Abstract
Background
Using a dextrose-containing solution, instead of normal saline, to maintain the patency of an arterial cannula results in the admixture of glucose in line samples. This can misguide the clinician down an inappropriate treatment pathway for hyperglycaemia.
Methods
Following a near-miss and subsequent educational and training efforts at our institution, we conducted two simulations: (1) to observe whether 20 staff would identify a 5% dextrose/0.9% saline flush solution as the cause for a patient’s refractory hyperglycaemia, and (2) to compare different arterial line sampling techniques for glucose contamination.
Results
(1) Only 2/20 participants identified the incorrect dextrose-containing flush solution, with the remainder choosing to escalate insulin therapy to levels likely to risk fatality, and (2) glucose contamination occurred regardless of sampling technique.
Conclusion
Despite national guidance and local educational efforts, this is still an under-recognised error. Operator-focussed preventative strategies have not been effective and an engineered solution is needed.
Keywords: Arterial line, glucose sampling error, patient safety, human factors, intensive care, anaesthesia, simulation
Introduction
Arterial lines are commonly used in the intensive care unit (ICU) for continuous monitoring of various physiological variables and arterial blood gas (ABG) samples are regularly taken from the line to check blood gases, electrolytes and blood glucose. Normal saline (0.9% sodium chloride) is the recommended flush solution for maintaining the patency of an arterial catheter and ensuring that blood does not clot within the line. 1 However, other solutions may be wrongly used as the flush due to a lack of staff awareness, misreading of the label or inadequate checking of the solution administered.2,3 When a glucose-containing solution, such as 5% dextrose, is used inadvertently against national guidelines, admixture of the flush solution and the patient’s blood may result in an apparently raised glucose level in ABG samples drawn from the arterial line. A clinician may then be dangerously misled down an inappropriate treatment pathway to maintain normoglycaemia, potentially resulting in repeated insulin administration and hypoglycaemic fatality. 4
The UK National Patient Safety Agency (NPSA) cascaded a Rapid Response Report in July 2008 to all British Hospitals which identified 41 incidents where arterial lines were kept patent using a glucose-containing flush solution, one of which directly led to a patient’s death. 1 The report mandated that the flush fluid should be prescribed by a doctor and double-checked by nursing staff prior to administration, at regular intervals and during shift handover. However, a further 169 incidents were reported to NPSA in the four years following the report, prompting the Medicines and Healthcare products Regulatory Authority to issue a Drug Safety Update in 2012 which reiterated the need for vigilance about this error. 5 More recently, in 2014, the Association of Anaesthetists of Great Britain & Ireland (AAGBI) recommended rigorous checking processes prior to administering flush solutions, raising staff awareness of the error and altering blood sampling technique to reduce the error rate. 6 Despite these national reports and guidelines, this error still continues to occur and in 2020 has gained widespread media attention in the United Kingdom after it was implicated in the death of a patient at a hospital in the South East of England. 7
Different sampling techniques have been shown to influence glucose contamination of the sample drawn from the arterial line. 8 Before obtaining the sample, the ‘dead-space volume’ of fluid between the sampling port and the tip of the arterial cannula must be removed, as it will contain flush solution. An open system technique may be used, where a single port is used to remove both the dead-space volume containing flush solution followed by the blood sample. As 5% dextrose has a glucose concentration of 277 mmol/L, even minimal sample contamination will produce falsely high blood glucose levels. Consequently, it has been recommended that three times the dead space volume of fluid should be removed prior to sampling. 8 However, one study demonstrated that when using the open sampling technique, significant glucose contamination from dextrose-containing flush fluids occurred even when five times the dead-space volume was removed prior to sampling. 9 As a result, the AAGBI guidelines recommended that a closed sampling technique should be used instead, in which the dead space volume is initially removed from a separate port distal to the sampling port, which is then used only to extract the blood sample itself. 6 This should theoretically reduce the risk of sample contamination by flush fluid and also reduces wastage of blood, as any dead-space blood initially removed at the distal port can be aseptically returned to the circulation without opening the system, which is not possible with an open sampling approach.10,11 Given that some ICU patients require multiple ABG samples daily, the ability to conserve blood each time is beneficial. 