Abstract
Background
As Parkinson's disease progresses the control of the symptoms often requires the addition of other drugs to levodopa. The principle aim of COMT inhibitor therapy is to increase the duration of effect of the levodopa dose and thus reduce the time patients spend in the relatively immobile 'off' phase.
Objectives
To compare the efficacy and safety of adjuvant COMT inhibitor therapy versus active comparators in patients with Parkinson's disease, already established on levodopa and suffering from motor complications.
Search methods
Electronic searches of the Cochrane Controlled Trials Register (The Cochrane Library Issue 1, 2003), MEDLINE (1966‐2003), EMBASE (1974‐2003), were conducted. Grey literature was hand searched and the reference lists of identified studies and reviews examined. The manufacturers of COMT inhibitors were contacted.
Selection criteria
Randomised controlled trials of adjuvant COMT inhibitor therapy versus an active comparator in patients with a clinical diagnosis of idiopathic Parkinson's disease and long‐term complications of levodopa therapy.
Data collection and analysis
Data was abstracted independently by the authors and differences settled by discussion. The outcome measures used included Parkinson's disease rating scales, levodopa dosage, 'off' time measurements and the frequency of withdrawals and adverse events.
Main results
Two trials were found that examined the efficacy of a COMT inhibitor against an active comparator (n = 349). Koller 1998 compared the efficacy of tolcapone versus pergolide (n = 203) over 12 weeks and TSG 1999 compared the efficacy of tolcapone versus bromocriptine (n = 146) over 8 weeks. No trials were found that compared entacapone with active comparators.
Tolcapone produced similar benefits to bromocriptine in 'off' time reduction, motor impairment and disability ratings over three months of therapy. Tolcapone produced a greater reduction in levodopa dosage than bromocriptine. Tolcapone produced similar benefits to pergolide in levodopa dose reduction, motor impairment and disability ratings, and in generic health‐related quality of life scales over the first two months of therapy. Tolcapone produced a greater improvement in the disease‐specific quality of life scale PDQ‐39 than pergolide. Nausea, constipation and orthostatic complaints were greater with agonist therapy, but otherwise the frequency of adverse events and withdrawals from treatment were similar with the two classes of adjuvant medication. One patient had significantly elevated liver enzymes whilst on tolcapone, but otherwise the frequency of adverse events and withdrawals from treatment were similar.
Authors' conclusions
The two trials comparing tolcapone with the dopamine agonists bromocriptine and pergolide were underpowered to detect clinically relevant differences between them. This is based on medium‐term evidence. No evidence was found comparing entacapone with active comparators. Further larger and longer‐term trials are required to compare tolcapone with entacapone and COMT inhibitor therapy with alternative adjuvant classes of drug in later Parkinson's disease such as dopamine agonists and monoamine oxidase inhibitors.
Keywords: Humans, Catechol O-Methyltransferase Inhibitors, Antiparkinson Agents, Antiparkinson Agents/adverse effects, Antiparkinson Agents/therapeutic use, Benzophenones, Benzophenones/therapeutic use, Bromocriptine, Bromocriptine/therapeutic use, Dopamine Agonists, Dopamine Agonists/therapeutic use, Levodopa, Levodopa/adverse effects, Nitrophenols, Nitrophenols/therapeutic use, Parkinson Disease, Parkinson Disease/drug therapy, Pergolide, Pergolide/therapeutic use, Tolcapone
Plain language summary
Insufficient data are available on the benefits of the COMT inhibitor tolcapone compared with the dopamine agonists bromocriptine and pergolide in relieving the symptoms of later Parkinson's disease.
As Parkinson's disease progresses the control of the symptoms often requires the addition of other drugs to levodopa. The principle aim of COMT inhibitor therapy is to increase the duration of effect of each levodopa dose and thus reduce the time patients spend in the relatively immobile 'off' phase. However other drugs such as dopamine agonists can also be used at this stage of the disease. This review found that the COMT inhibitor tolcapone as an adjuvant to levodopa treatment had a similar level of benefits as two dopamine agonists, bromocriptine and pergolide. There was no significant difference in efficacy between the adjuvant tolcapone and adjuvant bromocriptine or pergolide in the medium‐term. Tolcapone produced nausea less often than these agonists but there was some evidence of liver function abnormalities with tolcapone. Post‐marketing surveillance identified three cases of fatal hepatic toxicity in patients treated with tolcapone. As a result, tolcapone has been withdrawn from some countries and severe restrictions on its use have been imposed in others.
