Abstract
Background
Obesity develops due to an imbalance in energy homeostasis, wherein energy intake exceeds energy expenditure. Accumulating evidence shows that manipulations of dietary protein and their component amino acids affect the energy balance, resulting in changes in fat mass and body weight. Amino acids are not only the building blocks of proteins but also serve as signals regulating multiple biological pathways.
Scope of review
We present the currently available evidence regarding the effects of dietary alterations of a single essential amino acid (EAA) on energy balance and relevant signaling mechanisms at both central and peripheral levels. We summarize the association between EAAs and obesity in humans and the clinical use of modifying the dietary EAA composition for therapeutic intervention in obesity. Finally, similar mechanisms underlying diets varying in protein levels and diets altered of a single EAA are described. The current review would expand our understanding of the contribution of protein and amino acids to energy balance control, thus helping discover novel therapeutic approaches for obesity and related diseases.
Major Conclusions
Changes in circulating EAA levels, particularly increased branched-chain amino acids (BCAAs), have been reported in obese human and animal models. Alterations in dietary EAA intake result in improvements in fat and weight loss in rodents, and each has its distinct mechanism. For example, leucine deprivation increases energy expenditure, reduces food intake and fat mass, primarily through regulation of the general control nonderepressible 2 (GCN2) and mammalian target of rapamycin (mTOR) signaling. Methionine restriction by 80% decreases fat mass and body weight while developing hyperphagia, primarily through fibroblast growth factor 21 (FGF-21) signaling. Some effects of diets with different protein levels on energy homeostasis are mediated by similar mechanisms. However, reports on the effects and underlying mechanisms of dietary EAA imbalances on human body weight are few, and more investigations are needed in future.
Keywords: Essential amino acid, Energy balance, Protein, GCN2, mTOR, FGF21
Highlights
-
•
Dietary Essential Amino Acids (EAA) alterations affect energy homeostasis via distinct mechanisms.
-
•
Alterations in dietary EAA intake can reduce fat mass and body weight.
-
•
Increased circulating BCAAs have been observed in obese human and animal models.
1. Introduction
In recent decades, obesity has become a global public health concern. Between 1975 and 2016, the prevalence of obesity increased at an alarming rate in children and adolescents from 0.7% to 5.6% in boys and 0.9% to 7.8% in girls worldwide [1]. The prevalence of obesity increased from 3.2% to 10.8% in adult men and from 6.4% to 14.9% in adult women between 1975 and 2014 [1]. Obesity has also been associated with many other diseases, including diabetes, cardiovascular diseases, and hypertension. These conditions lead to reduced life quality and several social problems. One of the important factors that contribute to obesity is the dietary macronutrients, including fat, carbohydrate, and protein. Therefore, studies on effective dietary interventions to address obesity are gaining momentum.
Among the three macronutrients, fat and carbohydrate contents were thought particularly relevant in obesity previously. Increasing evidence has shown that dietary protein is also important in regulating body weight. Both low- and high-protein diets have been shown to promote weight loss. It seems controversial that the two diets do not demonstrate opposite effects and remains unknown how the two diets bring about similar changes to body weight. After consumption, proteins are hydrolyzed into single amino acid and peptides. The effects of a protein depend on its various constituent amino acids. It has been postulated that certain amino acids may mediate the metabolic effects of diets with different protein levels [[2], [3], [4], [5], [6]]. Alterations of certain amino acid intake could attenuate the effects of these protein diets on body weight. In addition, diets lacking or supplemented with a single amino acid may produce a similar physiological response to that observed following low- or high-protein diets. In this respect, manipulating the dietary composition of amino acids merits careful investigation.
Amino acids are classified as essential and non-essential. Dietary proteins are the key sources of essential amino acids (EAAs). Humans can synthesize non-essential amino acids endogenously. There are nine EAAs namely, leucine (Leu), isoleucine (Ile), valine (Val), phenylalanine (Phe), threonine (Thr), tryptophan (Trp), methionine (Met), lysine (Lys), and histidine (His). Among all EAAs, Leu, Ile, and Val are known as branched-chain amino acids (BCAAs), which have aliphatic side chains (a central carbon atom bound to ≥3 carbon atoms). Accounting for around 40% of the total amino acid requirement in the body, BCAAs have received considerable attention over the last decade because of their ability to promote protein synthesis and to affect metabolism. Recently, it has been proven that EAAs are not only the building blocks of proteins but also work as signaling molecules regulating multiple biological processes.
Obesity is often considered an outcome of energy imbalance with excessive energy intake and insufficient energy consumption [7]. Several central and peripheral factors are involved in energy homeostasis. A better understanding of mechanisms underlying the effects of EAAs in energy homeostasis will help to provide new intervention strategies for obesity treatments. This review aims to present the currently available knowledge on the key role played by individual EAA in body weight and energy balance as signaling molecules. Notably, the mentioned metabolic aspects here refer to adult mammals, as the relative importance of EAAs differs for growing mammals. This review will deepen understanding of the physiological function of EAAs and the related underlying mechanisms.
2. The effects of essential amino acid deprivation or restriction on body weight and energy balance
Numerous studies have reported that dietary EAA deprivation or restriction causes profound alterations in energy balance, resulting in remarkable changes in fat mass and body weight. Researchers have noticed that animals fed a diet devoid of an EAA exhibited a loss of body weight a long time ago. In rats, the extent of weight loss differs upon omission of different amino acids [8]. In these early studies, researchers have mainly focused on the effect of EAA deprivation on protein metabolism. During the last 20 years, the role of amino acids in regulating energy homeostasis has emerged. Here, we review the literature related to the effects of EAA deprivation or restriction on body weight and energy balance (Table 1).
Table 1.
The effects of essential amino acids on energy balance.
| Diet | The effects on energy balance | Studies |
|---|---|---|
| His deprivation |
Chow diet BW and fat mass↓ |
[8] |
| Ile deprivation |
Chow diet BW and fat mass↓ Food intake↓ Energy expenditure↑ BAT UCP1↑ |
[10] |
| Leu deprivation |
Chow diet BW and fat mass↓ Food intake↓ Energy expenditure↑ WAT lipolysis and browning↑ BAT UCP1↑ |
[9,40,63] |
| Lys deprivation |
Chow diet BW and abdominal fat↓ Food intake↓ Energy expenditure↑ |
[12] |
| Met deprivation |
Chow diet or high-fat, high-sucrose diet BW and fat mass↓ Energy expenditure↑ |
[11,12] |
| Phe deprivaiton |
Chow diet BW and fat mass↓ Food intake↓ Energy expenditure↑ |
[12] |
| Thr deprivation |
Chow diet BW and fat mass↓ Food intake↓ Energy expenditure↑ |
[12] |
| Trp deprivation |
Chow diet BW and fat mass↓ Food intake↓ Energy expenditure↑ |
[8,12] |
| Val deprivation |
Chow diet BW and fat mass↓ Food intake↓ Energy expenditure↑ BAT UCP1↑ |
[10,13] |
| Ile restriction |
Chow diet or high-fat, high-sucrose diet BW and fat mass↓ Food intake↑ Energy expenditure↑ WAT browning↑ |
[4] |
| Leu restriction |
Chow diet BW and fat mass↓ |
[[29], [30], [31]] |
| Met restriction |
Chow diet or high-fat diet: BW and fat mass↓ Food intake↑ Energy expenditure↑ WAT lipogenesis and lipolysis↑ BAT UCP1↑ |
[[18], [19], [20],26,29,34,35] |
| Thr restriction |
Chow diet BW and fat mass↓ Food intake↑ Energy expenditure↑ |
[6] |
| Val restriction |
High-fat, high-sucrose diet BW and fat mass↓ Food intake↑ Energy expenditure↑ |
[4] |
| His supplementation |
Chow diet or high-fat diet BW and fat mass↓ Food intake↓ |
[108,109] |
| Ile supplementation |
High-fat diet BW and fat mass↓ Food intake— WAT browning↑ |
[114,119] |
| Leu supplementation |
High-fat diet BW and fat mass↓ Food intake— Energy expenditure↑ WAT browning↑ |
[2,114,116,117] |
| Lys supplementation |
Chow diet BW and food intake↓ |
[106,107] |
| Phe supplementation |
High-fat diet BW and food intake↓ |
[110] |
| Thr supplementation |
High-fat diet BW and fat mass↓ Food intake— BAT UCP1↑ |
[111] |
2.1. Essential amino acid deprivation or restriction and body weight
Dietary deprivation of any single EAA is known to reduce fat mass and body weight in rodents [[8], [9], [10], [11], [12], [13]]. Diets devoid of BCAA have received utmost attention, as numerous studies have shown increased circulating BCAA levels in obese human and animal models [12,[14], [15], [16]]. Given that the complete deprivation of one EAA has adverse long-term health effects, people have investigated the optimal concentration of dietary EAAs that can reduce body weight without causing severe negative effects. To date, most work on the dietary restriction of an EAA has focused on Met. The effects of dietary Met restriction are well established. Much of the work about Met restriction began with the original report in 1993 that a diet low in Met (0.17% of diet [w/w] compared with 0.86% in controls) increased the life span of rats [17]. Accumulating evidence has demonstrated that in addition to enhancing longevity, 80% Met restriction decreases fat mass and body weight while developing hyperphagia [[18], [19], [20]]. Short-term (4–12 weeks) or long-term (80 weeks) consumption of the 80% Met restricted diet could produce the above-mentioned effects in growing, adult, or aging rats [[20], [21], [22]]. While most of the early studies on dietary Met restriction were performed in rats, subsequent studies with mice have shown that the responses to Met restriction are comparable in almost every respect [23]. To date, the effects of Met restriction on metabolic health have been the subject of several reviews [[23], [24], [25], [26]].
Studies have demonstrated that decreased consumption of BCAAs promotes fat loss and weight normalization [4,5,27,28]. Furthermore, restriction of only Leu [[29], [30], [31]] or Ile [4] has been shown to reduce fat accumulation and body weight. However, a recently published study reported that restriction of Leu did not produce these beneficial metabolic effects [4]. An important reason responsible for the different observations may be the different Leu concentrations in control diets. The earlier studies [[29], [30], [31]] used a control diet containing 1.11%–1.2% Leu, while the Leu concentration was 2.54% in the control diet in a latter study [4]. Moreover, to make all the diets isocaloric with equal fat levels, the carbohydrate level of the Leu-restriction diet was increased in the previous study, while the non-EAA levels of the Leu-restriction diet were increased in the latter study. Thus, the metabolic phenotype of the control mice might have shown a difference, and the degree of Leu restriction was not the same in these studies. Besides Met and BCAAs, some other EAAs have also been evaluated. For example, dietary Trp or Thr restriction decreases fat mass and body weight in rodents [6,32].
2.2. Essential amino acid deprivation or restriction and energy balance
Diets deficient in an EAA have long been known to reduce food intake in animals [9,10,12,13,33]. Studies on Leu deprivation included a pair-fed group by feeding mice the control diet in the amounts consumed by the Leu-deprivation group [9]. A minor decrease in average body weight was observed in the pair-fed mice, but the fat mass was similar to that in the control mice, suggesting that the observed weight reductions in Leu-deprived mice are primarily due to the increased energy expenditure, rather than due to the small reduction in food intake [9]. However, it remains unclear whether this is also the case for other EAA deprivation. In contrast, dietary Met [19,34,35], Ile [4] or Thr [6] restriction significantly increase food consumption, although these diets induce weight loss. This implies that Met, Ile, or Thr restriction reduces fat deposition without calorie restriction, and the excess food intake is largely dissipated as heat rather than incorporated as fat.
Dietary limitations of EAAs also affect whole-body energy expenditure, as observed using data from metabolic cages [4,[9], [10], [11], [12],19,20,27,28]. Deprivation of a single EAA [[9], [10], [11], [12]] and restriction of Met, Ile, or Thr [4,6,19,20,36] have been shown to increase energy expenditure. The higher energy expenditure may be caused by the activation of thermogenesis and mediated by brown adipose tissue (BAT) and the browning of white adipose tissue (WAT). Generally, adipocytes can be divided into white, brown, and beige fat cells [37]. BAT is a key site of heat production (thermogenesis) in mammals [38]. Some brown fat-like cells may appear in the WAT under external stimulation, including some nutrients. These inducible cells are called “beige cells”, and this process is known as white fat browning [39]. WAT stores chemical energy as triglycerides. Both brown and beige fat cells contain uncoupling protein-1 (UCP1), which functions to generate heat via uncoupling respiration from adenosine diphosphate (ADP) phosphorylation in the mitochondria [38]. Increasing energy expenditure through stimulation of BAT and WAT browning has been considered as a good strategy to promote weight loss. Deprivation of a BCAA [9,10,13,40] and restriction of Met or Ile [4,20] have been proven to upregulate UCP1 expression in BAT and WAT, suggesting increased thermogenesis. Additionally, Met restriction could not increase energy expenditure or reduce adiposity in Ucp1/- mice [41]. These findings strengthen the view that changes in BAT and WAT thermogenesis account for the increased energy expenditure and fat loss under these diets.
