Abstract
Purpose:
To examine the role age plays in the treatment and prognosis of locally advanced head and neck cancer (LAHNC) treated definitively with radiation alone or combined modality therapy.
Methods:
A retrospective analysis was performed of three NRG/RTOG trials examining either radiation alone or combined radiation and systemic therapy for LAHNC. The effect of age (≥70 yrs.) on cause-specific survival (CSS), overall survival (OS), and toxicity was evaluated.
Results:
A total of 2688 patients were analyzed, of whom 309 patients (11.5%) were ≥70. For all studies combined, the hazard ratio (HR) for CSS for patients age ≥70 vs. those <70 was 1.33 (95%CI: 1.14–1.55, p<0.001). For OS, the HR for patients age ≥70 vs. those < 70 for all studies combined was 1.55 (95% CI 1.35–1.77, p<0.001). After adjustment for all covariates, age ≥70 was associated with worse OS regardless of adjustment for smoking and p16 status. The survival difference was more pronounced in those receiving combined radiation and systemic therapy. Hematologic and renal toxicities were increased in combined modality trials in patients ≥70 years old.
Conclusions:
Patients age ≥70 with LAHNC were underrepresented in these clinical trials. Their CSS and OS proved inferior to patients <70 years old.
Keywords: Locally advanced head and neck cancer, Chemotherapy, Radiation, Older Adult, Age, Toxicity, CSS, OS, Retrospective Analysis
1. Introduction
Head and neck cancer most often occurs in the 5th and 6th decade. More than 50% of patients are over 60 years of age, and 28% are over 70 years of age at diagnosis [1]. In addition, the oropharynx cancer epidemic is shifting from a younger population (< 60 years) to an older one (> 65 years). This increase will have substantial implications on clinical care. If these trends continue, it is predicted that from 2016 to 2029 the incidence of oropharyngeal cancer will increase in older white men (65–74 years) from 40.7 per 100,000 to 71 per 100,000 [2]. For men 75–84 years, the incidence will rise from 25 to 50 per 100,000 [2]. Concomitantly, SEER data from 2000 to 2012 have indicated a decrease in the incidence of tobacco-related HNC in older adult patients [3]. Additionally, the number of U.S. citizens older than 65 years is expected to increase in the next decade [4,5]. Even as causation seems to have shifted, the effect of age on cancer outcomes is still a major issue, particularly as the oropharyngeal cancer epidemic shifts from a younger population (< 60 years) to an older one (> 65 years). These factors have created an opportunity to examine the specific effect of age on treatment outcomes in patients with locally advanced LAHNC. To date, most retrospective reviews on this topic have been single institution efforts with varied treatment regimens and no standard eligibility criteria. These studies, though informative, do not provide definitive guidance for treatment. Some previous analyses have shown that older adult patients can achieve comparable outcomes if treated with similar therapies as younger patients [6]. Alternatively, other studies have shown higher mortality and greater toxicities in older adults treated with standard of care therapies compared to younger patients. [7–9] To date, age as an independent prognostic variable in the treatment of head and neck cancer has not been addressed in the context of major cooperative group trials. Hence, we endeavored to assess the impact of age on outcome in three large serial phase III trials of radiation, either alone or combined with systemic therapy in LAHNC conducted under the aegis of the Radiation Therapy Group (RTOG) and the NRG Oncology.
2. Methods
In an effort to identify the relevance of age, a retrospective analysis of 2688 patients enrolled in three national RTOG trials (RTOG 0129, 0522 and 9003) was performed. Patients were evaluated for survival and toxicity by age (≥ 70 vs. < 70 years). Other factors were also assessed within the demographic profile. See Table 1.
Table 1.
Patient and tumor characteristics.
| Age < 70 (n = 2379) | Age ≥ 70 (n = 309) | Total (n = 2688) | |
|---|---|---|---|
| Trial, p < 0.001 [2] | |||
| RTOG 9003 | 869 (36.5%) | 207 (67.0%) | 1076 (40.0%) |
| RTOG 0129 | 673 (28.3%) | 48 (15.5%) | 721 (26.8%) |
| RTOG 0522 | 837 (35.2%) | 54 (17.5%) | 891 (33.1%) |
| Gender, p < 0.001 [3] | |||
| Male | 2011 (84.5%) | 229 (74.1%) | 2240 (83.3%) |
| Female | 368 (15.5%) | 80 (25.9%) | 448 (16.7%) |
| Race, p = 0.70 [3] | |||
| White | 1922 (80.8%) | 253 (81.9%) | 2175 (80.9%) |
| Non-white | 447 (18.8%) | 55 (17.8%) | 502 (18.7%) |
| Unknown | 10 (0.4%) | 1 (0.3%) | 11 (0.4%) |
| Zubrod performance status, p < 0.001 [4] | |||
| 0 | 1505 (63.3%) | 156 (50.5%) | 1661 (61.8%) |
| 1 | 844 (35.5%) | 132 (42.7%) | 976 (36.3%) |
| 2 | 30 (1.3%) | 21 (6.8%) | 51 (1.9%) |
| Smoking history: pack-years [1], p < 0.001 [4] | (n = 2054) | (n = 264) | (n = 2318) |
| Mean | 35.5 | 46.5 | 36.8 |
| Std. Dev. | 31.79 | 40.47 | 33.07 |
| Median | 31.75 | 49 | 33 |
| Min – Max | 0–247.5 | 0–224 | 0–247.5 |
| Q1 - Q3 | 7.5–52 | 8–66 | 7.5–54 |
| Primary site, p < 0.001 [2] | |||
| Oral cavity | 125 (5.3%) | 30 (9.7%) | 155 (5.8%) |
| Oropharynx | 1534 (64.5%) | 174 (56.3%) | 1708 (63.5%) |
| Hypopharynx | 216 (9.1%) | 45 (14.6%) | 261 (9.7%) |
| Larynx | 504 (21.2%) | 60 (19.4%) | 564 (21.0%) |
| p16 status, p < 0.001 [3] | (n = 1087) | (n = 107) | (n = 1194) |
| Negative | 537 (49.4%) | 73 (68.2%) | 610 (51.1%) |
| Positive | 550 (50.6%) | 34 (31.8%) | 584 (48.9%) |
| T stage, p = 0.72 [4] | |||
| T1 | 53 (2.2%) | 9 (2.9%) | 62 (2.3%) |
| T2 | 729 (30.6%) | 78 (25.2%) | 807 (30.0%) |
| T3 | 922 (38.8%) | 143 (46.3%) | 1065 (39.6%) |
| T4 | 674 (28.3%) | 79 (25.6%) | 753 (28.0%) |
| Tis | 1 (0.0%) | 0 (0.0%) | 1 (0.0%) |
| N stage, p < 0.001 [4] | |||
| Age < 70 (n = 2379) | Age ≥ 70 (n = 309) | Total (n = 2688) | |
|
| |||
| N0 | 387 (16.3%) | 88 (28.5%) | 475 (17.7%) |
| N1 | 351 (14.8%) | 51 (16.5%) | 402 (15.0%) |
| N2a | 217 (9.1%) | 24 (7.8%) | 241 (9.0%) |
| N2b | 616 (25.9%) | 68 (22.0%) | 684 (25.4%) |
| N2c | 605 (25.4%) | 54 (17.5%) | 659 (24.5%) |
| N3 | 203 (8.5%) | 24 (7.8%) | 227 (8.4%) |
Std. Dev., standard deviation; Q1, first quartile; Q3, third quartile.
