Abstract
The development of acid-resistant and efficient corrosion inhibitors is of great significance for metal protection in many industrial processes. In this work, eight cases of sandwich-type polyoxometalate (POM)-based inorganic–organic hybrids, namely, carboxyethyltin and transition metal (TM) cofunctionalized tungstoantimonates and tungstobismuthates, formulated as NaxK10–x[(SnR)2(TM(H2O)3)2(B-β-SbW9O33)2]·mH2O and NayK10–y[(SnR)2(TM(H2O)3)2(B-β-BiW9O33)2]·nH2O (abbreviated as SbW9-TM-SnR and BiW9-TM-SnR; TM = Mn, Co, Ni, and Zn; m = 18, 24, 24, and 22; n = 30, 25, 20, and 21; SnR = Sn(CH2CH2COO)) are first used as green corrosion inhibitors for 20# carbon steel in 0.5–2.0 M HCl solutions. Weight loss and electrochemical experiments prove that the corrosion inhibition efficiency is all above 81% for these POM-based corrosion inhibitors at 150 mg L–1, and SbW9-Mn-SnR shows the highest efficiency of 96.9% at 150 mg L–1 after immersion in a 0.5 M HCl solution for 10 h. Scanning electron microscopy–energy-dispersive X-ray spectroscopy and X-ray photoelectron spectroscopy analyses show that these POM-based inhibitors form films on the carbon steel and the adsorption mechanism obeys the Langmuir adsorption model. The thermodynamic activation parameters were calculated, proving the occurrence of both chemical and physical adsorptions. The film-forming mechanism was also analyzed. This work provides guidance for synthesizing new lacunary POM-based materials to protect metals from corrosion in HCl pickling.
Introduction
Metal anticorrosion is of great significance in fields of industrial production and environmental protection because oxygen or acid corrosion of metals is a spontaneous chemical reaction, which will increase energy consumption of equipment and cause leakage of oil or gas from pipelines in industry. Several strategies have been used to protect metals against corrosion, such as photoinduced cathodic protection and adding corrosion inhibitors.1 Adsorptive corrosion inhibitors have attracted intensive attention because of their excellent performance and operational ease. In addition, it is well-known that the fastest and most effective way to remove rust and scale from equipment is acid pickling, and hydrochloric acid is one of the most commonly used pickling solution. However, HCl solution is highly corrosive to metallic equipment, so a suitable corrosion inhibitor needs to be added in a chemical acidic cleaning process of equipment.2,3 In the early stage, some simple oxysalts were used for metal corrosion inhibition, but they are mostly used in neutral media rather than acidic solution.4,5 For organic corrosion inhibitors, they have more obvious advantages in acidic media.6 For instance, ionic liquids (ILs) have shown their outstanding performance in corrosion inhibition of steels in the acidic environment owing to many attractive characteristics such as high stability, high solubility, and low environmental hazards, although they suffer from the high production cost sometimes.7,8 Moreover, plant extract corrosion inhibitors have been widely studied as green corrosion inhibitors owing to their high inhibition efficiency, chemical stability, and environmental friendliness. However, their application is limited by the difficulty of separation.9−11 In recent years, intelligent corrosion inhibitors with pH responsivity, self-assembly, and self-healing properties have attracted more and more attention of scientists.12−16 Despite the above achievements, the development of new sustainable, environmentally friendly, and highly efficient corrosion inhibitors in acidic media is still an important topic in metal protection.
POMs, as a kind of well-defined and nano-sized anionic metal-oxygen cluster possessing low toxicity, high electron affinity, and structural stability, have been widely applied in energy-related fields.17,18 Such excellent properties also make them attractive as oxidizing and film-forming corrosion inhibitors.19 In 1994, Lomakina et al. used several heteropolytungstates as corrosion inhibitors of aluminum and alloys in high-temperature water.20 Liang et al. found that Na3PW12O40 and H4PW11VO40 could effectively retard the corrosion of carbon steel in 55% LiBr solution.21,22 Hamdani et al. reported that hexa-ammonium heptamolybdate tetrahydrate could act as an anode corrosion inhibitor for 304 stainless steel in 0.5 M HCl solution, and the inhibition efficiency could reach above 90%.23 In addition, research on POM-based composites as metal corrosion inhibitors has been increasingly conducted. Cases Iborra et al. reported that polypyrrole/PW12O403– coatings protect carbon steel electrodes against corrosion in chloride aqueous solutions.24 According to the work reported by Rao et al., the ferrocene POM hybrid molecular materials show significant corrosion inhibition performance when coated on stainless steel plates (SS, 316 grade) in 0.5 M H2SO4 and Ringer’s solutions.19 The hydrophobic POM-ILs composed of organic bulk cations and inorganic anions through weak interaction are also excellent corrosion-protection coating of metal surfaces in acetic acid or H2SO4 solutions.25,26 They can also protect typical building stones from corrosion (weathering) and biofilm formation (biodeterioration).27 Although many POMs or POM-based composite materials as metal corrosion inhibitors have been studied, relatively few studies have been conducted on POM inhibitors for carbon steel in HCl pickling, especially for lacunary POMs.
As everyone knows, the introduction of organic or organometallic groups can enhance POM’s functions and stabilize the structures. Organotin, including alkyltin and estertin/carboxyltin, possesses some unique properties and is the most widely used organometallic compounds. In 1989, Mourad et al. found that dimethyltin dichloride could form an adsorption film on an aluminum surface in a HCl medium, exhibiting effective corrosion inhibition.28 In view of low toxicity, high stability, functionality of estertin/carboxyltin, and the versatility of POMs, the first single crystal of carboxyethyltin-modified POM was obtained in 2010.29 After that, sandwich-type tungstoarsenate and tungstogermanate containing carboxyethyltin groups were synthesized and studied as corrosion inhibitors on carbon steel in circulating cooling water systems.30,31 In order to further investigate the corrosion inhibition behavior of this series of sandwich-type POMs to carbon steel in acidic solutions, in this work, two new sandwich-type tungstobismuthates Na5K5[(Sn(CH2CH2COO))2(Ni(H2O)3)2(B-β-BiW9O33)2]·20H2O (BiW9-Ni-SnR) and Na5K5[(Sn(CH2CH2COO))2(Zn(H2O)3)2(B-β-BiW9O33)2]·21H2O (BiW9-Zn-SnR) were newly synthesized in aqueous media. The corrosion inhibition activity of the two new compounds and other six sandwich-type POMs (SbW9-TM-SnR, TM = Mn, Co, Ni, and Zn; BiW9-TM-SnR, TM = Mn and Co),32,33 was all evaluated by weight loss, potentiodynamic polarization testing, electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM), and energy-dispersive X-ray spectrometry (EDX). Moreover, the corrosion inhibition mechanism was further studied.
Results and Discussion
Structural Analysis
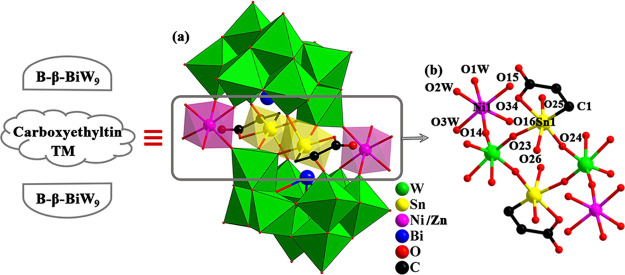
Single-crystal X-ray structural analysis indicates that the polyoxoanions of BiW9-Ni-SnR and BiW9-Zn-SnR are isomorphic, and the configurations of the two POM anion skeletons are the same as those of BiW9-Mn/Co-SnR. That is, they also consist of two trivacant Keggin [B-β-BiW9O33]9– (B-β-BiW9) subunits, sandwiching two Ni2+/Zn2+ ions and two SnR groups, displaying the well-known Keggin sandwich-type structural features (Figure 1a). The detailed crystallographic data, data collection, and structural refinement parameters of BiW9-Ni/Zn-SnR are summarized in the supporting materials (Table S1). In the synthesis of the two POMs, it is also found that some similar phenomena occur, i.e., the configuration of the starting material B-α-BiW9 changes to B-β-BiW9, and the raw material estertin [SnCH2CH2COOCH3]3+ hydrolyzes to carboxyethyltin [SnCH2CH2COO]2+ (Scheme S1), but its original five-membered ring is not opened. As shown in Figure 1b, in the central belt of the sandwich-type POM anion, each Ni/Zn center is six-coordinated with three terminal O atoms (O14, O15, and O16) from three WO6 octahedra and three other O atoms (O1W, O2W, and O3W) of three H2O molecules. Each Sn atom also displays a six-coordinated configuration with four terminal O atoms (O23, O24, O25, and O26) from four WO6 octahedra and one C atom (C1) and one O atom (O34) from a [SnCH2CH2COO]2+ group. In the two POMs, the adjacent polyoxoanions are connected by electrostatic interaction with counterions Na+ and K+ and hydrogen bondings with free H2O molecules to form 3-D network structures (Figure S1). The selected bond lengths and angles of the two POMs are listed in Tables S2 and S3.
Figure 1.
Polyhedral and ball-and-stick representation of the BiW9-Ni/Zn-SnR polyoxoanion (a) and ball-and-stick diagram corresponding to the central belt (b) (H atoms, K+, Na+, and free H2O molecules have been omitted for clarity).
The structure and thermal stability of BiW9-Ni-SnR and BiW9-Zn-SnR were further characterized by IR, powder X-ray diffraction patterns (PXRD), and thermogravimetric (TG) analysis (Figures S2–S4). The detailed analysis can be found in the supplementary materials. These analytical results are consistent with the results of single-crystal structure analysis, and TG results show that the main POM skeletons of the two new compounds still exist at about 750 °C.
Gravimetric Evaluation of Metal Corrosion
The weight loss method was used to evaluate the corrosion rate and inhibition efficiency (IEw) of 20# carbon steel in 0.5–2.0 M HCl solutions at room temperature without and with different POM-based corrosion inhibitors. The corrosion rate was calculated according to eq 1:34
| 1 |
where CR (mg cm–2 h–1) is the corrosion rate; Δm (mg) and Δt (h) are the average weight loss of 20# carbon steel immersed in HCl solutions by multiple tests and the immersion time, respectively; s (12.6 cm2) is the area of the 20# carbon steel sample; IEw was obtained using eq 2:34
| 2 |
Wcor and Wcoro are the weight losses with and without the POM-based inhibitor, respectively.
Taking SbW9-Mn-SnR for example, the optimal concentration of the POM-based corrosion inhibitor for 20# carbon steel in 0.5 M HCl solution for 6 h at room temperature was investigated (Table 1). As shown in Table 1, with increasing the inhibitor concentration (from 25 to 300 mg L–1), the value of IEw gradually increases (from 80.2 to 90.3%). However, at higher concentrations, i.e., the concentration of SbW9-Mn-SnR is from 150 to 300 mg L–1, the value of IEw only increases by 0.4% (from 89.9 to 90.3%).
Table 1. Corrosion Parameters Obtained from the Weight Loss Measurements for 20# Carbon Steel Immersed in 0.5 M HCl Solution with Different Concentrations of SbW9-Mn-SnR for 6 h at Room Temperature (298 K).
| concentration (mg L–1) | Δm (g)a | CR (mg cm–2 h–1) | IEw (%) |
|---|---|---|---|
| 0.0474 | 0.627 | ||
| 25 | 0.0094 | 0.124 | 80.2 |
| 75 | 0.0062 | 0.082 | 86.9 |
| 150 | 0.0048 | 0.063 | 89.9 |
| 300 | 0.0046 | 0.044 | 90.3 |
Note: Δm is the average weight loss by multiple tests.