Besides, a link between dietary limiting EEAs and lipid metabolism has been demonstrated. Individual BCAA deprivation stimulates lipolysis and the expression of β-oxidation genes and decreases the expression of lipogenic genes and the activity of fatty acid synthase (FAS) in WAT, consistent with increased use and decreased synthesis of fatty acids, respectively [9,10]. Recent studies have shown that Met [42] and Leu [30] restriction also alters lipid metabolism, including lipid synthesis and lipolysis pathway in WAT. Particularly, Met restriction increases both lipogenesis and lipolysis in WAT, leading to a lipid–futile cycle [26]. The enhanced lipid cycling consumes more potential energy as heat, which could partially explain the increased energy expenditure [29].
2.3. Underlying mechanisms in the regulation of EAA deprivation or restriction on energy balance
To date, much progress has been made in identifying the molecular sensors that detect changes in amino acid levels and the mechanisms linking dietary amino acid deprivation or restriction to the metabolic phenotype. These mechanisms are involved at both the central and peripheral levels. The central nervous system (CNS), particularly the hypothalamus, plays a key role in sensing nutrient levels and integrating the signaling network to regulate energy homeostasis [[43], [44], [45]]. Energy homeostasis is maintained by a balance between calorie intake and expenditure. The CNS can sense nutrient alterations directly or detect nutritional signals from peripheral tissues [[43], [44], [45]]. The hypothalamus controls energy balance via special nuclei, including the arcuate nucleus (ARC), paraventricular nucleus (PVN), and other hypothalamic nuclei [46,47]. In particular, two distinct neuronal populations have been identified as important mediators of food intake—one is orexigenic neurons that coproduce agouti-related peptide (AgRP) and neuropeptide Y (NPY), and the other is anorexigenic neurons that contain cocaine- and amphetamine-regulated transcript (CART) and proopiomelanocortin (POMC)-derived peptides [48]. Other brain regions such as the nucleus of the solitary tract (NTS) are also involved. Reportedly, Leu sensing in the NTS modulates non-aversive suppression of feeding via inhibiting AgRP neurons [49]. On the other hand, the hypothalamus regulates energy expenditure by affecting thermogenesis via regulating norepinephrine (NE) secretion from the sympathetic nervous system (SNS) and UCP1 expression in BAT and lipolysis in WAT [48,50]. Accumulating evidence has demonstrated that CNS is crucial to the modulation of energy balance under EAA deprivation or restriction. Neuronal activity in specific neurons and neuropeptides mediating energy homeostasis are changed under these conditions [13]. Intracerebroventricular (i.c.v) administration of Leu attenuated the fat loss and energy expenditure induced by dietary Leu deprivation [51]. In addition, Leu deprivation increased the expression of β-3 adrenergic receptors in adipose tissues and the serum NE levels, and Leu deprivation-induced fat loss was blocked in β-less mice [51], suggesting the involvement of the SNS.
Peripheral organs such as adipose tissues and intestines can also sense changes in amino acid concentrations and integrate these signals to control energy metabolic processes. For example, amino acids can regulate adipocyte differentiation and lipogenesis directly [52]. There are many amino acid sensors in the gut [53]. Amino acids can stimulate intestinal endocrine cells to release multiple gut hormones through these sensors, and these hormones trigger alterations in energy homeostasis via the nervous system or act on adipose tissues directly [53].
Over the past few decades, immense efforts in understanding the signaling initiated by amino acids alterations in the CNS and peripheral tissues to regulate energy balance have largely deepened our understanding of the physiological significance of amino acids. Here, we describe the current knowledge on the signaling pathways that bridge amino acid sensing with the control of energy balance (Figure 1).
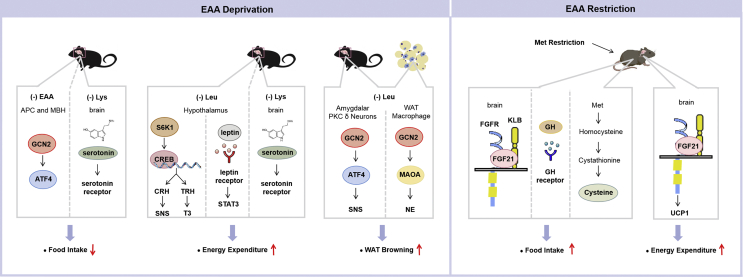
Figure 1.
Mechanisms involved in the regulation of essential amino acid deprivation or restriction on energy balance. Deprivation of a single essential amino acid (EAA) reduces food intake, increases energy expenditure, and induces white adipose tissue (WAT) browning. Activated GCN2 in the anterior piriform cortex (APC) and mediobasal hypothalamus (MBH) is one of the mechanisms mediating animals' response to reject a diet deficient in EAA. The Lys deprivation-decreased food intake and -increased energy expenditure is possibly mediated by enhanced serotonin signaling. Dietary Leu deprivation inhibits hypothalamic S6K1 activity, which in turn increases the activity of cAMP-response element-binding protein (CREB). In the nucleus, corticotrophin-releasing hormone (CRH) and thyrotropin-releasing hormone (TRH) transcriptions are stimulated by CREB. After translation and modification, CRH is secreted and activates the sympathetic nervous system (SNS) to stimulate energy expenditure in the whole organism. TRH in turn stimulates the biosynthesis of triiodothyronine (T3) to increase energy expenditure under Leu deprivation. Leptin signaling is also involved in Leu deprivation-increased energy expenditure. Leu deprivation induces WAT browning by GCN2 in PKC-δ neurons of the amygdale and adipose tissue macrophages (ATMs). Activated GCN2 in the amygdale stimulates activating transcription factor 4 (ATF4) expression and increases the PKC-δ neuronal activity to promote WAT browning via the sympathetic nerve; GCN2 activation in ATMs reduces the expression of monoamine oxidase A (MAOA), resulting in increased norepinephrine (NE) secretion from macrophages to adipocytes, and this results in enhanced WAT browning. Met restriction increases energy intake and energy expenditure. These effects are mediated by multiple mechanisms, including fibroblast growth factor (FGF) 21 signaling, growth hormone (GH) signaling, and Met-derived cysteine.
2.3.1. General control nonderepressible 2 (GCN2) signaling
GCN2, also known as eukaryotic translation initiation factor 2α kinase 4 (EIF2AK4), is a serine protein kinase that functions as a key sensor of amino acid starvation. GCN2 is known to be activated by uncharged transfer ribonucleic acids (tRNAs) under amino acid starvation conditions. Activated GCN2 increases the phosphorylation of eukaryotic initiation factor 2 α (eIF2α), leading to the inhibition of protein synthesis. The phosphorylation of eIF2α selectively upregulates genes involved in the amino acid synthesis and transport, thus inducing the adaptive response to amino acid deficiency. GCN2 is expressed in most mammalian tissues. Researchers have investigated the physiological function of GCN2 mainly using global (Gcn2−/−) and tissue-specific knockout mice. Gcn2−/− mice are viable, fertile, and they exhibit no phenotypic abnormalities under standard growth conditions [54]. However, when pregnant Gcn2−/− mice are reared on diets with limited EAA, fetal development is subsequently impaired [54].
Omnivorous animals choose foods to obtain a diet that maintains an appropriate balance of EAAs [55]. Rats and other omnivores reject diets lacking a single EAA. This is mediated by the CNS, particularly the anterior piriform cortex (APC). Animals with APC lesions failed to reduce their intake of EAA-deficient diets [56], and administration of EAA into the APC selectively reversed this phenomenon [57]. Evidence has shown that depletion of EAAs is first sensed by the APC via GCN2 signaling [58], the output cells of which project to the mediobasal hypothalamus (MBH) [59]. After ingestion of a meal lacking Leu, phosphorylation of eIF2α was increased in the APC and MBH of mice [59,60]. Although wild-type (WT) mice readily rejected a Leu-deficient diet, Gcn2−/− mice showed a significantly blunted aversive response. Similar results were observed with a brain-specific GCN2 knockout mice model or following MBH-specific knockdown of GCN2 [59,60]. In contrast, activation of GCN2 in the APC by the injection of the amino alcohol L-leucinol increased the uncharged tRNA levels, causing WT mice to reject diets containing basal levels of Leu [58]. A similar mechanism was observed in Drosophila larvae [61]. The larvae rejected an amino acid-imbalanced diet in favor of increased wandering. Knockdown of GCN2 in dopaminergic neurons reduced this aversive response in larvae via regulation of gamma-aminobutyric acid (GABA) signaling [61,62]. These common findings in vertebrates and invertebrates highlight that central GCN2 signaling is an ancient nutrient-sensitive pathway for maintaining energy homeostasis by governing feeding behavior.
Recent studies have shown that the activation of GCN2 signaling in amygdalar protein kinase C (PKC)-δ neurons and macrophages promote WAT browning under Leu deprivation [40,63]. Researchers have found that Leu deficiency-induced WAT browning could be blocked by GCN2 deletion in amygdalar PKC-δ neurons, which is reversed by the over-expression of amino acid-responsive gene activating transcription factor 4 (ATF4) and is mediated by amygdalar PKC-δ neurons activity and the SNS [40]. Macrophages, a type of white blood cells located in monocyte-derived tissues, are an important component of the immune system. Several studies have demonstrated the role of macrophages in WAT browning [64]. Recent work has shown that Leu deprivation decreases the accumulation and changes the polarization of adipose tissue macrophages (ATMs). Ablation of ATMs and myeloid-specific abrogation of GCN2 in mice blocks Leu deprivation-induced WAT browning. Further analyses revealed that GCN2 activation in macrophages reduces the expression of monoamine oxidase A (MAOA), resulting in increased NE secretion from macrophages to adipocytes, and an enhanced WAT browning. In addition, GCN2 signaling in ATMs similarly mediates Leu deficiency-increased WAT lipolysis [63].
Overall, these findings illustrate that GCN2 signaling mediates many metabolic effects of EAA deprivation, especially Leu. In contrast, Met restriction-induced energy expenditure is not mediated by GCN2 signaling. The absence of GCN2 in mice does not affect the ability of dietary Met restriction to increase energy expenditure and reduce body weight [65]. Instead, noncanonical glutathione (GSH)-dependent mechanism contributes to the effects of dietary Met restriction on energy expenditure [65]. These studies show that dietary EAA deprivation and restriction do not share a common mechanism in energy homeostasis, and show involvement of mechanisms other than GCN2 mediated signaling.
2.3.2. Mammalian target of rapamycin complex 1 (mTORC1) signaling
In many model systems, mTORC1 activity is regulated by specific amino acids, especially Leu. The three core components of mTORC1 are the mTOR, regulatory associated protein of mTOR (Raptor), and mammalian lethal with Sec13 protein 8 (mLST8) [66,67]. The mTOR protein is an evolutionarily conserved Ser/Thr kinase and the catalytic subunit of mTORC1. This complex has been identified as the convergence point of nutrient-driven signaling essential for protein synthesis, growth, development, and proliferation [66,67]. How mTORC1 senses the nutrient status of the cell is a long-standing question in the field [66,67]. It is known that amino acids signal to mTORC1 through the Rag guanosine triphosphatases (GTPase). Several factors regulate the Rags: GATOR1, a GTPase-activating protein that inhibits mTORC1 activation; GATOR2, a positive regulator of mTORC1 as it inhibits GATOR1; Sestrin2, a GATOR2-interacting protein that inhibits mTORC1. Sestrin2 is a direct Leu sensor; Leu disrupts the Sestrin2-GATOR2 interaction by binding to Sestrin2, ultimately activating mTORC1 [68]. Leucyl-tRNA synthetase (LARS) is also an intracellular Leu sensor for the mTORC1 signaling pathway; it binds directly to the Rag GTPase [69]. The two key downstream targets of mTORC1 are the proteins p70-S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1). These two proteins, when phosphorylated by mTORC1, lead to mRNA translation initiation and protein synthesis.