A pack-year is defined as the equivalent of smoking one pack of cigarettes a day for 1 year.
Pearson chi-square test.
Fisher’s exact test. Unknown race excluded.
Wilcoxon rank-sum test. Stage Tis excluded.
RTOG 9003 was a four-arm phase III trial testing three altered fractionation schedules against standard once-daily radiation administration with accrual between 9/91 and 8/97. No systemic therapy was involved. Patients with locally advanced head and neck cancer of the oral cavity, oropharynx, hypopharynx, and supraglottic larynx were included.
RTOG 0129 was a two-arm phase III trial evaluating standard fractionation of radiation with concurrent high dose cisplatin (100 mg/m2 every 21d for 3 cycles) versus accelerated fractionation by a concomitant boost with concurrent high dose cisplatin (2 cycles). Patients with locally advanced cancers of oral cavity, oropharynx, hypopharynx, and larynx were eligible and were accrued between 07/02 and 06/05.
RTOG 9003 was a four-arm phase III trial testing three altered fractionation schedules against standard once-daily radiation administration with accrual between 9/91 and 8/97. No systemic therapy was involved. Patients with locally advanced head and neck cancer of the oral cavity, oropharynx, hypopharynx, and supraglottic larynx were included.
RTOG 0129 was a two-arm phase III trial evaluating standard fractionation of radiation with concurrent high dose cisplatin (100 mg/m2 every 21d for 3 cycles) versus accelerated fractionation by a concomitant boost with concurrent high dose cisplatin (2 cycles). Patients with locally advanced cancers of oral cavity, oropharynx, hypopharynx, and larynx were eligible and were accrued between 07/02 and 06/05.
RTOG 0522 was a phase III trial testing the addition of cetuximab to standard high dose cisplatin delivered concurrently with accelerated radiation vs high dose cisplatin alone plus accelerated radiation. Eligible patients had locally advanced squamous cell carcinomas of the oropharynx, hypopharynx, and larynx and were accrued between 11/05 and 03/09.
Toxicities in RTOG 9003 were graded by the RTOG (acute) and RTOG/EORTC (late) criteria. Toxicities in RTOG 0129 were graded by Common Toxicity Criteria (CTC) version 2.0 (acute radiation and chemotherapy at any time) and RTOG/EORTC (late radiation) criteria. Toxicities in RTOG 0522 were graded by Common Terminology Criteria for Adverse Events (CTCAE) version 3.0; events unrelated to or unlikely to be related to protocol treatment were excluded from the analysis. Only acute toxicities within 90 days of the start of treatment were included in this analysis. The persistence of feeding tubes at 6, 12, and 24 months was also included as an endpoint. These studies were each officially reviewed by individual institutional review boards at each participating site, and therefore met the requirements for the protection of human subjects.
Statistical analyses:
Overall survival was defined as the time from randomization to death (event) or last follow-up. Failure for cause-specific survival was defined in two ways:
Any cause of death following local, regional, or distant progression, or cause of death due to study cancer, second primary, protocol treatment, or unknown.
Any cause of death following local, regional, or distant progression, or cause of death due to study cancer, second primary, or protocol treatment.
Survival rates were estimated using the Kaplan-Meier method. Hazard ratios were estimated by the Cox model. All analyses that included multiple trials were stratified by trial. Pearson chi-square test and Fisher’s exact test were used to compare categorical variables, Wilcoxon rank-sum test was used to compare continuous variables. Local-regional failure and distant metastasis rates were estimated by the cumulative incidence method and groups were compared by Gray’s test.
3. Results
This secondary analysis included 2688 patients (n = 1076, 721, 891 for each trial). A total of 309 patients (11.5%) were 70 years of age or older with the breakdown as follows: RTOG 9003: 207pts (19.2%); RTOG 0129: 48 pts. (6.7%); and RTOG 0522: 54 pts. (6.1%). The median follow-up for all surviving patients was 5.2 years (range 0.01 to 20.3); 14.1 years in RTOG 9003; 7.9 years in RTOG 0129 and 4.6 years in RTOG 0522.
Patients ≥70 years were more likely to be female, have a poorer performance status, and a more pronounced smoking history (Table 1). Distributions of the primary site, p16 status, and N stage also differed by age group. Oropharyngeal cancer was the predominant site across trials occurring in 64.5% of patients <70 years old and 56.3% of patients ≥70 years old. Forty-four percent (44%)(1194/2688) of patients had p16 testing performed; of these 48.9% were p16 positive. Fifty-one percent (51%) of the patients <70 years were p16+ compared to 31.8% of those patients ≥70 years.