In order to further investigate the effects of concentration and immersion time on the inhibitory behaviors of inhibitors, the inhibition performances of various inhibitors including SbW9-TM-SnR, BiW9-TM-SnR (TM = Mn, Co, Ni, and Zn), and their parent compounds (Na-SbW9 and Na-BiW9) at higher concentrations (300 and 500 mg L–1) toward the corrosion of 20# carbon steel immersed in 0.5 M HCl solution for 6 and 10 h at room temperature were evaluated (Table 2 and Table S4). As seen from Table 2, for SbW9-TM-SnR and BiW9-TM-SnR, when the inhibitor concentration increases from 300 to 500 mg L–1 after immersion in 0.5 M HCl solution for 6 h, there are no significant increases on the values of IEw for all inhibitors, which may be attributed to the saturation adsorption of inhibitors on the surface of 20# carbon steel. When the immersion time extends from 6 to 10 h, the IEw values of SbW9-TM-SnR (TM = Mn, Co, Ni, and Zn) at 300 mg L–1 increase from 92.9, 91.5, 92.3, and 90.4% to 93.2, 93.1, 93.8, and 94.0% and the IEw values of BiW9-TM-SnR (TM = Mn, Co, Ni, and Zn) increase from 84.3, 82.2, 86.0, and 85.0% to 87.8, 87.4, 86.7, and 87.5%, respectively. Meanwhile, the CR values of carbon steel all decrease for the eight inhibitors, indicating that the adsorption films of inhibitors are stable. For Na-SbW9 and Na-BiW9, the IEw values are far less than those of SbW9-TM-SnR and BiW9-TM-SnR (Table S4). Based on the above experimental results and energy consumption reduction consideration, the latter experiments were performed at 298 K, an immersion time of 6 h, and an inhibitor concentration of 150 mg L–1. Under the optimal conditions, the corrosion inhibition effect of different POM-based inhibitors on 20# carbon steel immersed in 0.5–2.0 M HCl solutions was evaluated and is summarized in Table 3.
Table 2. Corrosion Parameters Obtained from Weight Loss Measurement for 20# Carbon Steel Immersed in 0.5 M HCl Solution Containing Various Inhibitors at Different Concentrations for 6 and 10 h at Room Temperature (301 K).
| inhibitor | concentration (mg L–1) | time (h) | Δm (g)a | CR (mg cm–2 h–1) | IEw (%) |
|---|---|---|---|---|---|
| blank | 6 | 0.0520 | 0.688 | ||
| 10 | 0.0912 | 0.724 | |||
| SbW9-Mn-SnR | 300 | 6 | 0.0037 | 0.049 | 92.9 |
| 10 | 0.0062 | 0.049 | 93.2 | ||
| 500 | 6 | 0.0042 | 0.056 | 91.9 | |
| SbW9-Co-SnR | 300 | 6 | 0.0044 | 0.058 | 91.5 |
| 10 | 0.0063 | 0.050 | 93.1 | ||
| 500 | 6 | 0.0046 | 0.061 | 91.2 | |
| SbW9-Ni-SnR | 300 | 6 | 0.0040 | 0.053 | 92.3 |
| 10 | 0.0057 | 0.045 | 93.8 | ||
| 500 | 6 | 0.0038 | 0.050 | 92.7 | |
| SbW9-Zn-SnR | 300 | 6 | 0.0050 | 0.066 | 90.4 |
| 10 | 0.0055 | 0.044 | 94.0 | ||
| 500 | 6 | 0.0047 | 0.062 | 90.7 | |
| BiW9-Mn-SnR | 300 | 6 | 0.0082 | 0.108 | 84.3 |
| 10 | 0.0102 | 0.081 | 87.8 | ||
| 500 | 6 | 0.0080 | 0.106 | 85.9 | |
| BiW9-Co-SnR | 300 | 6 | 0.0093 | 0.123 | 82.2 |
| 10 | 0.0106 | 0.084 | 87.4 | ||
| 500 | 6 | 0.0077 | 0.102 | 86.4 | |
| BiW9-Ni-SnR | 300 | 6 | 0.0073 | 0.097 | 86.0 |
| 10 | 0.0111 | 0.088 | 86.7 | ||
| 500 | 6 | 0.0082 | 0.108 | 85.6 | |
| BiW9-Zn-SnR | 300 | 6 | 0.0078 | 0.103 | 85.0 |
| 10 | 0.0104 | 0.083 | 87.5 | ||
| 500 | 6 | 0.0068 | 0.090 | 88.0 |
Note: Δm is the average weight loss by multiple tests.
Table 3. Corrosion Parameters Obtained from the Weight Loss Measurement for 20# Carbon Steel Immersed in 0.5, 1.0, and 2.0 M HCl Solutions Containing 150 mg L–1 Different Inhibitors for 6 h at 298 K.
| inhibitor | HCl solution concentration (M) | Δm (g)a | CR (mg cm–2 h–1) | IEw (%) |
|---|---|---|---|---|
| blank | 0.5 | 0.0473 | 0.626 | |
| 1.0 | 0.0615 | 0.813 | ||
| 2.0 | 0.0829 | 1.097 | ||
| SbW9-Mn-SnR | 0.5 | 0.0048 | 0.063 | 89.9 |
| 1.0 | 0.0039 | 0.052 | 93.6 | |
| 2.0 | 0.0109 | 0.144 | 86.8 | |
| SbW9-Co-SnR | 0.5 | 0.0048 | 0.063 | 89.8 |
| 1.0 | 0.0045 | 0.060 | 92.7 | |
| 2.0 | 0.0121 | 0.160 | 85.4 | |
| SbW9-Ni-SnR | 0.5 | 0.0046 | 0.061 | 90.3 |
| 1.0 | 0.0056 | 0.074 | 90.9 | |
| 2.0 | 0.0115 | 0.152 | 86.1 | |
| SbW9-Zn-SnR | 0.5 | 0.0049 | 0.065 | 89.6 |
| 1.0 | 0.0053 | 0.070 | 91.4 | |
| 2.0 | 0.0106 | 0.140 | 87.2 | |
| BiW9-Mn-SnR | 0.5 | 0.0084 | 0.111 | 82.2 |
| 1.0 | 0.0061 | 0.081 | 90.1 | |
| 2.0 | 0.0112 | 0.148 | 86.5 | |
| BiW9-Co-SnR | 0.5 | 0.0086 | 0.114 | 81.8 |
| 1.0 | 0.0061 | 0.081 | 90.1 | |
| 2.0 | 0.0118 | 0.156 | 85.8 | |
| BiW9-Ni-SnR | 0.5 | 0.0074 | 0.098 | 84.4 |
| 1.0 | 0.0054 | 0.071 | 91.2 | |
| 2.0 | 0.0120 | 0.159 | 85.5 | |
| BiW9-Zn-SnR | 0.5 | 0.0073 | 0.097 | 84.6 |
| 1.0 | 0.0052 | 0.069 | 91.5 | |
| 2.0 | 0.0111 | 0.147 | 86.6 | |
| Na-SbW9 | 0.5 | 0.0419 | 0.554 | 11.4 |
| 1.0 | 0.0565 | 0.747 | 8.1 | |
| 2.0 | 0.0609 | 0.805 | 26.5 | |
| Na-BiW9 | 0.5 | 0.0430 | 0.569 | 9.1 |
| 1.0 | 0.0509 | 0.067 | 17.2 | |
| 2.0 | 0.0545 | 0.720 | 34.3 | |
| Cl3Sn(CH2)2COOCH3 | 0.5 | 0.0060 | 0.079 | 87.3 |
| 1.0 | 0.0055 | 0.073 | 91.1 | |
| 2.0 | 0.0127 | 0.168 | 84.7 |
Note: Δm is the average weight loss by multiple tests.
As shown in Table 3, in 0.5–2.0 M HCl solutions, the IEw values of these sandwich-type POM-based inhibitors containing the same SnR and different TMs are similar and all higher than 81%, which is obviously higher than those of parents Na-SbW9 (11.4–26.5%) and Na-BiW9 (9.1–34.3%). The above results show that these POM-based inhibitors have good corrosion inhibition performance for carbon steel in acidic solutions, and the type of TM component has little effect on the corrosion inhibition property. It is noted that the IEw values of SbW9-TM-SnR and BiW9-TM-SnR are slightly reduced in 2.0 M HCl compared to those in 1.0 M HCl, which is presumed to be because (i) a high concentration of Cl– penetrates the anticorrosive film and causes repitting corrosion and (ii) the corrosion film dissolves or decomposes.35 In addition, the corrosion inhibition performance of SbW9-TM-SnR is slightly better than that of BiW9-TM-SnR. It can also be seen from Table 3 that Cl3Sn(CH2)2COOCH3 has a good corrosion inhibition effect with IEw values of 87.3, 91.1, and 84.7% in 0.5, 1.0, and 2.0 M HCl solutions, respectively, which shows that the organotin component plays an important role in the corrosion protection of carbon steel although its content in the POM system is low. However, when the organotin is used as a corrosion inhibitor alone, compared with the POM-estertin derivatives, the film-forming speed for Cl3Sn(CH2)2COOCH3 is faster, while its formed film is not dense and easy to rub off. Therefore, the combination of organic and inorganic components is obviously beneficial to improving the performance of POM-based inhibitors.
Potentiodynamic Polarization Measurement
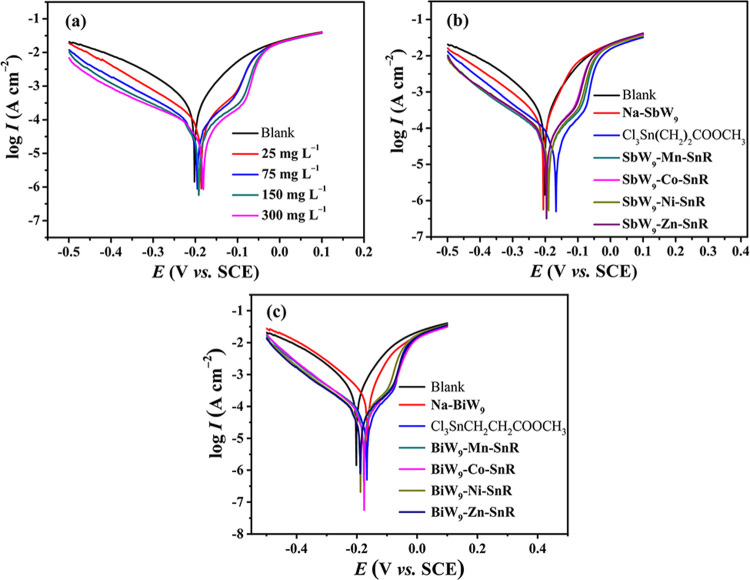
Potentiodynamic polarization curves for 20# carbon steel in 0.5 M HCl in the presence of different concentrations of SbW9-Mn-SnR and different inhibitors Na-SbW9/Na-BiW9, Cl3Sn(CH2)2COOCH3, and SbW9-TM-SnR/BiW9-TM-SnR (TM = Mn, Co, Ni, and Zn) with the same concentration (150 mg L–1) at 298 K are shown in Figure 2a–c, respectively. Generally, the corrosion on the surface of metals occurs through two paths, namely, the anodic reaction and the cathodic reaction, in which the anodic reaction involves the oxidation of iron atoms and the cathodic reaction involves the reduction of H+. The addition of corrosion inhibitors can usually inhibit the anodic or cathodic or both reactions by forming a protective film. From the potentiodynamic polarization plots, the corrosion current density (Icorr) and the corrosion potential (Ecorr) could be obtained by finding out the intersection of two tangents sketched from the cathodic and anodic curves. In addition, the anodic Tafel slope (βa) and the cathodic Tafel slope (βc) could be obtained. The corrosion inhibition efficiency (IEi) was calculated from eq 3:36
| 3 |
where Icorr and Icorro are the corrosion current densities with and without the POM-based inhibitors, respectively. Consequently, the corrosion parameters including Icorr, Ecorr, βa, βc, and IEi were calculated and are summarized in Table 4.