12 However, while the evidence supporting closed, blood-conserving arterial sampling in the context of reducing catheter tip colonisation and associated blood stream infection is established, 13 there is a lack of evidence on its beneficial impact in reducing glucose contamination in the event of a dextrose flush solution being inadvertently connected to the line. The AAGBI guidelines were based on a single bench study which demonstrated that closed arterial sampling prevented ‘clinically significant’ glucose contamination when a 5% glucose flush bag was connected to the circuit, defined by the authors as a glucose concentration of >1 mmol/L. This is in contrast to open sampling techniques which resulted in sample glucose concentrations of >1 mmol/L regardless of dead space volumes removed. 9 However, the authors also found that just 0.004 ml of 5% glucose would be required to increase the measured glucose concentration in 1 ml arterial blood samples by 1 mmol/L, with the required volume likely to be considerably smaller if glucose 20% or even 50% was used. Combined with the author’s findings that ‘clinically insignificant’ glucose contamination still occurred with closed sampling techniques in their model, further studies in this area are indicated. 9
Despite the numerous recommendations, implementation of the guidance varies and cases of hypoglycaemia and neuroglycopaenia continue to occur due to inadvertent treatment of falsely elevated ABG blood glucose readings.14–18 Indeed, a previous unpublished analysis of the English National Health Service National Learning and Reporting System (NHS NRLS) database conducted at our institution revealed 299 reported incidents of the error between 2005 and 2015, equating to an average of one reported error every two weeks. The real number of unreported errors is likely to be considerably more than this, with one postal study showing that 30% of the 241 adult ICUs across the United Kingdom have reported errors associated with incorrect flush fluid use in arterial lines, with 5% dextrose being the most frequently cited incorrect fluid. 16 Clinical outcomes in the NHS NRLS database included two reports of severe harm to patients and there have also been case reports of patient deaths as a direct result of this error causing fatal hypoglycaemia.1,4,17 In line with the NPSA and AAGBI recommendations, a range of preventative actions have been suggested by units in the NHS NRLS database following the error, with raising staff awareness and reinforcing checking procedures being by far the most common (Table 1). Informal analysis of the reports submitted to the NRLS database demonstrated that staff education and training efforts were typically direct, utilising face-to-face classroom-style training sessions, staff meetings, handover meetings and departmental morbidity and mortality forums or indirect, utilising email cascades, staff newsletters and posters in staff rooms to raise awareness of the error. Some units employed both direct and indirect strategies; however, of the 299 reports analysed, simulation and eLearning were not mentioned by any units in their retraining efforts.
Table 1.
Data from the English National Health Service National Reporting and Learning System from 2005 to 2015 showing incidence, clinical outcomes and preventative actions reported for arterial line glucose errors.
| Reports | Total |
|---|---|
| Incidents | 299 |
| Near misses | 5 |
| Clinical outcomes | |
| Inflammation/irritation at arterial line site | 7 |
| Hypoglycemic episodes | 6 |
| Necessitated MRI head | 1 |
| ‘Severe patient harm’ | 2 |
| Preventative actions | |
| Raising staff awareness | 117 |
| Reinforcing checking procedures | 90 |
| Staff education/training | 20 |
| Removing dextrose from the department | 17 |
| Doctors to prescribe flush fluid | 15 |
| Disciplinary/suspension | 10 |
| Future monitoring/audit | 2 |
| Remove 3× dead-space | 1 |
| Confirmatory capillary blood glucose | 1 |
In June 2016, we had a near miss at our hospital where a patient was admitted from the operating theatre with an arterial line primed with 5% dextrose solution. The error was fortunately noticed by nursing staff on the ICU before the patient came to any harm; however, all staff underwent additional face-to-face and email training immediately following the incident. This training was delivered in June 2016 by the ICU’s lead clinical governance nurse who had more than 10 years clinical experience working on the institution’s ICU, with support from a similarly experienced Consultant Intensivist on the unit. The training interventions consisted of an email cascade which informed all medical and nursing ICU staff of the near miss and encouraged vigilance in checking flush solutions by including a visual prompt demonstrating how dextrose-saline and normal saline arterial flush solutions can look similar, particularly when contained within a semi lucent pressure bag. The email also directed staff towards the AAGBI recommendations on closed sampling technique, and informed staff that newly updated local departmental guidance on arterial sampling would now mandate that staff follow the AAGBI recommendations. Similarly, the near miss, visual prompt and updated guidance on closed sampling were all displayed and discussed in depth at all staff meetings immediately following the error as a further form of classroom-style education utilising PowerPoint, and the email was also printed and placed on an educational board in the staff room. Six months later in December 2016, we sought to assess the beneficial impact of these educational efforts by conducting two simulation studies: (1) to assess staff awareness of the error, and (2) to investigate whether glucose contamination could be eliminated by altering sampling technique.