In univariate analysis, patients 70 years and older had worse survival compared to patients <70 years both in each individual trial and when the trial results were combined; the overall HR was 1.55 (95%CI: 1.35–1.77) for combined trials, 1.34 (95%CI: 1.15–1.57) for RTOG 9003, 2.34 (95%CI:1.68–3.26) for RTOG 0129, and 2.45 (95%CI: 1.69–3.53) for RTOG 0522, respectively. The p values were < 0.001 for all tests. Survival for all three (3) trials combined is shown in Fig. 1a. The survival curves for each individual trial are shown in Fig. 1b, 1c, and 1d.
Fig. 1.
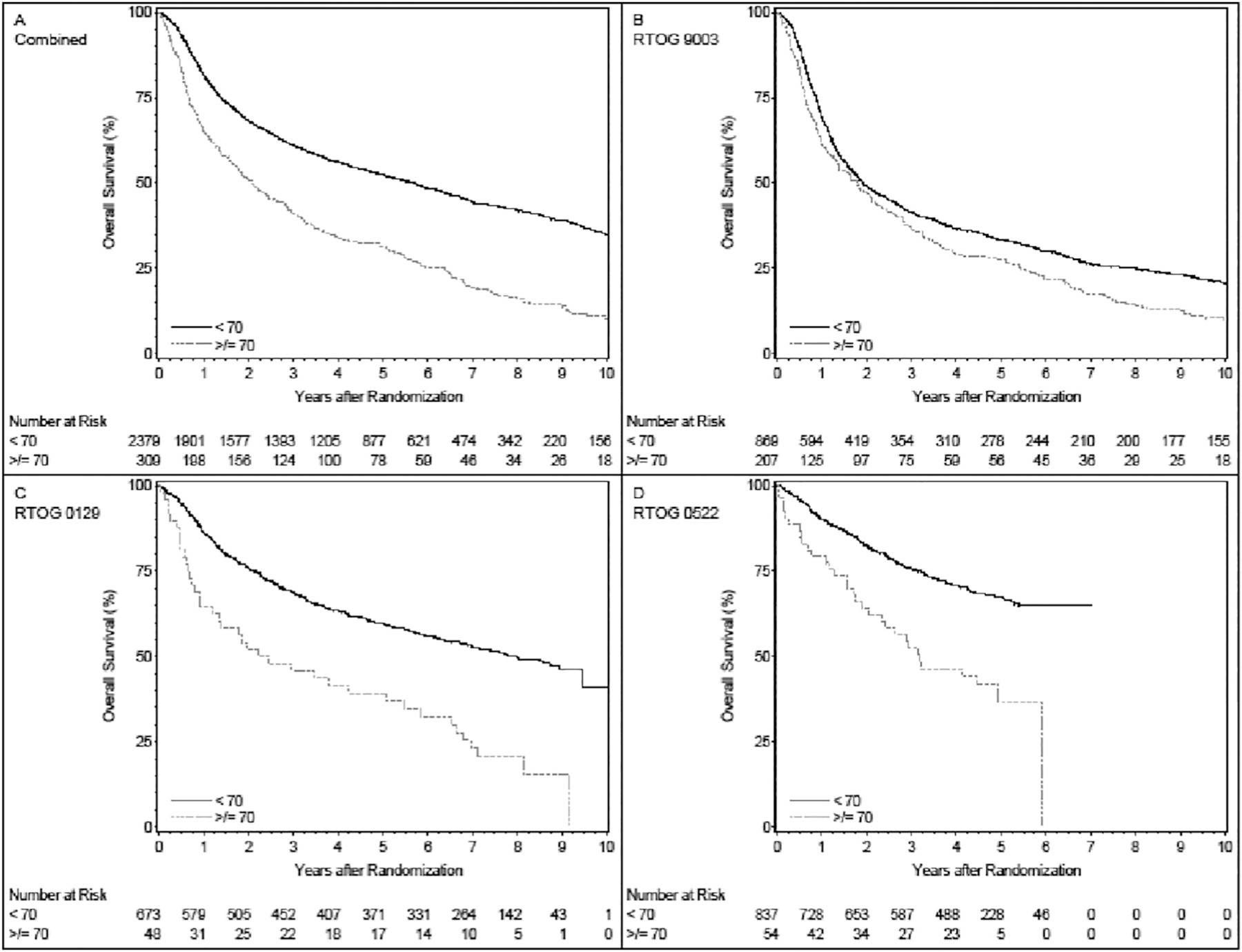
Fig. 1A: overall survival for the three trials combined. Patients age ≥ 70 years have significantly worse survival relative to patients age < 70 (p < 0.001). Five-year survival estimates are 31.3% (95%CI 26.1 to 36.6) and 52.4% (95%CI 50.4 to 54.5) for the ≥70 (309 patients, 270 deaths) and < 70 (2379 patients, 1341 deaths) groups, respectively. Fig. 1B: overall survival for RTOG 9003: Patients age ≥ 70 years have significantly worse survival relative to patients age < 70 (p < 0.001). Five-year survival estimates are 27.4% (95%CI 21.3 to 33.5) and 33.4% (95%CI 30.2 to 36.5) for the ≥70 (207 patients, 199 deaths) and < 70 (869 patients, 759 deaths) groups, respectively. Fig. 1C: overall survival for RTOG 0129: Patients age ≥ 70 years have significantly worse survival relative to patients age < 70 (p < 0.001). Five-year survival estimates are 39.3% (95%CI 25.3 to 53.2) and 59.7% (95%CI 55.9 to 63.4) for the ≥70 (48 patients, 39 deaths) and < 70 (673 patients, 327 deaths) groups, respectively. Fig. 1D: overall survival for RTOG 0522: Patients age ≥ 70 years have significantly worse survival relative to patients age < 70 (p < 0.001). Five-year survival estimates are 36.7% (95%CI 21.4 to 52.0) and 67.4% (95%CI 64.0 to 70.8) for the ≥70 (54 patients, 32 deaths) and < 70 (837 patients, 255 deaths) groups, respectively.