No evidence was found comparing entacapone with other adjuvant drugs for Parkinson's disease.
Background
Over 20 years after its introduction, levodopa remains the most effective therapy in Parkinson's disease. However, with long‐term treatment, patients develop side effects comprised of motor and psychiatric complications. The former consist of involuntary writhing movements of the limbs and trunk (choreoathetosis), painful cramps often affecting the feet (dystonia) and a shortened response to each dose of levodopa (end‐of‐dose deterioration). These affect 50% of patients after 6 years of therapy (Rajput 1984) and 100% of young onset patients (Quinn 1986).
To reduce fluctuations in dopaminergic stimulation of the striatum various therapeutic strategies can be adopted. These include using more frequent smaller doses of levodopa, controlled release levodopa formulations, additional dopamine agonists or continuous subcutaneous infusions of apomorphine. Catechol‐O‐methyltransferase (COMT) is an enzyme that catalyses the metabolism of levodopa to 3‐O‐methyldopa. Therefore drugs that inhibit COMT prolong the maintenance of serum levodopa levels and produce a longer and more stable clinical levodopa response (Bonifati 1999). There are two COMT inhibitors used in clinical practice at present: tolcapone and entacapone.
The efficacy and safety of COMT inhibitors have been examined in early and advanced Parkinson's disease. Trials in later disease when motor complications have developed have lead to COMT inhibitors being licensed for this indication in the expectation of a reduction in 'off' time and improved motor function. There has been some controversy over the licensing of tolcapone. Concerns over several deaths from liver toxicity thought to be induced by tolcapone led to the withdrawal of the European drug license, although the drug is still available in the USA provided stringent hepatic monitoring is performed (EAEM 1998).
Whilst a separate review examines the effects of adjuvant COMT inhibitor therapy versus placebo, clinicians are more interested in whether COMT inhibitors are more effective than existing therapies. Hence the present systematic review examines all randomised controlled trials of adjuvant COMT inhibitor therapy compared with active comparators in later Parkinson's disease with motor complications to establish its efficacy and tolerability.
Objectives
To compare the efficacy and safety of adjuvant COMT inhibitor therapy compared with active comparators in patients with Parkinson's disease, already established on levodopa and suffering from motor complications.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials comparing adjuvant COMT inhibitors with active comparators were considered for inclusion in the study.
Types of participants
Patients with a clinical diagnosis of idiopathic Parkinson's disease who had developed long‐term motor complications of dyskinesia and/or end‐of‐dose deterioration. All ages were included. Any duration of levodopa therapy was included.
Types of interventions
Oral tolcapone or entacapone therapy or any dopamine agonist, MAO inhibitor, anticholinergic, amantadine. Trial durations of greater than 4 weeks were included.
Types of outcome measures
1. Improvement in the time patients spend in the immobile 'off' state.
2. Changes in dyskinesia rating scales and the prevalence of dyskinesia.
3. Changes in Parkinsonian rating scales.
4. Reduction in levodopa dose.
5. Frequency of adverse events
6. Number of withdrawals due to lack of efficacy and/or side‐effects.
Search methods for identification of studies
1. The review was based on the search strategy of the Movement Disorders Group. This included computerised searches of MEDLINE (1966‐2003) and EMBASE (1974‐2003) and hand searching of appropriate neurology journals. Relevant trials were included on the Group's specialised register of randomised controlled trials. Further details are available in the Group's module on the Cochrane Database of Systematic Reviews.
2. The Cochrane Controlled Trials Register (The Cochrane Library Issue 1, 2003) was also searched for relevant trials.
3. The reference lists of located trials and of other COMT inhibitor reviews were searched.
4. Additional assistance was provided by the drug manufacturers, Orion Pharmacia and Roche
Data collection and analysis
The two authors (CC, KD) independently assessed the studies identified by the search strategy. Disagreements about inclusions were resolved by discussion. The full papers were assessed for methodological quality by recording the method of randomisation and blinding, whether an intention to treat analysis was used and the number of patients lost to follow up.
