Abstract
Triple-negative breast cancer (TNBC) has a high rate of metastasis, which is associated with breast cancer stem-like cells (CSCs). Although Taxol (micelle formulation of paclitaxel) is the first line chemotherapy to treat TNBC, it increases CSCs in residual tumors. Abraxane, albumin nanopartide of paclitaxel, showed lower plasma concentration compared to Taxol in both human and animal models, but it is not clear why Abraxane showed superior efficacy to Taxol in treatment of metastatic breast cancer in humans. In this study, we intend to investigate if Abraxane eliminates CSCs for its better efficacy. The results showed that Abraxane showed similar cytotoxicity in SUM149 cells in comparison with Taxol. Although Abraxane showed 3- to 5-fold lower blood drug concentration compared to Taxol, it achieved similar tumor drug concentration and 10-fold higher tumor/plasma ratio in SUM149 xenograft NOD/SCID mouse model. In addition, Abraxane and Taxol showed similar efficacy to shrink the tumor size in orthotopic breast cancer NOD/SCID mouse model. However, Abraxane decreased breast CSCs frequency by 3- to 9-fold, while Taxol increased breast CSCs frequency in an orthotopic breast cancer NOD/SCID mouse model. Furthermore, Abraxane increased 3- to 15-fold intracellular uptake in both ALDH+ CSCs and differentiated ALDH– cells in comparison with Taxol, which provides a mechanism for Abraxane’s superior efficacy to eliminate CSCs in comparison with Taxol. Our data suggest albumin nanopartide Abraxane may have a broad implication to enhance drug’s efficacy by eliminating breast cancer stem cells for treatment of metastatic diseases.
Keywords: Abraxane, Taxol, breast cancer stem cells, triple negative breast cancer, pharmacokinetics
Graphical Abstract

INTRODUCTION
Breast cancer remains the most common malignancy and the leading cause of cancer death among females worldwide.1 Based on the status of hormone receptors (estrogen receptor (ER) and progesterone receptor (PR)) and human epidermal growth factor receptor-2 (HER2)), breast cancers have been classified into at least four subtypes: luminal A (ER+ or PR+, Her2–), luminal B (ER+ or PR+, Her2+), Her2 amplified (ER– and PR–, Her2+), and triple-negative (ER–, PR–, Her2–).2,3 Triple-negative breast cancer (TNBC) represents about 20% of all breast cancers, with higher metastasis rate and poorer survival. Although significant advance has been made for treatment of ER+ and Her2+ breast cancer to improve patient survival, there is still lack of effective treatment for TNBC.
A new hypothesis suggests that breast cancer has a small subset of malignant cells with stem-like properties (~1–5% of the population), designated as cancer stem-like cells (CSCs).4-7 Cumulative compelling evidence suggests that breast cancer stem cells (CSCs) are responsible for tumor initiation and metastasis.8-19 CSCs possess abilities of both self-renewal, to maintain their own population, and differentiation, to produce more differentiated cancers to constitute the whole tumor. Two sets of markers have been used to identify breast cancer stem cells. One set of marker is high levels of aldehyde dehydrogenase 1 (ALDH1),9 which has been correlated significantly with node metastasis, poor prognoses, and poor survival in breast cancers.20,21 The other set of marker is CD44+/CD24−, which is a combination of cell surface markers with properties of driving tumorigenesis and generating tumor cell heterogeneity.4
Chemotherapy using paclitaxel is the first line therapy for the treatment for TNBC. 2-29 Taxol, as micelle formulation of paclitaxel using Kolliphor EL, is used in TNBC to reduce overall tumor burden predominantly by killing differentiated subsets of cancer cells while actually increasing subsets of stem -like cells (CSCs) by 2–4-fold.14-19,30-33 Expanding the subset of cells with stem -like functions elevates the potential for recurrent local and/or metastatic disease.8-10,34-48 Different mechanisms are proposed to explain this phenomenon. Some studies attribute increases in CSCs to inherent resistance of these cells to chemotherapy,49 while others suggest chemotherapy causes differentiated cancer cells to dedifferentiate to stem-like cells, resulting in expansion of CSCs.
Very interestingly, Abraxane, an albumin-bound nanoparticle of paclitaxel, had been approved by the FDA as the first line therapy for metastatic breast cancer, in addition to as the first-line treatment of advanced nonsmall cell lung cancer and late-stage (metastatic) pancreatic cancer. Abraxane showed superior efficacy (response rate 21.5%) to Taxol (response rate 11.1%) in metastatic breast cancer patients.50 Pooled-analysis of multiple clinical trials indicated that pathological complete response (pCR) in breast cancer patients treated with Abraxane was significantly higher than patients treated with Taxol.51-53
However, it is surprising that Abraxane’s superior efficacy cannot be explained by the plasma concentration and plasma pharmacokinetics since Abraxane achieved lower plasma concentration as compared to Taxol in both humans and mice.54-57
It is not clear why Abraxane showed superior efficacy to Taxol in metastatic breast cancer patients. It is unknown whether Abraxane has superior efficacy to eliminate breast cancer stem cells, which is associated with its clinical superior efficacy in metastatic breast cancer. Given Abraxane encapsulates the same drug (paclitaxel) as the clinically used micelle formulation (Kolliphor EL), the mechanism of action of drug to inhibit cancer cells is unlikely to be changed. Therefore, the superior efficacy of abraxane is likely due to the nanoformulation change, which needs to be explored.
In this report, we intend to investigate the efficacy and mechanism of Abraxane to eliminate cancer stem-like cells in vitro and in vivo in comparison with Taxol. We first compared the drug concentrations of tumor and plasma in xenograft model following the same dose of Abraxane and Taxol. We also compared the efficacy of Abraxane and Taxol in vitro of 2D cell culture, 3D mammosphere, and in vivo xenograft model using two different treatment regimens. The efficacies of Abraxane and Taxol on cancer stem-like cells were evaluated using five different gold standard cancer stem cells assays. Finally, we evaluated the cellular uptake of Abraxane, Taxol, and a simple mixture of paclitaxel and albumin in cancer stem-like cells and differentiated cancer cells to explore its mechanism to eliminate cancer stem-like cells. Our data provide a rationale to use an appropriate nanodelivery system (such as Abraxane) to eliminate breast cancer stem cells, which may have broad implication to improve drug’s efficacy to treat metastatic disease.
MATERIALS AND METHODS
Drugs.
Taxol (Hospira, clinical used Kolliphor EL formulation) and Abraxane (Celgene) were obtained through the University of Michigan Cancer Center Pharmacy (Ann Arbor, MI). The particle size and zeta potential were measured by dynamic fight scattering (DLS) using a Zetasizer Nano (Malvern,Worcestershire, UK) (Figure S1).
Cell Culture and Reagents.
SUM149 cells were cultured in Ham’s F-12 supplemented with 5% fetal bovine serum, 5 μg/mL insulin, 2 μg/mL Hydrocortisone, 4 μg/mL Gentamycin, and 1% antibiotic-antimycotic under a 5% CO2 cell culture incubator with saturated humility. All cell culture media and reagents were purchased from Thermo Fisher Scientific or Sigma unless stated elsewhere.
MTS Cell Viability Assay.
SUM149 cells were plated on 96-well plates at cell density of 3000 cells/well and cultured under a 5% CO2 cell culture incubator with saturated humility. Cells were treated with various concentrations of paclitaxel and Abraxane for 72 h. MTS assay (Promega, Madison, WI) was used to assess cell viability according to the manufacturer’s instruction. The IC50s of cytotoxicity were calculated with GraphPad Prism 7 software (GraphPad Software Inc., La Jolla, CA 92037 USA).
Cellular Uptake Assays.
