Abstract
Background and Aims
Initiation of cluster roots in white lupin (Lupinus albus) under phosphorus (P) deficiency requires auxin signalling, whereas flavonoids inhibit auxin transport. However, little information is available about the interactions between P deficiency and flavonoids in terms of cluster-root formation in white lupin.
Methods
Hydroponic and aeroponic systems were used to investigate the role of flavonoids in cluster-root formation, with or without 75 μm P supply.
Key Results
Phosphorus-deficiency-induced flavonoid accumulation in cluster roots depended on developmental stage, based on in situ determination of fluorescence of flavonoids and flavonoid concentration. LaCHS8, which codes for a chalcone synthase isoform, was highly expressed in cluster roots, and silencing LaCHS8 reduced flavonoid production and rootlet density. Exogenous flavonoids suppressed cluster-root formation. Tissue-specific distribution of flavonoids in roots was altered by P deficiency, suggesting that P deficiency induced flavonoid accumulation, thus fine-tuning the effect of flavonoids on cluster-root formation. Furthermore, naringenin inhibited expression of an auxin-responsive DR5:GUS marker, suggesting an interaction of flavonoids and auxin in regulating cluster-root formation.
Conclusions
Phosphorus deficiency triggered cluster-root formation through the regulation of flavonoid distribution, which fine-tuned an auxin response in the early stages of cluster-root development. These findings provide valuable insights into the mechanisms of cluster-root formation under P deficiency.
Keywords: Phosphorus deficiency, cluster root, flavonoid, chalcone synthase, auxin, white lupin
INTRODUCTION
Improving phosphorus (P)-acquisition efficiency is a critical strategy in crop production. The precipitation, sorption and occlusion of inorganic phosphate (Pi) in soil make it poorly available for most crop plants (Hinsinger, 2001; Shen et al., 2011; Heuer et al., 2017). Plants growing in strongly P-sorbing soil must be able to acclimate to P deficiency by expressing efficient Pi-acquisition strategies such as mobilizing sorbed P and acquiring Pi. Unlike most plants, white lupin (Lupinus albus) is a non-mycorrhizal species that is unable to associate with mycorrhizal fungi, but develops cluster roots that are bottlebrush-like root structures with short, dense lateral roots induced by P deficiency (Gardner et al., 1981; Dinkelaker et al., 1989; Li et al., 2008). One of the most effective strategies of white lupin to cope with P deficiency is to produce cluster roots, which release large amounts of exudates into the rhizosphere, such as protons, carboxylates, flavonoids as well as acid phosphatases, and effectively ‘mine’ sorbed soil P and organic P when very little inorganic P is in solution (Gardner et al., 1981; Vance et al., 2003; Tomasi et al., 2008).
Advances in the understanding of cluster-root formation as an adaptation to low-P soils in terms of physiology and ecology have been made in many studies (Skene, 1998; Neumann and Martinoia, 2002; Lamont, 2003; Shane and Lambers, 2005; Lambers et al., 2006; Zhou et al., 2018; Gallardo et al., 2019). The initiation of cluster roots begins with cell divisions from the pericycle opposite protoxylem poles of lateral roots, followed by differentiation of the cluster-root primordia, giving rise to determinate growth of lateral rootlets. Distinct from common lateral roots, cluster rootlets of white lupin exhibit determinate growth, and cluster roots are developed sequentially along a lateral root, with dense short rootlets, covered with abundant root hairs (Neumann and Martinoia, 2002). Cluster-root formation involves several regulators such as phytohormones, nitric oxide, sucrose and peptides (Zhou et al., 2008; B. Wang et al., 2010; Z. Wang et al., 2015; Zhou et al., 2018; Xu et al., 2020). For white lupin, auxin modulation is important for cluster-root formation under P-deficient conditions, since cluster-root formation is promoted by auxin application and suppressed by auxin transport inhibitors (Gilbert et al., 2000; Meng et al., 2013; Wang et al., 2015). Further studies showed that a series of auxin synthesis and signalling elements, including LaABCG36 (ATP-binding cassette G36), LaTIR1b (transport inhibitor response 1b), LaARF5 (auxin response factor 5) and LaIAA14 (auxin/indole-3-acetic acid repressors), are involved in regulating cluster-root formation (Gallardo et al., 2019; Hufnagel et al., 2020; Xu et al., 2020). However, the mechanism underlying auxin-mediated cluster-root formation still needs to be determined.