Figure 2.
Potentiodynamic polarization curves for 20# carbon steel in 0.5 M HCl solution containing different concentrations (25, 75, 150, and 300 mg L–1) of SbW9-Mn-SnR (a) and 150 mg L–1Na-SbW9/Na-BiW9, Cl3Sn(CH2)2COOCH3, and SbW9-TM-SnR/BiW9-TM-SnR (TM = Mn, Co, Ni, and Zn) corrosion inhibitor (b,c) at 298 K (0.5 M HCl solution was used as the blank).
Table 4. Corrosion Parameters Derived from Potentiodynamic Polarization Curves of 20# Carbon Steel in 0.5 M HCl Solution in the Absence (Blank) and in the Presence of Different Inhibitors at 298 K.
| inhibitor | inhibitor concentration (mg L–1) | βa (mV dec–1) | βc (mV dec–1) | Ecorr (V vs SCE) | Icorr (μA cm–2) | IEi (%) |
|---|---|---|---|---|---|---|
| blank | 109.6 | –139.2 | –0.208 | 429.5 | ||
| SbW9-Mn-SnR | 25 | 51.8 | –132.9 | –0.186 | 79.4 | 81.5 |
| 75 | 47.8 | –149.1 | –0.195 | 47.9 | 88.8 | |
| 150 | 43.0 | –165.6 | –0.192 | 35.7 | 91.7 | |
| 300 | 39.2 | –188.7 | –0.181 | 28.1 | 93.5 | |
| SbW9-Co-SnR | 150 | 45.6 | –168.3 | –0.196 | 45.3 | 89.5 |
| SbW9-Ni-SnR | 150 | 43.7 | –165.4 | –0.190 | 39.9 | 90.7 |
| SbW9-Zn-SnR | 150 | 44.0 | –168.7 | –0.196 | 47.3 | 89.0 |
| BiW9-Mn-SnR | 150 | 52.1 | –154.8 | –0.177 | 47.3 | 88.9 |
| BiW9-Co-SnR | 150 | 49.5 | –143.2 | –0.176 | 46.3 | 89.2 |
| BiW9-Ni-SnR | 150 | 46.5 | –150.8 | –0.188 | 40.3 | 90.6 |
| BiW9-Zn-SnR | 150 | 49.4 | –156.5 | –0.189 | 44.5 | 89.6 |
| Na-SbW9 | 150 | 106.0 | –146.0 | –0.206 | 273.0 | 36.4 |
| Na-BiW9 | 150 | 126.4 | –132.7 | –0.164 | 432.5 | |
| Cl3Sn(CH2)2COOCH3 | 150 | 45.9 | –146.4 | –0.146 | 53.2 | 87.6 |
Figure 2a shows that both the anodic branch and the cathodic branch move to lower current densities as the concentration of SbW9-Mn-SnR increases, indicating a better corrosion protection of 20# carbon steel by increasing the concentration of SbW9-Mn-SnR. Moreover, the value of Ecorr moves to a more positive position when SbW9-Mn-SnR is added. In general, the shift magnitude of Ecorr must exceed 85 mV to classify a corrosion inhibitor as a cathodic or anodic one. Therefore, it is concluded that SbW9-Mn-SnR acts as a mixed-type inhibitor in view of the small shift of Ecorr (<30 mV). Furthermore, it is noted that the cathodic branch with corrosion inhibitors shows the typical Tafel behavior, while the anodic branch displays a kink, which may be related to the degree of corrosion inhibitor coverage on the surface of carbon steel.37 Meanwhile, it can be observed that the anodic branch curves tend to coincide at higher polarization potentials. This is probably because corrosion inhibitor desorption occurs when the polarization potential exceeds the desorption potential, which accelerates the metal dissolution.38
As shown in Figure 2b,c, when 150 mg L–1SbW9-TM-SnR or BiW9-TM-SnR is added, the lower values of corrosion current density in both anodic and cathodic parts imply that SbW9-TM-SnR or BiW9-TM-SnR considerably inhibits the corrosion reaction of 20# carbon steel. In addition, the values of Ecorr for the inhibited solution move toward the positive direction relative to the value of Ecorr in the blank, and the shifts are less than 85 mV. These findings indicate that the POM-based inhibitors behave as mixed-type inhibitors, which inhibit both the anode metal corrosion and cathode H+ reduction.39
As can be seen from Table 4, Icorr decreases with the increase in the concentration of the SbW9-Mn-SnR inhibitor, while the IEi increases. When the concentration of SbW9-Mn-SnR is 300 mg L–1, the IEi can reach to 93.5%. In addition, when the dosage is only 25 mg L–1, the IEi can reach 81.5%, indicating that these inhibitors have obvious inhibition ability for 20# carbon steel in 0.5 M HCl at low concentration. For eight inhibitors SbW9-TM-SnR and BiW9-TM-SnR (TM = Mn, Co, Ni, and Zn) with a concentration of 150 mg L–1, compared with starting material Na-SbW9/Na-BiW9, they all exhibit IEi around 90%. The comparison results also show that SnR as a main functional group improves the corrosion inhibition performance of Na-SbW9- or Na-BiW9-based POMs.
In addition, the polarization curve of 20# carbon steel in 1.0 and 2.0 M HCl was also tested, and the results are shown in Table S5 and Figures S5 and S6. In the high concentration of HCl corrosion solutions, SbW9-TM-SnR and BiW9-TM-SnR can still form an adsorption film on the metal surface, and the IEi is above 87.6%. It shows that the corrosion inhibitors have excellent acid resistance.
Thermodynamic Activation Parameters
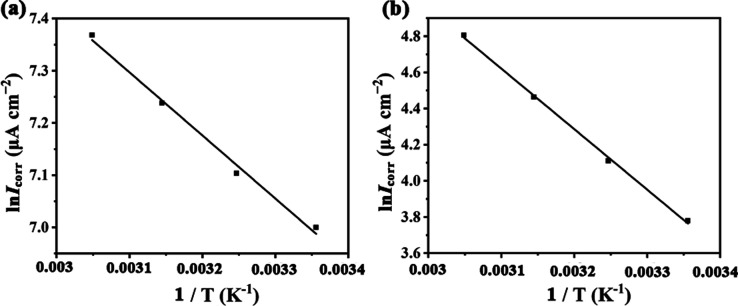
Polarization measurements were further carried out at 298, 308, 318, and 328 K to study the influence of temperature on the corrosion process. The potentiodynamic polarization curves and corrosion parameters are shown in Figure S7 and Table S6, respectively. Accordingly, the Arrhenius plots were drawn and are presented in Figure 3, from which the apparent activation energy (Ea) could be calculated from Arrhenius equation (eq 4):9
| 4 |
where Ea is the activation energy at absolute temperature T (K), R epitomizes the universal gas constant of 8.314 J K–1 mol–1, and A is the Arrhenius factor. Ea can be computed by a linear regression between ln Icorr and 1/T (Figure 3).
Figure 3.
Arrhenius curve for 20# carbon steel in the presence (a) and absence (b) of SbW9-Mn-SnR in 0.5 M HCl solution.
It is concluded that in the absence of inhibitors, the value of Ea is 10.05 kJ mol−1, while in the presence of SbW9-Mn-SnR, the value of Ea reaches 27.85 kJ mol−1. This indicates that the corrosion reaction process needs to overcome a higher energy barrier in the presence of inhibitors, which form an adsorption film covering the 20# carbon steel surface, thus slowing down the corrosion rate.11 Moreover, the activation enthalpy (ΔH) and activation entropy (ΔS) were attained using the transition state equation (eq 5):9
| 5 |
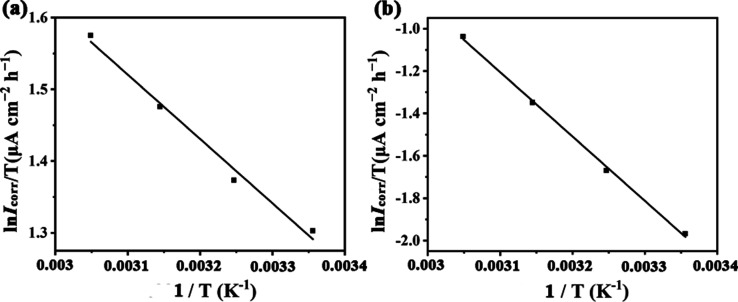
where h is Planck’s constant and N is Avogadro’s number. Figure 4 shows the plot of ln Icorr/T vs 1/T, from which the values of ΔS and ΔH have been calculated. All of the thermodynamic parameters are presented in Table 5.
Figure 4.
Transition plot for 20# carbon steel in the presence (a) and absence (b) of SbW9-Mn-SnR in 0.5 M HCl solution.
Table 5. Thermodynamic Parameters for 20# Carbon Steel in the Presence and Absence of SbW9-Mn-SnR in 0.5 M HCl Solution.
| Ea (kJ mol–1) | ΔH (kJ mol–1) | ΔS (J mol–1 K–1) | |
|---|---|---|---|
| blank | 10.05 | 7.46 | –276.32 |
| SbW9-Mn-SnR | 27.85 | 25.26 | –243.39 |
It can be seen from Table 5 that the values of ΔS and ΔH in the inhibited system are significantly increased compared with those in the uninhibited system. The values of ΔH for both inhibited and uninhibited systems are positive, reflecting that the dissolution process of carbon steel is endothermic. The increase in the ΔH value in the presence of SbW9-Mn-SnR again proves that the energy barrier of the corrosion reaction is increased.40 The value of ΔS in the presence of an inhibitor (−243.39 J mol−1 K−1) is higher than that in blank solution (−276.32 J mol−1 K−1), which is because the value of ΔS is the algebraic sum of the adsorption of inhibitors and desorption of water molecules.41
Electrochemical Impedance Spectroscopy (EIS) Analysis
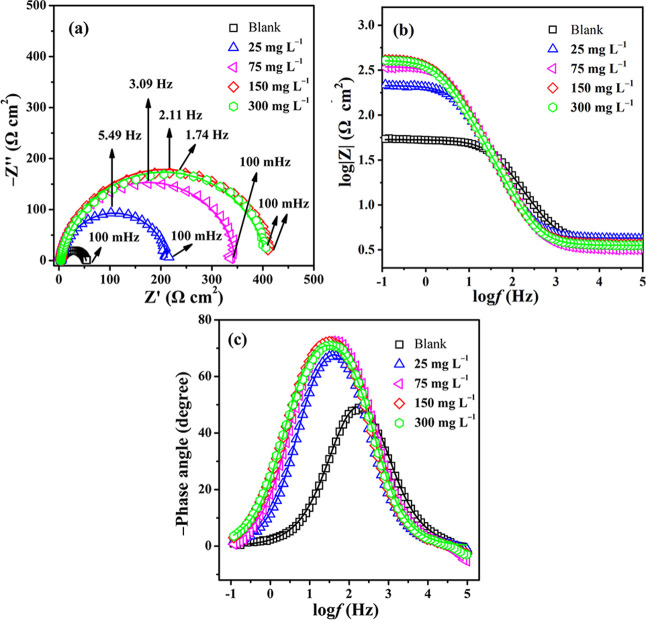
EIS measurements were performed to study the corrosion behavior of 20# carbon steel in 0.5 M HCl solution at 298 K in the absence and presence of the POM-based inhibitors in the frequency range from 100 mHz to 100 kHz.42 Taking SbW9-Mn-SnR for example, the Nyquist, Bode modulus, and phase plots for 20# carbon steel in 0.5 M HCl solution in the presence of various concentrations (0–300 mg L–1) of SbW9-Mn-SnR are shown in Figure 5. As seen from Figure 5a, the Nyquist graphic exhibits a depressed semicircle, which can be explained by the inhomogeneities and roughness of the carbon steel surface, and the semicircle diameter is related to the corrosion resistance of 20# carbon steel in 0.5 M HCl solution.43 It is found that as the inhibitor concentration increases, the diameter of the arc gradually increases, indicating a better protection of the metal surface. Moreover, the Nyquist plots show a single capacitive loop, and the Bode plots exhibit only one peak attributed to one time constant for all samples, which indicates that the charge transfer process controls the corrosion reaction of carbon steel in HCl solution.44 The impedance modulus is a measure of the protection against corrosion. The Bode modulus diagrams in Figure 5b show that the impedance modulus at the lowest frequency increases with the increase in the inhibitor concentration. Generally, the higher the impedance modulus at the lowest frequency, the higher the corrosion inhibition ability of the sample.16 In addition, the increasing phase angle may be attributed to the improvement of uniformity, which is mainly due to the formation of an adsorption layer at the metal interface. A higher coverage results in a greater phase shift. As shown in the Bode phase plots in Figure 5c, the values of the phase angle are higher for carbon steel in 0.5 M HCl solution containing SbW9-Mn-SnR than that in blank solution. The phase angle increases when the concentration of SbW9-Mn-SnR increases and reaches the maximum (∼74°) at 150 mg L–1.