Aberrant mTOR/S6K1 has been linked to many diseases including obesity. The mTOR pathway is elevated in many organs of obese animal models, whereas the absence of S6K1 protects against diet-induced obesity [70]. This signaling pathway also mediates the effects of Leu deprivation in energy balance. Dietary Leu deprivation inhibits hypothalamic S6K1 activity, which in turn increases the G protein-coupled melanocortin-4 receptor (MC4R) protein levels and stimulates guanine nucleotide–binding protein (Gs) activity. These events increase intracellular cyclic adenosine monophosphate (cAMP) levels, which stimulate protein kinase A (PKA)–mediated phosphorylation and the activation of cAMP-response element-binding protein (CREB). In the nucleus, corticotrophin-releasing hormone (CRH) transcription is stimulated by the binding of phosphorylated CREB at the cAMP-responsive element site in its promoter region. After translation and modification, CRH is secreted as a polypeptide and it activates the SNS to stimulate energy expenditure and thermogenesis in the whole organism [71]. In addition, CREB stimulates the thyrotropin-releasing hormone (TRH) pathway, which in turn stimulates the biosynthesis of triiodothyronine (T3) to increase UCP1 in BAT and energy expenditure under Leu deprivation [72].
2.3.3. Leptin signaling
Leptin is a peptide hormone encoded by the obese (ob) gene and secreted from the adipose tissue. Leptin acts via the leptin receptor, a single transmembrane protein belonging to the cytokine receptor family. The leptin receptor is highly expressed in the hypothalamus, and leptin signaling plays a critical role in the regulation of energy balance. Although serum leptin is decreased in mice fed on a Leu-deficient diet, Leu deprivation enhances leptin singling in the hypothalamus [73]. Leu deprivation-increased energy expenditure and fat loss are blocked in leptin receptor-deficient (db/db) or mutant (Y3F) mice [73]. Therefore, leptin signaling is required for the Leu deprivation-enhanced energy expenditure. In addition, impairment of leptin signaling is closely linked with obesity, and a high-fat diet (HFD) has been shown to cause leptin resistance. Leu deprivation could restore the leptin responses in HFD mice [73].
2.3.4. Fibroblast growth factor 21 (FGF 21) signaling
FGF 21, a member of the FGF family, binds to the FGF receptor and is involved in a variety of biological processes [74]. Many nutritional conditions affect circulating and tissue FGF21 levels [74]. Most metabolic effects of Met restriction are FGF21-dependent. Dietary Met restriction could increase serum FGF21 concentration in WT mice [65], and Met restriction-induced increase in energy intake and expenditure and the activation of thermogenesis in WAT and BAT are lost in Fgf21−/− mice [75]. Another study has demonstrated that the increase in FGF21 induced by Met restriction primarily acts in the brain to increase energy intake and expenditure, as brain-specific FGF21 coreceptor knockout mice cannot respond to the effects of Met restriction on energy intake and expenditure or the expression of UCP1 in the adipose tissue [76]. In contrast, adipose tissue-specific deletion of FGF21 coreceptor does not abrogate the ability of Met restriction to increase energy intake and expenditure and reduce fat accumulation [76]. Given that vegan and vegetarian diets exhibit a lower Met intake than an omnivore diet, plasma FGF21 levels are robustly increased in vegan and vegetarian subjects compared with omnivore subjects [77]. Moreover, a vegetarian diet for four days could upregulate plasma FGF21 levels in omnivore humans [77], suggesting that vegan and vegetarian diets in humans may offer metabolic benefits via lower Met intake and increasing circulating FGF21 levels. Besides, Ile [4] or Thr [6] restriction also increases FGF21 circulating levels. Ile restriction-increased food consumption and energy expenditure, but not –reduced fat mass and body weight, are blunted in Fgf21−/− mice [4]. Therefore, the metabolic effects of a low Ile diet are partially dependent on FGF21. The effects of Thr restriction on increasing food intake and energy expenditure are abrogated following liver FGF21 silencing, suggesting the role of liver-derived FGF21 in the metabolic remodeling induced by dietary Thr restriction [6].
2.3.5. Microbiota signaling
Gut microbiota or gut flora is the complex community of microorganisms that live in the digestive tracts of humans and animals. In humans, the gut microbiota has the largest number of bacteria and the maximum number of species compared to the other areas of the body [78]. Obesity is associated with changes in the composition and diversity of the gut microbiota [79,80]. For example, the relative proportion of Bacteroidetes is decreased in obese people compared with that of lean people, and this proportion increases with weight loss on a low-calorie diet [79].
Accumulating evidence has supported the idea of the involvement of the gut microbiota in the regulation of host energy homeostasis via modulation of lipid, glucose, and protein metabolism. For example, gut microbiota contributes to energy homeostasis through the production of short-chain fatty acids (SCFAs) that are produced by colonic fermentation, which involves the anaerobic breakdown of dietary fiber, protein, and peptides [81]. SCFAs can act as energy sources and can regulate gut hormones such as peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) via their receptors to protect against diet-induced obesity [82,83]. Gut microbiota was also shown to play a role in the regulation of bile acids and cholesterol metabolism in both humans and animals [81]. Both Firmicutes and Bacteroidetes promptly engage in the regulation of bile acid modification and govern bile acid-controlled endocrine functions, including triglyceride and cholesterol homeostasis [84]. In addition, obesity is characterized by low-grade inflammation [85]. Inflammatory cytokines pathways are activated by the production of lipopolysaccharides (LPS) that are a major component of the outer membrane of Gram-negative bacteria that is produced in the gut [81]. Changes of gut microbiota induced by an antibiotic treatment could reduce adipose tissue inflammation in HFD-fed mice [86].
It has been shown that dietary factors have a profound impact on altering the gut microbiota of humans and animals, including amino acids [87,88]. Dietary Met restriction improves the gut microbiota and reduces intestinal permeability and inflammation in HFD mice [18]. Under HFD, Met restriction increases the abundance of SCFA-producing bacteria Bifidobacterium, Lactobacillus, Bacteroides, Roseburia, Coprococcus, and Ruminococcus and inflammation-inhibiting bacteria Oscillospira and Corynebacterium. Met restriction also decreases the abundance of inflammation-producing bacteria Desulfovibrio in colonic contents. Moreover, Met restriction improves intestinal barrier function, inflammatory response, and oxidative stress levels [18]. However, whether the gut microbiota is a consequence or a causal factor, and which key gut microbial species are responsible for the alterations in energy balance under EAA deprivation or restriction remains unknown and needs further investigation. Changes in the food absorption efficiency in response to different diets and their effects on body weight remain largely unexplored. Moreover, there is a dearth of direct data on alterations in food absorption efficiency under EAA deprivation or restriction diet. It is well known that gut microbiota plays an important role in food absorption efficiency. Some of the above-mentioned microbes, such as Bifidobacterium [89,90] and Lactobacillus [91], are associated with absorption of several nutrients from food, including fat, calcium, iron, etc. Thus, we hypothesize that the food absorption efficiency may change under dietary Met restriction. This hypothesis is supported by studies showing that dietary Met restriction reduces the absorption of xanthine from the intestine [18]. Nevertheless, it remains unclear whether changes in microbiota affect the total food absorption efficiency under Met restriction or other diets, thus needs further study.
2.3.6. Metabolite and growth hormone-related signaling
In early studies, two EAAs of interest in the characterization of energy balance control were the neurotransmitter precursors Trp, a precursor of serotonin (5-hydroxytryptamine or 5-HT), and His, a precursor of histamine. Trp is the only precursor of serotonin, which is produced both centrally and peripherally. Some indirect evidence indicates the role of serotonin in energy balance under Trp restriction. In mammals, serotonin produced within the CNS inhibits food intake and promotes energy expenditure [92]. By binding to its receptor within the brain, serotonin suppresses food intake via influencing the activity of the melanocortin system and increases energy expenditure through enhancing the sympathetic drive to BAT. Several studies have shown that Trp restriction decreases brain serotonin levels [93,94], suggesting the role of decreased serotonin in reduced energy expenditure by Trp restriction [32]. Lys deficiency causes an increase in serotonin level in the brain [95]. Lys was shown to act like a partial serotonin receptor antagonist and inhibit the serotonin-mediated downstream pathway [96]. These observations indicate enhanced serotonin signaling under Lys deprivation; serotonin may mediate Lys deficient-suppressed food intake and increased energy expenditure. Of the nine EAAs, His is a semi-essential amino acid (children should obtain it from food) needed in humans for growth and tissue repair. His can be metabolized to the neurotransmitter histamine. In rats, the highest concentration of brain histamine is in the hypothalamus. By activating its receptor, an increase in hypothalamic histamine concentration suppresses food intake, accelerates lipolysis, and upregulates UCP proteins in the adipose tissue. Further, histamine is known as a neuromodulator because it regulates the release of other neurotransmitters such as acetylcholine and NE. Thus, histamine is an essential regulator of energy homeostasis [97]. His levels are elevated in the plasma and brain, while the other eight EAAs are limited in diets [98]. So histamine may mediate the effects of EAA deprivation in energy intake and energy expenditure. Somatostatin is involved in the anorexia of mice fed on a Val-deficient diet [99]. Somatostatin is an endogenous peptide hormone and was originally identified as a growth hormone (GH) inhibiting factor. Valine deficiency increased the hypothalamic somatostatin levels, and i.c.v injection of somatostatin decreased food intake [99].
Both GH signaling and downstream metabolite cysteine mediate the effects of Met restriction. Animals without GH signaling due to GH deficiency or resistance do not respond to Met restriction in terms of body weight and food intake [100]. On the other hand, Met is the precursor of homocysteine, which in turn donates a sulfur group to serine to form cysteine. Plasma cysteine levels are positively associated with obesity in humans. The researchers showed that dietary Met restriction decreases plasma cysteine concentrations. The addition of cysteine reverses the effects of Met restriction on fat and weight loss [101], indicating that lower cysteine synthesis is responsible for the anti-obesity effects of Met restriction. Notably, cysteine supplementation of a control diet does not affect body weight and tends to decrease adiposity [101]. Therefore, the effects of cysteine supplementation depend on the underlying diet. These findings support the view that besides EAA themselves, the flux of their catabolites is also key regulator for the metabolic response.
Some other signaling pathways may also participate in the regulation of energy balance under the above conditions. For instance, changes in the activity of AMP-activated protein kinase (AMPK) are often observed [26,102]. AMPK, a fuel sensor, is controlled by nutrient availability. However, direct evidence using knockout mice or inhibitors/agonists is needed to confirm its role under different diets. Furthermore, some mechanisms could be combined with others. It has been shown that GCN2 functions as an upstream inhibitor of mTOR under Leu deprivation [102]; it sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2 [103]. Exploring the integrated regulation of these signals is crucial to understand the effects of EAA on global metabolism.
3. The effects of essential amino acid supplementation on body weight and energy balance
Certain EAA supplementation is also a recognized intervention to reduce body weight (Table 1). Dietary supplementation is a promising intervention because it may improve diet qualities and is convenient to manufacturer as compared to other interventions. EAA supplementations have been extensively used in individuals with certain diseases. It appears that most EAA supplements are generally safe in the recommended dosages and the appropriate indications. However, possible adverse effects cannot be ignored for heavy dosage and long-term use. For instance, Met-rich diet-induced hyperhomocysteinemia (Hhcy), an intermediate generated from the demethylation of Met, is a risk factor for a variety of diseases, including cardiovascular diseases and fatty liver. The major negative effect of Phe is observed in patients with phenylketonuria. Excess phenylalanine intake is associated with neurological disorders in individuals.
3.1. Essential amino acid supplementation and body weight
Overall, except for Met [104] and Val [105], supplementation with other EAAs has been reported to reduce body weight under certain conditions [2,[106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119]]. However, the results are inconsistent. Possible reasons for these inconsistencies could be the diet background (e.g., the percentage of fat in the diet), additive amount, duration of treatment, animal strain, animal sex, etc. For example, most works have reported that oral Leu [2,[114], [115], [116], [117], [118]] or Ile [114,119] supplementation reduces fat mass and body weight in mice consuming an HFD simultaneously but not in already obese mice [120,121] or rodents on the chow diet [[116], [117], [118],122]. Some other researchers did not observe these effects [123,124]. One reason for this may be the different animal strains, reflecting underlying differences in genetic variants. Oral Leu supplementation could induce weight loss in mice but not in rats under HFD [123,124], suggesting that the response to Leu supplementation varies in different species. The sex of the animal may be another factor. While most studies have been done on male animals, female animals are often shown to respond differently to dietary challenges [[125], [126], [127]]. For instance, supplementation with HFD (45% fat) and 1.5% Leu in drinking water decreases abdominal fat in female mice, but not in male mice [127].