Flavonoids inhibit auxin transport and affect localized auxin accumulation in plants (Brown et al., 2001; Buer et al., 2013; Maloney et al., 2014; Ng et al., 2015a). Flavonoids are ubiquitous plant secondary metabolites, and comprise a vast variety of phenolic compounds (such as flavonols, anthocyanins and isoflavonoids) synthesized via the flavonoid branch of the phenylpropanoid pathway, which begins with chalcone synthesis catalysed by chalcone synthase (CHS) (Williams and Grayer, 2004; Routaboul, 2006; Buer et al., 2010). Flavonoids play crucial roles in root development involving auxin-related mechanisms by affecting auxin transport and redistribution through ABCB-type auxin transporters and PIN proteins (Geldner et al., 2001; Peer et al., 2004; Peer and Murphy, 2007; Santelia et al., 2008; Ng et al., 2015b). An optimal concentration of flavonoids is necessary to regulate auxin-controlled developmental processes such as lateral root growth and nodule formation, as demonstrated by using mutants of Arabidopsis thaliana, Solanum lycopersicum and Medicago truncatula that do not accumulate flavonoids (Brown et al., 2001; Buer and Muday, 2004; Maloney et al., 2014; Ng et al., 2015a).
Auxin is pivotal for cluster-root formation (Gallardo et al., 2019), and flavonoids inhibit auxin transport and affect auxin local accumulation in roots of Arabidopsis and Medicago (Brown et al., 2001; Imin et al., 2007; Grunewald et al., 2012). However, the mechanism underlying the effects of flavonoids on P-deficiency-induced cluster-root formation remains unknown. We hypothesized that the interactions of flavonoids and auxin regulate cluster-root formation under P deficiency in white lupin.
MATERIALS AND METHODS
Plant growth
White lupin (Lupinus albus L. ‘Kiev Mutant’) seeds were sterilized by 30 % (v/v) H2O2 prior to germination and grown in plastic pots containing 3.5 L of an aerated nutrient solution (three plants per pot). The solution was composed of (μm): Ca(NO3)2 (2000), K2SO4 (700), MgSO4 (500), KCl (100), H3BO3 (10), ZnSO4 (0.5), MnSO4 (0.5), CuSO4 (0.2), (NH4)6MO7O24 (0.01) and Fe-EDTA (20). The P-sufficient (+P) and P-deficient (−P) solutions contained 75 μm KH2PO4 and no Pi addition, respectively. The solution was renewed every 3 d, and the pH of the solution was adjusted to 5.8 using NaOH. Plants were grown in a controlled environment with a light:dark regime of 14:10 h, a temperature of 28:22 °C and a light intensity of 230 μmol m−2 s−1.
Flavonoid staining and fluorescence in roots
To examine the effect of P deficiency on flavonoid production during cluster-root development, the cluster roots were divided into different developmental stages. Plants were harvested at 21 d after P treatments. Different root segments including root apex, juvenile (just emergence of the rootlets of clusters) and mature cluster roots (all of the rootlets reach 0.5–1.0 cm) were excised from P-deficient roots; the same order of cluster root was collected as control for P-sufficient plants according to our previous study (Wang et al., 2010).
The endogenous flavonoid accumulation was determined by adopting the fluorescent probe diphenylboric acid 2-aminoethyl ester (DPBA; fluoresces upon binding flavonoids) as described by Buer and Muday (2004). The excised root segments were incubated with staining solution containing 0.25 % (w/v) DPBA and 0.005 % (v/v) Triton X-100 for 5 min. The root segments were then washed for 5 min with 50 mm sodium phosphate buffer containing 0.005 % (v/v) Triton X-100, pH 7.0. The fluorescence was excited with a 488-nm argon–krypton laser and emission signals were collected at 515 nm by using a laser confocal scanning microscope (Te2000-E; Nikon, Tokyo, Japan). The intensity of fluorescence was quantified using ImageJ software.