Figure 5.
Nyquist plots (a), Bode modulus plots (b), and Bode phase plots (c) of 20# carbon steel immersed in 0.5 M HCl solution in the absence (blank) and presence of different concentrations (25, 75, 150, and 300 mg L–1) of the SbW9-Mn-SnR inhibitor at room temperature (the solid line shows fitted results).
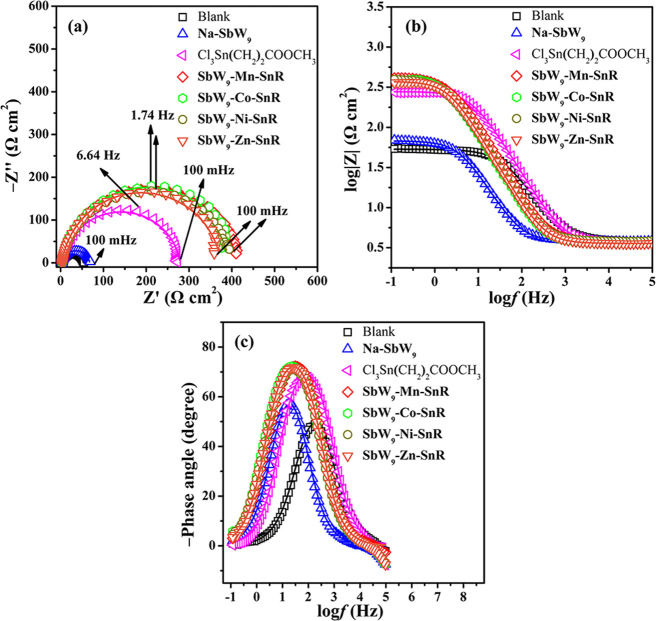
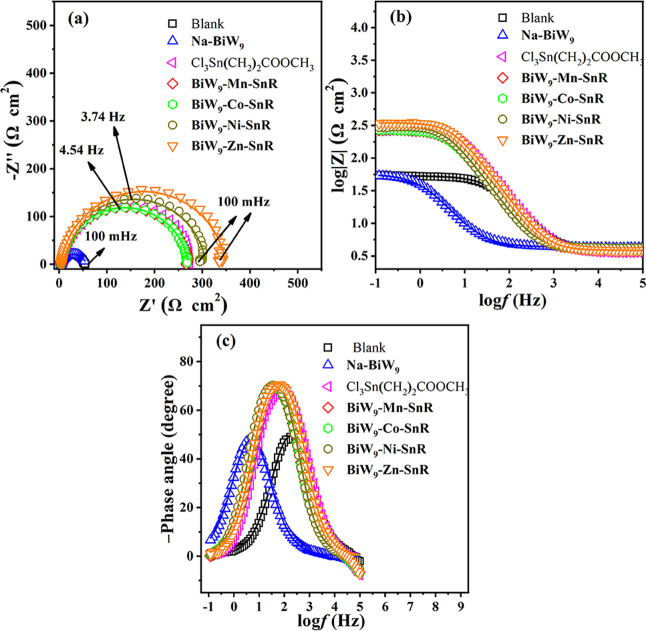
For comparison, at a concentration of 150 mg L–1, the inhibition effect of SbW9-TM-SnR/BiW9-TM-SnR (TM = Mn, Co, Ni, and Zn), Na-SbW9, Na-BiW9, and Cl3Sn(CH2)2COOCH3 on 20# carbon steel immersed in 0.5 M HCl solution at room temperature was also evaluated by the EIS plots (Figures 6 and 7). As evident from Figures 6a and 7a, the diameters of the semicircles in the presence of Cl3Sn(CH2)2COOCH3 and SbW9-TM-SnR/BiW9-TM-SnR are larger than that of Na-SbW9/Na-BiW9, indicating that the corrosion behavior between the interface of the metal and HCl solution is prevented, and Cl3Sn(CH2)2COOCH3 and SbW9-TM-SnR/BiW9-TM-SnR have an obviously better corrosion inhibition property for carbon steel. For different inhibitors SbW9-TM-SnR/BiW9-TM-SnR containing SnR and different TMs, their corrosion inhibition effect on carbon steel is not obviously different, and this result is confirmed again by the Bode modulus and phase plots (Figures 6b,c and 7b,c). Therefore, SnR is a more important factor for improving the corrosion inhibition effect of these POM-estertin derivatives.
Figure 6.
Nyquist plots (a), Bode modulus plots (b), and Bode phase plots (c) of 20# carbon steel immersed in 0.5 M HCl solution in the absence (blank) and presence of 150 mg L–1Na-SbW9/Cl3Sn(CH2)2COOCH3/SbW9-TM-SnR (TM = Mn, Co, Ni, and Zn) inhibitors at room temperature (the solid line shows fitted results).
Figure 7.
Nyquist plots (a), Bode modulus plots (b), and Bode phase plots (c) of 20# carbon steel immersed in 0.5 M HCl solution in the absence (blank) and presence of 150 mg L–1Na-BiW9/Cl3Sn(CH2)2COOCH3/BiW9-TM-SnR (TM = Mn, Co, Ni, and Zn) inhibitors at room temperature (the solid line shows fitted results).
The impedance diagrams were analyzed using ZView 2 software in terms of the equivalent circuit (Figure 8), where Rs is the solution resistance, Rct is the charge transfer resistance, and CPE is the constant phase element. The inhibition efficiency (IER) was computed using eq 6:45
| 6 |
where Rcto and Rct are charge transfer resistances without and with inhibitors, respectively.
Figure 8.
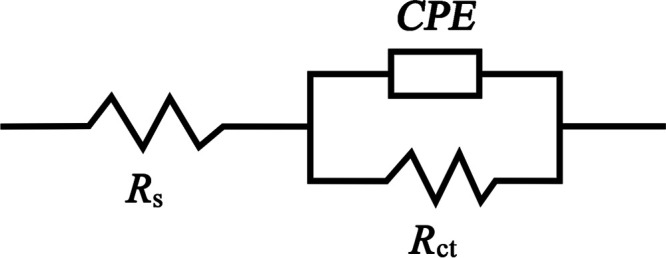
Equivalent circuit model used for fitting electrochemical impedance data.
Because the carbon steel/solution interface does not act as an ideal capacitor, the CPE is used to deal with nonideal capacitance response.43,46 The impedance of the CPE and the double-layer capacitance (Cdl) can be described using eqs 7 and 8,47,48 respectively.
| 7 |
| 8 |
Yo is the CPE constant, j is the imaginary value (j2 = −1), ω is the angular frequency (rad s–1), and n is a phase shift, which represents a measure of surface inhomogeneity. When the value of n is 1, 0, and −1, CPE represents the capacitance (C), resistance (R), and inductance (L), respectively. So, the value of n is an important parameter to show the surface character at the interface of carbon steel and acidic solution.49fmax is the frequency corresponding to the maximum value of the imaginary part in the impedance spectrum.
The corresponding impedance parameters were obtained by fitting the EIS plot using ZView 2 software and are listed in Table 6. It can be seen from the table that the λ2 values lie between 0.0007 and 0.0081, indicating that the equivalent circuit and experimental data can be well-fitted.
Table 6. Electrochemical Impedance Spectroscopy Parameters for 20# Carbon Steel in 0.5 M HCl in the Absence (Blank) and Presence of Inhibitors at Room Temperature (298 K).
| inhibitor | inhibitor concentration (mg L–1) | Rs (Ω cm2) | Rct (Ω cm2) | Cdl (μF cm–2) | Y0 (μF cm–2) | λ2 | n | IER (%) |
|---|---|---|---|---|---|---|---|---|
| blank | 3.8 | 49.6 | 85.3 | 182.0 | 0.0015 | 0.86 | ||
| SbW9-Mn-SnR | 25 | 4.3 | 208.7 | 138.9 | 193.8 | 0.0007 | 0.93 | 76.2 |
| 75 | 3.2 | 342.8 | 150.3 | 183.3 | 0.0023 | 0.93 | 85.5 | |
| 150 | 3.7 | 407.2 | 185.3 | 212.7 | 0.0014 | 0.92 | 87.8 | |
| 300 | 3.6 | 403.6 | 226.6 | 229.2 | 0.0020 | 0.91 | 87.7 | |
| SbW9-Co-SnR | 150 | 3.6 | 398.8 | 229.4 | 261.2 | 0.0036 | 0.92 | 87.6 |
| SbW9-Ni-SnR | 150 | 3.8 | 390.0 | 234.5 | 265.7 | 0.0031 | 0.91 | 87.3 |
| SbW9-Zn-SnR | 150 | 3.6 | 370.5 | 246.9 | 247.4 | 0.0034 | 0.92 | 86.6 |
| BiW9-Mn-SnR | 150 | 4.0 | 265.2 | 132.2 | 64.1 | 0.0029 | 0.93 | 81.0 |
| BiW9-Co-SnR | 150 | 4.1 | 265.5 | 132.0 | 168.9 | 0.0032 | 0.93 | 81.1 |
| BiW9-Ni-SnR | 150 | 4.2 | 297.7 | 142.9 | 164.2 | 0.0028 | 0.93 | 83.1 |
| BiW9-Zn-SnR | 150 | 3.9 | 343.6 | 84.4 | 113.2 | 0.0038 | 0.92 | 85.4 |
| Na-SbW9 | 150 | 3.8 | 64.0 | 664.9 | 828.7 | 0.0051 | 0.92 | 22.5 |
| Na-BiW9 | 150 | 4.3 | 52.6 | 2564.2 | 3746.0 | 0.0081 | 0.85 | 4.5 |
| Cl3Sn(CH2)2COOCH3 | 150 | 3.6 | 275.6 | 71.6 | 109.7 | 0.0041 | 0.91 | 82.0 |
Compared with the blank (0.5 M HCl solution), the values of Rct gradually increase with the concentrations of inhibitors increasing; moreover, the corrosion inhibition effect for SbW9-TM-SnR/BiW9-TM-SnR (TM = Mn, Co, Ni, and Zn) is obviously better than that of parent Na-SbW9/Na-BiW9 and also better than those of some reported surfactant inhibitors.50 The n values are also significant parameters to evaluate the surface property at the metal–acid interface. In the investigation, the values of n are within the range of 0.85 to 0.93, implying the inhomogeneity or roughness of the carbon steel surface, which causes the slight deviation from an ideal capacitance.35,48 These results again prove that SbW9-TM-SnR/BiW9-TM-SnR possess good corrosion inhibition behavior for 20# carbon steel in 0.5 M HCl solution.