Methods
Ethical approval to conduct this research was granted by the Institutional Review Board at the Queen Elizabeth Hospital, Kings Lynn. All participants in the observational simulation study provided written and signed informed consent.
Simulation 1: Assessing staff awareness of the glucose sampling error in a forced error simulation
Ten doctors and 10 nurses with ICU experience, capable and expected to obtain and interpret ABG samples from an arterial line, self-selected for the study (n = 20, none excluded). All 10 doctors were at either registrar or consultant level in ICU, and all 10 nurses were at band 5 level or above. All participants had worked on the ICU for at least 12 months and were working at the time of the near-miss incident 6 months prior to the study, following which they all received the additional training highlighted. Signed and informed consent was taken from all participants. An intubated manikin model (Laerdal, Gatesville, USA) was mechanically ventilated in a simulated ICU environment and was set up with a peripheral arterial line running 0.9% sodium chloride/5% dextrose as a flush solution, as well as a peripheral venous line running an insulin infusion at six units/h (Actrapid). The 0.9% sodium chloride/5% dextrose solution flush bag was placed in a transparent pressure bag with the labelling clearly visible. Participants were presented with a clinical scenario of a known non-insulin-dependent diabetic gentleman admitted following a road traffic accident, and they were expected to manage the patient’s routine and emergency care. This included evaluating the ventilation circuit and conducting endotracheal tube cuff pressure checks, used as distractor tasks in order to simulate a high-load ICU environment and to mask the data points of interest from participants (Figure 1). It was felt that if the simulation had been limited to include only questions 5–8 pertaining to the glucose error, this may have introduced a bias in participant responses and they may have been unduly directed towards a forced error. By introducing distractor tasks in questions 1–4, the error was embedded into a wider clinical scenario and this more accurately represents how such an error may manifest in clinical practice (Figure 1). Importantly, questions were asked sequentially not concomitantly such that during the forced error part of the simulation (questions 6–8), this was the sole focus of participants without any further distractors.
Figure 1.
The scenario and questions used in the first simulation study, with questions 1–4 being used as distractors and participant responses to questions 5–8 being recorded for the purpose of the study.
The simulation lasted 10 min and was undertaken in an ICU side room with two assessors and one participant present. The two assessors were rotational final year medical students who had spent a total of six weeks in the ICU but were otherwise independent of the department (their medical school was not based at our institution). Both assessors received training in the setup of ventilation circuits, artificial humidification systems, endotracheal tube cuff pressure management and different arterial line sampling techniques on a manikin model (Laerdal, Gatesville, USA) prior to conducting the simulation study. This training was delivered by an ICU consultant who also closely supervised and periodically observed the simulations to ensure data accuracy. During each simulation, one assessor introduced the scenario and asked all eight questions, while the other remained silent and recorded responses to questions 5–8. For question 5, each participant’s preference for an open or closed blood sampling technique was recorded and for questions 6–8, record was made of whether or not, and when, the dextrose-containing flush fluid running through the arterial line was identified as the cause of the simulated patient’s persistent and refractory hyperglycaemia. Participants were offered the hospital’s insulin infusion protocol for hyperglycaemia if they asked for this in their management. Failure to identify the error and instead repeatedly escalating the insulin infusion rate following three sequentially raised blood glucose readings was taken as a likely adverse outcome.