3.2. Essential amino acid supplementation and energy balance
In general, when there are effects of an EAA supplementation on weight loss, the underlying mechanisms could involve decreased food intake and/or increased energy expenditure. Lys [106,107], His [108,109], and Phe [110] supplementation suppresse food intake. Although excess Leu intake [[114], [115], [116]] does not affect food intake under HFD, it increases energy expenditure. Additionally, these diets impact thermogenesis and lipid metabolism. Supplementation of Leu, Ile [114,115], His [108], or Thr [111] upregulates UCP1 in BAT and induces WAT browning. Leucine [115] and Thr [111] supplementation was shown to increase the expression of lipolysis-related proteins, including phosphorylated (p)-hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL).
3.3. Mechanisms involved in the regulation of essential amino acid supplementation on energy balance
Several mechanisms have been proposed to explain the weight-losing effects of EAA supplementation. Amino acid sensors in both the CNS and peripheral tissues contribute to these effects (Figure 2), but several knowledge gaps need to be filled to complete the understanding of these processes.
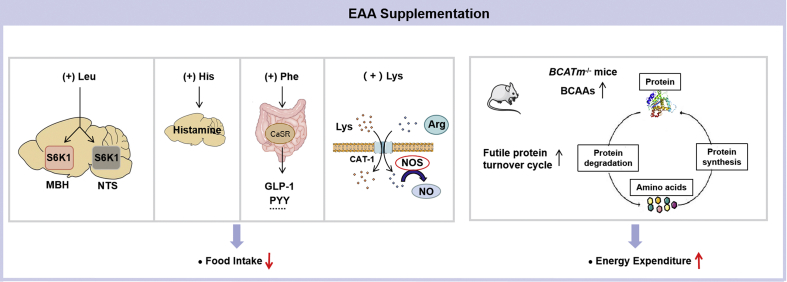
Figure 2.
Mechanisms involved in the regulation of essential amino acid supplementation on energy balance. Central Leu infusion acutely decreases food intake. S6K1 in the mediobasal hypothalamus (MBH) and the nucleus of the solitary tract (NTS) are required for this response. Dietary supplementation of His, Phe, or Lys reduces food intake. His suppresses food intake through its conversion into neuronal histamine. Phe stimulates the gastrointestinal hormones glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) release via the calcium-sensing receptor (CaSR); GLP-1 and PYY reduce food intake by affecting circuits mainly in the hypothalamus. Lys and Arg compete for the same cellular transporter CAT-1, therefore excess Lys could result in intracellular Arg deficiency. As Arg is converted into nitric oxide (NO) by nitric oxide synthase (NOS), Lys supplementation leads to NO reduction. Raising circulating branched-chain amino acids (BCAAs) in mice lacking mitochondrial branched chain aminotransferase (BCATm), the first enzyme in breakdown of BCAAs, drives a futile cycle of protein synthesis and degradation that contributes to higher energy expenditure.
3.3.1. mTORC1 signaling
Oral Leu supplementation does not change the energy intake in most studies. However, central Leu administration reduces the food intake in rodents, suggesting the role of Leu in inhibiting food intake by directly affecting the CNS [128]. For example, i.c.v administration of Leu decreased food intake via stimulating the mTOR activity in 24-h fasted rats [129]. This was accompanied by reduced NPY levels in the ARC, and rapamycin could block these effects [129]. In contrast, i.c.v administration of Val did not produce this effect, as Val did not increase the mTOR signaling under the same condition [129]. Studies have also shown that MBH [130] and the caudomedial nucleus of the solitary tract (cmNTS) [131] Leu administration reduce food intake. An acute increase in MBH Leu levels engages a forebrain/hindbrain neurocircuit to decrease food intake by activating MBH POMC neurons, PVN oxytocin neurons, and NTS neurons [130]. The S6K1 in the cmNTS mediates the effects of Leu in reducing food intake [131]. The divergent results caused by oral or central Leu supplementation may be explained by two reasons. The first reason is the capacity of Leu to cross the blood–brain barrier (BBB) and thus reach the CNS [128]. Whether oral Leu supplementation could significantly increase the Leu concentration in the CNS to reduce food intake remains poorly understood [128]. The second reason may be the abnormal signaling under some conditions. For instance, mTOR activity is aberrant, and POMC neuronal sensitivity to peripheral effectors is reduced in HFD-induced obese mice. Thus, Leu supplementation under HFD does not change the energy intake. Furthermore, it seems controversial that dietary Leu deprivation could reduce food intake through inhibiting mTOR signaling, as compared with the scenario discussed here. The possible reason may be that central Leu infusion is an acute treatment with a high dosage in a short time, while dietary Leu deprivation is a comparatively chronic mild treatment for a longer time. In addition, central Leu administration and oral Leu intake induce neuronal activation in different brain regions, which may have different effects on food intake [128].
3.3.2. Hormone and metabolite signaling
Phe was shown to suppress food intake in rodents, probably via modulation of the gut hormone release and the calcium-sensing receptor (CaSR) [110]. Phe could stimulate the release of the gastrointestinal hormones GLP-1 and PYY in vitro [132] and in vivo [110]. GLP-1 and PYY reduce food intake by affecting circuits mainly in the hypothalamus. The secretion of these hormones has been extensively investigated, and the mechanisms involve increased Ca2+ levels. The CaSR is a cell surface receptor from the family of G-protein-coupled receptors and is expressed in several tissues, including the intestine. It is colocalized in enteroendocrine cells with gut hormones such as GLP-1 and PYY. Evidence shows that the CaSR is activated not only by Ca2+ but also by l-amino acids, and Phe is the most potent amino acid activator of CaSR. The gut peptide response and anorectic effect induced by Phe is blocked by the CaSR inhibitor [110,132], indicating the role of CaSR in sensing amino acids to regulate feeding. Dietary supplementation and the intraperitoneal (i.p.) and i.c.v injection of His elevate brain histamine levels and depress food intake [108,133]. Blocking the conversion of His into histamine attenuates the suppressive effect of His on food intake [133]. These findings suggest that His suppresses food intake through its conversion into neuron histamine.
Two mechanisms have been proposed for the effects of Lys supplementation [106,107]. First, the effects of Lys supplementation may be partially a result of intracellular l-arginine (Arg) deficiency, as the addition of Arg could reverse the effects of Lys supplementation on food intake and body weight reduction. This is because Lys and Arg share the same transport systems, thus competing for cellular uptake. Excess Lys intake could result in intracellular Arg deficiency. Furthermore, Arg is the substrate for nitric oxide (NO) formation. NO is a signaling molecule in neurons that stimulates feeding in many species. Arg increases the food intake in mice, while inhibition of NO production decreases the food intake [134]. Lys was shown to decrease NO production, depending on the uptake of Arg. Therefore, the reduction in food intake and body weight by Lys supplementation may be due to the antagonism between Lys and Arg and the reduced NO production. Second, studies have revealed that Lys supplementation increases the serum levels of pancreatic polypeptide (PP), a hormone mainly produced by the pancreas in response to ingestion of food. Overexpression of PP decreases body weight and food intake [107]. These indicate that increased PP may mediate the effects of Lys supplementation on hypophagia and weight loss.
3.3.3. Futile protein turnover cycle
Many biological processes demand energy, and the energy cost of protein turnover may lead to fat loss. BCAA could regulate protein synthesis and degradation as a nutrient signal via mTORC1 [135]. A study using BCATm−/− mice suggested that the effects of BCAAs on energy balance may be primarily caused by the stimulation of protein turnover [128,135]. The first step in BCAA metabolism is the reversible transamination catalyzed by BCAA transaminase (BCAT), which transfers the α-amino group of BCAAs to α-ketoglutarate (α-KG), forming glutamate and the respective branched-chain α-keto acids (BCKAs) from Leu, Ile, and Val. BCAT can be localized in the cytosol (BCATc) or mitochondria (BCATm). BCATm is the main form in most peripheral tissues. Neither isoform is expressed in the liver or gut; thus, BCAAs are not metabolized following their intestinal absorption. Therefore, circulating BCAA levels rise significantly after meals, and tissues sense BCAAs intake quickly, whereas other amino acids are highly metabolized by the gut or liver before reaching the systemic circulation [128]. BCATm−/− mice exhibit elevated plasma BCAAs but are expected to have low BCAAs use. In addition, this BCATm−/− mice demonstrate decreased adiposity and body weight, along with increased energy expenditure and protection from diet-induced obesity [136]. The increased energy expenditure is not because of altered uncoupling proteins, sympathetic activity, or thyroid hormones, but it results from an active futile cycle of increased protein degradation and synthesis in most tissues [136]. Therefore, elevated BCAAs and/or loss of BCAA catabolism in peripheral tissues play a vital role in regulating energy expenditure.
4. Clinical and translational implications in humans
Based on the growing body of work demonstrating the beneficial metabolic effects of amino acids in animal models, it has attracted extensive interest from scientists to investigate the relevance of amino acids in weight loss in humans. Clinical studies have shown that circulating levels of some EAAs are associated with obesity in individuals. Serum His, Thr, Lys, and Trp concentrations are lower in a few obese subjects [137,138]. Among the EAAs, the BCAAs are of particular interest because they appear to have unique obesity-related signatures. Several studies have shown increased circulating BCAA levels in obese humans [12,[14], [15], [16]], which decrease after weight loss [139]. For example, studies have indicated a different BCAA-related amino acid metabolic signature between obese and lean subjects in a Western population [15]. Levels of four EAAs (BCAAs and Phe) were found elevated in obese humans compared with those in lean controls in a Chinese Han population [12]. In some cases, BCAAs might have been elevated simply because of an increased protein intake. Another potential explanation for the rise in BCAA levels is increased protein catabolism and/or reduced protein synthesis. Evidence has indicated that elevated BCAA levels result at least, in part, from lower rates of BCAA metabolism in some tissues, especially adipose tissue [16,[140], [141], [142]]. It has been observed that the expression and activity of many BCAA-degrading enzymes (such as BCKDH) in the adipose tissue are aberrant in animal models and humans with obese phenotype [16,[140], [141], [142]]. Another possibility is that the gut microbiome could contribute to alterations in the host's circulating BCAA levels because many bacterial species are capable of de novo synthesis of BCAAs [143]. Although there is a strong correlation between BCAAs and obesity, the current evidence for whether BCAAs are a causal driver or a consequence of obesity is equivocal.
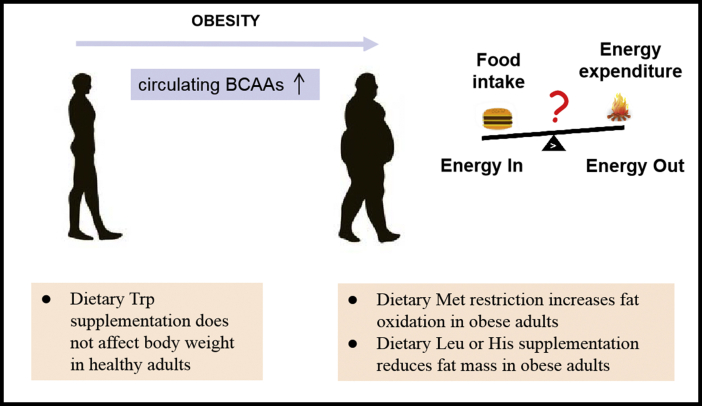
Results of population-based studies on dietary EAAs intake and body weight have been inconsistent. Some researchers have observed that dietary Ile or Leu levels were positively associated with obesity in humans [4,144], while Qin et al. reported that higher dietary BCAA intake was associated with a lower prevalence of overweight status/obesity [145]. Only limited clinical research has been done to examine the direct effects of EAA restriction or supplementation on body weight. A 16-week dietary Met restriction increased fat oxidation in obese adults with metabolic syndrome [146]. Dietary Leu [147,148] or His [149] supplementation could reduce fat mass in obese subjects. In contrast, supplementing healthy women with Trp did not affect body weight [150]. Therefore, restriction or supplementation with a specific EAA is helpful for body weight control in certain populations (Figure 3). More clinical investigation is needed to explore the potential therapeutic use of diets with different EAA levels to treat obesity.
Figure 3.
Essential amino acids and obesity in humans. Circulating branched-chain amino acids (BCAAs) levels are increased in obese humans. Limited clinical research has been carried out to examine the effects of modifying dietary essential amino acid composition in humans, including dietary Met restriction, Leu, His, or Trp supplementation.