The corrosion action of 20# carbon steel in 0.5 M HCl solution in the absence and presence of SbW9-Mn-SnR at different immersion times (6, 10, 24, and 48 h) was also investigated by the EIS measurements at room temperature. The Nyquist, Bode modulus, and phase plots are shown in Figure S8, and the EIS parameters are listed in Table 7. It is found that the diameter of the semicircle increases at all immersion times in the presence of SbW9-Mn-SnR compared to that in the blank (Figure S8a,d). The highest value of Rct in the inhibitor-containing solution is achieved after 10 h of exposure (Table 7). Afterward, the extended immersion time (24 and 48 h) results in the decreased values of Rct and n, indicating that the corrosion behavior recovers, which may be due to the increase in the number of microcracks in the adsorption film on the metal surface.
Table 7. EIS Parameters for 20# Carbon Steel in 0.5 M HCl in the Absence (Blank) and Presence of Inhibitors at Room Temperature (298 K).
| inhibitor | time (h) | Rs (Ω cm2) | Rct (Ω cm2) | Cdl (μF cm–2) | CPE-T (μF cm–2) | n | IER (%) |
|---|---|---|---|---|---|---|---|
| blank | 6 | 3.41 | 39.29 | 340.12 | 596.78 | 0.88487 | |
| 10 | 3.323 | 15.37 | 1283.0 | 2071.2 | 0.86419 | ||
| 24 | 3.951 | 39.02 | 614.02 | 1084.1 | 0.86987 | ||
| 48 | 4.266 | 34.23 | 1242.57 | 1799.6 | 0.8795 | ||
| SbW9-Mn-SnR | 6 | 3.168 | 389.1 | 615.75 | 705.64 | 0.92858 | 89.9 |
| 10 | 2.14 | 495.6 | 1259.39 | 1337 | 0.93404 | 96.9 | |
| 24 | 3.098 | 339.8 | 3956.01 | 4093.6 | 0.91716 | 88.5 | |
| 48 | 4.114 | 139.3 | 11699.44 | 11298.0 | 0.83694 | 75.4 |
Adsorption Isotherm
In order to clarify the adsorption mechanism of these inhibitors on the surface of carbon steel in 0.5 M HCl solution, taking SbW9-Mn-SnR for example, different adsorption models including Langmuir, Frumkin, Temkin, Freundlich, Flory–Huggins, and El-Awady isotherms were separately used to analyze the adsorption behaviors of POM-based inhibitors.7,40 It is found from Figure 9 that the experimental data could be well-fitted with the Langmuir adsorption isotherm with R2 > 0.99. The other tested isotherms are shown in Figure S9. It is proven that SbW9-Mn-SnR forms a monomolecular adsorption layer on the surface of 20# carbon steel, which prevents the corrosion behavior of metals. The Langmuir adsorption isotherm can be described using eq 9:51
| 9 |
Cinh is the inhibitor concentration, θ is the surface coverage (θ = IE/100), and Kads is the adsorption/desorption equilibrium constant.
Figure 9.
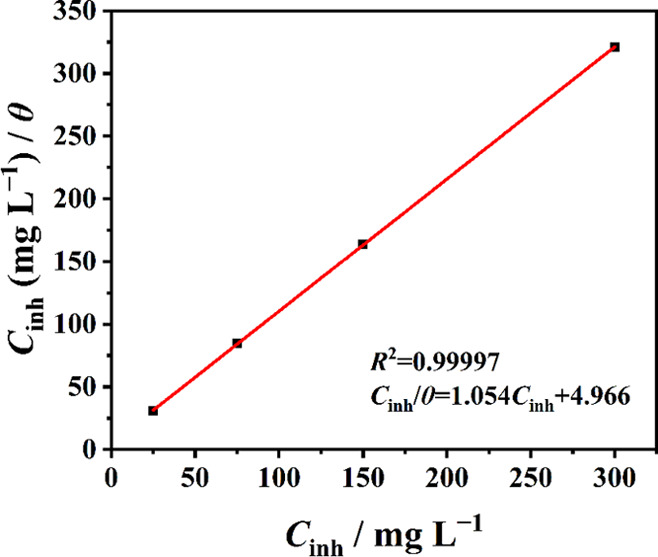
Langmuir adsorption isotherm of SbW9-Mn-SnR on 20# carbon steel in 0.5 M HCl solution at 298 K.
The Kads value can be calculated from the intercept of the line on the Cinh/θ axis. Accordingly, the standard free energy (ΔGads0) can be obtained from the following equation (eq 10):52
| 10 |
in which T is the thermodynamic temperature (298 K), R (8.314 J mol–1 K–1) is the gas constant, and 55.5 (M) is the molar concentration of water in the solution. In this case, the calculated values of Kads and ΔGads0 are 1156.06 L mol–1 and −27.43 kJ mol–1, respectively. The high value of Kads indicates that SbW9-Mn-SnR is robustly adsorbed on the 20# carbon steel surface. It is generally acknowledged that the negative value of ΔGads shows that the formation of the above POM-based protective film on the metal surface is a spontaneous adsorption process. According to the literature,30,53 when ΔGads0 > −20 kJ mol–1, the adsorption of the inhibitor on the metal surface can be considered as physical adsorption; if −40 kJ mol–1 < ΔGads < −20 kJ mol–1, then the interaction between inhibitor molecules and metal atoms can be attributed to physical adsorption and chemical adsorption. Hence, the ΔGads0 of −27.43 kJ mol–1 for this paper suggests that physical and chemical adsorption has taken place between SbW9-Mn-SnR and 20# carbon steel, but it is mainly physical adsorption.
Surface Morphology and Composition of 20# Carbon Steel
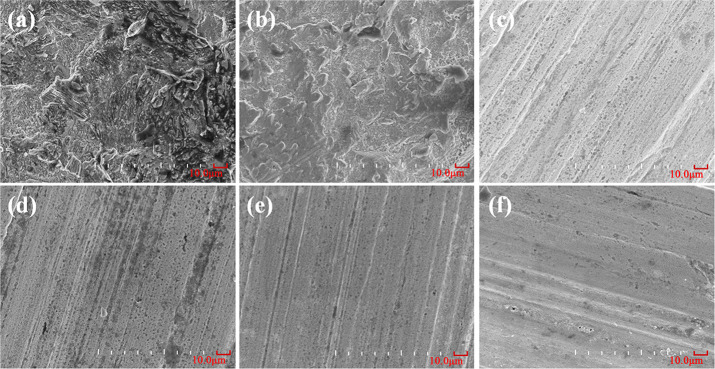
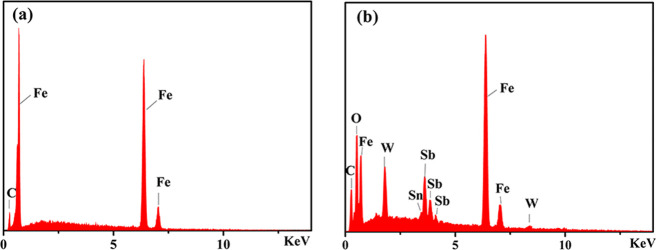
The surface morphology of 20# carbon steel after corrosion testing was observed by SEM (Figure 10). As expected, it can be seen from Figure 10a,b that severe corrosive attack occurred on the surface of carbon steel in 0.5 M HCl solution without the inhibitor and with the raw material Na-SbW9, respectively. Conversely, the damage degree of carbon steel in 0.5 M HCl solution with SbW9-TM-SnR (TM = Mn, Co, Ni, and Zn) inhibitors (Figure 10c–f) is weaker, and the surfaces are smoother. This phenomenon further indicates that these sandwich-type POMs modified by SnR and TMs can be used as good corrosion inhibitors to protect carbon steel well in 0.5 M HCl solution. Figure 11a,b shows EDX diagrams for 20# carbon steel immersed in 0.5 M HCl solution containing no inhibitor and the inhibitor SbW9-Mn-SnR, respectively. Compared with Figure 11a, Figure 11b exhibits the existence of O, W, Sb, and Sn in addition to C and Fe elements on the film surface of carbon steel; the result proves that the POM-based corrosion inhibitor exists on the carbon steel surface. However, no Mn was detected in the EDX spectrum, which may be caused by the following reasons: (1) the content of TM is relatively low; (2) two TM ions are located at the active sites on both sides of the intermediate belt in the sandwich-type structures, so they are easily replaced by the formed Fe2+/Fe3+ ions, forming a protective film on the carbon steel surface. This substitution reaction also often occurred in other sandwich-type POMs.54−56
Figure 10.
SEM images of the carbon steel surface after 6 h of immersion in 0.5 M HCl solution without an inhibitor (a) and with Na-SbW9 (b) and SbW9-TM-SnR (TM = Mn, Co, Ni, and Zn) (c–f).
Figure 11.
EDX analysis of carbon steel immersed in 0.5 M HCl solution without an inhibitor (a) and with the SbW9-Mn-SnR inhibitor (b).
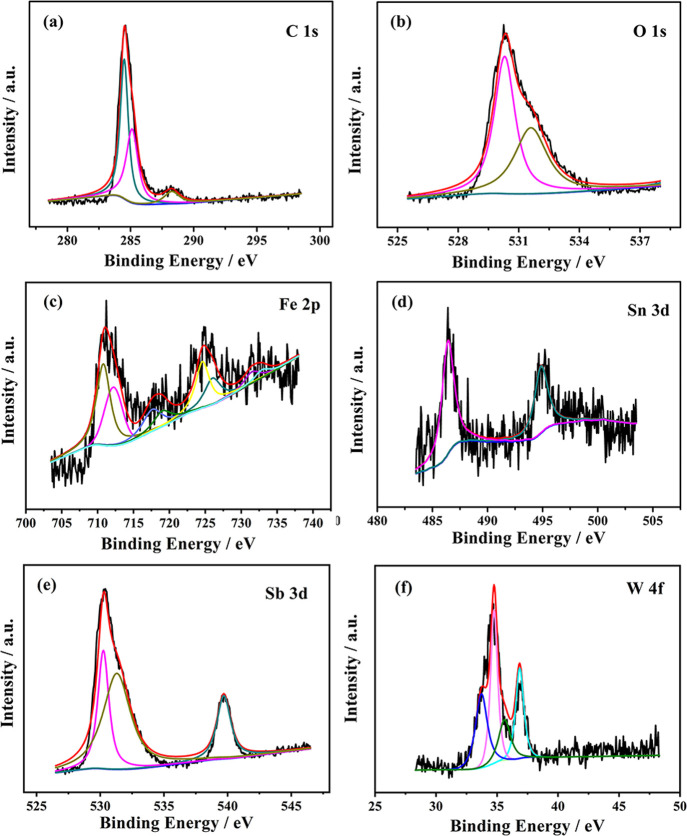
In order to characterize the oxidation states of various elements existing on the carbon steel surface, taking SbW9-Mn-SnR as an example, XPS analysis of 20# carbon steel after immersion in 0.5 M HCl containing 150 mg L–1SbW9-Mn-SnR for 6 h was performed (Figure 12). The signal of Mn was not detected because of its low content. As shown in Figure 12a, XPS peaks of C 1s can be fitted with three peaks at binding energies of 284.5, 285.12, and 288.27 eV, which are ascribed to C–C/C–H species, C–O, and C=O/O–C=O bonds in SnR groups, respectively.57 As seen from Figure 12b, O 1s shows signals at binding energies of 530.3 and 531.6 eV, which correspond to O2– and OH–, respectively.58,59 As for Fe 2p, in Figure 12c, the peak positions of Fe 2p1/2 and Fe 2p3/2 for Fe2+ are located at 724.6 and 710.8 eV, with their associated satellite peaks at 731.5 and 717.6 eV, respectively. In addition, the XPS peaks with binding energies of 726 and 712.2 eV are assigned to Fe 2p1/2 and Fe 2p3/2 for Fe3+, with their associated satellite peaks at 732.9 and 719 eV, respectively. The above test results show that Fe2+ and Fe3+ oxide films are formed on the surface of carbon steel.60 In Figure 12d, the binding energies of Sn 3d located at 486.4 and 494.85 eV are attributed to the Sn4+ oxidation state.58Figure 12e shows the XPS spectra of Sb 3d, in which the peaks at binding energies of 539.7 and 530.24 eV are ascribed to Sb 3d3/2 and Sb 3d5/2 for the Sb3+ oxidation state, in which there exists an overlap between the O 1s peak at 531.3 eV and the Sb 3d5/2 peak.32,61 In Figure 12f, the peaks at binding energies of 36.9 and 34.8 eV are attributed to W 4f7/2 and W 4f5/2 for the W6+ oxidation state, and the peaks at binding energies of 35.7 and 33.7 eV are assigned to W 4f7/2 and W 4f5/2 for the W5+ oxidation state, respectively, inferring that part of the W element in POM is reduced in the HCl media.32,62
Figure 12.