Simulation 2: Examining whether glucose contamination could be eliminated by altering sampling technique
A standard arterial line and sampling transducer system was set up on a manikin (Laerdal, Gatesville, USA); however, the saline flush solution was replaced by 5% dextrose and attached to an 8 cm 3Fr arterial cannula. The blood circulation was simulated using normal saline and the dead space from the cannula tip to the sampling Luer port was 1 mL, and to the distal transducer Luer port was a total of 3 mL. Aliquots of 1.5 mL were taken into a heparinised blood gas syringe following 1, 2, 3, 4 and 5 mL pre-drawing of waste solution at the sampling port (equivalent to 1, 2, 3, 4 and 5× dead space removal in an open system). Following this, a two-port blood conservation technique (closed system) was simulated by pre-drawing 4 mL at the distal transducer port before aliquots were then taken at the sampling Luer port immediately (‘conserving-1’) or following a further pre-draw of 1 mL at the Luer sampling port (‘conserving-2’). Ten samples were taken for each method and the glucose concentration of each sample was tested with a calibrated blood glucose monitor.
Results
Simulation 1: Assessing staff awareness of the glucose sampling error in a forced error simulation
Only 10% (2/20) of participants identified the dextrose-containing flush bag as the cause of the simulated patient’s persistent and refractory hyperglycaemia, both following the third and final ABG result; 90% (18/20) did not recognise the error and either maintained or increased the insulin infusion rate after each of the three sequential ABG samples. One participant in the latter group inspected the flush bag but did not realise that it was a dextrose-containing solution. Analysis of arterial sampling technique showed that 50% (10/20) of participants used a closed system, of whom 9/10 were nurses, while the remaining 50% (10/20) participants preferred to use an open system sampling technique, of whom 9/10 were doctors.
Simulation 2: Examining whether glucose contamination could be eliminated by altering sampling technique
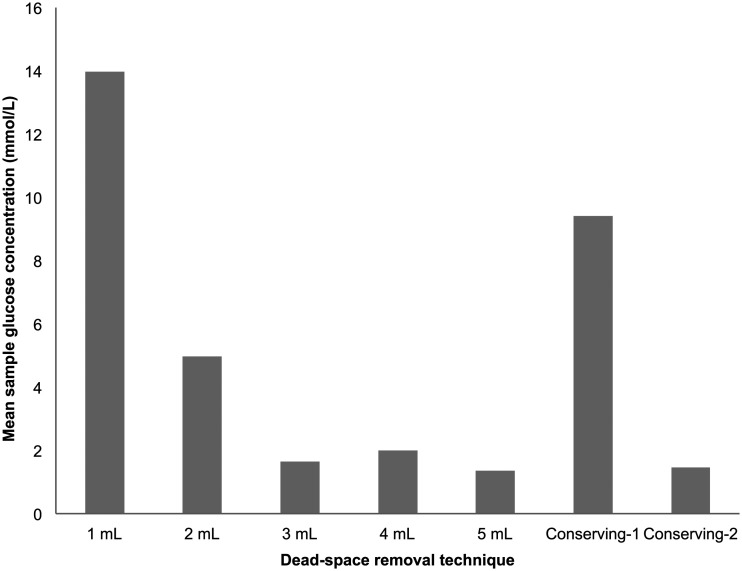
All sampling techniques resulted in glucose contamination (Figure 2, Table 2). Using an open system, removing three times the dead space volume or more substantially reduced but did not eliminate contamination. With a closed blood-conserving approach, contamination was substantially reduced but again not eliminated by additional aspiration of 1 mL at the sampling Luer port prior to obtaining a sample (‘conserving-2’).
Figure 2.
Mean sample glucose contamination (mmol/L) detected with different dead-space removal techniques.
Table 2.