SUM149 cells were plated on a 6-well plate 24 h prior to the uptake assays. The drugs were diluted into PBS and added to cells and cultured in a cell culture incubator. After removing the drugs and extensive washing with cold PBS, cells were lysed with RIPA buffer supplemented with proteinase inhibitors (Thermo Scientific Halt Protease Inhibitor Cocktail, EDTA-free) and collected for further analysis. Drug concentrations were determined using LC-MS/MS, and protein concentrations were measured using Pierce BCA Protein Assay Kit according to manufacturer’s instructions. The drug uptake was normalized by dividing the total drug amount with the total protein amount.
Flow Cytometry and Immunohistochemistry Staining of ALDH+ CSCs in Primary Tumors and SUM149 Cells.
ALDH+ CSCs in dissociated primary tumor cells and SUM149 cells were accessed using ALDEFLURO kits according to manufacturer’s instructions (StemCell Technogies, Inc., Vancouver, BC, Canada). Flow cytometry analysis was performed on a MoFlo Astrios (Beckman Coulter, Inc.) flow cytometer with a minimum of 10,000 live cells detected. IHC staining of ALDH1 (BD Biosciences, #611195) in sectioned paraffin embedded primary tumors slides was performed by In-Vivo Animal Core (IVAC) at University of Michigan using the Beckman intellipath system with standard protocol. Slides were scanned on Aperio At-2, and images were analyzed by IVAC faculty.
Flow Cytometry of CD44+/CD24− CSCs in Primry Tumor and SUM149 Cells.
Dissociated primary tumor cells or SUM149 cells were stained with anti-CD44 (G44–26 clone, BD Biosciences) and anti-CD24 (ML5 clone, BD Biosciences) antibodies. After extensive washing, samples were analyzed on a MoFlo Astrios (Beckman Coulter, Inc.) flow cytometer with a minimum of 10,000 live cells detected. Percentages of CD44+/CD24− CSCs were analyzed using Summit’s software.
Mammosphere Formation Assay.
SUM149 cells were dissociated into single cells and seeded at a density of 2000 cells/well in Mammocult (StemCell Technologies) in ultralow attachment 6-well plates (Coming Inc., Corning, NY, USA). The drugs were added to cells at indicated concentrations. After culturing for 14 days, primary mammospheres greater than 50 μm in diameter were counted using GelCount (Oxford Optronix Ltd.). All cells from primary culture were collected and further dissociated into single cells and seeded at a density of 500 cells/well in Mammocult (StemCell Technologies) in ultralow attachment 6-well plates (Corning Inc., Corning, NY, USA) for secondary mammosphere formation. After culturing for another 14 days, mammospheres greater than 50 μm in diameter were counted using GelCount (Oxford Optronix Ltd.). Mammosphere formation efficiency (MFE) was calculated as number of mammospheres per well/numbers of cells seeded per well ×100.
Mouse Orthotopic Xenograft Models.
All experimental studies involving the use of mice were performed with prior approval of Institutional Animal Care and Use Committee (IACUC) at the University of Michigan (Animal Protocols: PRO00005661, PRO00007728), in accordance with the guidelines established by the National Research Council Guide for the Care and Use of Laboratory Animals (NIH). 6–8 weeks old female nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice were obtained from Jackson Laboratory (Stock No: 001303). Orthotopic xenograft formation in NOD/SCID mouse model was generated by direct injection of SUM149 cells (1,000,000 cells/injection for advanced treatment model, 50,000 cells/injection for adjuvant treatment model), suspended in Matrigel Membrane Matrix (Corning, CB-40234), into the exposed fourth mammary fat pad. Taxol (clinically used Kolliphor EL micelle formulation, 10 mg/kg once every week) and Abraxane (10 mg/kg once every week) were administered intravenously 1 day after surgery for the adjuvant treatment model (4 doses in total) or when tumor volume reached approximately 150 mm3 for the advanced treatment model (5 doses in total). When control tumors reached approximately 1,500 mm3, mice were euthanized with CO2 inhalation and tumors were resected.
Secondary Tumor Reimplantation Assay.
Isolated primary tumors were dissociated into single cells using gentleMACS Octo Dissociator and tumor dissociation kit (Miltenyi Biotec) according to manufacturer’s instructions. Human tumor cells were then isolated using fluorescent activated cell sorting on a MoFlo Astrios (Beckman Coulter, Inc.) flow cytometer. NOD/SCID mice were transplanted with 100, 1,000, and 10,000 sorted human tumor cells for orthotopic xenograft formation as described above. Tumor formation rates in secondary mice were assessed 9–12 weeks following implanting cells by direct palpitation and measured with a digital caliber.
Paclitaxel Tumor Concentrations Measured by LC-MS/MS.
Orthotopic xenograft formation in NOD/SCID mouse model was generated as described above for advanced treatment model. When tumors reached 150–500 mm3, Taxol and Abraxane (10 mg/kg once) were administered intravenously. At designated time points after drug administration (0.08, 0.17, 0.25, 0.5, 0.75, 1, 2, 4, 7, 16, 24, 48, and 72 h), 3 mice from each treatment group were euthanized using isoflurane, and blood was immediately collected via cardiac puncture using a 25-G needle and 1 mL syringe (pretreated with sodium heparin). Plasma was collected after the blood was centrifuged at 14,500 rpm for 10 min. Tumors were removed from the mouse and rinsed extensively in phosphate-buffered saline (pH 7.4). The tumors were transferred to a tube from the Precellys CK28 Lysing Kit (Montigny-le-Bretonneux, France) and stored at −80 °C until further analysis with LC–tandem mass spectrometry (LC-MS/MS). Concentrations of paclitaxel were determined using an AB-5500 Qtrap (Sciex, Concord, ON, Canada) mass spectrometer with electrospray ionization source interfaced with a Shimadzu high-performance liquid chromatography system. Analyst Software (version 1.6) from Applied Biosystems (MDS SCIEX; Carlsbad, CA, USA) was used to control the LC-MS/MS system, as well as for data acquisition and processing. Separation was performed on an Xbridge C18 column (50 × 2.1 mm ID, 3.5 μm; Waters, Milford, MA, USA) at a flow rate of 0.4 mL/min. The mobile phase consisted of A (100% H2O with 0.1% formic acid) and B (100% acetonitrile with 0.1% formic acid). The gradient started with 25% B for 30 s, linearly increased to 65% B at 2 min, increased to 95% B at 2.5 min, was maintained at 95% B for 2 min, decreased to 25% B at 5 min, and was maintained at 25% for 2 min. The mass spectrometer was operated in a positive mode with multiple reaction monitoring for analysis. The gas temperature was 300 °C with an ion spray voltage of 5500 V, gas 1 and gas 2 of 60 psi, and curtain gas of 30 psi. The PK parameters of paclitaxel from pac-T, nab-P, m-nab-P, pac-P, and pac-G were compiled and calculated with Phoenix/WinNonlin (version 6.4; Phar-sight, Mountain View, CA, USA).
Statistical Analysis.
One-way ANOVA was used if the comparison involved more than two groups. Two-tailed Student’s t test was used to compare the statistical difference between two groups. A P-value < 0.05 was considered significant.
RESULTS
Abraxane Showed Similar Tumor Drug Concentration and Lower Blood Drug Concentration in SUM149 Xenograft NOD/SCID Mouse Compared to Taxol.