5. Discussion
5.1. Dietary amino acid imbalance could reduce body weight
Taken together, it seems that dietary EAA imbalances (deficiency, restriction, or excess) can result in improvements in fat and weight loss. The possible mechanisms may involve two aspects: first, alterations in EAA levels are detected by different sensors, subsequently activating or inhibiting distinct downstream pathways—the majority of which can lead to decreased energy intake or increased energy expenditure. Second, the effects produced by an EAA supplementation may be due to the reduction of another amino acid in the cells and vice versa, as some amino acids share the same transport systems.
5.2. Manipulation of dietary amino acid mimics the diets with different protein levels in weight loss and energy balance
Both low- and high-protein diets are used for weight loss. Generally speaking, low protein diets could reduce fat mass and body weight and increase energy expenditure while increasing food intake; high protein diets are thought to produce enhanced weight loss, increased energy expenditure (especially thermogenesis), and increased satiety with a reduction in energy intake [151]. However, inadequate protein intake for a long time may cause skeletal muscle wasting and nutrient deficiency. Moreover, chronic high protein intake may have harmful effects on bone and kidney function in some populations. The above studies suggest that even a single EAA deprivation/restriction or supplementation could produce similar metabolic effects in energy balance and weight loss. Changing just a single amino acid may be more precise to control and have side effects to a lesser extent.
In addition, the effects of diets varying in protein levels and diets with altered EAAs are mediated through some similar mechanisms, both involving peripheral signals and central targets that influence the energy balance.
5.2.1. Low-protein diet
The effects of low protein diets in energy balance have been associated with several signaling pathways similar to that of EAA deficiency or restriction, including (ⅰ) FGF21 and GCN2 signaling: in both humans and rodents, serum FGF21 levels are induced by a low protein diet [5,152]. While protein restriction altered food intake, energy expenditure, and body weight gain in WT mice, the whole-body deletion of FGF21 [152,153] or deletion of the FGF21 co-receptor βKlotho (Klb) from the brain [154] abrogated these responses. These findings suggest that FGF21 acts in the brain to coordinate energy homeostatic responses to protein restriction [154]. In addition, prior data implicate GCN2 as the amino acid sensor linking protein restriction to FGF21 induction. Fgf21−/− mice were fully resistant to low protein diet-induced changes in energy expenditure and body weight for 2 or 27 weeks [155]. Although GCN2 connects reduced protein intake to increased circulating FGF21, GCN2 contributes to the induction of FGF21 by a low protein diet only at the onset. Over longer periods, additional mechanisms compensate for GCN2 absence. Thus, while the induction of serum FGF21 by a low protein diet was markedly blunted in Gcn2−/− mice at 2 weeks, FGF21 levels progressively rose in Gcn2−/− mice fed with a low protein diet at 12 and 27 weeks. In agreement with these observations, the effects of a low protein diet on energy expenditure and body weight were transiently blocked in Gcn2−/− mice and subsequently appeared after two weeks [155]. Therefore, FGF21 may mediate other effects of GCN2, and further investigation is required to understand its complete role. (ⅱ) serotonergic signaling: this signaling is associated with low protein-induced energy expenditure [156]. Administration of a non-selective 5-HT receptor antagonist, metergoline, and 5-HT3 receptor antagonist, ondansetron, can reduce energy expenditure in rats fed protein-restricted diets [156]. However, whether central or peripheral serotonergic signaling contributes to these processes remains to be studied.
5.2.2. High-protein diet
Current opinion on the mechanisms underlying high protein diet-induced satiety involves elevated circulating amino acids levels, increased diet-induced thermogenesis, and stimulation of gut hormones [151,157,158]. The changes in gut hormones include increased anorexigenic hormones (i.e., GLP-1, PYY) and decreased orexigenic hormones (i.e., ghrelin). According to the aminostatic hypothesis, there is a satiety center in the brain. After reaching a certain point, serum amino acids could serve as satiety signals sensed by the CNS directly or sensed by peripheral organs innervating satiety centers in the brain [158], causing suppression of hunger. According to the studies on the effect of EAA supplementation discussed above, elevated circulating EAAs may also mediate protein-induced thermogenesis and gut hormone secretion. With respect to the effects on energy expenditure, high protein intake stimulates protein turnover by increasing protein synthesis and breakdown, given that the protein storage capacity of the body is limited [157]. This is also the case in BCATm−/− mice. Consistently, “complete” proteins containing all EAAs show more satiating effects and larger increases in energy expenditure than those of “incomplete proteins”, i.e. proteins that lack some EAAs [159].
In addition to the abovementioned different mechanisms used by low- and high-protein diets, these two diets may share some common pathways, such as mTOR signaling. mTOR pathway is shown activated in response to high protein diets in tissues such as the liver [160], whereas low protein diets decrease mTOR activity in tissues such as kidneys [161], liver and adipose tissue [162,163]. However, whether mTOR signaling mediates the effects of these two diets in energy balance remains unclear. Based on the available literature, we speculate that mTOR signaling may play a role in energy homeostasis under these two diets [164,165]. On the one hand, the activation of mTOR signaling pathway in the hypothalamus reduces food intake via inhibiting AgRP and NPY [129,166]. In contrast, reduced hypothalamic mTOR signaling contributes to hyperphagia [166,167]. Therefore, hypothalamic mTORC signaling may mediate the effects of high- and low-protein diets in food intake. On the other hand, the function of mTOR signaling in energy expenditure and thermogenesis remains unclear. For instance, the i.c.v injection of adenoviruses expressing a constitutively active mutant of S6K (CA-S6K) does not affect energy expenditure [71], whereas MBH CA-S6K injection decreases energy expenditure [166] under normal conditions. Additionally, adipose tissue-specific depletion of Raptor in mice (RaptorAdipoq-Cre), which inhibits mTOR signaling, increases UCP1 expression and browning in WAT, but decreases expression of thermogenic genes in BAT [168]. Similarly, S6K1−/− mice show increased UCP1 expression in WAT [70]. However, activation of mTOR signaling via tuberous sclerosis complex 1 (TSC1) deletion inhibits the expression of UCP1 and thermogenic genes in BAT [169]. These findings imply that the function of mTOR signaling in energy expenditure and thermogenesis varies in different tissues and cells under the different stimuli, rather than responding in a uniform manner. Therefore, mTOR signaling in some tissues may account for the increased energy expenditure by high protein diet, while in other tissues, it may contribute to the altered energy expenditure in response to low protein diet.
6. Conclusions and perspectives
EAAs serve as important nutrient signals and regulators of energy balance via various mechanisms. Although substantial progress has been registered in amino acids and obesity-related studies, molecular events have not been completely established. In addition, cross-talks occur among these signaling pathways. Much work remains to be done to further illustrate how these mechanisms are combined to develop these metabolic effects, and this research will allow the design of novel therapeutic approaches for obesity.
As mentioned above, interventions aimed at lowering or increasing the intake of specific EAAs can be beneficial in preventing obesity in animal models, and each has its distinct mechanism. The clinical studies have also demonstrated an association between circulating EAAs levels and obesity in humans. However, studies determining the direct effects and underlying mechanisms of dietary EAA alterations on human body weight are limited, and more investigations are needed. Moreover, humans live in a more complex environment, thereby making it difficult to maintain one type of diet long-term. Alternatively, some other strategies can be considered for the control of certain amino acid levels. For example, in Met restriction, most plant proteins contain too low Met. In humans, dietary Met restriction may be achieved with a predominately vegan diet [170], as vegetarianism is considered to be a “mild” form of Met restriction. Met degradation with methionase (METase) is found to be another strategy. METase is a pyridoxal phosphate dependent enzyme that catalyzes the disintegration of Met into ammonia, α-Keto glutarate, and methanethiol, and is used to reduce circulating Met levels in vivo. Oral recombinant METase was shown to prevent obesity in mice on an HFD [171]. Besides, an intermittent diet plan may also work. Leu deprivation every other day was shown to decrease body weight and increase energy expenditure in db/db mice [172].
Some points should be considered when altering dietary amino acids as a nutritional tool. First, it is important to determine whether this diet could reduce body weight without side effects such as neurological disorders. Second, dietary alteration of a single amino acid often changes other amino acid concentrations in the plasma. Thus, adjustments are needed at times to antagonize the effect of the changes in other amino acid concentrations. Third, manipulation of the amino acid and its metabolite is required in some cases. Recent studies suggest that dietary cysteine supplementation can eliminate many beneficial effects of Met restriction [101]. As most diets contain Met and cysteine, limiting both amino acids in diets may be required for full effects.
Acknowledgment
This work was supported by grants from the National Key R&D Program of China (2018YFA0800600), the National Natural Science Foundation (81970742, 81770852, 91957207, 31830044, 81870592, 81970731, and 82000764), Shanghai leading talent program, CAS Interdisciplinary Innovation Team, and Novo Nordisk-Chinese Academy of Sciences Research Fund (NNCAS-2008-10). Fei Xiao was sponsored by the Youth Innovation Promotion Association of CAS, Shanghai Rising Star Program and Sanofi-Aventis-SIBS scholarship.
Conflict of interest
The authors declare no competing interest.