Adjusting for covariates, patients ≥70 years had worse survival compared to patients <70 years, without adjustment (HR:1.53, 95%CI: 1.34–1.76, p < 0.001, Table 2 model #1) or with adjustment (HR:1.46, 95%CI: 1.14–1.88, p = 0.003, Table 2 model #2) for p16 status and smoking history. In all primary sites, the harmful effect of age ≥ 70 appeared worse in p16-positive patients (HR: 2.07, 95%CI: 1.31–3.28 vs. 1.30, 95%CI: 0.97–1.75; interaction p = 0.09; Table 2, model #3). Among patients with oropharyngeal cancers, the results were similar for the effect of age (HR: 1.82, 95%CI: 1.10–3.00 for p16-positive vs. 1.16, 95%CI: 0.74–1.82 for p16-negative; interaction p = 0.18). There were no significant interactions between age and primary tumor site or a differential effect of age by treatment arm in each trial.
Table 2.
Multivariate analysis of overall survival.
| Model#1 (not including pack-years or p16 status; n=2688; 1611 deaths) |
Model#2 (including p16 status and pack-years; n=1043; 556 deaths) |
Model#3 (including interaction between p16 status and age; n=1043; 556 deaths) |
||||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | HR (95%CI) | p-value | |
| Gender (male vs. female) | 1.17(1.03–1.33) | 0.02 | 1.10(0.88–1.37) | 0.39 | 1.12(0.90–1.40) | 0.30 |
| Race (non-white vs. white) | 1.18(1.05–1.33) | 0.007 | 0.92(0.74–1.14) | 0.44 | 0.92(0.74–1.14) | 0.44 |
| Zubrod performance status (1–2 vs. 0) | 1.74(1.57–1.94) | <0.001 | 1.55(1.30–1.84) | <0.001 | 1.54(1.29–1.83) | <0.001 |
| Primary site (non-OP vs. OP) | 1.37(1.24–1.52) | <0.001 | 0.98(0.81–1.19) | 0.83 | 0.99(0.82–1.90) | 0.92 |
| T stage (T4 vs. T1–3) | 1.55(1.39–1.72) | <0.001 | 1.58(1.32–1.90) | <0.001 | 1.58(1.31–1.89) | <0.001 |
| N stage (N2b-3 vs. N0–2a) | 1.36(1.23–1.51) | <0.001 | 1.51(1.26–1.80) | <0.001 | 1.52(1.27–1.81) | <0.001 |
| Smoking history (>10 vs. ≤ 10 pack-years) | - | - | 1.83(1.46–2.30) | <0.001 | 1.81(1.44–2.28) | <0.001 |
| p16 status (negative vs. positive) | - | - | 2.21(1.79–2.72) | <0.001 | - | - |
| p16 status (negative vs. positive) if age<70 | - | - | - | - | 2.34(1.88–2.91) | <0.001 |
| p16 status (negative vs. positive) if age≥70 | - | - | - | - | 1.47(0.88–2.44) | 0.14 |
| Age (≥ 70 vs. <70) | 1.53(1.34–1.76) | <0.001 | 1.46(1.14–1.88) | 0.003 | - | - |
| Age (≥ 70 vs. <70) if p16-positive | - | - | - | - | 2.07(1.31–3.28) | 0.002 |
| Age (≥ 70 vs. <70) if p16-negative | - | - | - | - | 1.30(0.97–1.75) | 0.08 |
| Interaction between p16 status and age | - | - | - | - | - | 0.09 |
Cox models were stratified by trial (9003, 0129, 0522); CI, confidence interval; OP, oropharynx.
In RTOG 0129, with or without adjusting for institutional accrual volume, the effect of age remained significant (HRs = 1.99, 1.88, p values = 0.02, 0.02).
Examination of cause-specific survival is detailed in Table 3. Without adjustment for covariates (including unknown causes), patients ≥70 had worse survival in all trials combined (HR = 1.33, 95%CI: 1.14–1.55, p < 0.001), in RTOG 0129 (HR = 2.52, 95%CI: 1.78–3.57, p < 0.001) and in RTOG 0522 individually (HR = 2.05, 95%CI: 1.34–3.16, p = 0.001), but not in RTOG 9003 (HR = 1.09, 95%CI: 0.90–1.31, p = 0.39). With adjustment for covariates in all three trials, age ≥ 70 was associated with worse survival compared to age < 70 years without (HR = 1.35, 95%CI: 1.15–1.58, p < 0.001) but not with adjustment for p16 and smoking status (HR = 1.26, 95%CI: 0.93–1.70, p = 0.14). The harmful effect of age ≥ 70 appeared to be worse in p16-positive patients (HR: 2.15, 95%CI: 1.26–3.67 for p16-postive vs. 1.05, 95%CI: 0.74–1.50 for p16-negative; interaction p = 0.027). This effect was more evident in RTOG 0129 and 0522 combined (HR: 3.76, 95% CI: 1.85–7.65 for p16-postive vs. HR: 1.68, 95%CI: 0.98–2.89 for p16-negative, interaction p = 0.08). Results for cause-specific survival that did not include unknown causes of death showed similar age effects (Table 3). For example, the harmful effect of age ≥ 70 appeared worse in p16-positive patients (HR: 1.86, 95%CI: 1.01–3.42 for p16-positive vs. 1.05, 95% CI: 0.73–1.52 for p16-negative; interaction p = 0.11). Again, this effect was more evident in 0129 and 0522 combined (HR: 3.33, 95%CI: 1.50–7.39 for p16-positive vs. HR: 1.81, 95%CI: 1.03–3.17 for p16-negative, interaction p = 0.22).
Table 3.