Quantitative analysis of flavonoids
The concentration of flavonoids in different root segments was measured by high-performance liquid chromatography (HPLC). To extract flavonoids from roots, root tissue (100 mg) was extracted in 1.0 mL methanol for 16 h with constant shaking in the dark. Extracts were then centrifuged at 6000 g for 30 min. The supernatant was removed and evaporated under a nitrogen atmosphere till dryness. The dried residue was resuspended in 300 μL of 2 m HCl in a water bath at 80 °C for 1.5 h. After the samples were cooled to room temperature, they were extracted with 600 μL ethyl acetate, followed by centrifuging at 6000 g for 10 min; the ethyl acetate layer was evaporated under nitrogen atmosphere till dryness. The dried residue was resuspended in 1.0 mL with 80 % aqueous methanol and filtered through a 0.45-µm polytetrafluoroethylene filter. The composition and concentrations of flavonoid aglycones in the samples were analysed using a reverse-phase HPLC system according to the modified method from a previous study (Dardanelli et al., 2008). Separation was conducted on a 250 × 4.6 mm reverse-phase column (Alltima C18, 5 Micron; Alltech Associates, Deerfield, IL, USA) at 36 °C. Solvent A consisted of 99.5 % water and 0.5 % acetic acid and solvent B consisted of 50 % methanol and 50 % acetonitrile. An isocratic elution program with 60 % A:40 % B (v/v) was maintained for 40 min with a flow rate of 1 mL min−1. The peaks were monitored at 370 nm. Naringenin, quercetin, kaempferol and genistein (Sigma–Aldrich, St Louis, MO, USA) were used as standards in the range of 0.05–1.0 mg ml−1.
Expression of CHS
Two full-length cDNA clones (GenBank no. MW538516, MW538517) were corresponding to a previously described expressed sequence tag (EST) detected in a cluster root cDNA library with homology to CHS (Vance et al., 2003). Total RNA was isolated using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA). The cDNA was synthesized from total RNA with the Superscript RT II enzyme (Invitrogen, Carlsbad, CA, USA) and oligo(dT)17 primer. TB Green™ Premix Ex Taq™ II (Tli RNaseH Plus; RR820A, Takara, Japan) was used to perform quantitative real-time (qRT) PCR on an iQTM5 real-time PCR detection systems (Bio-Rad) following the manufacturer’s protocols. The PCR amplification was performed at 95 °C for 15 s and 60 °C for 1 min. The reference gene was Laα-Tubulin. The primers used to quantify gene expression levels are listed in Supplementary Data Table S1.
Analysis of LaCHS8 RNAi-silenced plants
To create constructs for RNAi-mediated silencing of LaCHS8, a 472-bp fragment of the LaCHS8 mRNA sequence (bp 912–1384) was PCR-amplified from cDNA clones using the primer set CHS8Fi and CHS8Ri (Supplementary Data Table S1). The PCR products were cloned into the pENTR/D-TOPO entry vector following the manufacturer’s instructions (Invitrogen) and then recombined with the expression vector pK7GWIWG2 (II)-DsRED to produce the LaCHS8 RNAi construct. The pK7GWIWG2 (II)-DsRED vector with a fragment of the human myosin gene was used as a control. All resulting constructs were introduced into Agrobacterium rhizogenes A4TC24 by electroporation and used for white lupin transformation.
The A. rhizogenes-mediated transformation method of white lupin was modified from the description in Uhde-Stone et al. (2005). White lupin seeds were surface-sterilized with 95 % ethanol for 10 min, followed by rinsing with sterile water. Then seeds were sterilized with 30 % (v/v) H2O2 for 10 min and rinsed with sterilized water. Seeds were germinated in the dark for 2 d on 0.6 % agar plates at room temperature and then transformed with A. rhizogenes as follows: when emerging radicles reached a length of ~20 mm on the plates, seedlings were transplanted to wet vermiculite and kept in the dark at room temperature for 2 d, followed by 2 d in the dark at 4 °C. After the cold treatment, the primary root apex (~5–10 mm) was excised, and the cut end was inoculated with the A4TC24 containing the plasmid of interest. One hour after inoculation, seedlings were grown in an aeroponic system (EZ Clone Enterprises, Sacramento, CA, USA) filled with deionized water for the first week. Following that, they were supplied a nutrient solution with or without 75 µm Pi and harvested 4 weeks later.
Exogenous application of flavonoids on plants
To study the effect of flavonoids on cluster-root formation, plants were grown in a P-deficient solution with 10 µm naringenin, quercetin or kaempferol (Sigma–Aldrich, St Louis, MO, USA), and the treatment solution was renewed every 3 d. Plants were harvested after 14 d, and roots were collected and scanned using an Epson V800 scanner at a resolution of 400 dpi. Images were analysed by WinRhizo software (Regent Instruments, Quebec, QC, Canada) to obtain cluster rootlet length and density.
Analysis of DR5:GUS plants treated by exogenous application of flavonoids
DR5:GUS transgenic hairy roots were obtained by A. rhizogenes-mediated transformation of white lupin as described above. The DR5:GUS transgenic hairy roots were grown in P-deficient nutrition solution with mock or flavonoids (10 μm) for 24 h before experimental observation.