Glucose contamination (mmol/L) with different sampling techniques (Sampling method 1= 1 ml dead-space removal using an open system, 2= 2 ml, 3= 3 ml, 4= 4 ml and 5= 5 ml, 1–10= each of the 10 trials).
| Sampling method | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.6 | 18.4 | 4.8 | 14.3 | 15.7 | 17.0 | 12.0 | 18.8 | 15.1 | 17.0 | 13.97 (5.94) |
| 2 | 2.2 | 7.2 | 7.0 | 3.1 | 1.3 | 3.9 | 7.3 | 8.5 | 3.6 | 5.7 | 4.98 (2.76) |
| 3 | 0.9 | 2.1 | 1.3 | 0.0 | 3.5 | 0.0 | 2.9 | 0.0 | 3.9 | 1.8 | 1.64 (1.32) |
| 4 | 0.0 | 12.4 | 2.3 | 0.7 | 0.0 | 0.0 | 0.9 | 0.7 | 2.9 | 0.0 | 1.99 (5.29) |
| 5 | 0.7 | 3.1 | 0.0 | 3.8 | 2.3 | 0.0 | 0.0 | 0.0 | 1.7 | 1.9 | 1.35 (1.60) |
| Conserving-1 | 10.8 | 13.3 | 12.4 | 4.2 | 10.0 | 11.3 | 9.6 | 12.3 | 0.0 | 10.3 | 9.42 (3.56) |
| Conserving-2 | 6.3 | 1.6 | 1.6 | 1.2 | 1.0 | 0.0 | 0.0 | 1.1 | 1.9 | 0.0 | 1.47 (2.23) |
Note: Values in the final column are mean (SD).
Discussion
Hypoglycaemia has been shown to increase mortality and length of stay on the ICU.19,20 In one study, patients with iatrogenic hypoglycaemia had a mortality rate of 11.5% compared to 8% in patients with no recorded episodes (P < 0.001)), and iatrogenic hypoglycaemia also increased the ICU length of stay by 2.7 days. 19 Consequently, even clinically silent errors where insulin is administered following an arterial glucose sampling error leading to sub-clinical hypoglycaemia may have the potential to result in adverse outcomes for patients. This highlights the importance of prompt recognition by staff when arterial glucose sampling errors occur and in line with this, both the AAGBI guidelines and the NHS NRLS database suggest raising staff awareness of the error as a key preventative strategy. The first simulation study was conducted to test the robustness of this operator-focussed approach, and found that despite a near-miss at our institution six months prior to the study which was followed by concerted local efforts to raise staff awareness of the error, only 10% of staff identified the dextrose-containing flush solution in a forced error scenario. As the results demonstrate, clinicians are liable to forget training over time, especially given frequent rotation of staff between departments and changing responsibilities, and repeated training and educating is wasteful of time and resources. 21 The six-month interval between educational efforts at our institution following the near miss and the simulation study appears to be a short period over which clinicians may forget training. It is possible that the method of training (face-to-face classroom style training sessions and an email cascade) was insufficient to result in a sustained change in practice, such as clinicians automatically remembering to check flush solutions when presented with persistent and refractory hyperglycaemia on ABG samples. However, other authors have also noted similar results with educational programmes targeted at preventing never events. One institution found that that despite two cases of retained guidewires during central line insertion and a subsequent comprehensive multi-disciplinary intern education programme incorporating the use of hands-on simulation training, eLearning, online assessments requiring a passing mark as well as standardised in-person assessments, two more cases of retained guidewires occurred within a year of the residents involved having undergone the additional training.22,23 Similarly, another study demonstrated that implementing an education and retraining programme following four wrong-side regional anaesthesia procedures still led to a further wrong-side procedure shortly after. 24
Both the NPSA report and the AAGBI guidelines recommend reinforced checking procedures to prevent arterial glucose errors, and this is also the second most common preventative action undertaken by units in the NHS NRLS database (Table 1). However, in the first simulation, one participant carefully checked the flush bag indicating awareness of the error, but did not notice the dextrose-containing flush solution. The phenomenon of ‘creeping complacency’ may explain why checking practices ultimately falter, as the rare error of incorrectly using a dextrose flush solution occurs amongst many more safe arterial lines set up with a saline flush. 21 Additionally, in a high-pressure ICU environment where tasks are time-critical and constantly interrupted, creating additional routine checking jobs can increase the cognitive load on already burdened clinicians, leaving even straightforward procedures vulnerable to human error.17,25