Cause-specific survival.
| Definition #1 HR (95%CI) |
p-value | Definition #2 HR (95%CI) |
p-value | |
|---|---|---|---|---|
| Model 1: not including pack-years or p16 status (n = 2688) | ||||
| Gender (male vs. female) | 1.17(1.02–1.36) | 0.03 | 1.16(1.00–1.36) | 0.05 |
| Race (non-white vs. white) | 1.29(1.13–1.46) | <0.001 | 1.30(1.14–1.49) | <0.001 |
| Zubrod performance status (1–2 vs. 0) | 1.65(1.47–1.85) | <0.001 | 1.59(1.41–1.80 | <0.001 |
| Primary site (non-oropharynx vs. oropharynx) | 1.41(1.26–1.58) | <0.001 | 1.39(1.23–1.57) | <0.001 |
| T stage (T4 vs. T1–3) | 1.69(1.50–1.89) | <0.001 | 1.73(1.53–1.96) | <0.001 |
| N stage (N2b-3 vs. N0–2a) | 1.45(1.29–1.62) | <0.001 | 1.42(1.26–1.61) | <0.001 |
| Age (≥ 70 vs. <70) | 1.35(1.15–1.58) | <0.001 | 1.26(1.06–1.49) | 0.009 |
| Model 2: including pack-years and p16 status (n = 1043) | ||||
| Gender (male vs. female) | 1.06(0.84–1.35) | 0.61 | 1.06(0.82–1.37) | 0.64 |
| Race (non-white vs. white) | 1.01(0.80–1.27) | 0.96 | 1.02(0.80–1.30) | 0.86 |
| Zubrod performance status (1–2 vs. 0) | 1.52(1.25–1.84) | <0.001 | 1.47(1.20–1.81) | <0.001 |
| Primary site (non-oropharynx vs. oropharynx) | 1.05(0.85–1.29) | 0.67 | 1.10(0.88–1.38) | 0.39 |
| T stage (T4 vs. T1–3) | 1.73(1.42–2.11) | <0.001 | 1.81(1.47–2.23) | <0.001 |
| N stage (N2b-3 vs. N0–2a) | 1.51(1.24–1.83) | <0.001 | 1.50(1.22–1.85) | <0.001 |
| Smoking history (>10 vs. ≤ 10 pack- years) | 1.80(1.39–2.32) | <0.001 | 1.81(1.38–2.38) | <0.001 |
| p16 status (negative vs. positive) | 2.43(1.92–3.07) | <0.001 | 2.45(1.90–3.14) | <0.001 |
| Age (≥ 70 vs. <70) | 1.26(0.93–1.70) | 0.14 | 1.19(0.87–1.64) | 0.28 |
| Model 3: including pack-years, p16 status, and interaction between p16 status and age (n = 1043) | ||||
| Gender (male vs. female) | 1.09(0.86–1.39) | 0.47 | 1.08(0.84–1.39) | 0.55 |
| Race (non-white vs. white) | 1.00(0.80–1.27) | 0.97 | 1.02(0.80–1.30) | 0.86 |
| Zubrod performance status (1–2 vs. 0) | 1.51(1.24–1.83) | <0.001 | 1.47(1.20–1.80) | <0.001 |
| Primary site (non-oropharynx vs. oropharynx) | 1.06(0.86–1.31) | 0.58 | 1.11(0.89–1.39) | 0.35 |
| T stage (T4 vs. T1–3) | 1.72(1.41–2.10) | <0.001 | 1.80(1.46–2.22) | <0.001 |
| N stage (N2b-3 vs. N0–2a) | 1.51(1.24–1.85) | <0.001 | 1.51(1.23–1.86) | <0.001 |
| Smoking history (>10 vs. ≤ 10 pack- years) | 1.78(1.37–2.29) | <0.001 | 1.79(1.36–2.35) | <0.001 |
| p16 status (negative vs. positive) if age < 70 | 2.62(2.05–3.36) | <0.001 | 2.59(1.99–3.37) | <0.001 |
| p16 status (negative vs. positive) if age ≥ 70 | 1.28(0.70–2.34) | 0.42 | 1.47(0.75–2.87) | 0.26 |
| Age (≥ 70 vs. <70) if p16-positive | 2.15(1.26–3.67) | 0.005 | 1.86(1.01–3.42) | 0.05 |
| Age (≥ 70 vs. <70) if p16-negative | 1.05(0.74–1.50) | 0.78 | 1.05(0.73–1.52) | 0.78 |
| Interaction between p16 status and age | – | 0.03 | – | 0.11 |
Cox models were stratified by trial (9003, 0129, 0522); HR, hazard ratio; CI, confidence interval.
We also compared local-regional failure (5-year 34.5%, 95%CI: 32.5–36.4% age < 70 vs. 5-year 39.4%, 95%CI: 33.9–44.9% for age ≥ 70, p = 0.69) and distant metastases (5-year 13.4%, 95%CI: 12.1–14.9% vs. 5-year 10.2%, 95%CI: 7.1–13.9%, p = 0.12) between two age groups. These differences were not significant.
Causes of death are outlined in Table 4 for all trials. The distributions of the cause of death were significantly different between the age groups (p < 0.001). Deaths due to underlying cancer (local, regional, or distant) were 54.7% in patients <70 years and 41.9% in patients ≥70 years. Second primaries accounted for 9.5% (<70 years) and 8.1% (≥70 years) of deaths. Death due to other causes and protocol treatment combined, occurred in 20.7% of patients <70 years compared to 37.0% in patients ≥70 years. The majority of other causes of deaths were either cardiac or pulmonary.
Table 4.
Causes of death by age.
| Age < 70 | Age ≥ 70 | Total | |
|---|---|---|---|
| (n = 2379) | (n = 309) | (n = 2688) | |
| Due to this disease (local, regional, or distant) | 734 (54.7%) | 113 (41.9%) | 847 (52.6%) |
| Due to second primary or other malignancy | 128 (9.5%) | 22 (8.1%) | 150 (9.3%) |
| Due to protocol treatment | 17 (1.3%) | 6 (2.2%) | 23 (1.4%) |
| Due to other cause | 261 (19.5%) | 94 (34.8%) | 355 (22.0%) |
| Unknown | 201 (15.0%) | 35 (13.0%) | 236 (14.6%) |
3.1. Radiation Dose Delivery
In RTOG 0129, the median radiation dose for the standard fractionation arm was 69.7 Gy for patients <70 years and 70 Gy for patients ≥70 years; in the accelerated arm, it was 70 Gy for patients <70 years and 63 Gy for patients ≥70 years; however, this difference was not statistically significant. For RTOG 0522 the median radiation dose across the two age groups and treatment arms was 70 Gy. Likewise, the median RT dose was similar between the two age groups in RTOG 9003.