References
- 1.Bluher M. Obesity: global epidemiology and pathogenesis. Nature Reviews Endocrinology. 2019;15(5):288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 2.Freudenberg A., Petzke K.J., Klaus S. Comparison of high-protein diets and leucine supplementation in the prevention of metabolic syndrome and related disorders in mice. The Journal of Nutritional Biochemistry. 2012;23(11):1524–1530. doi: 10.1016/j.jnutbio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Layman D.K., Walker D.A. Potential importance of leucine in treatment of obesity and the metabolic syndrome. Journal of Nutrition. 2006;136(1 Suppl.):319S–323S. doi: 10.1093/jn/136.1.319S. [DOI] [PubMed] [Google Scholar]
- 4.Yu D., Richardsom N.E., Green C.L., Spicer A.B., Murphy M.E., Flores V., et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metabolism. 2021;33(5):905–922 e6. doi: 10.1016/j.cmet.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana L., Cummings N.E., Apelo S.I.A., Neuman J.C., Kasza I., Schmidt B.A., et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Reports. 2016;16(2):520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yap Y.W., Rusu P.M., Chan A.Y., Fam B.C., Jungmann A., Solon-Biet S.M., et al. Restriction of essential amino acids dictates the systemic metabolic response to dietary protein dilution. Nature Communications. 2020;11(1):2894. doi: 10.1038/s41467-020-16568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen K.Y., Brychta R.J., Sater Z.A., Cassimatis T.M., Cero C., Fletcher L.A., et al. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. Journal of Biological Chemistry. 2020;295(7):1926–1942. doi: 10.1074/jbc.REV119.007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokogoshi H., Ashida K. Comparison between the metabolic effects of tryptophan and histidine deficiencies in the rat. Journal of Nutrition. 1975;105(5):550–556. doi: 10.1093/jn/105.5.550. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y., Meng Q., Wang C., Li H., Huang Z., Chen S., et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes. 2010;59(1):17–25. doi: 10.2337/db09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Y., Meng Q., Zhang Q., Guo F. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT. Amino Acids. 2012;43(2):725–734. doi: 10.1007/s00726-011-1123-8. [DOI] [PubMed] [Google Scholar]
- 11.Yu D., Yang S.E., Miller B.R., Wisinski J.A., Sherman D.S., Brinkman J.A., et al. Short-term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. The FASEB Journal. 2018;32(6):3471–3482. doi: 10.1096/fj.201701211R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao F., Du Y., Lv Z., Chen S., Zhu J., Sheng H., et al. Effects of essential amino acids on lipid metabolism in mice and humans. Journal of Molecular Endocrinology. 2016;57(4):223–231. doi: 10.1530/JME-16-0116. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X., Krasnow S.M., Roth-Carter Q.R., Levasseur P.R., Braun T.P., et al. Hypothalamic signaling in anorexia induced by indispensable amino acid deficiency. American Journal of Physiology. Endocrinology and Metabolism. 2012;303(12):E1446–E1458. doi: 10.1152/ajpendo.00427.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felig P., Marliss E., Cahill G.F., Jr. Plasma amino acid levels and insulin secretion in obesity. New England Journal of Medicine. 1969;281(15):811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 15.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D., Lien L.F., et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabolism. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.She P., Horn C.V., Reid T., Hutson S.M., Cooney R.N., Lynch C.J., et al. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. American Journal of Physiology. Endocrinology and Metabolism. 2007;293(6):E1552–E1563. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orentreich N., Matia J.R., Defelice A., Zimmerman J.A. Low methionine ingestion by rats extends life span. Journal of Nutrition. 1993;123(2):269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y., Zhang Y., Xu Y., Luo T., Ge Y., Jiang Y., et al. Dietary methionine restriction improves the gut microbiota and reduces intestinal permeability and inflammation in high-fat-fed mice. Food and Function. 2019;10(9):5952–5968. doi: 10.1039/c9fo00766k. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y., Zhang J., Wu G., Sun J., Wang Y., Guo H., et al. Dietary methionine restriction regulated energy and protein homeostasis by improving thyroid function in high-fat diet mice. Food and Function. 2018;9(7):3718–3731. doi: 10.1039/c8fo00685g. [DOI] [PubMed] [Google Scholar]
- 20.Hasek B.E., Stewart L.K., Henagan T.M., Boudrean A., Lenard N.R., Black C., et al. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2010;299(3):R728–R739. doi: 10.1152/ajpregu.00837.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malloy V.L., Krajcik R.A., Bailey S.J., Hristopoulos G., Plummer J.D., Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 2006;5(4):305–314. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 22.Perrone C.E., Mattocks D.A.L., Hristopoulos G., Plummer J.D., Krajcik R.A., Orentreich N. Methionine restriction effects on 11-HSD1 activity and lipogenic/lipolytic balance in F344 rat adipose tissue. The Journal of Lipid Research. 2008;49(1):12–23. doi: 10.1194/jlr.M700194-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Anthony T.G., Morrison C.D., Gettys T.W. Remodeling of lipid metabolism by dietary restriction of essential amino acids. Diabetes. 2013;62(8):2635–2644. doi: 10.2337/db12-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green C.L., Lamming D.W. Regulation of metabolic health by essential dietary amino acids. Mechanism of Ageing and Development. 2019;177:186–200. doi: 10.1016/j.mad.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonsson W.O., Margolies N.S., Anthony T.G. Dietary sulfur amino acid restriction and the integrated stress response: mechanistic insights. Nutrients. 2019;11(6) doi: 10.3390/nu11061349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X., He L., Wan D., Yang H., Yao K., Wu G., et al. Methionine restriction on lipid metabolism and its possible mechanisms. Amino Acids. 2016;48(7):1533–1540. doi: 10.1007/s00726-016-2247-7. [DOI] [PubMed] [Google Scholar]
- 27.Cummings N.E., Williams E.M., Kasza I., Konon E.N., Schaid M.D., Schmidt B.A., et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. The Journal of Physiology. 2018;596(4):623–645. doi: 10.1113/JP275075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson N.E., Konon E.N., Schuster H.S., Mitchell A.T., Boyle C., Rodgers A.C., et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nature Aging. 2021;1(1):73–86. doi: 10.1038/s43587-020-00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lees E.K., Banks R., Cook C., Hill S., Morrice N., Grant L., et al. Direct comparison of methionine restriction with leucine restriction on the metabolic health of C57BL/6J mice. Scientific Reports. 2017;7(1):9977. doi: 10.1038/s41598-017-10381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Z., Yin H., Guo Y., Fang Y., Yuan F., Chen S., et al. A fifty percent leucine-restricted diet reduces fat mass and improves glucose regulation. Nutrition and Metabolism. 2021;18(1):34. doi: 10.1186/s12986-021-00564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanders D., Stone K.P., Dille K., Simon J., Pierse A., Gettys T.W. Metabolic responses to dietary leucine restriction involve remodeling of adipose tissue and enhanced hepatic insulin signaling. BioFactors. 2015;41(6):391–402. doi: 10.1002/biof.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zapata R.C., Singh A., Ajdari N.M., Chelikani P.K. Dietary tryptophan restriction dose-dependently modulates energy balance, gut hormones, and microbiota in obesity-prone rats. Obesity. 2018;26(4):730–739. doi: 10.1002/oby.22136. [DOI] [PubMed] [Google Scholar]
- 33.Kamata S., Yamamoto J., Kamijo K., Ochiai T., Morita T., Yoshitomi Y., et al. Dietary deprivation of each essential amino acid induces differential systemic adaptive responses in mice. Molecular Nutrition & Food Research. 2014;58(6):1309–1321. doi: 10.1002/mnfr.201300758. [DOI] [PubMed] [Google Scholar]
- 34.Han L., Wu G., Feng C., Yang Y., Li B., Ge Y., et al. Dietary methionine restriction improves the impairment of cardiac function in middle-aged obese mice. Food and Function. 2020;11(2):1764–1778. doi: 10.1039/c9fo02819f. [DOI] [PubMed] [Google Scholar]
- 35.Lees E.K., Krol E., Shearer K., Mody N., Gettys T.W., Delibegovic M. Effects of hepatic protein tyrosine phosphatase 1B and methionine restriction on hepatic and whole-body glucose and lipid metabolism in mice. Metabolism. 2015;64(2):305–314. doi: 10.1016/j.metabol.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orgeron M.L., Stone K.P., Wanders D., Cortez C.C., Van N.T., Gettys T.W. The impact of dietary methionine restriction on biomarkers of metabolic health. Progress in Molecular Biology and Translational Science. 2014;121:351–376. doi: 10.1016/B978-0-12-800101-1.00011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nature Medicine. 2013;19(10):1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 38.Li L., Li B., Li Min, Speakman J.R. Switching on the furnace: regulation of heat production in brown adipose tissue. Molecular Aspects of Medicine. 2019;68:60–73. doi: 10.1016/j.mam.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan F., Jiang H., Yin H., Jiang X., Jiao F., Ying H., et al. Activation of GCN2/ATF4 signals in amygdalar PKC-delta neurons promotes WAT browning under leucine deprivation. Nature Communications. 2020;11(1):2847. doi: 10.1038/s41467-020-16662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wanders D., Burk D.H., Cortez C.C., Van N.T., Stone K.P., Baker M., et al. UCP1 is an essential mediator of the effects of methionine restriction on energy balance but not insulin sensitivity. The FASEB Journal. 2015;29(6):2603–2615. doi: 10.1096/fj.14-270348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barcena C., Quiros P.M., Durand S., Mayoral P., Rodriguez F., Caravia X.M., et al. Methionine restriction extends lifespan in progeroid mice and alters lipid and bile acid metabolism. Cell Reports. 2018;24(9):2392–2403. doi: 10.1016/j.celrep.2018.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jais A., Bruning J.C. Endocr Rev; 2021. Arcuate nucleus-dependent regulation of metabolism – pathways to obesity and diabetes mellitus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li R.J.W., Zhang S.Y., Lam T.K.T. Interaction of glucose sensing and leptin action in the brain. Molecular Metabolism. 2020;39:101011. doi: 10.1016/j.molmet.2020.101011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers M.G., Jr., Olson D.P. Central nervous system control of metabolism. Nature. 2012;491(7424):357–363. doi: 10.1038/nature11705. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz M.W., Woods S.C., Porte D., Jr., Seeley R.J., Baskin D.G. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 47.Woods S.C., Seeley R.J., Cota D. Regulation of food intake through hypothalamic signaling networks involving mTOR. Annual Review of Nutrition. 2008;28:295–311. doi: 10.1146/annurev.nutr.28.061807.155505. [DOI] [PubMed] [Google Scholar]
- 48.Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 49.Tsang A.H., Nuzzaci D., Darwish T., Samudrala H., Blouet C. Nutrient sensing in the nucleus of the solitary tract mediates non-aversive suppression of feeding via inhibition of AgRP neurons. Molecular Metabolism. 2020;42:101070. doi: 10.1016/j.molmet.2020.101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 51.Cheng Y., Zhang Q., Meng Q., Xia T., Huang Z., Wang C., et al. Leucine deprivation stimulates fat loss via increasing CRH expression in the hypothalamus and activating the sympathetic nervous system. Molecular Endocrinology. 2011;25(9):1624–1635. doi: 10.1210/me.2011-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green C.R., Wallace M., Divakaruni A.S., Phillips S.A., Murphy A.N., Ciaraldi T.P., et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nature Chemical Biology. 2016;12(1):15–21. doi: 10.1038/nchembio.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J., Yu K., Zhu W. Amino acid sensing in the gut and its mediation in gut-brain signal transduction. Animal Nutrition. 2016;2(2):69–73. doi: 10.1016/j.aninu.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang P., McGrath B.C., Reinert J., Olsen D.S., Lei L., Gill S., et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Molecular and Cellular Biology. 2002;22(19):6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dever T.E., Hinnebusch A.G. GCN2 whets the appetite for amino acids. Molecular Cell. 2005;18(2):141–142. doi: 10.1016/j.molcel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 56.Leung P.M., Rogers Q.R. Importance of prepyriform cortex in food-intake response of rats to amino acids. American Journal of Physiology. 1971;221(3):929–935. doi: 10.1152/ajplegacy.1971.221.3.929. [DOI] [PubMed] [Google Scholar]
- 57.Russell M.C., Koehnle T.J., Barrett J.A., Blevins J.E., Gietzen D.W. The rapid anorectic response to a threonine imbalanced diet is decreased by injection of threonine into the anterior piriform cortex of rats. Nutritional Neuroscience. 2003;6(4):247–251. doi: 10.1080/1028415031000151567. [DOI] [PubMed] [Google Scholar]