The second simulation study was conducted in response to the 2014 AAGBI guidelines which recommended a closed sampling technique to reduce arterial glucose errors. This guidance was highlighted to staff at the additional training sessions undertaken following the near-miss incident at our institution six months prior to the study. However, there was an even split between open and closed sampling techniques amongst our participants indicating poor compliance to the AAGBI guidelines, with most doctors preferring an open sampling technique and most nurses preferring a closed sampling technique. This demonstrates the relative ineffectiveness of the training sessions given to staff following the near-miss incident and while compliance with the AAGBI guidelines on closed sampling technique was 90% amongst nursing staff, the 10% compliance amongst doctors highlights that a one-size-fits-all approach to operator-focussed training sessions can lack efficacy. Poor compliance amongst doctors in particular is partially explained by the fact that they undertake arterial sampling far less routinely than their bedside nursing colleagues, and many doctors may also view arterial glucose errors as a ‘nursing issue’ given that nurses are typically responsible for administering fluids and connecting flush solutions. Doctors have also been found to be less likely to follow strict guidelines and protocols in their clinical practice as compared to their nursing colleagues, with many instead relying more on their past experience and tacit knowledge.26,27 Indeed, one study demonstrated that senior doctors viewed guidelines and protocols as only useful for junior trainees, 26 and this attitude may explain why compliance was so poor amongst the medical staff in our simulation, who were all at registrar level or above. This further highlights the difficulties and limitations which may be encountered when delivering inter-professional training in order to prevent rare errors. Additionally, while altering sampling technique reduced glucose contamination, it was never fully eliminated in any of the sampling techniques tested (Figure 2, Table 2). Taken together, this suggests that guidance and training sessions to alter sampling technique in order to reduce sample contamination are insufficient, and ultimately aim only to mitigate rather than eliminate the underlying error of incorrectly using a dextrose-containing flush solution.
The solutions and preventative actions so far considered from various guidelines and highlighted in the NHS NRLS data all depend on clinicians to prevent the error.1,6 Indeed, 94% of preventative interventions mentioned by units were operator-focussed and of the nine categories of interventions identified in the NHS database, only removing dextrose from the department was identified as system-orientated (Table 1). Relying on humans alone to prevent rare errors ignores human factors science, which ranks such interventions unfavourably,28,29 and this questions the robustness of solutions in current guidelines that place the onus on staff awareness, checking and training to prevent the error. The simulations demonstrate that raising staff awareness and training them to alter their sampling technique both had a limited impact despite a near miss six months prior to the study. This is corroborated by a postal study which found that only 49% of British ICUs surveyed reported compliance with double checking of flush fluid procedures, in line with the NPSA and AAGBI guidelines. 16 Instead of relying on the operator, the system and environment in which clinicians work should be designed to prevent the error from occurring, as this is a safer and more reliable solution than depending on human behaviour.29,30
Reprimanding clinicians following rare errors is similarly a wholly operator-focussed approach that contradicts guidance from the NHS which recognises that a culture of blame after such incidents is often counterproductive, and instead encourages healthcare providers to focus on system change and develop environments which minimise the risks of humans making such errors. 31 Indeed, in healthcare systems with a blame culture, identifying individuals and enacting punishment can often be the endpoint of an investigation following an incident, and the opportunity for true learning is often lost. Instead, healthcare providers should acknowledge that a complex interdependence of factors exists between operators and systems when rare errors occur, and the identification of system, not individual, failures should take priority when designing future preventative strategies.31,32 Therefore, a simple, convenient engineered solution is not only favourable in the hierarchy of intervention effectiveness in human factors terms, it would also be in line with NHS guidance and would eliminate the need to increase staff workload. A gold-standard example of a system-orientated solution to the arterial glucose error would be to design arterial line transducers which can only connect to normal saline flush bags, and cannot physically connect to dextrose-containing flush solutions. However, this is unrealistic in clinical practice given the need for standardised and mass-producible connectors in a resource-limited healthcare system. A more pragmatic solution would perhaps be an arterial line transducer which clots or produces an unexpected colour change when connected to a dextrose-containing flush solution, thus alerting the operator to an equipment issue without the need for them to rely on previous experience or training alone when rare errors occur.