3.2. Chemotherapy Delivery
RTOG 0129 and RTOG 0522 featured systemic therapy. In RTOG 0129, chemotherapy delivery was dictated by protocol. The standard fractionation arm prescribed 3 cycles of cisplatin at a dose of 100 mg/m2 every 21 days. The accelerated arm stipulated 2 doses. The mean cisplatin dose in the standard fractionation arm was 255.6 mg/m2 in patients <70 years and 244.1 mg/m2 in those ≥70 years (p = 0.45). In the accelerated arm, the cisplatin mean dose was 184.6 mg/m2 in patients <70 years and 162 mg/m2 in those ≥70 years (p = 0.31).
RTOG 0522 investigated the role of cetuximab in combination with cisplatin and accelerated radiation. Two doses of cisplatin 100 mg/m2 q 21d were prescribed in both arms. The control arm featuring cisplatin alone yielded a mean dose of 193.2 mg/m2 in patients <70 years and 171.9 mg/m2 in those ≥70 years (p = 0.009). The experimental arm which included cetuximab yielded a mean cisplatin dose of 186.6 mg/m2 in patients <70 years and 173.4 mg/m2 in those ≥70 years (p = 0.9).
3.3. Toxicity
The most common toxicity recorded in the RT alone trial (RTOG 9003) was mucositis. The results were similar across age cohorts and treatment arms. Grade 3–5 mucositis was prevalent; the incidence was 31.8% in patients <70 years and 39.8% in patients >=70 years in the standard fractionation and accelerated fractionation with split (SFX/AFX-S) arms (p = 0.16). In the hyperfractionation and accelerated fractionation with concomitant boost (HFX/AFX-C) arms, patients <70 years experienced a 44.7% incidence of grade 3–5 mucositis whereas those >=70 years had a 45% incidence (p = 1.0). Grade 3–5 ototoxicity was minimal in this trial. None of the acute and late toxicities differed by age groups.
In the combined modality trials (RTOG 0129 and RTOG 0522), both of which featured high dose cisplatin, acute systemic toxicities were observed more frequently in patients ≥70 years. Although two versions of toxicity grading were used as noted, there was no substantial difference between the two studies. Anemia, thrombocytopenia, and nephrotoxicity were the most common toxicities. In RTOG 0129, there were no significant differences in grade 3–5 acute toxicities by age group overall within treatment arms. In RTOG 0522, the rate of grade 3–5 acute hemoglobin decrease was higher in patients ≥70 years (13% vs. 4.8%; p = 0.02). The rate of grade 3–5 acute platelet decrease was higher in patients ≥70 years (7.4% vs. 1.7%; p = 0.02). The rate of grade 1–5 acute serum creatinine increase was higher in ≥70 years group (35.2% vs. 19.6%; p = 0.009).
Feeding tubes were present at baseline for all studies combined in 16.3% of patients <70 years old versus 12.3% in patients ≥70 years (p = 0.07). There was no statistical difference in all studies combined for the presence of feeding tubes at 6, 12, and 24 months (p = 0.18, p = 0.59, p = 0.09, respectively). However, the presence of feeding tubes was consistently higher in patients ≥70 years old in trials that included systemic therapy (RTOG 0129, RTOG 0522). For example, in RTOG 0129 patients >=70 years old had a 54.1% rate of feeding tubes at 6 months, compared to 38.7% in patients <70 years old (p = 0.08). In RTOG 0522, the 6-month rate was 58.8% for patients ≥70 years old vs. 38.0% in patients <70 (p = 0.02). Even though the presence of feeding tubes was consistently higher in patients ≥70 years old in both studies, no statistical significance was found at 24 months (Supplemental Table 1–4).
4. Discussion
Age matters. This analysis strikingly demonstrates age as an independent prognostic variable in LAHNC in 3 separate, major cooperative group trials, either in combined analysis or individually by trial. Age is not merely chronology; it is a compilation of physiologic and functional factors. Classic performance status as a discriminant of functionality is insufficient. The majority of the patients in this study had a PS of 0–1; only 6.8% of those ≥70 years had a PS >1. Thus, chronologic age and classically defined performance status are inadequate guides for treatment decision-making in LAHNC.
Unlike this analysis of three large cooperative group trials, much of the pre-existing data have been based on retrospective evaluations of either single institution experiences or the SEER database. These analyses have no pre-specified eligibility criteria for treatment or consistently prescribed treatment plans.
Studies by Syrigos and Argiris have demonstrated that the concurrent chemotherapy and radiation regimens administered to HNC patients under age 70 can be effectively and safely given to those over 70 years of age [6,10]. Machtay and Pignon noted inferior outcomes for patients over 70 years of age treated with combined modality [11,12]. A recent (2017) retrospective review of 349 patients receiving concurrent chemotherapy and radiation by Strom et al. noted reduced survival in patients ≥70 years. Even though they received less toxic chemotherapy compared to younger patients, the older adult cohort experienced increased rates of hospitalization and increased acute mortality [13]. Our analysis included three distinct, large cooperative group trials. These were prospectively randomized trials with clearly defined entry criteria and treatment plans. Ultimately, the uniformity of eligibility and treatment approaches provided a more objective means to evaluate the effect of age on outcome. These trials spanned a 20-year time-period. The nature of the radiation treatment modalities has evolved as noted below. However, the systemic treatment has remained relatively static. Thus, the changes and outcomes would likely be attributable to underlying cancer biology as well as physiologic differences that occur with age. It is safe to assume a relatively high incidence of HPV-related tumors was present in the oropharynx cancer patients in our analysis and that this incidence was likely higher in more recent studies. Unfortunately, testing regarding p16 status was only available for 48% of patients accrued to these trials. It has been noted by Chung et al. that p16 positive cancers have consistently better outcomes than p16 negative [14]. Yet in our analysis, older adult patients with p16 (+) tumors fared worse than younger patients. The attenuation of the survival advantage of HPV positivity in older adult patients has also been noted by Rettig et al. [9]
Factors often cited for lower survival of HNC patients ≥70 years of age include comorbidities, increased toxicity, and inadequate treatment delivery compounded by an inherent age bias by health care providers [10,15,16]. The relatively small number of patients ≥70 years in our trials reflect that bias. Yet outcomes with respect to treatment delivery and toxicity showed little difference by age. Treatment delivery in both age cohorts was comparable across trials. Toxicities occurred as expected, with slightly more thrombocytopenia, anemia, and nephrotoxicity in those patients 70 years of age and older who received concurrent chemotherapy with radiation [11]. Although different toxicity criteria were used in each trial, results from trial-specific analysis with sufficient sample sizes show similar acute and late toxicities between two age groups. This strongly suggests a major role for co-morbidity and other factors in the inferior outcome of older patients, divorced from the original cancer diagnosis and its therapy. Alternatively, intrinsic cancer biology may be more aggressive in older patients.