- 58.Hao S., Sharp J.W., Ross-Inta C.M., McDaniel B.J., Anthony T.G., Wek R.C., et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307(5716):1776–1778. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- 59.Maurin A.C., Benani A., Lorsignol A., Brenachot X., Parry L., Carraro V., et al. Hypothalamic eIF2alpha signaling regulates food intake. Cell Reports. 2014;6(3):438–444. doi: 10.1016/j.celrep.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maurin A.C., Jousse C., Averous J., Parry L., Bruhat A., Cherasse Y., et al. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metabolism. 2005;1(4):273–277. doi: 10.1016/j.cmet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Herbert S.L., Ribeiro C. Nutrition: rejection is the fly's protection. Current Biology. 2014;24(7):R278–R280. doi: 10.1016/j.cub.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 62.Bjordal M., Arquier N., Kniazeff J., Pin J.P., Leopold P. Sensing of amino acids in a dopaminergic circuitry promotes rejection of an incomplete diet in Drosophila. Cell. 2014;156(3):510–521. doi: 10.1016/j.cell.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 63.Wang F., Xiao F., Du L., Niu Y., Yin H., Zhou Z., et al. Activation of GCN2 in macrophages promotes white adipose tissue browning and lipolysis under leucine deprivation. The FASEB Journal. 2021;35(6):e21652. doi: 10.1096/fj.202100061RR. [DOI] [PubMed] [Google Scholar]
- 64.Villarroya F., Cereijo R., Villarroya J., Navarro A.G., Giralt M. Toward an understanding of how immune cells control brown and beige adipobiology. Cell Metabolism. 2018;27(5):954–961. doi: 10.1016/j.cmet.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Wanders D., Stone K.P., Forney L.A., Cortez C.C., Dille K.N., Simon J., et al. Role of GCN2-independent signaling through a noncanonical PERK/NRF2 pathway in the physiological responses to dietary methionine restriction. Diabetes. 2016;65(6):1499–1510. doi: 10.2337/db15-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernandes Stephanie A., Demetriades Constantinos. The multifaceted role of nutrient sensing and mTORC1 signaling in physiology and aging. Frontiers in Aging. 2021;2 doi: 10.3389/fragi.2021.707372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim J., Guan K.L. mTOR as a central hub of nutrient signalling and cell growth. Nature Cell Biology. 2019;21(1):63–71. doi: 10.1038/s41556-018-0205-1. [DOI] [PubMed] [Google Scholar]
- 68.Wolfson R.L., Chantranupong L., Saxton R.A., Shen K., Scaria S.M., Cantor J.R., et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351(6268):43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han J.M., Jeong S.J., Park M.C., Kim G., Kwon N.H., Kim H.K., et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149(2):410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 70.Um S.H., Frigerio F., Watanabe M., Picard F., Joaquin M., Sticker M., et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 71.Xia T., Cheng Y., Zhang Q., Xiao F., Liu B, Chen S., et al. S6K1 in the central nervous system regulates energy expenditure via MC4R/CRH pathways in response to deprivation of an essential amino acid. Diabetes. 2012;61(10):2461–2471. doi: 10.2337/db11-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xia T., Zhang Q., Xiao Y., Wang C., Yu J., Liu H., et al. CREB/TRH pathway in the central nervous system regulates energy expenditure in response to deprivation of an essential amino acid. International Journal of Obesity. 2015;39(1):105–113. doi: 10.1038/ijo.2014.65. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Q., Liu B., Cheng Y., Meng Q., Xia T., Jiang L., et al. Leptin signaling is required for leucine deprivation-enhanced energy expenditure. Journal of Biological Chemistry. 2014;289(3):1779–1787. doi: 10.1074/jbc.M113.528943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Staiger H., Keuper M., Berti L., de Angelis M.H., Haring H.U. Fibroblast growth factor 21-metabolic role in mice and men. Endocrine Reviews. 2017;38(5):468–488. doi: 10.1210/er.2017-00016. [DOI] [PubMed] [Google Scholar]
- 75.Wanders D., Forney L.A., Stone K.P., Burk D.H., Pierse A., Gettys T.W. FGF21 mediates the thermogenic and insulin-sensitizing effects of dietary methionine restriction but not its effects on hepatic lipid metabolism. Diabetes. 2017;66(4):858–867. doi: 10.2337/db16-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forney L.A., Fang H., Sims L.C., Stone K.P., Vincik L.Y., Vick A.M., et al. Dietary methionine restriction signals to the brain through fibroblast growth factor 21 to regulate energy balance and remodeling of adipose tissue. Obesity. 2020;28(10):1912–1921. doi: 10.1002/oby.22919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castano-Martinez T., Schumacher F., Schumacher S., Kochlik B., Weber D., Grune T., Biemann R., et al. Methionine restriction prevents onset of type 2 diabetes in NZO mice. The FASEB Journal. 2019;33(6):7092–7102. doi: 10.1096/fj.201900150R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quigley E.M. Gut bacteria in health and disease. Gastroenterology & Hepatology (NY) 2013;9(9):560–569. [PMC free article] [PubMed] [Google Scholar]
- 79.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 80.Liu R., Hong J., Xu X., Feng Q., Zhang D., Gu Y., et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nature Medicine. 2017;23(7):859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 81.Baothman O.A., Zamzami M.A., Taher I., Abubaker J., Abu-Farha M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids in Health and Disease. 2016;15:108. doi: 10.1186/s12944-016-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin H.V., Frassetto A., Kowalik E.J., Jr., Nawrocki A.R., Lu M.M., Kosinski J.R., et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hwang I., Park Y.J., Kim Y.R., Kim Y.N., Ka S., Lee H.Y., et al. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. The FASEB Journal. 2015;29(6):2397–2411. doi: 10.1096/fj.14-265983. [DOI] [PubMed] [Google Scholar]
- 85.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 86.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 87.Patterson E., Ryan P.M., Cryan J.F., Dinan T.G., Ross R.P., Fitzgerald G.F., et al. Gut microbiota, obesity and diabetes. Postgraduate Medical Journal. 2016;92(1087):286–300. doi: 10.1136/postgradmedj-2015-133285. [DOI] [PubMed] [Google Scholar]
- 88.Brown K., DeCoffe D., Molcan E., Gibson D.L. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4(8):1095–1119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patterson E., Wall R., Lisai S., Ross R.P., Dinan T.G., Cryan J.F., et al. Bifidobacterium breve with alpha-linolenic acid alters the composition, distribution and transcription factor activity associated with metabolism and absorption of fat. Scientific Reports. 2017;7:43300. doi: 10.1038/srep43300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whisner C.M., Martin B.R., Schoterman M.H.C., Nakatsu C.H., McCabe L.D., McCabe G.P., et al. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial. British Journal of Nutrition. 2013;110(7):1292–1303. doi: 10.1017/S000711451300055X. [DOI] [PubMed] [Google Scholar]
- 91.Hoppe M., Onning G., Berggren A., Hulthen L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: a double-isotope cross-over single-blind study in women of reproductive age. British Journal of Nutrition. 2015;114(8):1195–1202. doi: 10.1017/S000711451500241X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yabut J.M., Crane J.D., Green A.F., Keating D.J., Khan W.I., Steinberg G.R. Emerging roles for serotonin in regulating metabolism: new implications for an ancient molecule. Endocrine Reviews. 2019;40(4):1092–1107. doi: 10.1210/er.2018-00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzalez E.M., Penedo L.A., Oliveira-Silva P., Campello-Costa P., Guedes R.C.A., et al. Neonatal tryptophan dietary restriction alters development of retinotectal projections in rats. Experimental Neurology. 2008;211(2):441–448. doi: 10.1016/j.expneurol.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 94.Kot M., Pilc A., Daniel W.A. Simultaneous alterations of brain and plasma serotonin concentrations and liver cytochrome P450 in rats fed on a tryptophan-free diet. Pharmacological Research. 2012;66(4):292–299. doi: 10.1016/j.phrs.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 95.Smriga M., Kameishi M., Unevama H., Torii K. Dietary L-lysine deficiency increases stress-induced anxiety and fecal excretion in rats. Journal of Nutrition. 2002;132(12):3744–3746. doi: 10.1093/jn/132.12.3744. [DOI] [PubMed] [Google Scholar]
- 96.Smriga M., Torii K. L-Lysine acts like a partial serotonin receptor 4 antagonist and inhibits serotonin-mediated intestinal pathologies and anxiety in rats. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15370–15375. doi: 10.1073/pnas.2436556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tabarean I.V. Histamine receptor signaling in energy homeostasis. Neuropharmacology. 2016;106:13–19. doi: 10.1016/j.neuropharm.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mercer L.P., Dodds S.J., Schweisthal M.R., Dunn J.D. Brain histidine and food intake in rats fed diets deficient in single amino acids. Journal of Nutrition. 1989;119(1):66–74. doi: 10.1093/jn/119.1.66. [DOI] [PubMed] [Google Scholar]
- 99.Nakahara K., Takata S., Ishii A., Nagao K., Bannai M., Takahashi M., et al. Somatostatin is involved in anorexia in mice fed a valine-deficient diet. Amino Acids. 2012;42(4):1397–1404. doi: 10.1007/s00726-011-0836-z. [DOI] [PubMed] [Google Scholar]
- 100.Brown-Borg H.M., Rakoczy S.G., Wonderlich J.A., Rojanathammanee L., Kopchick J.K., Armstrong V., et al. Growth hormone signaling is necessary for lifespan extension by dietary methionine. Aging Cell. 2014;13(6):1019–1027. doi: 10.1111/acel.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elshorbagy A.K., Valdivia-Garcia M., Mattocks D.A.L., Plummer J.D., Smith A.D., Drevon C.A., et al. Cysteine supplementation reverses methionine restriction effects on rat adiposity: significance of stearoyl-coenzyme A desaturase. The Journal of Lipid Research. 2011;52(1):104–112. doi: 10.1194/jlr.M010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiao F., Huang Z., Li H., Yu J., Wang C., Chen S., et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60(3):746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ye J., Palm W., Peng M., King B., Lindsten T., Li M.O., et al. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes & Development. 2015;29(22):2331–2336. doi: 10.1101/gad.269324.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ai Y., Sun Z., Peng C., Liu L., Xiao X., Li J. Homocysteine induces hepatic steatosis involving ER stress response in high methionine diet-fed mice. Nutrients. 2017;9(4) doi: 10.3390/nu9040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ma Q., Hu L., Zhu J., Chen J., Wang Z., Yue Z., et al. Valine supplementation does not reduce lipid accumulation and improve insulin sensitivity in mice fed high-fat diet. ACS Omega. 2020;5(48):30937–30945. doi: 10.1021/acsomega.0c03707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiao C.W., Wood C., Bertinato J. Dietary supplementation with L-lysine affects body weight and blood hematological and biochemical parameters in rats. Molecular Biology Reports. 2019;46(1):433–442. doi: 10.1007/s11033-018-4492-1. [DOI] [PubMed] [Google Scholar]
- 107.Xiao C.W., Faddoul C., Wood C. Dietary L-lysine supplementation altered the content of pancreatic polypeptide, enzymes involved in glutamine metabolism, and beta-actin in rats. Amino Acids. 2018;50(12):1729–1737. doi: 10.1007/s00726-018-2648-x. [DOI] [PubMed] [Google Scholar]
- 108.Kasaoka S., Tsuboyama-Kasaoka N., Kawahara Y., Inoue S., Tsuji M., Ezaki O., et al. Histidine supplementation suppresses food intake and fat accumulation in rats. Nutrition. 2004;20(11–12):991–996. doi: 10.1016/j.nut.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 109.Sun X., Feng R., Li Y., Lin S., Zhang W., Li Y., et al. Histidine supplementation alleviates inflammation in the adipose tissue of high-fat diet-induced obese rats via the NF-kappaB- and PPARgamma-involved pathways. British Journal of Nutrition. 2014;112(4):477–485. doi: 10.1017/S0007114514001056. [DOI] [PubMed] [Google Scholar]
- 110.Alamshah A., Spreckley E., Norton M., Kinsey-Jones J.S., Amin A., Ramgulam A., et al. l-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. International Journal of Obesity. 2017;41(11):1693–1701. doi: 10.1038/ijo.2017.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma Q., Zhou X., Sun Y., Hu L., Zhu J., Shao C., et al. Threonine, but not lysine and methionine, reduces fat accumulation by regulating lipid metabolism in obese mice. Journal of Agricultural and Food Chemistry. 2020;68(17):4876–4883. doi: 10.1021/acs.jafc.0c01023. [DOI] [PubMed] [Google Scholar]
- 112.Shipelin V.A., Trusov N.V., Apryatin S.A., Shumakova A.A., Balakina A.S., Riger N.A., et al. Effects of tyrosine and tryptophan in rats with diet-induced obesity. International Journal of Molecular Sciences. 2021;22(5) doi: 10.3390/ijms22052429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coskun S., Ozer C., Gonul B., Take G., Erdogan D. The effect of repeated tryptophan administration on body weight, food intake, brain lipid peroxidation and serotonin immunoreactivity in mice. Molecular and Cellular Biochemistry. 2006;286(1–2):133–138. doi: 10.1007/s11010-005-9103-5. [DOI] [PubMed] [Google Scholar]
- 114.Ma Q., Zhou X., Hu L., Chen J., Zhu J., Shan A. Leucine and isoleucine have similar effects on reducing lipid accumulation, improving insulin sensitivity and increasing the browning of WAT in high-fat diet-induced obese mice. Food and Function. 2020;11(3):2279–2290. doi: 10.1039/c9fo03084k. [DOI] [PubMed] [Google Scholar]
- 115.Jiao J., Han S.F., Zhang W., Xu J.Y., Tong X., Yin X.B., et al. Chronic leucine supplementation improves lipid metabolism in C57BL/6J mice fed with a high-fat/cholesterol diet. Food & Nutrition Research. 2016;60:31304. doi: 10.3402/fnr.v60.31304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y., Guo K., LeBlanc R.E., Loh D., Schwartz G.J., Yu Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56(6):1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- 117.Binder E., Bermudez-Silva F.J., Andre C., Elie M., Romero-Zerbo S.Y., Leste-Lasserre T., et al. Leucine supplementation protects from insulin resistance by regulating adiposity levels. PLoS One. 2013;8(9):e74705. doi: 10.1371/journal.pone.0074705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li H., Xu M., Lee J., He C., Xie Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. American Journal of Physiology. Endocrinology and Metabolism. 2012;303(10):E1234–E1244. doi: 10.1152/ajpendo.00198.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nishimura J., Masaki T., Arakawa M., Seike M., Yoshimatsu H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARalpha and uncoupling protein in diet-induced obese mice. Journal of Nutrition. 2010;140(3):496–500. doi: 10.3945/jn.109.108977. [DOI] [PubMed] [Google Scholar]
