 10.1002/bjs.11168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 19.Tetzlaff MT, Messina JL, Stein JE, et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol. 2018;29(8):1861-1868. doi: 10.1093/annonc/mdy226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Akkooi AC, Bouwhuis MG, van Geel AN, et al. Morbidity and prognosis after therapeutic lymph node dissections for malignant melanoma. Eur J Surg Oncol. 2007;33(1):102-108. doi: 10.1016/j.ejso.2006.10.032 [DOI] [PubMed] [Google Scholar]
- 21.de Vries M, Vonkeman WG, van Ginkel RJ, Hoekstra HJ. Morbidity after axillary sentinel lymph node biopsy in patients with cutaneous melanoma. Eur J Surg Oncol. 2005;31(7):778-783. doi: 10.1016/j.ejso.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 22.de Vries M, Vonkeman WG, van Ginkel RJ, Hoekstra HJ. Morbidity after inguinal sentinel lymph node biopsy and completion lymph node dissection in patients with cutaneous melanoma. Eur J Surg Oncol. 2006;32(7):785-789. doi: 10.1016/j.ejso.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 23.Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072-1078. doi: 10.1200/JCO.2015.64.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Noordaa MEM, van Duijnhoven FH, Straver ME, et al. Major reduction in axillary lymph node dissections after neoadjuvant systemic therapy for node-positive breast cancer by combining PET/CT and the MARI procedure. Ann Surg Oncol. 2018;25(6):1512-1520. doi: 10.1245/s10434-018-6404-y [DOI] [PubMed] [Google Scholar]
- 25.Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263(4):802-807. doi: 10.1097/SLA.0000000000001375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Yao L, Jin P, et al. MRI and PET/CT for evaluation of the pathological response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. Breast. 2018;40:106-115. doi: 10.1016/j.breast.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 27.Heil J, Kuerer HM, Pfob A, et al. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: current evidence and future challenges. Ann Oncol. 2020;31(1):61-71. doi: 10.1016/j.annonc.2019.10.012 [DOI] [PubMed] [Google Scholar]
- 28.Stein JE, Soni A, Danilova L, et al. Major pathologic response on biopsy (MPRbx) in patients with advanced melanoma treated with anti-PD-1: evidence for an early, on-therapy biomarker of response. Ann Oncol. 2019;30(4):589-596. doi: 10.1093/annonc/mdz019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blank CU, Reijers ILM, Pennington T, et al. First safety and efficacy results of PRADO: A phase II study of personalized response-driven surgery and adjuvant therapy after neoadjuvant ipilimumab (IPI) and nivolumab (NIVO) in resectable stage III melanoma. J Clin Oncol. 2020;38(15_suppl):10002. doi: 10.1200/JCO.2020.38.15_suppl.10002 [DOI] [Google Scholar]