XPS analysis of C 1s, O 1s, Fe 2p, Sn 3d, Sb 3d, and W 4f (a–f) on the 20# carbon steel surface after immersion in 0.5 M HCl solution containing SbW9-Mn-SnR for 6 h.
Stability Analysis of Corrosion Inhibitors
The stability of inhibitors in the corrosion test was studied by IR and UV–vis absorption spectra. To evaluate the stability of these POMs after the corrosion tests, iron powder was used instead of the carbon steel plate to simulate the corrosion test. Taking SbW9-Mn-SnR for example, the IR spectra of the solid samples recrystallized from the following HCl solutions were tested (Figure S10): (1) 150 mg L–1SbW9-Mn-SnR in 0.5 M HCl solution and excessive Fe powder, 6 h (curve a); (2) 150 mg L–1SbW9-Mn-SnR in 0.5 M HCl solution and no Fe powder, 6 h (curve b). As seen from curves a and b in Figure S10, the main peaks appearing at 2950–2800, 1650, and 1000–600 cm–1 are the characteristic peaks of CH2, COO, and POM, respectively,30 which is consistent with that of the pure original SbW9-Mn-SnR (curve c). This result proves that the skeleton of the POM is still stable in the acidic solution. In addition, UV–vis absorption spectra of SbW9-Mn-SnR dissolved in 0.5 M HCl solution for 0 and 6 h (curves a and b in Figure S11), and with adding Fe powder (curve c in Figure S11) for 6 h, were recorded to identify the inhibitor stability. As shown in Figure S11, two peaks in the UV region of 200–300 nm attributed to O → W charge transfer transitions are observed in curve a and unchanged in curves b and c. In order to further evaluate the stability of corrosion inhibitors in more acidic solutions, similar experiments were carried out in 1.0 and 2.0 M HCl solutions, respectively. As can be seen from Figures S12 and S13, the characteristic peaks of IR and UV–vis absorption spectra are consistent with Figures S10 and S11, which indicate that the POM skeleton still exists in 1.0–2.0 M HCl media. However, the 119Sn NMR spectrum (Figure S14) shows that the inhibitor breaks down in 2.0 M HCl because the signal (δ = −424.06 ppm) is similar to that of pure SnR (δ = −416.84 ppm). This verifies the above speculation that the IEw of the corrosion inhibitor in 2 M HCl is lower than that in 1 M HCl because of decomposition of anionic POM. To sum up, these sandwich-type POM-based corrosion inhibitors containing SnR and TMs remain stable in 0.5–1.0 M HCl solutions.
Corrosion Inhibition Mechanism
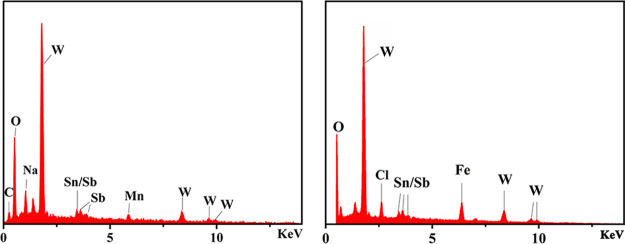
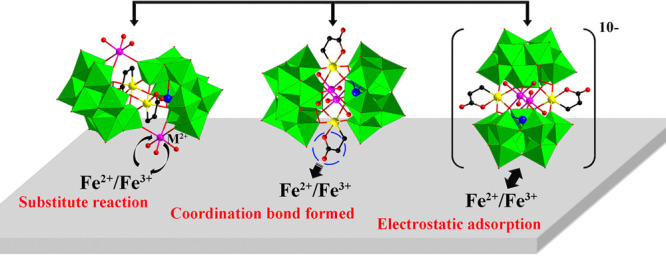
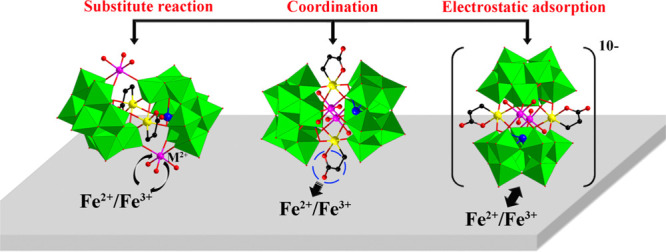
In order to further explore the film-forming mechanism, EDX analysis (Figure 13) was also carried out on the above described SbW9-Mn-SnR recrystallized from HCl solution treated with Fe powder. Compared with the pure original SbW9-Mn-SnR (Figure 13a), the Mn content of the solid sample recrystallized from 0.5 M HCl solution illustrated in Figure 13b is actually reduced, and other elements have not changed significantly. Importantly, the appearance of the Fe signal peak further confirms the conjecture that the Fe0 on the surface of carbon steel is oxidized to Fe2+/Fe3+ in HCl solution, and the formed Fe2+/Fe3+ further replaces TM ions situated on either side of the middle part in SbW9-TM-SnR/BiW9-TM-SnR (TM = Mn, Co, Ni, and Zn), thereby preventing further corrosion of Fe in the carbon steel. In addition, Fe2+/Fe3+ on the carbon steel surface possesses two/three positive charges in HCl solution, while [(TM(H2O)3)2(SnCH2CH2COO)2(SbW9O33)2]10–/[(TM(H2O)3)2(SnCH2CH2COO)2(BiW9O33)2]10– in SbW9-TM-SnR/BiW9-TM-SnR is a large polyoxoanion cluster with 10 negative charges, so the POMs can be easily adsorbed on the surface of carbon steel by electrostatic adsorption. Furthermore, the exposed COO group of SnR in the POM corrosion inhibitor can provide lone electron pairs to interact with the empty 3d orbital of Fe atoms, forming the coordination covalent bond,63 which resulted in a denser protective film on the surface of carbon steel through chemical and physical adsorption. Consequently, the combined actions of physical adsorption and chemical adsorption delay the corrosion of carbon steel (Scheme 1).
Figure 13.
EDX analysis of pure SbW9-Mn-SnR (left) and a solid sample obtained by recrystallization for several times in 0.5 M HCl solution containing 150 mg L–1SbW9-Mn-SnR and Fe powder (right).
Scheme 1. Schematic Diagram of the Corrosion Inhibition Mechanism of SbW9-TM-SnR/BiW9-TM-SnR (TM = Mn, Co, Ni, and Zn) on 20# Carbon Steel in the HCl Solution.
Conclusions
This work first reports the inhibition behaviors of SbW9-TM-SnR and BiW9-TM-SnR (TM = Mn, Co, Ni, and Zn) in a HCl medium. The inhibition performance of the lacunary POMs is greatly improved by carboxyethyltin functionalization. The inhibition efficiency of SbW9-TM-SnR is slightly higher than that of BiW9-TM-SnR. The electrochemical and surface analyses show that SbW9-TM-SnR and BiW9-TM-SnR act as mixed-type inhibitors, forming stable and protective films on the surface of carbon steel by physical and chemical adsorption. The adsorption process follows the Langmuir adsorption isotherm. This work also shows the high acid resistance of the sandwich-type POM-estertin derivatives with Sb/Bi as a heteroatom for the first time.
Materials and Experimental Methods
Materials
All chemicals and reagents (analytical grade) were commercially available. Na9[B-α-SbW9O33]·19.5H2O (Na-SbW9), Na9[B-α-BiW9O33]·16H2O (Na-BiW9), and Cl3Sn(CH2)2COOCH3 were synthesized according to the reported procedures.64−66 HCl solution (0.5 M) was prepared by diluting 37% HCl solution with water. All tests were performed at room temperature, and the water used in all experiments was secondary distilled water.
Weight Loss Measurement
The new 20# carbon steel plates (the size is 4 cm × 1 cm × 0.2 cm with the compositions of C, 0.23; Si, 0.29; Mn, 0.60; and S, 0.028 in wt % and Fe in balance) were washed with acetone and ethanol and then dried in a desiccator for more than 24 h to a constant weight before immersion in the test solutions. The specific test procedures used in weight loss measurement were as follows: the carbon steel plates were immersed in 0.5, 1.0, and 2.0 M HCl solutions containing different corrosion inhibitors. The concentrations of corrosion inhibitors were 25–500 mg L–1, and the corrosion test time at room temperature was 6 and 10 h, respectively. After the steel specimen was taken out from HCl solutions, its surface was cleaned with water and ethanol and then dried for more than 24 h to a constant weight. Three sets of parallel experiments were performed for each test to ensure the accuracy of the experiment.
Electrochemical Measurements
Electrochemical measurements were conducted in a three-electrode system on a CHI604B electrochemical workstation by using a platinum plate (20 mm × 60 mm × 0.1 mm) as the counter electrode, a saturated calomel electrode (SCE) as the reference electrode, and a 20# carbon steel sample with an exposed area of 1 cm2 as the working electrode. To reach a steady state, the working electrode should be immersed in the measured solution for 1 h before the measurement. As for polarization tests, the Tafel curve was obtained in the potential range from −0.5 to 0.1 V, and the scan rate was 1 mV s–1. The corrosion potential (Ecorr) and corrosion current density (Icorr) were obtained by Tafel extrapolation of anode and cathode polarization curves. The electrochemical impedance test was performed with an AC amplitude of 5 mV at the corrosion potential and a frequency range of 100 mHz to 100 kHz; all impedance data were fitted to the relevant impedance parameters by ZView 2 software.
Surface Analysis
The morphology and composition of the surfaces of 20# carbon steel samples in 0.5 M HCl without and with different inhibitors were investigated by a HITACHI SU8010 serious microscope equipped with EDX after weight loss tests. X-ray photoelectron spectroscopy (XPS) analysis was performed on an ESCALAB-MKII X-ray spectrometer with a Mg Kα (1253.6 eV) X-ray source.