Limitations to this study include the small sample size of 20 participants in the first simulation study, chosen because of the need to complete the simulation in one day for all participants to prevent the effect of participants sharing details of the study to each other. Importantly, all 20 staff had at least 12 months of ICU experience and were aware of, and had additional training following, the near-miss which happened on our ICU six months prior to the study. Signs and symptoms of systemic hypoglycaemia such as reduced consciousness may also in some instances help staff realise an error earlier due to the incongruence of symptoms with sample glucose readings, 33 and this facet is difficult to simulate. Simulation cannot fully replicate real clinical scenarios but given the nature of the error and its clinical implications, forced error simulation remains the safest method to observe participant responses to rare errors.
Conclusion
Iatrogenic hypoglycaemia continues to occur as a result of arterial line glucose sampling errors despite national Rapid Response Reports and the AAGBI recommendations. Current guidelines and solutions to prevent the error are largely operator-focussed and hence fallible. Our simulations demonstrate this, with poor staff awareness of the error and poor compliance with recommended sampling techniques despite a near miss and concerted educational efforts at our institution six months prior to the study. Altering sampling technique to a closed system approach in line with AAGBI recommendations also fails to eliminate the error in simulated conditions. A robust systems-based, safety-engineered solution which does not rely on human behaviour is the safest and most effective way to protect patients from iatrogenic harm.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: VP, NS, MB, MM – no conflicting interests declared.
PY is the inventor on a patent (EP3294375A2) belonging to the Queen Elizabeth Hospital NHS Trust.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Vikesh Patel https://orcid.org/0000-0002-8298-9232
References
- 1.National Patient Safety Agency. Rapid Response Report, PSA/2008/RRR006, www.weahsn.net/wp-content/uploads/Problems-with-infusions-and-sampling-from-arterial-lines-NPSA-Rapid-Response-Report-2008.pdf (2008, accessed 30 April 2020).
- 2.Bates DW. Unexpected hypoglycemia in a critically ill patient. Ann Intern Med 2002; 137: 110–116. [DOI] [PubMed] [Google Scholar]
- 3.Panchagnula U, Thomas AN. The wrong arterial line flush solution. Anaesthesia 2007; 62: 1077–1078. [DOI] [PubMed] [Google Scholar]
- 4.Sinha S, Jayaram R, Hargreaves CG. Fatal neuroglycopaenia after accidental use of a glucose 5% solution in a peripheral arterial cannula flush system. Anaesthesia 2007; 62: 615–620. [DOI] [PubMed] [Google Scholar]
- 5.United Kingdom Government. Glucose solutions: false blood glucose readings when used to flush arterial lines, www.gov.uk/drug-safety-update/glucose-solutions-false-blood-glucose-readings-when-used-to-flush-arterial-lines (2014, accessed 30 April 2020).
- 6.Woodcock TE, Cook TM, Gupta KJ, et al. Arterial line blood sampling: preventing hypoglycaemic brain injury 2014. The Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2014; 69: 380–385. [DOI] [PubMed] [Google Scholar]
- 7.The Guardian. Anonymous letter prompts police inquiry into hospital death, www.theguardian.com/uk-news/2020/jan/16/anonymous-letter-prompts-coroner-to-call-for-hospital-death-inquiry (2020, accessed 30 April 2020).