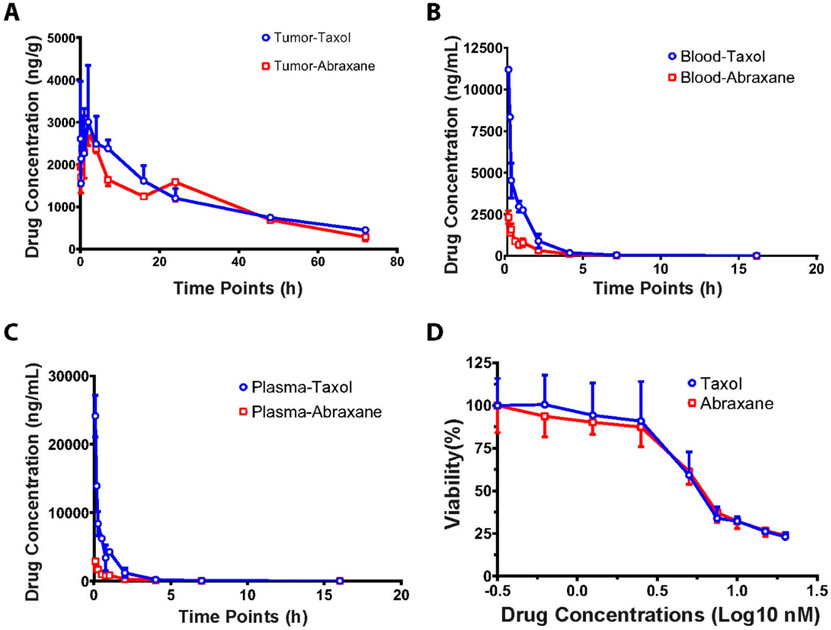
Although Abraxane demonstrated its superior efficacy to Taxol for metastatic breast cancer patients,50,52,58 plasma drug concentration is 3- to 5-fold lower in humans after dosing with Abraxane in comparison to Taxol,54-56 which cannot explain its superior efficacy. In order to test if Abraxane’s superior efficacy to Taxol may be attributed by higher drug accumulation in tumors, we measured the drug concentrations in tumor tissues using orthotopic breast cancer mouse model (SUM149 cells implanted in fat pad in NOD/SCOID mouse) dosed with Abraxane and Taxol intravenously. Surprisingly, we did not observe significant difference of drug concentration in tumors treated with Abraxane or Taxol (Figure 1A). In contrast, we observed that the drug concentrations in blood and plasma of Abraxane were 5-fold lower than that of Taxol (Figure 1B, 1C), which is similar to our previous studies.57 These results are consistent with clinical observation for the plasma concentration of Abraxane and Taxol in breast cancer patients.54-56 These results cannot explain why Abraxane has better efficacy than Taxol in metastatic breast cancer.
Figure 1.
Abraxane showed similar cytotoxicity in vitro, similar tumor drug concentration, and lower blood drug concentration in SUM149 xenograft NOD SCID mouse model compared to Taxol. For in vivo assay, Abraxane and Taxol were administrated intravenously in SUM 149 xenograft NOD SCID mice and the blood, plasma, and tumor samples were taken at 13 time points. The drug concentrations were measured with LC-MS/MS. The drug concentrations in tumors were similar in both Abraxane and Taxol groups as shown in (A). The drug concentrations in blood (B) and plasma (C) were much lower in the Abraxane group than those in the Taxol group. (D) SUM149 cells were treated with various concentrations of Abraxane and Taxol in vitro. The cell viability was measured with MTS assays at 72 h.
Abraxane and Taxol Showed Similar Cytotoxicity in SUM149 Cells in Vitro, Similar Efficacy to Inhibit Tumor Size in Orthotopic Breast Cancer Xenograft in NOD/SCID Mouse in Advanced Treatment Regime.
To further test if Abraxane has superior efficacy to inhibit TNBC cell proliferation than Taxol, we used MTS assay to measure the IC50 of both Abraxane and Taxol to SUM149 cell lines. The data showed that Abraxane and Taxol showed similar IC50s, with 6.57 nM for Abraxane and 6.61 nM for paclitaxel (Figure 1D).
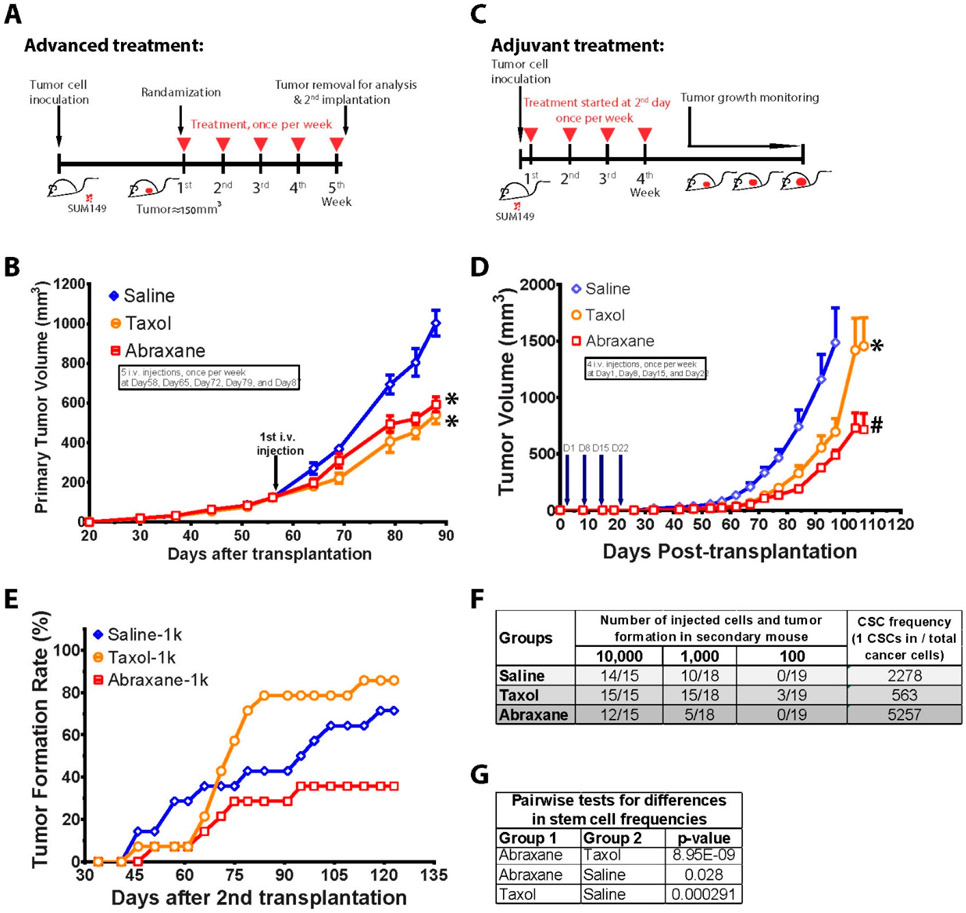
To test whether Abraxane has better efficacy than Taxol to inhibit TNBC tumor growth, we used orthotopic breast cancer xenograft in NOD/SCID mouse model using human TNBC cells (SUM 149) in an advanced treatment setting (Figure 2A). A total of 1,000,000 SUM149 cells were injected into the fourth mammary fat pad of NOD/SCID mouse, and the drug treatment started when tumor sizes reached to about 150 mm3. Tumor sizes were recorded every 5 days. Interestingly, we found both Taxol and Abraxane treatment showed similar efficacy to inhibit tumor growth (Figure 2B). Yet there was no difference between Abraxane and Taxol treatment groups to inhibit primary tumor size in the orthotopic breast cancer model. The pictures and tumor weight after treatment of Abraxane and Taxol are showed in Supporting Information Figure S2.
Figure 2.