The β-glucuronidase (GUS) histochemical staining of white lupin hairy roots was performed as previously described (Cheng et al., 2011). Transgenic roots harbouring the GUS reporter gene were incubated at 37 °C for 2 h in GUS staining solution with the substrate 1 mm X-Gluc (5-bromo-4-chloro-3-indolyl-β-D-GlcA cyclohexyl-ammonium). Before microscopic examination using a Zeiss Axioskop, these samples were destained with 95 % (v/v) ethanol.
Statistical analyses
SPSS 19.0 for Windows (SPSS version 19.0, IBM SPSS, Chicago, IL, USA) was used for statistical analysis of the data. After one-way ANOVA, Fisher’s LSD multiple comparison was used to test for significant differences in cluster rootlet density and cluster root length with different exogenous flavonoid treatments. An independent sample Student’s t-test was used to test for significant differences in flavonoid concentrations, relative expressions of genes under different P levels, and the relative expressions of LaCHS8, fluorescence intensity and lateral rootlet density in different transgenic hairy roots.
RESULTS
Flavonoid distribution during formation of P-deficiency-induced cluster roots
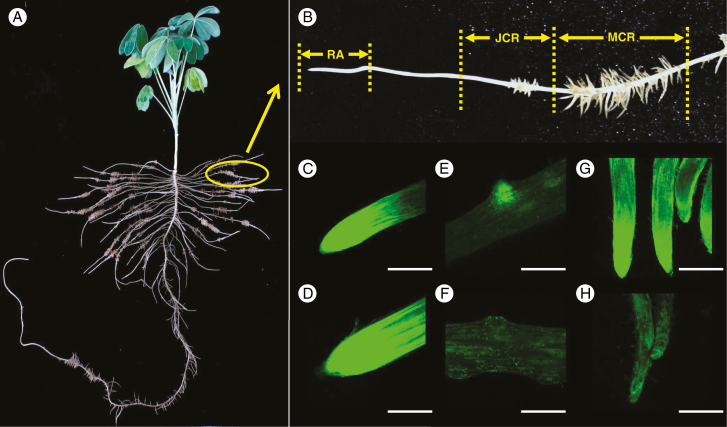
White lupin formed large number of cluster roots under P deficiency (Fig. 1A) though some cluster roots were also observed in the treatment of P-sufficient plants (data not shown). In the present study, cluster roots were separated into root apices (RAs), juvenile cluster roots (JCRs) and mature cluster roots (MCRs) (Fig. 1B). An intensive fluorescence signal was observed in root apices of both P-sufficient and P-deficient plants (Fig. 1C, D). The fluorescence signal in juvenile and mature cluster roots was much stronger in P-deficient plants than in P-sufficient ones (Fig. 1E–H and Supplementary Data Fig. S1). At the early stage, when cluster rootlets just emerged from axial roots, there was a greater fluorescence signal in P-deficient rootlets (Fig. 1E, F). In addition, when the rootlets were mature, greater flavonoid accumulation was found in the apices of rootlets, especially under P-deficient conditions (Fig. 1G, H).
Fig. 1.
Flavonoid fluorescence in roots of white lupin grown under P-sufficient and P-deficient conditions. Plants were grown in hydroponic solution with 75 μm P (+P) or 0 μm P (−P) for 21 d. Root segments were stained with DPBA. Green fluorescence was detected by confocal laser scanning microscopy, which shows accumulation of flavonoids. (A) White lupin plant grown under −P conditions formed a large number of cluster roots. (B) Cluster roots were separated into root apex (RA), juvenile cluster roots (JCR) and mature cluster roots (MCR). (C–H) Images of flavonoid fluorescence in (C) −P root apex, (D) +P root apex, (E) −P juvenile cluster root, (F) +P juvenile cluster root, (G) −P mature cluster root and (H) +P mature cluster root. Scale bar = 1.0 mm.