- 120.O’Rielly R., Li H., Lim S.M., Yazbeck R., Kritas S., Ullrich S.S., et al. The effect of isoleucine supplementation on body weight gain and blood glucose response in lean and obese mice. Nutrients. 2020;12(8) doi: 10.3390/nu12082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Binder E., Bermudez-Silva F.J., Elie M., Leste-Lasserre T., Belluomo I., Clark S., et al. Leucine supplementation modulates fuel substrates utilization and glucose metabolism in previously obese mice. Obesity. 2014;22(3):713–720. doi: 10.1002/oby.20578. [DOI] [PubMed] [Google Scholar]
- 122.Kawabe M., Takesada Y., Tamano S., Hagiwara A., Ito N., Shirai T. Subchronic toxicity study of L-isoleucine in F344 rats. Journal of Toxicology and Environmental Health. 1996;47(5):499–508. doi: 10.1080/009841096161645. [DOI] [PubMed] [Google Scholar]
- 123.Yuan X.W., Han S.F., Zhang J.W., Xu J.Y., Qin L.Q. Leucine supplementation improves leptin sensitivity in high-fat diet fed rats. Food & Nutrition Research. 2015;59:27373. doi: 10.3402/fnr.v59.27373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li X., Wang X., Liu R., Ma Y., Guo H., Hao L., et al. Chronic leucine supplementation increases body weight and insulin sensitivity in rats on high-fat diet likely by promoting insulin signaling in insulin-target tissues. Molecular Nutrition & Food Research. 2013;57(6):1067–1079. doi: 10.1002/mnfr.201200311. [DOI] [PubMed] [Google Scholar]
- 125.Medrikova D., Jilkova Z.M., Bardova K., Janovska P., Rossmeisl M., Kopecky J. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. International Journal of Obesity. 2012;36(2):262–272. doi: 10.1038/ijo.2011.87. [DOI] [PubMed] [Google Scholar]
- 126.Babygirija R., Lamming D.W. The regulation of healthspan and lifespan by dietary amino acids. Translational Medicine of Aging. 2021;5:17–30. doi: 10.1016/j.tma.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Drgonova J., Jacobsson J.A., Han J.C., Yanovski J.A., Fredriksson R., Marcus C., et al. Involvement of the neutral amino acid transporter SLC6A15 and leucine in obesity-related phenotypes. PLoS One. 2013;8(9):e68245. doi: 10.1371/journal.pone.0068245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pedroso J.A., Zampieri T.T., Donato J., Jr. Reviewing the effects of L-leucine supplementation in the regulation of food intake, energy balance, and glucose homeostasis. Nutrients. 2015;7(5):3914–3937. doi: 10.3390/nu7053914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cota D., Proulx K., Smith K.A.B., Kozma S.C., Thomas G., Woods S.C., et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 130.Blouet C., Jo Y.H., Li X., Schwartz G.J. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. Journal of Neuroscience. 2009;29(26):8302–8311. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blouet C., Schwartz G.J. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metabolism. 2012;16(5):579–587. doi: 10.1016/j.cmet.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mace O.J., Schindler M., Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. The Journal of Physiology. 2012;590(12):2917–2936. doi: 10.1113/jphysiol.2011.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yoshimatsu H., Chiba S., Tajima D., Akehi Y., Sakata T. Histidine suppresses food intake through its conversion into neuronal histamine. Experimental Biology and Medicine (Maywood) 2002;227(1):63–68. doi: 10.1177/153537020222700111. [DOI] [PubMed] [Google Scholar]
- 134.Morley J.E., Flood J.F. Evidence that nitric oxide modulates food intake in mice. Life Sciences. 1991;49(10):707–711. doi: 10.1016/0024-3205(91)90102-h. [DOI] [PubMed] [Google Scholar]
- 135.Fried S.K., Watford M. Leucing weight with a futile cycle. Cell Metabolism. 2007;6(3):155–156. doi: 10.1016/j.cmet.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 136.She P., Reid T.M., Bronson S.K., Vary T.C, Hajnal A., Lynch C.J., et al. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metabolism. 2007;6(3):181–194. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Niu Y.C., Feng R.N., Hou Y., Li K., Kang Z., Wang J., et al. Histidine and arginine are associated with inflammation and oxidative stress in obese women. British Journal of Nutrition. 2012;108(1):57–61. doi: 10.1017/S0007114511005289. [DOI] [PubMed] [Google Scholar]
- 138.Breum L., Rasmussen M.H., Hilsted J., Fernstrom J.D. Twenty-four-hour plasma tryptophan concentrations and ratios are below normal in obese subjects and are not normalized by substantial weight reduction. American Journal of Clinical Nutrition. 2003;77(5):1112–1118. doi: 10.1093/ajcn/77.5.1112. [DOI] [PubMed] [Google Scholar]
- 139.Laferrere B., Reilly D., Arias S., Swerdlow N., Gorroochurn P., Bawa B., et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Science Translational Medicine. 2011;3(80):80re2. doi: 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shin A.C., Fasshauer M., Filatova N., Grundell L.A., Zielinski E., Zhou J.Y., et al. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metabolism. 2014;20(5):898–909. doi: 10.1016/j.cmet.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.White P.J., McGarrah R.W., Grimsrud P.A., Tso S.C., Yang W.H., Haldeman J.M., et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metabolism. 2018;27(6):1281–1293 e7. doi: 10.1016/j.cmet.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou M., Shao J., Wu C.Y., Shu L., Dong W., Liu Y., et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes. 2019;68(9):1730–1746. doi: 10.2337/db18-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Newgard C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metabolism. 2012;15(5):606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kahleova H., Fleeman R., Hlozkova A., Holubkov R., Barnard N.D. A plant-based diet in overweight individuals in a 16-week randomized clinical trial: metabolic benefits of plant protein. Nutrition & Diabetes. 2018;8(1):58. doi: 10.1038/s41387-018-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qin L.Q., Xun P., Bujnowski D., Daviglus M.L., Horn L.V., Stamler J., et al. Higher branched-chain amino acid intake is associated with a lower prevalence of being overweight or obese in middle-aged East Asian and Western adults. Journal of Nutrition. 2011;141(2):249–254. doi: 10.3945/jn.110.128520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Plaisance E.P., Greenway F.L., Boudreau A., Hill K.L., Johnson W.D., Krajcik R.A., et al. Dietary methionine restriction increases fat oxidation in obese adults with metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism. 2011;96(5):E836–E840. doi: 10.1210/jc.2010-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zemel M.B., Bruckbauer A. Effects of a leucine and pyridoxine-containing nutraceutical on body weight and composition in obese subjects. Diabetes, Metabolic Syndrome and Obesity. 2013;6:309–315. doi: 10.2147/DMSO.S49623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zemel M.B., Bruckbauer A. Effects of a leucine and pyridoxine-containing nutraceutical on fat oxidation, and oxidative and inflammatory stress in overweight and obese subjects. Nutrients. 2012;4(6):529–541. doi: 10.3390/nu4060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Feng R.N., Niu Y.C., Sun X.W., Li Q, Zhao C., Wang C., et al. Histidine supplementation improves insulin resistance through suppressed inflammation in obese women with the metabolic syndrome: a randomised controlled trial. Diabetologia. 2013;56(5):985–994. doi: 10.1007/s00125-013-2839-7. [DOI] [PubMed] [Google Scholar]
- 150.Hiratsuka C., Fukuwatari T., Sano M., Saito K., Sasaki S., Shibata K. Supplementing healthy women with up to 5.0 g/d of L-tryptophan has no adverse effects. Journal of Nutrition. 2013;143(6):859–866. doi: 10.3945/jn.112.173823. [DOI] [PubMed] [Google Scholar]
- 151.Cuenca-Sanchez M., Navas-Carrillo D., Orenes-Pinero E. Controversies surrounding high-protein diet intake: satiating effect and kidney and bone health. Advances in Nutrition. 2015;6(3):260–266. doi: 10.3945/an.114.007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Laeger T., Henagan T.M., Albarado D.C., Redman L.M., Bray G.A., Noland R.C., et al. FGF21 is an endocrine signal of protein restriction. Journal of Clinical Investigation. 2014;124(9):3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hill C.M., Laeger T., Albarado D.C., McDougal D.H., Berthoud H.R., Munzberg H., et al. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Scientific Reports. 2017;7(1):8209. doi: 10.1038/s41598-017-07498-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hill C.M., Laeger T., Dehner M., Albarado D.C., Clarke B., Wanders D., et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Reports. 2019;27(10):2934–2947 e3. doi: 10.1016/j.celrep.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Laeger T., Albarado D.C., Burke S.J., Trosclair L., Hedgepeth J.W., Berthoud H.R., et al. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Reports. 2016;16(3):707–716. doi: 10.1016/j.celrep.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pezeshki A., Chelikani P.K. Low protein diets and energy balance: mechanisms of action on energy intake and expenditure. Frontiers in Nutrition. 2021;8:655833. doi: 10.3389/fnut.2021.655833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Westerterp-Plantenga M.S. Challenging energy balance – during sensitivity to food reward and modulatory factors implying a risk for overweight - during body weight management including dietary restraint and medium-high protein diets. Physiology & Behavior. 2020;221:112879. doi: 10.1016/j.physbeh.2020.112879. [DOI] [PubMed] [Google Scholar]
- 158.Drummen M., Tischmann L., Gatta-Cherifi B., Adam T., Westerterp-Plantenga M. Dietary protein and energy balance in relation to obesity and co-morbidities. Frontiers in Endocrinology. 2018;9:443. doi: 10.3389/fendo.2018.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Westerterp-Plantenga M.S., Nieuwenhuizen A., Tome D., Soenen S., Westerterp K.R. Dietary protein, weight loss, and weight maintenance. Annual Review of Nutrition. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 160.Chotechuang N., Azzout-Marniche D., Bos C., Chaumontet C., Gausseres N., Steiler T., et al. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. American Journal of Physiology. Endocrinology and Metabolism. 2009;297(6):E1313–E1323. doi: 10.1152/ajpendo.91000.2008. [DOI] [PubMed] [Google Scholar]
- 161.Kitada M., Ogura Y., Suzuki T., Monno I., Kanasaki K., Watanabe A., et al. A low-protein diet exerts a beneficial effect on diabetic status and prevents diabetic nephropathy in Wistar fatty rats, an animal model of type 2 diabetes and obesity. Nutrition and Metabolism. 2018;15:20. doi: 10.1186/s12986-018-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo J.H., Wang H.Y., Zhang Q., Feng M., Zhang L.J. The low protein diet affects the nonspecific inflammatory response of middle-aged and old mice through mTOR. European Review for Medical and Pharmacological Sciences. 2018;22(21):7551–7561. doi: 10.26355/eurrev_201811_16297. [DOI] [PubMed] [Google Scholar]
- 163.Maida A., Chan J.S.K., Sjøberg K.A., Zota A., Schmoll D., Kiens B., et al. Repletion of branched chain amino acids reverses mTORC1 signaling but not improved metabolism during dietary protein dilution. Molecular Metabolism. 2017;6(8):873–881. doi: 10.1016/j.molmet.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Haissaguerre M., Saucisse N., Cota D. Influence of mTOR in energy and metabolic homeostasis. Molecular and Cellular Endocrinology. 2014;397(1–2):67–77. doi: 10.1016/j.mce.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 165.Zhang Z., Yang D., Xiang J., Zhou J., Cao H., Che Q., et al. Non-shivering thermogenesis signalling regulation and potential therapeutic applications of brown adipose tissue. International Journal of Biological Sciences. 2021;17(11):2853–2870. doi: 10.7150/ijbs.60354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Blouet C., Ono H., Schwartz G.J. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metabolism. 2008;8(6):459–467. doi: 10.1016/j.cmet.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cota D., Matter E.K., Woods S.C., Seeley R.J. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. Journal of Neuroscience. 2008;28(28):7202–7208. doi: 10.1523/JNEUROSCI.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang X., Luo Y., Wang C., Ding X., Yang X., Wu D., et al. Adipose mTORC1 suppresses prostaglandin signaling and beige adipogenesis via the CRTC2-COX-2 pathway. Cell Reports. 2018;24(12):3180–3193. doi: 10.1016/j.celrep.2018.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xiang X., Lan H., Tang H., Yuan F., Xu Y., Zhao J., et al. Tuberous sclerosis complex 1-mechanistic target of rapamycin complex 1 signaling determines brown-to-white adipocyte phenotypic switch. Diabetes. 2015;64(2):519–528. doi: 10.2337/db14-0427. [DOI] [PubMed] [Google Scholar]
- 170.Cavuoto P., Fenech M.F. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treatment Reviews. 2012;38(6):726–736. doi: 10.1016/j.ctrv.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 171.Tashiro Y., Han Q., Tan Y., Sugisawa N., Yamamoto J., Nishino H., et al. Oral recombinant methioninase prevents obesity in mice on a high-fat diet. In Vivo. 2020;34(2):489–494. doi: 10.21873/invivo.11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wei S., Zhao J., Wang S., Huang M., Wang Y., Chen Y. Intermittent administration of a leucine-deprived diet is able to intervene in type 2 diabetes in db/db mice. Heliyon. 2018;4(9):e00830. doi: 10.1016/j.heliyon.2018.e00830. [DOI] [PMC free article] [PubMed] [Google Scholar]