Abraxane and Taxol showed the same efficacy to inhibit tumor size, but Abraxane decreased breast cancer stem cells, while Taxol increased breast cancer stem cells, in orthotopic breast cancer mouse model. (A) Advanced treatment scheme: SUM149 cells were inoculated into the fourth fat pad of NOD SCID mice. Tumors were developed until the average size reached to about 150 mm3. The mice were randomized into three groups, and treatment started with administration of Taxol and Abraxane intravenously using the same dose (10 mg/kg), once per week, 5 times in total. One day after the last drug administration, mice were sacrificed and tumors were removed and dissociated into single cell suspension for further analysis. (B) Both Abraxane and Taxol showed similar efficacy to shrink tumors compared to saline control. * P < 0.05 compared to control group. (C) Adjuvant treatment scheme: SUM149 cells were inoculated into the fourth fat pad of NOD SCID mice. One day after inoculation, treatment started with administration of Taxol and Abraxane intravenously, once per week, 4 times in total. The tumor growth was monitored every 5 days until the end of the experiment. (D) Abraxane is superior to Taxol in adjuvant treatment setting in orthotopic breast cancer NOD SCID mouse model. Tumor growth was delayed in both Abraxane and Taxol groups compared to saline control as shown in tumor volumes. *P < 0.05 compared to control group; #P < 0.05 compared to Taxol group. (E). Cancer stem cell frequency was assessed by reimplantation of sorted single tumor cells from primary residual tumors (from 2B) into new NOD SCID mice. Tumor formation rates were shown with reimplantation of 1,000 sorted primary tumor cells from indicated treatment groups. (F) Estimated CSC frequencies and (G) pairwise test in Abraxane, Taxol, and saline control groups.
Abraxane Decreased Breast Cancer Stem Cells, While Taxol Increased Breast Cancer Stem Cells, in Orthotopic Breast Cancer Xenograft NOD/SCID Mouse in Advanced Treatment.
Since Abraxane showed better efficacy than Taxol in metastatic breast cancer, while breast cancer metastasis is associated with breast cancer stem cells, we postulated that Abraxane may reduce breast cancer stem cells more efficiently than Taxol.
In the xenograft model for evaluation of drug anticancer efficacy, the treatment is usually started when tumor size reaches 50 to 150 mm3. Tumor size shrinkage is used as the gold standard as measurement of a drug’s efficacy. However, tumor size shrinkage is mainly contributed by the reduction of bulk differentiated cancer cells. Since cancer stem-like cells are only a small portion of the total tumor mass (1–5%), the tumor size change can not reflect the drug’s efficacy to eliminate cancer stem-like cells. In contrast, Taxol increases cancer stem-like cells while reducing tumor size.8-10,14-19,30-48 Therefore, the gold standard assays to evaluate cancer stem-like cell frequency are using secondary implantation by extreme limiting dilution from the residual tumors of the primary implantation.
Therefore, we used secondary implantation to evaluate Abraxane’s efficacy to eliminate cancer stem-like cells. Even though Abraxane did not show any difference to Taxol to shrink the tumor size in primary tumor implantation (Figure 2B), we collected the primary tumors and then dissociated into single cell suspension for flow cytometry sorting and analysis. The sorted SUM149 cells were reimplanted to the fat pad of mammary gland of new NOD/SCID mice (secondary reimplantation) to assess the frequencies of CSCs in primary tumors. Consistent with the previous studies,14,59-62 taxol indeed significantly increased the tumor regrowth rate in secondary implantation from the residual tumor of primary tumor (Figure 2E), and increased 4-fold CSCs frequency compared to the control group (Figure 2F and 2G). However, Abraxane did not increase secondary tumor regrowth rate (or may decrease) and decreased CSC frequency by more than 2-fold and 9-fold compared to the control group and the Taxol group, respectively (Figure 2F, 2G).
To examine the efficacy of Abraxane on ALDH+ CSCs, we measured the percentage of ALDH+ CSCs in primary tumors using both FACS (Figure 3A) and IHC staining with anti-ALDH1 antibody (Figure 3C, D). As shown in Figure 3, the percentage of ALDH+ CSCs in tumors from the Taxol treatment group is 3-fold higher than that of the saline control group, whereas there was no significant difference between the Abraxane and saline control groups. IHC staining confirmed the same finding from flow analysis of ALDH+ CSCs in primary tumors. In addition, we also evaluated the drug’s efficacy on another type of CSCs (CD44+/CD24−) in the residual tumors in vivo using FACS. The data showed that Taxol (10 mg/kg) increased CD44+/CD24− CSCs, but Abraxane (10 mg/kg) did not alter CD44+/CD24− CSCs compared to control group (Figure 3B). Taken together, our data demonstrated that Abraxane eliminates breast CSCs, while Taxol increases breast CSCs in TNBC in vivo.
Figure 3.
Abraxane decreased breast cancer stem cells in primary tumors in SUM 149 xenograft NOD/SCID mouse model in advanced treatment regime. Abraxane decreased breast CSCs in primary tumors compared to Taxol treatment with both Flow analysis (A, B) and IHC analysis of primary tumors (C, D). Scale bar in C is 200 μm. *P < 0.05 compared to control group. **P < 0.01 compared to control group.
Abraxane is Superior to Taxol in Adjuvant Treatment to Delay Tumor Growth in Orthotopic Breast Cancer Xenograft NOD/SCID Mouse Model.
Another model to evaluate drug’s efficacy to eliminate cancer stem cells is to use the adjuvant setting treatment, where tumor regrowth is observed after cessation of drug treatment. If the drug enriches CSCs population, the tumor would be rapidly regrown after cessation of drug treatment. In contrast, if the drug inhibits the CSCs, the regrowth rate of the residual tumor will be slower.
Therefore, we also evaluated if Abraxane has better efficacy than Taxol in the adjuvant setting. Interestingly, Abraxane showed better efficacy than Taxol to delay tumor regrowth after cessation of treatment in the orthotopic breast cancer xenograft NOD/SCID mouse model in an adjuvant treatment setting (Figure 2C). A total of 50,000 SUM149 cells were injected into the fourth mammary fat pad of NOD/SCID mouse, and the drug treatment started 1 day after the cancer cell implantation. After four doses (10 mg/kg, once a week) treatment, tumor growth was observed for 120 days. Tumor sizes were recorded every 5 days. The data showed that both Taxol and Abraxane treatment showed significant tumor size shrinkage compared to the saline control group (Figure 2D). More importantly, Abraxane treatment showed 3-fold better efficacy than the Taxol group to delay tumor growth (Figure 2D).
Abraxane Showed Superior Activity in Inhibiting CSCs in Vitro and Mammosphere Formation in Comparison to Taxol.
To confirm these in vivo data, we also tested the in vitro activity of Abraxane and Taxol against both ALDH+ CSCs and CD44+/CD24− CSCs in SUM149 cells by FACS. The data showed that Abraxane (5, 10 nM) reduced ALDH+ CSCs by 3- to 4-fold, which is superior to Taxol (Figure 4A, 4B). However, neither Abraxane nor Taxol inhibited the CD44+/CD24− CSCs in vitro (Figure 4C, 4D).
Figure 4.
Abraxane showed superior activity in inhibiting CSCs in vitro and mammosphere formation in comparison to Taxol. The SUM 149 cells were incubated with Abraxane or Taxol at 1, 5, and 10 nM for 72 h. The ALDH+ CSCs were measured by Aldeflour assay (A and B). CD44+/CD24− CSCs were measured by FACS with antibody staining (C and D). T-1, T-5, T-10: Taxol 1, 5, 10 nM. A-1, A-5, A-10: Abraxane 1, 5, 10 nM. The SUM 149 cells were cultured in suspension to form 3D mammosphere. The mammosphere formation efficiencies are shown in E (primary) and F (secondary). T-0.2, T-1, T-5: Taxol 0.2, 1, 5 nM. A-0.2, A-1, A-5: Abraxane 0.2, 1, 5 nM.