Eligible data was abstracted onto standardised forms by the authors independently, checked for accuracy and amalgamated. A weighted estimate (fixed effect model) of the typical treatment effect across trials was calculated for continuous (weighted mean difference) and dichotomous (Peto odds ratio) variables such as 'off' time and prevalence of adverse events. Since multiple comparisons of adverse events were examined statistically, the results were interpreted cautiously using 99% confidence intervals.
Results
Description of studies
See also Characteristics of Included Studies Table and Results of Included Studies.
Two trials fulfilled the inclusion criteria with a total of 349 patients with Parkinson's disease and motor fluctuations included in these studies. Both trials examined tolcapone compared to an active comparator. One trial (Koller 1998) with 203 participants examined tolcapone against pergolide whilst the other (TSG 1999) with 146 participants examined tolcapone against bromocriptine. Both studies were randomised open‐label parallel‐group studies. The trials were open label because both active comparators required titration phases. Koller 1998 used a blinded rater. Levodopa dose reduction was allowed in both of the trials.
Patients were well balanced across the arms of the studies in terms of age and Hoehn and Yahr score (see Characteristics of Included Studies).
In Koller 1998 participants received 100 mg tolcapone tid with a possible increase to 200 mg tid, or pergolide titrated to a maximum dose of 5 mg/day by week 9, with a final mean dose of 2.2 mg/day pergolide. In TSG 1999 participants received either 200 mg tolcapone tid, or bromocriptine titrated to a maximum dose of 30 mg/day by day 24, with a mean final dose of 22.4 mg/day. These dosages are comparable to those used in clinical practice: 3 mg pergolide per day, 100‐200 mg tolcapone three times a day, and 10 ‐ 30 mg bromocriptine per day (BNF45 2003; FDA 1998).
Risk of bias in included studies
Koller 1998 compared the efficacy of tolcapone against pergolide. The method of randomisation and concealment of allocation were not stated which could have led to selection bias. The participants had at least two of the three cardinal features of idiopathic Parkinson's disease. The participants baseline characteristics and other medications were described. The data was analysed on an intention to treat basis. Number of withdrawals were stated and reasons given. Some of the results were presented in a manner amenable to meta‐analysis.
TSG 1999 compared the efficacy of tolcapone against bromocriptine. The method of randomisation and concealment of allocation were described. Patients satisfied the clinical criteria for Parkinson's disease from the United Kingdom Parkinson's Disease Brain Bank (Gibb 1988). The participants baseline characteristics and other medications were described. The data was analysed on an intention to treat basis. Number of withdrawals were stated and reasons given. Some of the results were presented in a manner amenable to meta‐analysis.
Effects of interventions
Two trials were found that examined the efficacy of a COMT inhibitor against an active comparator (n = 349). Koller 1998 compared the efficacy of tolcapone against pergolide (n = 203) over 12 weeks and TSG 1999 compared the efficacy of tolcapone against bromocriptine (n = 146) over 8 weeks. No trials were found that compared entacapone with active comparators.
Koller 1998 presented data for the baseline and 4 and 12 week time points; we will only discuss data from the later time point since this is the most clinically meaningful. Data on efficacy were not presented in a manner suitable for meta‐analysis although adverse event frequencies were available. TSG 1999 presented most data in a manner amenable to meta‐analysis.
Levodopa dose reduction was allowed in both trials. In Koller 1998 the levodopa dose decreased by a mean of 108 mg in the tolcapone group compared with 92 mg in the pergolide group which was not statistically significant. At week 8 in TSG 1999 the total daily levodopa dose decreased by a mean of 124 mg in the tolcapone group compared to 30 mg in the bromocriptine group which was statistically significant (P<0.01). No data on standard deviations were available from either trial so meta‐analysis cannot be performed for this outcome.
The results for total 'off' and 'on' times were not presented in Koller 1998. In TSG 1999 tolcapone produced a non‐significant decrease of 36 minutes in 'off' time compared to bromocriptine (Figure 1). This was mirrored by a non‐significant increase of 42 minutes in 'on' time with tolcapone compared to bromocriptine (Figure 2).