The incidence of feeding tubes long-term appeared higher in the older age group. This has been previously noted by Strom et al. in older adult patients receiving combined modality therapy [13]. This may speak to increased risk for aspiration, poor underlying nutritional status, weakened immune system, and higher risk of frailty. However, this relationship cannot really be evaluated further at this point in the context of these long-completed trials.
The factors elucidated in comprehensive geriatric assessment (CGA), which was not employed in these trials, might identify older adults at greater risk for cancer treatment toxicities [17–21].
The comprehensive geriatric assessment (CGA) covers multiple domains (medical, psychologic, functional) and is traditionally performed by a multidisciplinary team that includes a geriatrician, nurse, and social worker [22,23]. It may not be practical for use in most cancer treatment trials. However, a briefer form, the GA, could be tested to learn if it can more effectively evaluate an older adult patient’s fitness for combined modality treatment and/or a clinical trial enrollment [23,24]. A recent publication from virtual ASCO2020 demonstrated the positive effect on outcomes when a geriatric assessment precedes treatment [25,26]. Similarly, in our analysis of these three (3) RTOG trials, more toxicities were observed in the combined modality trials as expected. Two separate predictive geriatric models for chemotherapy toxicity could be evaluated [27,28] in future trials to test their value in assessing the risks of combined modality treatment in HNC and potentially inform appropriate dose adjustments [16].
There are several limitations to this study in applying the findings to modern patients age ≥ 70. These trials spanned over a period of 20 years, during which the radiation treatment techniques have evolved from 3-dimensional conformal radiotherapy (3DCRT) in RTOG 9003 and RTOG 0129 to intensity-modulated radiotherapy (IMRT) in RTOG 0522. Since then, IMRT treatment plans continue to be refined with better software and reduced margins, leading to more protection of normal tissues. In addition, the accelerated fractionation regimens that were employed in several arms of these trials are not often used in the clinic, especially when given concurrently with chemotherapy. Most importantly, the percentage of older patients with HPV(+) oropharyngeal carcinoma is much larger now than when these trials were actively enrolling patients. These drawbacks make it difficult to extrapolate our findings to current older adult patients, raising the need for targeted modern trials in this patient population.
Ultimately, despite better assessments and predictive models, a clearer understanding of the role of chronologic age in head and neck cancer therapy will require clinical trials enriched for the older adult population to provide an adequate evidence base. To date, older adults have been consistently under-represented in prospective clinical trials that are agnostic to age. Trials specific to older individuals are sparse, particularly in HNC [27]. Although patients ≥65 years constitute 63% of all patients with cancer in the USA, only 25% of those enrolled in major cooperative group trials are 65 years of age or older. When 70 years of age is used as the cut-off, only 13% of those enrolled in oncologic trials are in this age group, although this age cohort constitutes 47% of the population at large with cancer [28]. This will present a particular clinical challenge as the number and age of patients with head and neck cancer in general and oropharyngeal cancer in particular increases over the coming years [4]. To address this issue, NRG Oncology has launched a randomized phase II-III trial (NCT03258554), in which LAHNC patients 70 years of age or older, as well as those deemed cisplatin-ineligible based on co-morbidities or other vulnerabilities, are randomized to either radiation plus cetuximab or radiation plus durvalumab, a checkpoint inhibitor that has shown activity in HNC [29,30]. Although this trial is open to all age groups, the elimination of cisplatin in the treatment regimen will likely facilitate the enrollment of older adult patients.
The Institute of Medicine (IOM) report, “Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis,” has highlighted the need to improve the evidence base in older adults with cancer. ASCO has established specific recommendations to address this problem [27]. Most notable are the recommendations to conduct clinical trials that are older adult-specific as well as strategies to increase the accrual of older adult cancer patients to existing trials to define the evidence base.
Our analysis clearly supports the need for older adult-specific prospective trials to establish the evidence base for optimal head and neck cancer treatment of older adult individuals.
Supplementary Material
Funding/Support
This project was supported by grants U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), UG1CA189867 (NRG Oncology NCORP), from the National Cancer Institute (NCI) and Eli Lilly & Co. This project was funded, in part, under a grant 4100062200 with the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Role of Funding
The National Cancer Institute (NCI), the Pennsylvania Department of Health, and Eli Lilly & Co. had no role in the study design; in the collection, analysis and interpretation of data in the writing of the report; and in the decision to submit the article for publication.