Furthermore, CSCs have self-renewal ability which can be assessed in 3D primary and secondary mammosphere assays. Usually many chemotherapeutic drugs would inhibit primary mammosphere formation. However, if the drug does not eliminate CSCs, it would not interfere with the secondary mammosphere formation when the drug is not present in the cell culture media. As shown in Figure 4F, Abraxane (5, 10 nM) inhibited secondary mammosphere formation while Taxol increased secondary mammosphere formation, although both drugs inhibited primary mammosphere formation (Figure 4E). These data suggest Abraxane is able to inhibit CSCs while Taxol increases CSCs.
Abraxane Enhanced Drug Uptake in Both Differentiated Cells and Cancer Stem Cells in TNBC.
Although drug tumor concentrations are similar after the same dose of Abraxane and Taxol, Abraxane has 5-fold lower plasma drug concentration than Taxol. Therefore, when the drug tumor concentration was normalized to its plasma concentration, the concentration ratio between tumor and plasma in the Abraxane group is much higher than that of the Taxol group, which may suggest better drug uptake and better penetration into tumors compared to Taxol (Figure 5A). This is consistent with previous observations in mouse model and clinical trial data.54-57 Therefore, we tested if Abraxane can enhance drug uptake in TNBC cells in vitro.
Figure 5.
Abraxane enhanced drug uptake in both differentiated cancer cells and cancer stem cells in TNBC. (A) Abraxane achieved higher tumor to plasma concentration ratio compared to Taxol. (B) SUM149 cells took up more Abraxane than Taxol when treated with 2 μM drugs for 5, 10, and 60 min. **P < 0.01 compared to Taxol treatment. (C) Both ALDH+ CSCs and ALDH− non-CSCs took up more Abraxane than Taxol when SUM149 cells were treated at 2 μM drugs for 60 min. **P < 0.01 compared to Taxol treatment. ****P < 0.001 compared to Taxol treatment. (D) Simple mix of albumin and paclitaxel did not enhance uptake of paclitaxel in comparison to Abraxane. ***P < 0.001 compared to Taxol treatment. ##P < 0.01 compared to simple mix of HSA treatment. ###P < 0.001 compared to simple mix of HSA treatment.
As shown in Figure 5B, Abraxane was taken up by SUM 149 cells significantly (8- to 15-fold) more than Taxol (clinically used Taxol micelle formulation with Kolliphor EL) at the same concentration at 2 μM for 5, 10, and 60 min incubation periods. Since Abraxane eliminated ALDH+ CSCs in SUM149 xenograft NOD/SCID mouse model and SUM149 cell line, we also investigated the cellular uptake of Abraxane and Taxol in ALDH+ and ALDH− cells. We sorted out both ALDH+ and ALDH− population using FACS for cellular uptake assays to distinguish whether ALDH+ CSCs and ALDH− non-CSCs uptake the drug in a different manner. As shown in Figure 4C, both ALDH+ and ALDH− SUM149 cells took up more Abraxane than Taxol (clinically used micelle formulation) at 2 μM concentration when incubated for 60 min. These data suggest that nanoparticle formulation in Abraxane enhanced intracellular uptake of the drug compared to Taxol (clinically used micelle formulation with Kolliphor EL).
A nanoparticle of Abraxane is not stable in the plasma, which is dissociated within a few minutes. Therefore, it is suspected that Abraxane solely increased solubility and avoided the side effect of Kolliphor EL compared to Taxol. Since paclitaxel has high protein binding (95%) to human serum albumin (HSA), thus Abraxane, once dissociated, would behave similarly to Taxol once they are injected to the bloodstream, which will bind to HSA. However, this explanation would not explain why Abraxane showed superior efficacy to Taxol in treating metastatic breast cancer, lung cancer, and pancreatic cancer. One hypothesis is that a higher dose of Abraxane (260 mg/m2 for 30 min infusion) is used than Taxol (175 mg/m2 for 3 h infusion) to achieve better efficacy. However, the counter argument is that the plasma concentration from the Abraxane regimen (260 mg/m2 for 30 min infusion) is lower than that of the Taxol dose regimen (175 mg/m2 for 3 h infusion) in clinical patients, which contradicts the observation of Abraxane’s superior clinical efficacy in three types of cancers. We previously reported that Abraxane increased drug distribution/penetration potential in the breast, lung, and pancreatic tissues,57 which may also explain its superior efficacy in these three types of cancers.
To test if Abraxane nanopartide formulation is different from a simple mixture of albumin and paclitaxel for cellular uptake, we compared uptake of Abraxane, clinically used Taxol, and a simple mixture of paclitaxel (dissolved in DMSO) and human serum albumin (w:w of drug/HSA = 1:9) (Figure 5D). In contrast to Abraxane, the simple mixture of paclitaxel (in DMSO) and human serum albumin did not change the cellular uptake of paclitaxel. These data suggest that the Abraxane nanoparticle and formulation process play a critical role to enhance intracellular uptake of paclitaxel in both differentiated cancer cells and cancer stem cells, which is associated with Abraxane’s superior efficacy in metastatic breast cancer.
DISCUSSION
Due to its superior efficacy to Taxol, Abraxane had been approved by FDA for treatment of metastatic breast cancer, advanced nonsmall cell lung cancer, and late-stage (metastatic) pancreatic cancer in the past two decades.50,63,64 Yet its mechanisms remain elusive. In the current study, we showed that Abraxane can be used to treat TNBC in both adjuvant and advanced settings in SUM 149 xenograft NOD/SCID mouse models. In adjuvant settings, Abraxane is superior to Taxol in delaying tumor growth. This is consistent with the reported clinical studies.51
In TNBC, ALDH+ CSCs had been associated with tumor initiation, progression, metastasis, and chemo-resistance.65-68 Both in vitro and in vivo data demonstrated that Abraxane eliminates ALDH+ CSCs whereas Taxol increases ALDH+ CSCs in TNBC. This is the first study showing Abraxane reduces ALDH+ CSCs in TNBC, which is attributed to its superior efficacy to Taxol in metastatic TNBC. Given the fact that ALDH1 has been a valid CSC marker in other solid tumors,69-75 it would be interesting to explore further whether Abraxane is superior to Taxol in these cancer patients such as nonsmall cell lung cancer, and late-stage (metastatic) pancreatic cancer since ALDH1 is the marker for CSCs in both cases.70,72
It is worth noting that we did not observe any difference for tumor size inhibition in orthotopic breast cancer model by either Abraxane or Taxol. However, previous reports using a xenograft model (using various cell lines, including lung, breast, ovarian, prostate, colon, rhabdomyosarcoma, osteosarcoma, neuroblastoma, esophageal adenocarcinoma) in the flanks of the mice showed Abraxane had better tumor inhibition than Taxol.76-79 These data suggest that the xenograft model may exaggerate the efficacy of nanodelivery system, and a more clinically relevant cancer model should be used to evaluate the nanodelivery system. Regardless, these data further highlight the importance of Abraxane to eliminate cancer stem cells for better efficacy in clinical settings.
There may be two possibilities for why Abraxane reduces breast CSCs in TNBC tumors. First, at the tissue level, although equal doses of Abraxane and Taxol achieved similar drug tumor concentrations, the ratio of tumor/plasma concentration in the Abraxane group was significantly higher than that of Taxol. This indicates that Abraxane may penetrate better into tumor tissue. Our data are consistent with previous reports on pharmacokinetic modeling based on human clinical data and drug concentration measurement in normal breast tissues in animal models.50,54-57 It has been hypothesized that albumin-mediated delivery resulted in enhanced transport of nab-paclitaxel to tumors.79 Second, at the cellular level, our data showed that the Abraxane facilitated significantly higher paclitacel uptake by TNBC cells, which is different from the simple mixture of paclitaxel and human serum albumin (Figure 5).
These data suggest that nanoformulation of albumin and the process are critical in order to achieve higher cellular uptake. The efficient uptake by both cancer stem cells and differentiated cancer cells provided adequate intracellular concentration to effectively eliminate breast cancer stem cells.