Functional improvements were measured using the UPDRS ADL and motor subsections in both studies. In Koller 1998 the UPDRS ADL improved by 1.9 points with tolcapone and 1.6 points with pergolide which was not statistically significant. In TSG 1999 the UPDRS ADL improved by 0.9 points with tolcapone and 0.1 points with bromocriptine which was not significant. In Koller 1998 the UPDRS motor scores improved by 3.3 points with tolcapone and 2.7 points with pergolide which was not significant. In TSG 1999 the UPDRS motor score changed by 3.1 points with tolcapone and 3.3 points with bromocriptine which was not significant.
Koller 1998 also assessed quality of life with the Sickness Impact Profile (SIP) and Parkinson's Disease Questionnaire 39 (PDQ 39). The SIP score improved by 4.1 points in the tolcapone group and 3.5 points in the pergolide group which was not significant. The PDQ‐39 score improved by 7.1 points in the tolcapone group and 4.5 points in the pergolide group which was statistically significant (P= 0.005).
Since there were a large number of types of adverse events recorded in these trials, there is a significant risk of finding positive results by chance during meta‐analysis. Therefore we have taken results with P=0.01 or less to be statistically significant. As both trials compared ergot‐derived dopamine agonists which have similar side‐effect profiles we have combined the results in Figure 5. This shows significantly more nausea (OR=0.42, P=0.0003), constipation (OR=0.26, P=0.00007) and orthostatic complaints (OR=0.24, P=0.0002) with ergot derived dopamine agonists than tolcapone.
Abnormalities in liver function tests were only reported in Koller 1998. One patient on tolcapone had raised aspartate aminotransferase (AST) levels at greater than three time the upper level of normal with no related symptoms. The enzyme levels returned to normal after withdrawal of tolcapone.
Only Koller 1998 provided data for withdrawals due to adverse events and there was a trend for more withdrawals in the pergolide group (Peto odds ratio 0.34, P=0.02 Figure 6). However neither study showed any significant differences in all cause withdrawal rates (Figure 7).
Discussion
Two trials were found that compared tolcapone with active comparators (n=349). Both were short‐term studies (8 and 12 weeks) and consequently of little value in assessing the efficacy of long‐term treatment of this chronic condition. No trials were found that examined entacapone with active comparators therefore its relative efficacy compared to other adjuvant medications cannot be determined.
The principle aim of COMT inhibitor therapy is to increase the duration of effect of the levodopa dose and thus reduce the time patients spend in the relatively immobile 'off' phase. Tolcapone and bromocriptine both reduced off time in TSG 1999. However, although a trend towards more reduction in 'off' time was seen with tolcapone, this did not reach statistical significance. Levodopa dose reduction was comparable with the tolcapone and pergolide, but it was claimed that tolcapone produced a significantly greater reduction in levodopa dosage than bromocriptine, although insufficient data was provided for us to confirm this assertion.
Tolcapone, bromocriptine and pergolide all produced improvements in motor impairments and disability as measured with the UPDRS ADL and motor scales. However there was no benefit in using tolcapone over bromocriptine or pergolide. Only the Koller 1998 trial examined quality of life. The generic SIP scale did not detect any differences between the tolcapone and pergolide. The disease‐specific PDQ‐39 showed greater benefit with tolcapone, however the clinical significance of a 2.6 point difference in PDQ‐39 is uncertain. The ongoing PD MED trial is using a 6 point difference in the motor subscale of PDQ 39 as a clinically meaningful difference.
Bromocriptine and pergolide increased the incidence of nausea, constipation and orthostasis compared to tolcapone. Koller 1998 described a patient whose AST level rose to 14 times the upper limit of normal. This returned to normal after withdrawal of the tolcapone, strongly suggesting that it was the causative agent. Otherwise adverse events and withdrawals were similar with both tolcapone and dopamine agonists.
These trials were underpowered to detect clinically relevant differences between drugs that have individually been shown to be effective adjuvant therapies for complications in Parkinson's disease (Deane 2003; Clarke 2003; Hilten 2003).
Authors' conclusions
Implications for practice.