Corrosion Inhibitor Stability Analysis
Taking SbW9-Mn-SnR as a sample, the stability of the corrosion inhibitor was analyzed by IR and UV spectroscopy. The FT-IR spectra were measured on a Bruker AXS TENSOR-27 spectrometer using KBr pellets at 4000–400 cm–1. Using a PerkinElmer Lambda 35 spectrometer with a wavelength range of 200–800 nm, the UV–vis absorption spectra of SbW9-Mn-SnR solutions were obtained. NMR spectra were recorded at room temperature on a 500 MHz Bruker AVANCE 500 spectrometer. An inner tube containing D2O was used as an instrumental lock. Tin chemical shifts were referenced to Cl3Sn(CH2)2COOCH3.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 21671091 and 21601077), the Natural Science Foundation of Liaoning Province (no. 20180550544), the Scientific Research Project of the Liaoning Normal University (GD19L005), and the High-Level Talent Innovation Support Program of Dalian (nos. 2020RQ046 and 2019RQ133).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c06276.
Author Contributions
# X.-.F.W. and X.-Y.L. contributed equally to this work and should be considered as co-first authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Yang Y.; Cheng Y. F. Visible light illuminated high-performance WO3-TiO2-BiVO4 nanocomposite photoanodes capable of energy self-storage for photo-induced cathodic protection. Corros. Sci. 2020, 164, 108333. 10.1016/j.corsci.2019.108333. [DOI] [Google Scholar]
- Dandia A.; Gupta S. L.; Singh P.; Quraishi M. A. Ultrasound assisted synthesis of pyrazolo(3,4-b) pyridines as potential corrosion inhibitors for mild steel in 1.0 M HCl. ACS Sustainable Chem. Eng. 2013, 1, 1303–1310. 10.1021/sc400155u. [DOI] [Google Scholar]
- Ye Y.; Yang D.; Chen H.; Guo S.; Yang Q.; Chen L.; Zhao H.; Wang L. A high-efficiency corrosion inhibitor of N-doped citric acid-based carbon dots for mild steel in hydrochloric acid environment. J. Hazard. Mater. 2020, 381, 121019. 10.1016/j.jhazmat.2019.121019. [DOI] [PubMed] [Google Scholar]
- Bastos A. C.; Ferreira M. G.; Simões A. M. Corrosion inhibition by chromate and phosphate extracts for iron substrates studied by EIS and SVET. Corros. Sci. 2006, 48, 1500–1512. 10.1016/j.corsci.2005.05.021. [DOI] [Google Scholar]
- Alentejano C. R.; Aoki I. V. Localized corrosion inhibition of 304 stainless steel in pure water by oxyanions tungstate and molybdate. Electrochim. Acta 2004, 49, 2779–2785. 10.1016/j.electacta.2004.01.039. [DOI] [Google Scholar]
- Goyal M.; Kumar S.; Bahadur I.; Verma C.; Ebenso E. E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. 10.1016/j.molliq.2018.02.045. [DOI] [Google Scholar]
- Aslam R.; Mobin M.; Huda; Shoeb M.; Murmu M.; Banerjee P. Proline nitrate ionic liquid as high temperature acid corrosion inhibitor for mild steel: Experimental and molecular-level insights. J. Ind. Eng. Chem. 2021, 100, 333–350. 10.1016/j.jiec.2021.05.005. [DOI] [Google Scholar]
- San Y.; Sun J.; Wang H.; Jin Z. H.; Gao H. J. Synthesis of 1,8-naphthyridines by the ionic liquid-catalyzed friedlander reaction and application in corrosion inhibition. ACS Omega 2021, 6, 28063–28071. 10.1021/acsomega.1c04103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanra A.; Srivastava M.; Rai M. P.; Prakash R. Application of unsaturated fatty acid molecules derived from microalgae toward mild steel corrosion inhibition in HCl solution: a novel approach for metal-inhibitor association. ACS Omega 2018, 3, 12369–12382. 10.1021/acsomega.8b01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngouné B.; Pengou M.; Nouteza A. M.; Nanseu-Njiki C. P.; Ngameni E. Performances of alkaloid extract from rauvolfia macrophylla stapf toward corrosion inhibition of C38 steel in acidic media. ACS Omega 2019, 4, 9081–9091. 10.1021/acsomega.9b01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra N.; Bhat J. I. Natural products for material protection: an interesting and efficacious anticorrosive property of dry arecanut seed extract at electrode (aluminum)–electrolyte (hydrochloric acid) interface. J. Bio. Tribo Corros. 2016, 2, 21. 10.1007/s40735-016-0051-2. [DOI] [Google Scholar]
- Wang Y. N.; Dong C. F.; Zhang D. W.; Ren P. P.; Li L.; Li X. G. Preparation and characterization of a chitosan-based low-pH-sensitive intelligent corrosion inhibitor. Int. J. Min. Met. Mater. 2015, 22, 998–1004. 10.1007/s12613-015-1161-4. [DOI] [Google Scholar]
- Zhang F.; Ju P.; Pan M.; Zhang D.; Huang Y.; Li G.; Li X. Self-healing mechanisms in smart protective coatings: a review. Corros. Sci. 2018, 144, 74–88. 10.1016/j.corsci.2018.08.005. [DOI] [Google Scholar]
- Wang J.; Tang J.; Zhang H.; Wang Y.; Wang H.; Lin B.; Hou J.; Zhang H. A CO2-responsive anti-corrosion ethyl cellulose coating based on the pH-response mechanism. Corros. Sci. 2021, 180, 109194. 10.1016/j.corsci.2020.109194. [DOI] [Google Scholar]
- Attaei M.; Calado L. M.; Morozov Y.; Taryba M. G.; Shakoor R. A.; Kahraman R.; Marques A. C.; Montemor M. F. Smart epoxy coating modified with isophorone diisocyanate microcapsules and cerium organophosphate for multilevel corrosion protection of carbon steel. Prog. Org. Coat. 2020, 147, 105864. 10.1016/j.porgcoat.2020.105864. [DOI] [Google Scholar]
- Asaldoust S.; Ramezanzadeh B. Synthesis and characterization of a high-quality nanocontainer based on benzimidazole-zinc phosphate (ZP-BIM) tailored graphene oxides; A facile approach to fabricating a smart self-healing anti-corrosion system. J Colloid. Interface Sci. 2020, 564, 230–244. 10.1016/j.jcis.2019.12.122. [DOI] [PubMed] [Google Scholar]
- Katsoulis D. E. A Survey of applications of polyoxometalates. Chem. Rev. 1998, 98, 359–388. 10.1021/cr960398a. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Liu J.; Li S. L.; Su Z. M.; Lan Y. Q. Polyoxometalate-based materials for sustainable and clean energy conversion and storage. EnergyChem. 2019, 1, 100021. 10.1016/j.enchem.2019.100021. [DOI] [Google Scholar]
- Sruthi G.; Shakeela K.; Shanmugam R.; Rao G. R. Corrosion inhibition of stainless steel by ferrocene-polyoxometalate hybrid molecular materials-experimental and first principle studies. Phys. Chem. Chem. Phys. 2020, 22, 3329–3344. 10.1039/C9CP06284J. [DOI] [PubMed] [Google Scholar]
- Lomakina S. V.; Shatova T. S.; Kazansky L. P. Heteropoly anions as corrosion inhibitors for aluminium in high temperature water. Corros. Sci. 1994, 36, 1645–1651. 10.1016/0010-938X(94)90059-0. [DOI] [Google Scholar]
- Liang C. H.; Hu X. Q.; Ma L. Effects of Na3PW12O40 on the corrosion behavior of carbon steel in 55% LiBr solution. Mater. Corros. 2007, 58, 39–43. 10.1002/maco.200603981. [DOI] [Google Scholar]
- Hu X. Q.; Liang C. H.; Wu X. N. Corrosion behaviors of carbon steel in 55% LiBr solution containing PWVA inhibitor. Mater. Corros. 2011, 62, 444–448. 10.1002/maco.200905528. [DOI] [Google Scholar]
- Ait Albrimi Y.; Ait Addi A.; Douch J.; Souto R. M.; Hamdani M. Inhibition of the pitting corrosion of 304 stainless steel in 0.5 M hydrochloric acid solution by heptamolybdate ions. Corros. Sci. 2015, 90, 522–528. 10.1016/j.corsci.2014.10.023. [DOI] [Google Scholar]
- Bonastre J.; Garcés P.; Huerta F.; Quijada C.; Andión L. G.; Cases F. Electrochemical study of polypyrrole/PW12O403– coatings on carbon steel electrodes as protection against corrosion in chloride aqueous solutions. Corros. Sci. 2006, 48, 1122–1136. 10.1016/j.corsci.2005.05.004. [DOI] [Google Scholar]
- Herrmann S.; Kostrzewa M.; Wierschem A.; Streb C. Polyoxometalate Ionic Liquids as Self-Repairing Acid-Resistant Corrosion Protection. Angew. Chem., Int. Ed. 2014, 53, 13596–13599. 10.1002/anie.201408171. [DOI] [PubMed] [Google Scholar]
- Al-Dawsari A.; Turkustani A. M.; Bannani F. Acid-resistant corrosion protection polyoxometalate ionic liquids as anticorrosion coatings. J. Mater. Environ. Sci. 2019, 10, 1135–1151. [Google Scholar]; ISSN 2028-2508
- Misra A.; Franco Castillo I.; Müller D. P.; Gonzalez C.; Eyssautier-Chuine S.; Ziegler A.; de la Fuente J. M.; Mitchell S. G.; Streb C. Polyoxometalate-Ionic Liquids (POM-ILs) as Anticorrosion and Antibacterial Coatings for Natural Stones. Angew. Chem., Int. Ed. 2018, 57, 14926–14931. 10.1002/anie.201809893. [DOI] [PubMed] [Google Scholar]
- Mourad M. Y.; Seliman S. A.; Ibrahim E. H. The inhibitive action of dimethyltin dichloride towards the corrosion of aluminium in hydrochloric acid and sodium hydroxide solutions. J. Chem. Technol. Biotechnol. 1989, 46, 27–40. 10.1002/jctb.280460104. [DOI] [Google Scholar]
- Zhang L. C.; Zheng S. L.; Xue H.; Zhu Z. M.; You W. S.; Li Y. G.; Wang E. B. New tetra(organotin)-decorated boat-like polyoxometalate. Dalton Trans. 2010, 39, 3369–3371. 10.1039/b925822a. [DOI] [PubMed] [Google Scholar]
- Wang Z. X.; Sang X. J.; Yang H.; Ji L. P.; Zhang L. C.; Zhu Z. M.; You W. S. A new carboxyethyltin functionalized sandwich-type tungstoarsenate: Synthesis, catalytic activity and corrosion inhibition behavior for carbon steel. Inorg. Chem. Commun. 2017, 83, 44–48. 10.1016/j.inoche.2017.06.016. [DOI] [Google Scholar]
- Sang X. J.; Wang Z. X.; Li J. S.; Wang H. D.; Su F.; Liu Z. B.; Zhang L. C.; Zhu Z. M. Corrosion protection of carbon steel in circulating cooling water by open-chain carboxyethyltin and transition metal co-functionalized tungstogermanates. ChemistrySelect 2018, 3, 7358–7362. 10.1002/slct.201800789. [DOI] [Google Scholar]