CONCLUSION
Although Abraxane showed similar cytotoxicity in SUM149 cells in comparison with Taxol, while Abraxane showed 3- to 5-fold lower blood drug concentration compared to Taxol, it achieved similar drug tumor concentration and 10-fold higher tumor/plasma ratio in SUM149 xenograft NOD/SCID mouse model. In addition, Abraxane and Taxol showed similar efficacy to shrink tumor size in orthotopic breast cancer xenograft cancer in NOD/SCID mouse in advanced treatment regime. However, Abraxane is superior to Taxol (3-fold) in adjuvant treatment to delay tumor growth in orthotopic breast cancer xenograft NOD SCID mouse model. Furthermore, the secondary implantation assay and FACS analyses showed Abraxane decreased breast CSCs frequency by 3- to 9-fold, while Taxol increased breast CSCs frequency in orthotopic breast cancer xenograft in NOD/SCID mouse. The in vitro cell uptake assay showed that Abraxane increased intracellular uptake (3- to 15-fold) in both ALDH+ CSCs and ALDH− differentiated cells, which provides a mechanism for the superior efficacy of Abraxane to eliminate cancer stem cells compared to Taxol. Taken together, our data suggest an albumin nanopartide Abraxane enhanced drug efficacy to eliminate breast cancer stem cells, which may have broad implication for treatment of metastatic disease.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was partially supported by the National Cancer Institutes of Health under Award Number P30CA046592 by the use of the following Cancer Center Shared Resource(s): Flow Cytometry; Pharmacokinetics).
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.molpharmaceut.9b01221.
Particle size and zeta potentials of Abraxane (albumin nanoparticles) and Taxol (paclitaxel micelle formulation with cremophor EL), tumor pictures and weight from orthotopical xenograft mice after treatment of Abraxane (10 mg/kg) and Taxol (paclitaxel micelle formulation with Kolhphor EL) (PDF)
REFERENCES
- (1).Torre LA; Bray F; Siegel RL; Ferlay J; Lortet-Tieulent J; Jemal A Global cancer statistics, 2012. Ca-Cancer J. Clin 2018, 65, 87–108. [DOI] [PubMed] [Google Scholar]
- (2).Dai X; Li T; Bai Z; Yang Y; Liu X; Zhan J; Shi B Breast cancer intrinsic subtype classification, clinical use and future trends. American journal of cancer research 2015, 5, 2929–2943. [PMC free article] [PubMed] [Google Scholar]
- (3).Hennigs A; Riedel F; Gondos A; Sinn P; Schirmacher P; Marme F; Jager D; Kauczor HU; Stieber A; Lindel K; Debus J; Golatta M; Schutz F; Sohn C; Heil J; Schneeweiss A Prognosis of breast cancer molecular subtypes in routine clinical care: A large prospective cohort study. BMC Cancer 2016, 16, 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Al-Hajj M; Wicha M; Benito-Hernandez A; Morrison S; Clarke M Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A 2003, 100, 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Korkaya H; Kim GI; Davis A; Malik F; Henry NL; Ithimakin S; Quraishi AA; Tawakkol N; D’Angelo R; Paulson AK; Chung S; Luther T; Paholak HJ; Liu S; Hassan KA; Zen Q; Clouthier SG; Wicha MS Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell 2012, 47, 570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Korkaya H; Paulson A; Charafe-Jauffret E; Ginestier C; Brown M; Dutcher J; Clouthier SG; Wicha MS Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009, 7, No. e1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Liu S; Cong Y; Wang D; Sun Y; Deng L; Liu Y; Martin-Trevino R; Shang L; McDermott SP; Landis MD; Hong S; Adams A; D’Angelo R; Ginestier C; Charafe-Jauffret E; Clouthier SG; Birnbaum D; Wong ST; Zhan M; Chang JC; Wicha MS Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014, 2, 78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Al-Hajj M; Wicha MS; Benito-Hernandez A; Morrison SJ; Clarke MF Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A 2003, 100, 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ginestier C; Hur MH; Charafe-Jauffret E; Monville F; Dutcher J; Brown M; Jacquemier J; Viens P; Kleer CG; Liu S; Schott A; Hayes D; Birnbaum D; Wicha MS; Dontu G ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell 2007, 1, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Prince ME; Sivanandan R; Kaczorowski A; Wolf GT; Kaplan MJ; Dalerba P; Weissman IL; Clarke MF; Ailles LE Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Li C; Heidt DG; Dalerba P; Burant CF; Zhang L; Adsay V; Wicha M; Clarke MF; Simeone DM Identification of pancreatic cancer stem cells. Cancer Res. 2007, 67, 1030–1037. [DOI] [PubMed] [Google Scholar]
- (12).Ho MM; Ng AV; Lam S; Hung JY Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007, 67, 4827–4833. [DOI] [PubMed] [Google Scholar]
- (13).Bertolini G; Roz L; Perego P; Tortoreto M; Fontanella E; Gatti L; Pratesi G; Fabbri A; Andriani F; Tinelli S; Roz E; Caserini R; Lo Vullo S; Camerini T; Mariani L; Delia D; Calabro E; Pastorino U; Sozzi G Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 16281–16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Park SY, Kim MJ, Park SA, Kim JS, Min KN, Kim DK, Lim W, Nam JS, Sheen YY Combinatorial TGF-beta attenuation with paclitaxel inhibits the epithelial-to-mesenchymal transition and breast cancer stem-like cells. Oncotarget 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Abubaker K; Luwor RB; Escalona R; McNally O; Quinn MA, Thompson EW, Findlay JK, Ahmed N Targeted Disruption of the JAK2/STAT3 Pathway in Combination with Systemic Administration of Paclitaxel Inhibits the Priming of Ovarian Cancer Stem Cells Leading to a Reduced Tumor Burden. Front. Oncol 2014, 4, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lee H, Kim JB, Park SY, Kim SS, Kim H Combination effect of paclitaxel and hyaluronic acid on cancer stem-like side population cells. J. Biomed. Nanotechnol 2013, 9, 299–302. [DOI] [PubMed] [Google Scholar]
- (17).Craveiro V, Yang-Hartwich Y, Holmberg JC, Sumi NJ; Pizzonia J; Griffin B, Gill SK; Silasi DA; Azodi M, Rutherford T, Alvero AB, Mor G Phenotypic modifications in ovarian cancer stem cells following Paclitaxel treatment. Cancer Med. 2013, 2, 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chen J; Chen Y; Chen Z MiR-125a/b regulates the activation of cancer stem cells in paclitaxel-resistant colon cancer. Cancer Invest. 2013, 31, 17–23. [DOI] [PubMed] [Google Scholar]
- (19).Hossain M, Banik NL, Ray SK Synergistic anti-cancer mechanisms of curcumin and paclitaxel for growth inhibition of human brain tumor stem cells and LN18 and U138MG cells. Neurochem. Int 2012, 61, 1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ohi Y, Umekita Y, Yoshioka T, Souda M, Rai Y, Sagara Y, Sagara Y, Sagara Y, Tanimoto A Aldehyde dehydrogenase 1 expression predicts poor prognosis in triple-negative breast cancer. Histopathology 2011, 59, 776–780. [DOI] [PubMed] [Google Scholar]
- (21).Kida K; Ishikawa T; Yamada A; Shimada K; Narui K; Sugae S, Shimizu D, Tanabe M, Sasaki T, Ichikawa Y, Endo I Effect of ALDH1 on prognosis and chemoresistance by breast cancer subtype. Breast Cancer Res. Treat 2016, 156, 261–269. [DOI] [PubMed] [Google Scholar]