The two trials comparing tolcapone with the dopamine agonists bromocriptine and pergolide were underpowered to detect clinically relevant differences between them. No evidence was found comparing entacapone with active comparators.
Implications for research.
Further trials are required to compare tolcapone with entacapone and COMT inhibitor therapy with alternative adjuvant classes of drug in later Parkinson's disease such as dopamine agonists and monoamine oxidase inhibitors.
In the future, adjuvant therapy trials in Parkinson's disease should:‐
Be reported using the CONSORT guidelines (CONSORT 2001).
Include valid sample size calculations which also take into account safety issues.
Provide full data on outcome measures including mean change and its standard deviation / error.
Express results in the original unit of measurement (hours rather than percentage off time).
Use firm diagnostic criteria (e.g. UK Parkinson's Disease Brain Bank Criteria, Gibb 1988).
What's new
| Date | Event | Description |
|---|---|---|
| 13 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 8 June 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Orion and Roche Pharmaceuticals.
Data and analyses
Comparison 1. TOLCAPONE VERSUS ACTIVE COMPARATOR.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 'Off' time reduction (hours) | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.67, 1.87] |
| 1.1 Tolcapone versus bromocriptine | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.67, 1.87] |
| 2 'On' time increase (hours) | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.53, 1.93] |
| 2.1 Tolcapone versus bromocriptine | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.53, 1.93] |
| 3 UPDRS ADL section (Part II) | Other data | No numeric data | ||
| 3.1 Tolcapone versus pergolide | Other data | No numeric data | ||
| 3.2 Tolcapone versus bromocriptine | Other data | No numeric data | ||
| 4 UPDRS Motor section (Part III) | Other data | No numeric data | ||
| 4.1 Tolcapone versus pergolide | Other data | No numeric data | ||
| 4.2 Tolcapone versus bromocriptine | Other data | No numeric data | ||
| 5 Adverse events ‐ Tolcapone versus Dopamine Agonist | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Adverse events ‐ Dyskinesia | 2 | 349 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.63 [1.05, 2.54] |
| 5.2 Adverse events ‐ Nausea | 2 | 349 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.26, 0.67] |
| 5.3 Adverse events ‐ Vomiting | 2 | 349 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.41, 2.50] |
| 5.4 Adverse events ‐ Diarrhoea | 1 | 203 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.27 [0.88, 5.82] |
| 5.5 Adverse events ‐ Constipation | 2 | 349 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.14, 0.51] |
| 5.6 Adverse events ‐ Confusion | 1 | 203 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.14, 1.19] |
| 5.7 Adverse events ‐ Hallucinations | 1 | 146 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.05, 0.88] |
| 5.8 Adverse events ‐ Orthostatic complaints (e.g. hypotension) | 2 | 349 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.11, 0.51] |
| 6 Adverse event withdrawal rate | 1 | 203 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.13, 0.84] |
| 6.1 Tolcapone versus Pergolide | 1 | 203 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.13, 0.84] |
| 7 All cause withdrawal rate | 2 | 349 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.37, 1.27] |
| 7.1 Tolcapone versus pergolide | 1 | 203 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.25, 1.18] |
| 7.2 Tolcapone versus bromocriptine | 1 | 146 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.37, 2.90] |
1.1. Analysis.
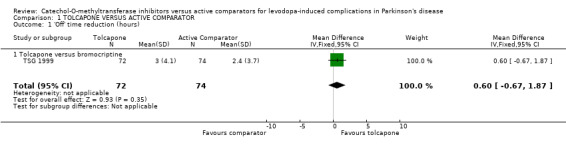
Comparison 1 TOLCAPONE VERSUS ACTIVE COMPARATOR, Outcome 1 'Off' time reduction (hours).
1.2. Analysis.
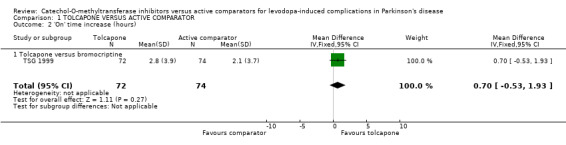
Comparison 1 TOLCAPONE VERSUS ACTIVE COMPARATOR, Outcome 2 'On' time increase (hours).