- Wang H. D.; Wang X. F.; Su F.; Li J. S.; Zhang L. C.; Sang X. J.; Zhu Z. M. Carboxyethyltin and transition metal co-functionalized tungstoantimonates composited with polypyrrole for enhanced electrocatalytic methanol oxidation. Dalton Trans. 2019, 48, 2977–2987. 10.1039/c8dt05118f. [DOI] [PubMed] [Google Scholar]
- Wang Z. J.; Zhang L. C.; Zhu Z. M.; Chen W. L.; You W. S.; Wang E. B. Two new sandwich-type tungstobismuthates constructed from trivacant Keggin units, estertin and transition metals. Inorg. Chem. Commun. 2012, 17, 151–154. 10.1016/j.inoche.2011.12.038. [DOI] [Google Scholar]
- Loganayagi C.; Kamal C.; Sethuraman M. G. Opuntiol: An Active Principle of Opuntia elatior as an Eco-Friendly Inhibitor of Corrosion of Mild Steel in Acid Medium. ACS Sustainable Chem. Eng. 2014, 2, 606–613. 10.1021/sc4003642. [DOI] [Google Scholar]
- Mahato P.; Mishra S. K.; Murmu M.; Murmu N. C.; Hirani H.; Banerjee P. A prolonged exposure of Ti-Si-B-C nanocomposite coating in 3.5wt% NaCl solution: Electrochemical and morphological analysis. Surf. Coat. Technol. 2019, 375, 477–488. 10.1016/j.surfcoat.2019.07.039. [DOI] [Google Scholar]
- Oguzie E. E.; Oguzie K. L.; Akalezi C. O.; Udeze I. O.; Ogbulie J. N.; Njoku V. O. Natural Products for Materials Protection: Corrosion and Microbial Growth Inhibition Using Capsicum frutescens Biomass Extracts. ACS Sustainable Chem. Eng. 2013, 1, 214–225. 10.1021/sc300145k. [DOI] [Google Scholar]
- Corrales-Luna M.; Le Manh T.; Romero-Romo M.; Palomar-Pardavé M.; Arce-Estrada E. M. 1-ethyl 3-methylimidazolium thiocyanate ionic liquid as corrosion inhibitor of API 5L X52 steel in H2SO4 and HCl media. Corros. Sci. 2019, 153, 85–99. 10.1016/j.corsci.2019.03.041. [DOI] [Google Scholar]
- Chen W.; Huang D. X.; Wei F. Inhibition Effect of Brainea Insignis Extract Against Carbon Steel Corrosion in HCl Solution. J Chin. Soc. Corr. Pro. 2021, 41, 376–382. 10.11902/1005.4537.2020.058. [DOI] [Google Scholar]
- Cao S.; Liu D.; Ding H.; Wang J.; Lu H.; Gui J. Task-specific Ionic Liquids as corrosion inhibitors on carbon steel in 0.5 M HCl solution: An experimental and theoretical study. Corros. Sci. 2019, 153, 301–313. 10.1016/j.corsci.2019.03.035. [DOI] [Google Scholar]
- Abdel-Gaber A. M.; Khamis E.; Abo-ElDahab H.; Adeel S. Inhibition of aluminium corrosion in alkaline solutions using natural compound. Mater. Chem. Phys. 2008, 109, 297–305. 10.1016/j.matchemphys.2007.11.038. [DOI] [Google Scholar]
- Singh A.; Ansari K. R.; Banerjee P.; Murmu M.; Quraishi M. A.; Lin Y. Corrosion inhibition behavior of piperidinium based ionic liquids on Q235 steel in hydrochloric acid solution: Experimental, density functional theory and molecular dynamics study. Colloids Surf., A 2021, 623, 126708. 10.1016/j.colsurfa.2021.126708. [DOI] [Google Scholar]
- Pathan S.; Ahmad S. s-Triazine Ring-Modified Waterborne Alkyd: Synthesis, Characterization, Antibacterial, and Electrochemical Corrosion Studies. ACS Sustainable Chem. Eng. 2013, 1, 1246–1257. 10.1021/sc4001077. [DOI] [Google Scholar]
- Solomon M. M.; Gerengi H.; Kaya T.; Umoren S. A. Performance Evaluation of a Chitosan/Silver Nanoparticles Composite on St37 Steel Corrosion in a 15% HCl Solution. ACS Sustainable Chem. Eng. 2017, 5, 809–820. 10.1021/acssuschemeng.6b02141. [DOI] [Google Scholar]
- Murmu M.; Saha S. K.; Bhaumick P.; Murmu N. C.; Hirani H.; Banerjee P. Corrosion inhibition property of azomethine functionalized triazole derivatives in 1mol L–1 HCl medium for mild steel: Experimental and theoretical exploration. J. Mol. Liq. 2020, 313, 113508. 10.1016/j.molliq.2020.113508. [DOI] [Google Scholar]
- Negm N. A.; Zaki M. F.; Said M. M.; Morsy S. M. Inhibitory action of biodegradable modified vanillin on the corrosion of carbon steel in 1 M HCl. Corros. Sci. 2011, 53, 4233–4240. 10.1016/j.corsci.2011.08.034. [DOI] [Google Scholar]
- Adesina A. Y.; Gasem Z. M.; Kumar A. M. Corrosion resistance behavior of single-layer cathodic arc PVD nitride-base coatings in 1M HCl and 3.5 pct NaCl solutions. Metall. Mater. Trans. B 2017, 48, 1321–1332. 10.1007/s11663-016-0891-7. [DOI] [Google Scholar]
- Abd El-Lateef H. M. Corrosion inhibition characteristics of a novel salycilidene isatin hydrazine sodium sulfonate on carbon steel in HCl and a synergistic nickel ions additive: A combined experimental and theoretical perspective. Appl. Surf. Sci. 2020, 501, 144237. 10.1016/j.apsusc.2019.144237. [DOI] [Google Scholar]
- Haruna K.; Saleh T. A.; Obot I. B.; Umoren S. A. Cyclodextrin-based functionalized graphene oxide as an effective corrosion inhibitor for carbon steel in acidic environment. Prog. Org. Coat. 2019, 128, 157–167. 10.1016/j.porgcoat.2018.11.005. [DOI] [Google Scholar]
- Murmu M.; Saha S. K.; Murmu N. C.; Banerjee P. Effect of stereochemical conformation into the corrosion inhibitive behaviour of double azomethine based Schiff bases on mild steel surface in 1 mol L–1 HCl medium: An experimental, density functional theory and molecular dynamics simulation study. Corros. Sci. 2019, 146, 134–151. 10.1016/j.corsci.2018.10.002. [DOI] [Google Scholar]
- Negm N. A.; Al Sabagh A. M.; Migahed M. A.; Bary H. M. A.; El Din H. M. Effectiveness of some diquaternary ammonium surfactants as corrosion inhibitors for carbon steel in 0.5 M HCl solution. Corros. Sci. 2010, 52, 2122–2132. 10.1016/j.corsci.2010.02.044. [DOI] [Google Scholar]
- Döner A.; Yüce A. O.; Kardaş G. Inhibition effect of Rhodanine-N-Acetic acid on copper corrosion in acidic media. Ind. Eng. Chem. Res. 2013, 52, 9709–9718. 10.1021/ie400160x. [DOI] [Google Scholar]
- Khaled K. F.; Al-Qahtani M. M. The inhibitive effect of some tetrazole derivatives towards Al corrosion in acid solution: chemical, electrochemical and theoretical studies. Mater. Chem. Phys. 2009, 113, 150–158. 10.1016/j.matchemphys.2008.07.060. [DOI] [Google Scholar]
- Solmaz R.; Kardaş G.; Çulha M.; Yazıcı B.; Erbil M. Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim. Acta 2008, 53, 5941–5952. 10.1016/j.electacta.2008.03.055. [DOI] [Google Scholar]
- Sang X. J.; Li J. S.; Zhang L. C.; Wang Z. J.; Chen W. L.; Zhu Z. M.; Su Z. M.; Wang E. B. A novel carboxyethyltin functionalized sandwich-type germanotungstate: synthesis, crystal structure, photosensitivity, and application in dye-sensitized solar cells. Appl. Mater. Interfaces 2014, 6, 7876–7884. 10.1021/am501192f. [DOI] [PubMed] [Google Scholar]
- Sang X. J.; Li J. S.; Zhang L. C.; Zhu Z. M.; Chen W. L.; Li Y. G.; Su Z. M.; Wang E. B. Two carboxyethyltin functionalized polyoxometalates for assembly on carbon nanotubes as efficient counter electrode materials in dye-sensitized solar cells. Chem. Commun. 2014, 50, 14678–14681. 10.1039/c4cc06211f. [DOI] [PubMed] [Google Scholar]
- Sun H.; Guo L. Y.; Li J. S.; Bai J. P.; Su F.; Zhang L. C.; Sang X. J.; You W. S.; Zhu Z. M. Two new armtype polyoxometalates grafted on titanium dioxide films: towards enhanced photoelectrochemical performance. ChemSusChem 2016, 9, 1125–1133. 10.1002/cssc.201600131. [DOI] [PubMed] [Google Scholar]
- Xu W.; Han E.-H.; Wang Z. Effect of tannic acid on corrosion behavior of carbon steel in NaCl solution. J. Mater. Sci. Technol. 2019, 35, 64–75. 10.1016/j.jmst.2018.09.001. [DOI] [Google Scholar]
- Guo L. Y.; Li Y.; Zhang Y.; Li J.-S.; You W.-S.; Su F.; Zhu Z.-M.; Sang X.-J.; Zhang L.-C. Extended visible photosensitivity of carboxyethyltin functionalized polyoxometalates with common organic dyes enabling enhanced photoelectric performance. RSC Adv. 2017, 7, 20685–20693. 10.1039/c7ra02353g. [DOI] [Google Scholar]
- Miao M. Y.; Wang J. H.; Hu W. B. Synthesis, characterization and inhibition properties of ZnAlCe layered double hydroxide intercalated with 1-hydroxyethylidene-1,1-diphosphonic acid. Colloids Surf., A 2018, 543, 144–154. 10.1016/j.colsurfa.2018.01.056. [DOI] [Google Scholar]
- Yamashita T.; Hayes P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. 10.1016/j.apsusc.2007.09.063. [DOI] [Google Scholar]
- Caglar Y.; Caglar M.; Ilican S. XRD, SEM, XPS studies of Sb doped ZnO films and electrical properties of its based Schottky diodes. Optik 2018, 164, 424–432. 10.1016/j.ijleo.2018.03.017. [DOI] [Google Scholar]
- Xie F. Y.; Gong L.; Liu X.; Tao Y. T.; Zhang W. H.; Chen S. H.; Meng H.; Chen J. XPS studies on surface reduction of tungsten oxide nanowire film by Ar+ bombardment. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 112–118. 10.1016/j.elspec.2012.01.004. [DOI] [Google Scholar]
- Wan K.; Feng P.; Hou B.; Li Y. Enhanced corrosion inhibition properties of carboxymethyl hydroxypropyl chitosan for mild steel in 1.0 M HCl solution. RSC Adv. 2016, 6, 77515–77524. 10.1039/c6ra12975g. [DOI] [Google Scholar]
- Bösing M.; Loose I.; Pohlmann H.; Krebs B. New strategies for the generation of large heteropolymetalate clusters: the β-B-SbW9 fragment as a multifunctional unit. Chem. – Eur. J. 1997, 3, 1232–1237. 10.1002/chem.19970030810. [DOI] [Google Scholar]
- Botar B.; Yamase T.; Ishikawa E. A highly nuclear vanadium-containing tungstobismutate: synthesis and crystal structure of K11H[(BiW9O33)3Bi6(OH)3(H2O)3V4O10]·25H2O. Inorg. Chem. Commun. 2000, 3, 579–584. 10.1016/S1387-7003(00)00146-5. [DOI] [Google Scholar]
- Hutton R. E.; Burley J. W.; Oakes V. β-substituted alkyltin halides: I. Monoalkyltin trihalides: synthetic, mechanistic and spectroscopic aspects. J. Organomet. Chem. 1978, 156, 369–382. 10.1016/S0022-328X(00)93543-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.