Footnotes
The effect of age on outcome in prospective, phase III NRG Oncology/RTOG trials of radiotherapy (XRT) +/− chemotherapy in locally advanced (LA) head and neck cancer (HNC). Julie Ann Kish, Qiang Zhang, Corey J. Langer, Felix Nguyen-Tan, David Ira Rosenthal, Randal S. Weber, Marcy A List, Stuart J. Wong, Adam S. Garden, Jay Scott Cooper, Andy Trotti, James A. Bonner, Christopher U Jones, Sue S Yom, Jeff M. Michalski, Christopher J. Schultz, John A Ridge, George Shenouda, and Quynh-Thu Le Journal of Clinical Oncology 2015 33:15_suppl, 6003–6003
Conflicts of Interest
Drs. Garden, Hu, Jones, Kish, List, Ridge, Rosenthal, Shenouda, Trotti, Weber, Wong, & Zhang, and Mr. Harris have nothing to disclose. Dr. Bonner reports personal fees from Bristol-Myers Squibb Company, personal fees from Eli Lilly and Company, personal fees from Merck Serono, personal fees from Cel-Sci, outside the submitted work. Dr. Langer reports advisory board & consulting personal fees from BMS, AstraZeneca, Eli Lilly, Merck, Roche/Genentech, Gilead, & Takeda, institutional grant support for ongoing research from Merck, Takeda, Advantagene, Inovio, & Trizell, and a role on the Data Safety Monitoring Boards with South-west Oncology Group, Amgen, & Eli Lilly, outside the submitted work. Dr. Le reports travel expenses from Merck, a preclinical research grant from Redhill, personal fees and a consultant role with GRAIL, clinical trial planning grant from Pharmapler, and travel expenses from BMS, Varian, & Genentech, outside the submitted work. Dr. Ngyen-Tan reports an advisory board role with Bristol Myers Squibb, outside the submitted work. Dr. Schultz reports institutional research support and travel expenses from Elekta AB, institutional research support and an advisory board role with Manteia Imaging Technologies, and institutional research support from Siemens Heathneers, & Accuray, outsidethe submitted work. Dr. Thorstad reports that an immediate family member works for Elekta Inc. Dr. Yom reports clinical trial grant support from Genentech, Bristol-Myers Squibb, Merck, & BioMimetix, book roaylties from Springer, and chapter royalties from UpToDate, outside the submitted work.
Appendix A. Supplementary Data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgo.2021.03.011.
References
- 1.Moye VA, et al. Elderly patients with squamous cell carcinoma of the head and neck and the benefit of multimodality therapy. Oncologist 2015;20(2):159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tota JE, et al. Evolution of the oropharynx cancer epidemic in the United States: moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J Clin Oncol 2019;37(18):1538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumsteg ZS, et al. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol 2016;2(12):1617–23. [DOI] [PubMed] [Google Scholar]
- 4.Adelstein DJ, et al. Role of treatment deintensification in the management of p16+ oropharyngeal cancer: ASCO provisional clinical opinion. J Clin Oncol 2019;37 (18):1578–89. [DOI] [PubMed] [Google Scholar]
- 5.Vespa J, Medina L, Armstrong D. Demographic turning points for the United States: Population projections for 2020–2060. Current population reports, P25–1144. 2020 Washington, DC: U.S. Census Bureau; 2020. [Google Scholar]
- 6.Bhattacharyya N A matched survival analysis for squamous cell carcinoma of the head and neck in the elderly. Laryngoscope 2003;113(2):368–72. [DOI] [PubMed] [Google Scholar]
- 7.Syrigos KN, et al. Head and neck cancer in the elderly: an overview on the treatment modalities. Cancer Treat Rev 2009;35(3):237–45. [DOI] [PubMed] [Google Scholar]
- 8.van der Schroeff MP, et al. The effect of age on survival and quality of life in elderly head and neck cancer patients: a long-term prospective study. Eur Arch Otorhinolaryngol 2007;264(4):415–22. [DOI] [PubMed] [Google Scholar]
- 9.Rettig EM, et al. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of human papillomavirus is attenuated among older patients: analysis of the national cancer database. Oral Oncol 2018;83:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Argiris A, et al. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin-based chemotherapy. J Clin Oncol 2004;22(2): 262–8. [DOI] [PubMed] [Google Scholar]
- 11.Machtay M, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 2008;26(21):3582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pignon JP, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92(1):4–14. [DOI] [PubMed] [Google Scholar]
- 13.Strom TJ, et al. Increased acute mortality with chemoradiotherapy for locally advanced head and neck cancer in patients >/=70years. J Geriatr Oncol 2017;8(1): 50–5. [DOI] [PubMed] [Google Scholar]
- 14.Chung CH, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol 2014;32(35):3930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardi D, et al. Treatment of head and neck cancer in elderly patients: state of the art and guidelines. Crit Rev Oncol Hematol 2005;53(1):71–80. [DOI] [PubMed] [Google Scholar]
- 16.Hurria A, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 2014;32(24):2587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurria A, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29(25):3457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Extermann M, et al. Predicting the risk of chemotherapy toxicity in older patients: the chemotherapy risk assessment scale for high-age patients (CRASH) score. Cancer 2012;118(13):3377–86. [DOI] [PubMed] [Google Scholar]
- 19.Wildes TM, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J Geriatr Oncol 2013;4(3): 227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramjaun A, et al. Improved targeting of cancer care for older patients: a systematic review of the utility of comprehensive geriatric assessment. J Geriatr Oncol 2013;4 (3):271–81. [DOI] [PubMed] [Google Scholar]
- 21.Extermann M, Wedding U. Comorbidity and geriatric assessment for older patients with hematologic malignancies: a review of the evidence. J Geriatr Oncol 2012;3 (1):49–57. [Google Scholar]
- 22.Cohen HJ, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med 2002;346(12):905–12. [DOI] [PubMed] [Google Scholar]
- 23.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25(14):1824–31. [DOI] [PubMed] [Google Scholar]
- 24.Hurria A, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol 2011;29(10):1290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleckner A, et al. Communication about comorbidities among 527 older patients with advanced cancer and their oncologists and caregivers: a multisite cluster-randomized controlled trial. J Clin Oncol 2020;38(15_suppl):12040. [Google Scholar]
- 26.Seedor RS, et al. Prospective study comparing self-administered geriatric assessment to provider’s routine clinical assessment of older patients with metastatic breast cancer treated at community oncology practices. J Clin Oncol 2020;38(15_suppl): 12029. [Google Scholar]
- 27.Hurria A, et al. Improving the evidence base for treating older adults with cancer: American society of clinical oncology statement. J Clin Oncol 2015;33(32):3826–33. [DOI] [PubMed] [Google Scholar]
- 28.Hutchins LF, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341(27):2061–7. [DOI] [PubMed] [Google Scholar]
- 29.Segal NH, et al. Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. J Clin Oncol 2015;33(15_suppl):3011. [Google Scholar]
- 30.Seiwert TY, et al. A phase 3, randomized, open-label study of first-line durvalumab (MEDI4736) ± tremelimumab versus standard of care (SoC; EXTREME regimen) in recurrent/metastatic (R/M) SCCHN: KESTREL. J Clin Oncol 2016;34(15_suppl): TPS6101. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