1.3. Analysis.
Comparison 1 TOLCAPONE VERSUS ACTIVE COMPARATOR, Outcome 3 UPDRS ADL section (Part II).
| UPDRS ADL section (Part II) | |
|---|---|
| Study | |
| Tolcapone versus pergolide | |
| Koller 1998 | Week 12, 'on' state. Tolcapone mean change 1.9 (SD not calculable), pergolide mean change 1.6 (SD not calculable). Not significant. |
| Tolcapone versus bromocriptine | |
| TSG 1999 | Week 8, 'on' state. Tolcapone mean change ‐0.9 (SD 4.2); bromocriptine mean change ‐0.1 (SD 3.4). Not significant. |
1.4. Analysis.
Comparison 1 TOLCAPONE VERSUS ACTIVE COMPARATOR, Outcome 4 UPDRS Motor section (Part III).
| UPDRS Motor section (Part III) | |
|---|---|
| Study | |
| Tolcapone versus pergolide | |
| Koller 1998 | Week 12, 'on' state. Tolcapone mean change 3.3 (SD not calculable), pergolide mean change 2.7 (SD not calculable). Not significant. |
| Tolcapone versus bromocriptine | |
| TSG 1999 | Week 8, 'on' state. Tolcapone mean change ‐3.1 (SD 8.5); pergolide mean change ‐3.3 (SD 8.6). Not significant. |
1.5. Analysis.
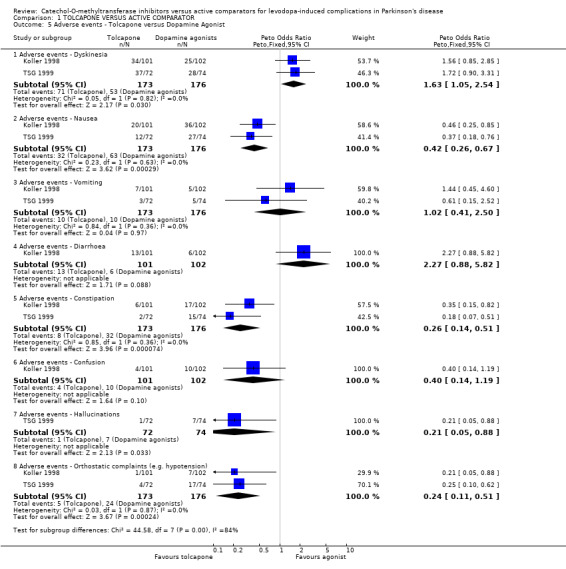
Comparison 1 TOLCAPONE VERSUS ACTIVE COMPARATOR, Outcome 5 Adverse events ‐ Tolcapone versus Dopamine Agonist.
1.6. Analysis.
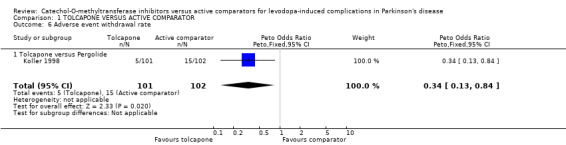
Comparison 1 TOLCAPONE VERSUS ACTIVE COMPARATOR, Outcome 6 Adverse event withdrawal rate.
1.7. Analysis.
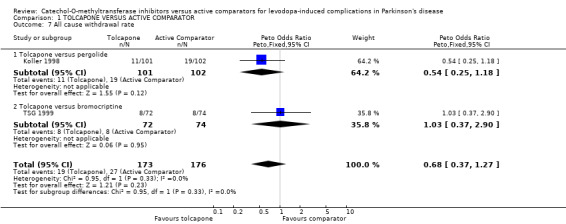
Comparison 1 TOLCAPONE VERSUS ACTIVE COMPARATOR, Outcome 7 All cause withdrawal rate.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Koller 1998.