- (22).Lebert JM, Lester R; Powell E, Seal M, McCarthy J Advances in the systemic treatment of triple-negative breast cancer. Curr. Oncol 2018, 25, S142–S150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Partridge AH, Rumble RB, Carey LA; Come SE, Davidson NE, Di Leo A; Gralow J; Hortobagyi GN, Moy B, Yee D, Brundage SB, Danso MA; Wilcox M; Smith IE Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol 2014, 32, 3307–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, Hammond EH, Kuderer NM, Liu MC, Mennel RG, Van Poznak C, Bast RC, Hayes DF, American Society of Clinical, O. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol 2016, 34, 1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Echeverria GV, Ge Z, Seth S, Zhang X, Jeter-Jones S, Zhou X, Cai S, Tu Y, McCoy A; Peoples M, Sun Y, Qiu H, Chang Q; Bristow C, Carugo A; Shao J; Ma X; Harris A; Mundi P, Lau R; Ramamoorthy V, Wu Y, Alvarez MJ; Califano A; Moulder SL, Symmans WF, Marszalek JR; Heffernan TP; Chang JT; Piwnica-Worms H Resistance to neoadjuvant chemotherapy in triple-negative breast cancer mediated by a reversible drug-tolerant state. Sci. Transl. Med 2019, 11, eaav0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Gradishar WJ; Anderson BO, Abraham J; Aft R; Agnese D, Allison KH, Blair SL; Burstein HJ; Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ; Isakoff SJ; Krishnamurthy J; Lyons J; Marcom PK; Matro J; Mayer IA; Moran MS, Mortimer J; O’Regan RM, Patel SA; Pierce LJ; Rugo HS, Sitapati A; Smith KL, Smith ML, Soliman H, Stringer-Reasor EM; Telli ML; Ward JH, Young JS, Burns JL; Kumar R Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Network 2020, 18, 452–478. [DOI] [PubMed] [Google Scholar]
- (27).Mayer EL, Burstein HJ Chemotherapy for Triple-Negative Breast Cancer: Is More Better? J. Clin. Oncol 2016, 34, 3369–3371. [DOI] [PubMed] [Google Scholar]
- (28).Pandy JGP, Balolong-Garcia JC, Cruz-Ordinario MVB; Que FVF Triple negative breast cancer and platinum-based systemic treatment: a meta-analysis and systematic review. BMC Cancer 2019, 19, 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wahba HA; El-Hadaad HA Current approaches in treatment of triple-negative breast cancer. Cancer Biol. Med 2015, 12, 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Burnett JP, Lim G, Li Y, Shah RB, Lim R; Paholak HJ, McDermott SP, Sun L; Tsume Y, Bai S, Wicha MS, Sun D; Zhang T Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 2017, 394, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Liu L; Yang L; Yan W, Zhai J; Pizzo DP, Chu P, Chin AR; Shen M, Dong C, Ruan X; Ren X; Somlo G, Wang SE Chemotherapy Induces Breast Cancer Stemness in Association with Dysregulated Monocytosis. Clin. Cancer Res 2018, 24, 2370–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Tanei T, Morimoto K; Shimazu K; Kim SJ; Tanji Y, Taguchi T, Tamaki Y, Noguchi S Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin. Cancer Res 2009, 15, 4234–4241. [DOI] [PubMed] [Google Scholar]
- (33).Zhang S, Zhang H, Ghia EM; Huang J, Wu L; Zhang J, Lam S, Lei Y, He J; Cui B, Widhopf GF 2nd, Yu J, Schwab R, Messer K, Jiang W, Parker BA, Carson DA, Kipps TJ Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc. Natl. Acad. Sci. U. S. A 2019, 116, 1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Bonnet D; Dick JE Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med 1997, 3, 730–737. [DOI] [PubMed] [Google Scholar]
- (35).Singh SK; Clarke ID; Terasaki M; Bonn VE; Hawkins C; Squire J; Dirks PB Identification of a cancer stem cell in human brain tumors. Cancer research 2003, 63, 5821–5828. [PubMed] [Google Scholar]
- (36).O’Brien CA; Pollett A; Gallinger S; Dick JE A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [DOI] [PubMed] [Google Scholar]
- (37).Schatton T; Murphy GF; Frank NY; Yamaura K; Waaga-Gasser AM; Gasser M; Zhan Q; Jordan S; Duncan LM; Weishaupt C; Fuhlbrigge RC; Kupper TS; Sayegh MH; Frank MH Identification of cells initiating human melanomas. Nature 2008, 451, 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Yang ZF; Ho DW; Ng MN; Lau CK; Yu WC; Ngai P; Chu PW; Lam CT; Poon RT; Fan ST Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 2008, 13, 153–166. [DOI] [PubMed] [Google Scholar]
- (39).Shipitsin M; Polyak K The cancer stem cell hypothesis: in search of definitions, markers, and relevance. Lab. Invest 2008, 88, 459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Reya T; Morrison SJ; Clarke MF; Weissman IL Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [DOI] [PubMed] [Google Scholar]
- (41).Liu S; Dontu G; Wicha MS Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005, 7, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Zhou BB; Zhang H; Damelin M; Geles KG; Grindley JC; Dirks PB Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat. Rev. Drug Discovery 2009, 8, 806–823. [DOI] [PubMed] [Google Scholar]
- (43).Sakariassen PO; Immervoll H; Chekenya M Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia 2007, 9, 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Tang C; Chua CL; Ang BT Insights into the cancer stem cell model of glioma tumorigenesis. Ann. Acad. Med. Singapore 2007, 36, 352–357. [PubMed] [Google Scholar]
- (45).Liu S; Dontu G; Mantle ID; Patel S; Ahn NS; Jackson KW; Suri P; Wicha MS Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006, 66, 6063–6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Dontu G; Jackson KW; McNicholas E; Kawamura M Abdallah WM; Wicha MS Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004, 6, R605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Smalley M; Dale TC Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 1999, 18, 215–230. [DOI] [PubMed] [Google Scholar]
- (48).Kakarala M; Wicha MS Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J. Clin. Oncol 2008, 26, 2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Prieto-Vila M; Takahashi RU; Usuba W; Kohama I; Ochiya T Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int. J. Mol. Sci 2017, 18, 2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Gradishar WJ; Tjulandin S; Davidson N; Shaw H; Desai N; Bhar P; Hawkins M; O’Shaughnessy J Phase III trial of nanoparticle albumin-bound pachtaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol 2005, 23, 7794–7803. [DOI] [PubMed] [Google Scholar]
- (51).Zong Y; Wu J; Shen K Nanoparticle albumin-bound paclitaxel as neoadjuvant chemotherapy of breast cancer: a systematic review and meta-analysis. Oncotarget 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Hamilton E; Kimmick G; Hopkins J; Marcom PK; Rocha G; Welch R; Broadwater G; Blackwell K Nab-pachtaxel/bevacizumab/carboplatin chemotherapy in first-line triple negative metastatic breast cancer. Clin. Breast Cancer 2013, 13, 416–420. [DOI] [PubMed] [Google Scholar]
- (53).Lobo C; Lopes G; Baez O; Castrellon A; Ferrell A; Higgins C; Hurley E; Hurley J; Reis I; Richman S; Seo P; Silva O; Slingerland J; Tukia K; Welsh C; Gluck S Final results of a phase II study of nab-pachtaxel, bevacizumab, and gemcitabine as first-line therapy for patients with HER2-negative metastatic breast cancer. Breast Cancer Res. Treat 2010, 123, 427–435. [DOI] [PubMed] [Google Scholar]