- 8.Burnett RW, Covington AK, Fogh-Andersen N, et al. Recommendations on whole blood sampling, transport, and storage for simultaneous determination of pH, blood gases, and electrolytes. International Federation of Clinical Chemistry Scientific Division. J Int Fed Clin Chem 1994; 6: 115–120. [PubMed] [Google Scholar]
- 9.Brennan KA, Eapen G, Turnbull D. Reducing the risk of fatal and disabling hypoglycaemia: a comparison of arterial blood sampling systems. Br J Anaesth 2010; 104: 446–451. [DOI] [PubMed] [Google Scholar]
- 10.O’Hare D, Chilvers RJ. Arterial blood sampling practices in intensive care units in England and Wales. Anaesthesia 2001; 56: 568–571. [DOI] [PubMed] [Google Scholar]
- 11.Silver MJ, Li YH, Gragg LA, et al. Reduction of blood loss from diagnostic sampling in critically ill patients using a blood-conserving arterial line system. Chest 1993; 104: 1711–1715. [DOI] [PubMed] [Google Scholar]
- 12.Barie PS. Phlebotomy in the intensive care unit: strategies for blood conservation. Crit Care 2004; 8: S34–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benedict A, Mayer A, Craven H. Closed arterial lab sampling devices: a study of compliance and best practice. Br J Nursing 2017; 26: 24–29. [DOI] [PubMed] [Google Scholar]
- 14.Brewer A, Williams G. Caution: arterial line error. Anaesthesia 2009; 64: 1142–1143. [DOI] [PubMed] [Google Scholar]
- 15.Thirugnanam M, French J. Accidental hypoglycaemia caused by an arterial flush drug error. Anaesthesia 2014; 69: 524–525. [DOI] [PubMed] [Google Scholar]
- 16.Leslie RA, Gouldson S, Habib N, et al. Management of arterial lines and blood sampling in intensive care: a threat to patient safety. Anaesthesia 2013; 68: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 17.Gupta KJ, Cook TM. Accidental hypoglycaemia caused by an arterial flush drug error: a case report and contributory causes analysis. Anaesthesia 2013; 68: 1179–1187. [DOI] [PubMed] [Google Scholar]
- 18.Thomas AN, Taylor RJ. Review of patient safety incidents reported from critical care units in North-West England in 2009 and 2010. Anaesthesia 2012; 67: 706–713. [DOI] [PubMed] [Google Scholar]
- 19.Cichosz SL, Redke F, Hejlesen OK. Spontaneous and iatrogenic hypoglycaemia related to mortality in the ICU. Diabetes Metab 2019; 45: 545–9. [DOI] [PubMed] [Google Scholar]
- 20.Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 2010; 85: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariyaselvam MZA, Catchpole KR, Menon DK, et al. Preventing retained central venous catheter guidewires: a randomized controlled simulation study using a human factors approach. Anesthesiology 2017; 127: 658–65. [DOI] [PubMed] [Google Scholar]
- 22.Vannucci A, Jeffcoat A, Ifune C, et al. Retained guidewires after intraoperative placement of central venous catheters. Anesth Analg 2013; 117: 102–108. [DOI] [PubMed] [Google Scholar]
- 23.Duncan JR, Henderson K, Street M, et al. Creating and evaluating a data-driven curriculum for central venous catheter placement. J Grad Med Educ 2010; 2: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green J, Butterworth J. “Never” events: anaesthesiology’s dirty little secret. Anesth Analg 2013; 117: 1–2. [DOI] [PubMed] [Google Scholar]
- 25.Valentin A, Capuzzo M, Guidet B, et al. Errors in administration of parenteral drugs in intensive care units: multinational prospective study. BMJ 2009; 338: b814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald R, Waring J, Harrison S, et al. Rules and guidelines in clinical practice: a qualitative study in operating theatres of doctors’ and nurses’ views. Qual Saf Health Care 2005; 14: 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutter J, Jordan S. Inter-professional differences in compliance with standard precautions in operating theatres: a multi-site, mixed methods study. Int J Nurs Stud 2012; 49: 953–968. [DOI] [PubMed] [Google Scholar]
- 28.Lum TE, Fairbanks RJ, Pennington EC, et al. Profiles in patient safety: misplaced femoral line guidewire and multiple failures to detect the foreign body on chest radiography. Acad Emerg Med 2005; 12: 658–662. [DOI] [PubMed] [Google Scholar]
- 29.Cafazzo JA, St-Cyr O. From discovery to design: the evolution of human factors in healthcare. Healthc Q 2012; 15: 24–29. [DOI] [PubMed] [Google Scholar]
- 30.Reason J. Human error: models and management. BMJ 2000; 320: 768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NHS Resolution. Being fair supporting a just and learning culture for staff and patients following incidents in the NHS, https://resolution.nhs.uk/wp-content/uploads/2019/07/NHS-Resolution-Being-Fair-Report-2.pdf (2019, accessed 28 June 2020).
- 32.Brenner R. Is it blame or accountability? www.chacocanyon.com/pointlookout/051221.shtml (2018, accessed 28 June 2020).
- 33.Thomas AN. Hypoglycaemia associated with the use of incorrect arterial flush solutions. Anaesthesia 2014; 69: 90–91. [DOI] [PubMed] [Google Scholar]