| Methods | Randomised, open‐label, blinded rater, parallel‐group trial. The method of randomisation was not stated. Three centres in USA, UK and Australia. Intention‐to‐treat data analysis. Duration: 12 weeks. | |
| Participants | 203 patients, 11 withdrew from the Tolcapone group, 5 due to adverse events. 19 withdrew from the pergolide group, 15 due to adverse events. Baseline characteristics: 112 males (55%), mean age 65 years, mean disease duration 7.5 years, mean levodopa dose 574 mg/day. Inclusion criteria: Patients at least 30 years old at onset of symptoms with at least two of the three cardinal features of IPD. Patients were levodopa responsive but had end‐of‐dose motor fluctuations and were considered to require additional drug therapy. They were on a stable levodopa regime for four weeks before screening and were taking at least four doses of levodopa daily (or at least 3 dose if two were controlled release formulations). The total daily levodopa dose was less than 1.5g. Patients receiving other antiparkinsonian drugs were included if the drug regime had been stable for at least 4 weeks. Six 18 hour screening diaries were completed in the 2 weeks before baseline. Of these at least four diaries had to be filled correctly and patients to have recorded at least two separate 'off' periods after the first 'on' period of the day in each of the three diaries. Exclusion criteria: Cases of secondary parkinsonism, pregnancy, lactation, or unreliable contraception (in women), previous treatment with dopamine agonists, COMT inhibitors, treatment with centrally acting dopamine antagonists in the previous 6 months (except antiemetics if stopped 2 months before baseline), treatment with irreversible MAO inhibitors in previous 2 months, reversible MAO inhibitors in the previous day (selegiline was permitted), or any investigational agent in the previous 4 weeks (or five half‐lives of the compound if longer), a history of drug or alcohol abuse in previous 2 years, unstable medical problems, and brain surgery in the previous year. | |
| Interventions | Patients received 100 mg tid tolcapone, with a possible increase to 200 mg tid, or pergolide titrated to a maximum dose of mg/day by week 9 (mean final dose 2.2 mg/day). Patients could also receive a stable regime of amantadine, anticholinergics, selegiline, antihistamines or beta‐adrenergic blockers. | |
| Outcomes | 'Off' time Levodopa dose SIP PDQ‐39 UPDRS Adverse events | |
| Notes | TOLCAPONE VS PERGOLIDE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
TSG 1999.
| Methods | Randomised, open‐label parallel‐group study. Randomisation was stratified by previous use of dopamine agonists and weighted every two patients in each stratum. To randomise a given patient the investigator called a vocal computer that reviewed his most important selection criteria and assigned treatment accordingly. Location: 19 centres in France. Intention‐to‐treat data analysis. Duration: 8 weeks | |
| Participants | 146 patients, 16 withdrew, eight in each group, due to adverse events or intercurrent illness, one patient in the bromocriptine group withdrew because of protocol violation. Baseline characteristics: 80 males (55%), mean age 63, mean duration of disease 9.6 years, mean levodopa dose 765 mg/day. Inclusion criteria: at least 30 years old at onset of symptoms and satisfied the clinical criteria for PD from the UK PD Brain Bank. They had to be taking at least three daily doses of levodopa and be experiencing clinical fluctuations. They were required to be able to keep reliable 'on/off' charts. Exclusion criteria: nonidiopathic parkinsonism, PSP, MSA, unpredictable fluctuations or prolonged severe dyskinesias that could interfere with daily activities, treatment with dopamine agonist in previous four weeks before randomisation, apomorphine in previous 6 months, or a MAO inhibitor (other than selegiline) during previous two months, a MMSE score of 24 or less, a history of psychotic illness or major depression during previous 6 months, unstable medical problems, a history of drug or alcohol abuse. Women were required to be sterile or to be using effective contraception. Patients treated with dopamine agonists (up to 20 mg/day bromocriptine, up to 1.2 mg/day lisuride, or up to 150 mg/day piribedil) could be included after a four‐week washout period. | |
| Interventions | Patients received either 200 mg tolcapone tid or bromocriptine titrated to a maximum of 30 mg/day by day 24 (mean final dose 22.4 mg/day). Other antiparkinsonian medication was allowed provided the dose had been stable for 1 months before entry into study and remained unchanged during study. Patients could receive medication for adverse events during the study, including the peripheral dopamine antagonist domperidone to treat nausea and vomiting. | |
| Outcomes | Levodopa dose 'On/off' time UPDRS ADL UPDRS motor Adverse events | |
| Notes | TOLCAPONE VS BROMOCRIPTINE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Lyytinen 1997 | Adjuvant entacapone was not compared to adjuvant selegiline. The study compared levodopa plus entacapone with or without the addition of selegiline. |
Sources of support
Internal sources
No sources of support supplied
External sources
Orion Pharmacia, UK.