- (54).Li Y; Chen N; Palmisano M; Zhou S Pharmacologic sensitivity of paclitaxel to its delivery vehicles drives distinct clinical outcomes of paclitaxel formulations. Mol. Pharmaceutics 2015, 12, 1308–1317. [DOI] [PubMed] [Google Scholar]
- (55).Chen N; Brachmann C; Liu X; Pierce DW; Dey J; Kerwin WS; Li Y; Zhou S; Hou S; Carleton M; Klinghoffer RA; Palmisano M; Chopra R Albumin-bound nanoparticle (nab) paclitaxel exhibits enhanced paclitaxel tissue distribution and tumor penetration. Cancer Chemother. Pharmacol 2015, 76, 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Chen N; Li Y; Ye Y; Palmisano M; Chopra R; Zhou S Pharmacokinetics and pharmacodynamics of nab-paclitaxel in patients with sohd tumors: disposition kinetics and pharmacology distinct from solvent-based paclitaxel. J. Clin. Pharmacol 2014, 54, 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Li F; Zhang H; He M; Liao J; Chen N; Li Y; Zhou S; Palmisano M; Yu A; Pai MP; Yuan H; Sun D Different Nanoformulations Alter the Tissue Distribution of Paclitaxel, Which Aligns with Reported Distinct Efficacy and Safety Profiles. Mol. Pharmaceutics 2018, 15, 4505–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Untch M; Jackisch C; Schneeweiss A; Conrad B; Aktas B; Denkert C; Eidtmann H; Wiebringhaus H; Kummel S; Hilfrich J; Warm M; Paepke S; Just M; Hanusch C; Hackmann J; Blohmer JU; Clemens M; Darb-Esfahani S; Schmitt WD; Dan Costa S; Gerber B; Engels K; Nekljudova V; Loibl S; von Minckwitz G; German Breast, G.; Arbeitsgemeinschaft Gynakologische Onkologie-Breast, I. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016, 17, 345–356. [DOI] [PubMed] [Google Scholar]
- (59).Mukherjee P; Gupta A; Chattopadhyay D; Chatterji U Modulation of SOX2 expression delineates an end-point for pachtaxel-effectiveness in breast cancer stem cells. Sci. Rep 2017, 7, 9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Larzabal L; El-Nikhely N; Redrado M; Seeger W; Savai R; Calvo A Differential effects of drugs targeting cancer stem cell (CSC) and non-CSC populations on lung primary tumors and metastasis. PLoS One 2013, 8, No. e79798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Samanta D; Gilkes DM; Chaturvedi P; Xiang L; Semenza GL Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc. Natl. Acad. Sci. U. S. A 2014, 111, E5429–5438. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (62).Fischer MM; Cancilla B; Yeung VP; Cattaruzza F; Chartier C; Murriel CL; Cain J; Tam R; Cheng CY; Evans JW; O’Young G; Song X; Lewicki J; Kapoun AM; Gurney A; Yen WC; Hoey T WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci. Adv 2017, 3, No. e1700090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Von Hoff DD; Ervin T; Arena FP; Chiorean EG; Infante J; Moore M; Seay T; Tjulandin SA; Ma WW; Saleh MN; Harris M; Reni M; Dowden S; Laheru D; Bahary N; Ramanathan RK; Tabernero J; Hidalgo M; Goldstein D; Van Cutsem E; Wei X; Iglesias J; Renschler MF Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med 2013, 369, 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Socinski MA; Bondarenko I; Karaseva NA; Makhson AM; Vynnychenko I; Okamoto I; Hon JK; Hirsh V; Bhar P; Zhang H; Iglesias JL; Renschler MF Weekly nab-Paclitaxel in Combination With Carboplatin Versus Solvent-Based Paclitaxel Plus Carboplatin as First-Line Therapy in Patients With Advanced Non-Small-Cell Lung Cancer: Final Results of a Phase III Trial. J. Clin. Oncol 2012, 30, 2055–2062. [DOI] [PubMed] [Google Scholar]
- (65).Charafe-Jauffret E; Ginestier C; Bertucci F; Cabaud O; Wicinski J; Finetti P; Josselin E; Adelaide J; Nguyen TT; Monville F; Jacquemier J; Thomassin-Piana J; Pinna G; Jalaguier A; Lambaudie E; Houvenaeghel G; Xerri L; Harel-Behan A; Chaffanet M; Viens P; Birnbaum D ALDHl-positive cancer stem cells predict engraftment of primary breast tumors and are governed by a common stem cell program. Cancer Res. 2013, 73, 7290–7300. [DOI] [PubMed] [Google Scholar]
- (66).Clark DW; Palle K Aldehyde dehydrogenases in cancer stem cells: potential as therapeutic targets. Annals of translational medicine 2016, 4, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Tomita H; Tanaka K; Tanaka T; Hara A Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Xu X; Chai S; Wang P; Zhang C; Yang Y; Yang Y; Wang K Aldehyde dehydrogenases and cancer stem cells. Cancer Lett. 2015, 369, 50–57. [DOI] [PubMed] [Google Scholar]
- (69).Ma S, Chan KW, Lee TKW, Tang KH, Wo JYH, Zheng BJ; Guan XY Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol. Cancer Res 2008, 6, 1146–1153. [DOI] [PubMed] [Google Scholar]
- (70).Dembinski JL, Krauss S Characterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinoma. Clin. Exp. Metastasis 2009, 26, 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM Aldehyde Dehydrogenase 1 Is a Marker for Normal and Malignant Human Colonic Stem Cells (SC) and Tracks SC Overpopulation during Colon Tumorigenesis. Cancer Res. 2009, 69, 3382–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Ucar D; Cogle CR; Zucali JR; Ostmark B, Scott EW, Zori R; Gray BA, Moreb JS Aldehyde dehydrogenase activity as a functional marker for lung cancer. Chem.-Biol. Interact 2009, 178, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Deng S, Yang XJ, Lassus H, Liang S, Kaur S, Ye QR, Li CS, Wang LP, Roby KF, Orsulic S, Connolly DC, Zhang YC, Montone K, Butzow R, Coukos G, Zhang L Distinct Expression Levels and Patterns of Stem Cell Marker, Aldehyde Dehydrogenase Isoform 1 (ALDH1), in Human Epithelial Cancers. PLoS One 2010, 5, e10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Penumatsa K, Edassery SL, Barua A, Bradaric MJ, Luborsky JL Differential expression of aldehyde dehydrogenase 1a1 (ALDH1) in normal ovary and serous ovarian tumors. J. Ovarian Res 2010, 3, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, Chen DT, Tai LK; Yung MC, Chang SC, Ku HH, Chiou SH, Lo WL Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem. Biophys. Res. Commun 2009, 385, 307–313. [DOI] [PubMed] [Google Scholar]
- (76).Zhang L, Marrano P, Kumar S, Leadley M, Elias E, Thorner P; Baruchel S Nab-paclitaxel is an active drug in preclinical model of pediatric solid tumors. Clin. Cancer Res 2013, 19, 5972–5983. [DOI] [PubMed] [Google Scholar]
- (77).Shao H, Tang H, Salavaggione OE, Yu C, Hylander B, Tan W, Repasky E, Adjei AA, Dy GK Improved response to nab-paclitaxel compared with cremophor-solubilized paclitaxel is independent of secreted protein acidic and rich in cysteine expression in non-small cell lung cancer. J. Thorac. Oncol 2011, 6, 998–1005. [DOI] [PubMed] [Google Scholar]
- (78).Hassan MS, Awasthi N, Li J; Williams F, Schwarz MA, Schwarz RE, von Holzen U Superior Therapeutic Efficacy of Nanoparticle Albumin Bound Paclitaxel Over Cremophor-Bound Paclitaxel in Experimental Esophageal Adenocarcinoma. Transl Oncol 2018, 11, 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Desai N, Trieu V, Yao Z, Louie L; Ci S, Yang A, Tao C, De T, Beals B, Dykes D, Noker P, Yao R; Labao E, Hawkins M, Soon-Shiong P Increased antitumor activity, intra-tumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin. Cancer Res 2006, 12, 1317–1324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.