The Cadbury Foundation, UK.
The Movement Disorders Research Charity, UK.
Declarations of interest
The department of Dr C E Clarke has received unrestricted grants (£3000 from each) from Orion Pharmaceuticals and Roche Pharmaceuticals.
Edited (no change to conclusions)
References
References to studies included in this review
Koller 1998 {published data only}
- Koller W, Lees A, Doder M, Hely M, and the Tolcapone/Pergolide study group. Randomized trial of Tolcapone versus Pergolide as add‐on to levodopa therapy in Parkinson's disease patients with motor fluctuations. Movement Disorders 2001;16(5):858‐866. [DOI] [PubMed] [Google Scholar]
- Koller W, Lees A, Morris J, Sterman A. A multicentre trial comparing the efficacy, tolerability, and safety of tolcapone vs pergolide in Parkinson's patients with motor fluctuations. Movement Disorders 1998;13(Supplement 2):52. [Google Scholar]
TSG 1999 {published data only}
- Agid Y, Destee A, Durif F, Montastruc J‐L, Pollak P, on behalf of the French Tolcapone Study Group. Tolcapone, bromocriptine, and Parkinson's disease. The Lancet 1997;350:712‐713. [DOI] [PubMed] [Google Scholar]
- The Tolcapone Study Group. Efficacy and tolerability of tolcapone compared with bromocriptine in levodopa‐treated Parkinsonian patients. Movement Disorders 1999;14(1):38‐44. [PubMed] [Google Scholar]
References to studies excluded from this review
Lyytinen 1997 {published data only}
- Lyytinen J, Kaakkola S, Ahtila S, Tuomainen P, Teravainen H. Simultaneous MAO‐B and COMT Inhibition in L‐Dopa‐treated patients with Parkinson's disease. Movement Disorders 1997;12(4):497‐505. [DOI] [PubMed] [Google Scholar]
Additional references
BNF45 2003
- Joint Formulary Committee. British National Formulary. 45. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2003. [Google Scholar]
Bonifati 1999
- Bonifati V, Meco G. New, selective catechol‐O‐methytransferase inhibitors as therapeutic agents in Parkinson's disease. Pharmacological Therapies 1999;81(1):1‐36. [DOI] [PubMed] [Google Scholar]
Clarke 2003
- Clarke C E, Speller J M. Pergolide for levodopa‐induced complications in Parkinson's disease (Cochrane Review). The Cochrane Library 2003, Issue 3. [DOI] [PubMed] [Google Scholar]
CONSORT 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. The Lancet 2001;357:1191‐1194. [PubMed] [Google Scholar]
Deane 2003
- Deane KHO, Spieker S, Clarke CE. COMT‐Inhibitors for levodopa‐induced complications in Parkinson's disease (Cochrane Review). The Cochrane Library 2003, Issue 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
EAEM 1998
- The European Agency for the Evaluation of Medicines. CPMP/2457/98: Recommendation for the suspension of the marketing authorisation for Tasmar (Tolcapone). Press release (London) 17th November 1998:EU/1/97/044/001‐006.
FDA 1998
- Federal Drug Administration. http://www.fda.gov/cder/consumerinfo/druginfo/tasmar.htm 1998:Accessed on 24th July 2003.
Gibb 1988
- Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. The Journal of Neurology, Neurosurgery and Psychiatry 1988;51:745‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hilten 2003
- Hilten JJ van, Ramaker C, Beek WJT van de, Finken MJJ. Bromocriptine for levodopa‐induced motor complications in Parkinson's disease (Cochrane Review). The Cochrane Library 2003, Issue 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinn 1986
- Quinn N, Critchley P, Parkes D, Marsden CD. When should levodopa be started?. Lancet 1986;ii:985‐986. [DOI] [PubMed] [Google Scholar]
Rajput 1984
- Rajput AH, Stern W, Laverty WH. Chronic low‐dose levodopa therapy in Parkinson's disease. Neurology 1984;34:991‐996. [DOI] [PubMed] [Google Scholar]