Expression of LaCHS2 and LaCHS8 in cluster roots
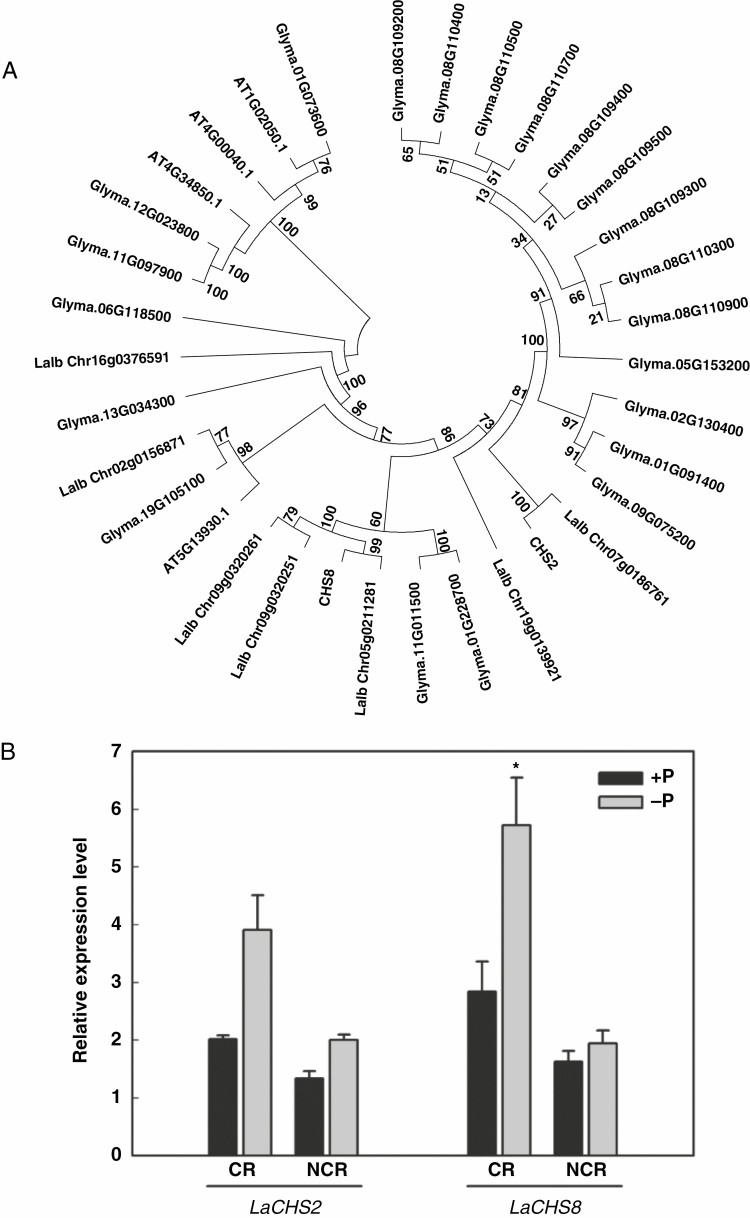
To further investigate the role of flavonoids in cluster-root formation induced by P deficiency, we determined the expression of LaCHS, which encodes a CHS that catalyses the first step in the biosynthetic pathway of all flavonoids. Two full-length cDNA clones that have homology with CHS ESTs detected in a cluster-root cDNA library of white lupin (established in Vance’s laboratory; Vance et al., 2003) were isolated and sequenced. The identified cDNAs were designated as LaCHS2 and LaCHS8, present in a separate clade in the tree (Fig. 2A); their amino acid sequences shared 91 % sequence identity with each other (Supplementary Data Fig. S2).
Fig. 2.
Phylogenetic tree based on sequences of CHS proteins and expression of LaCHS2 and LaCHS8 in roots of white lupin grown under P-sufficient and P-deficient conditions. (A) Sequences were derived from NCBI and WHITE LUPIN GENOME (https://www.whitelupin.fr/index.html). The alignments were saved and executed using MEGA to generate a neighbour-joining tree. (B) qRT–PCR analysis of CHS expression in different root types of white lupin grown under P-sufficient and P-deficient conditions. Plants were grown for 21 d with 75 μm P (+P) or 0 μm P (−P). Cluster roots (CR) and non-cluster roots (NCR) were harvested and used for RNA isolation and cDNA synthesis. qRT–PCR was used to assess LaCHS2 and LaCHS8 transcript abundance in response to P treatments. Data are expressed as relative values based on the LaCHS expression level in +P non-cluster roots. Asterisk in (B) indicates significant difference between −P and +P by Student’s t-test: *P < 0.05. Significant difference was based on four biological replicates. Bars on the graph are standard errors.
Quantitative RT–PCR was used to analyse LaCHS2 and LaCHS8 transcripts in root tissues of P-sufficient and P-deficient plants. Both LaCHS2 and LaCHS8 showed significantly greater transcript abundance in cluster roots than in non-cluster roots of P-deficient plants. Particularly, the expression of LaCHS8 in cluster roots was significantly upregulated by P deficiency, and increased by 101 % in P-deficient plants compared with P-sufficient plants (Fig. 2B).
Silencing of CHS in white lupin roots reduced flavonoid accumulation and impaired lateral root development
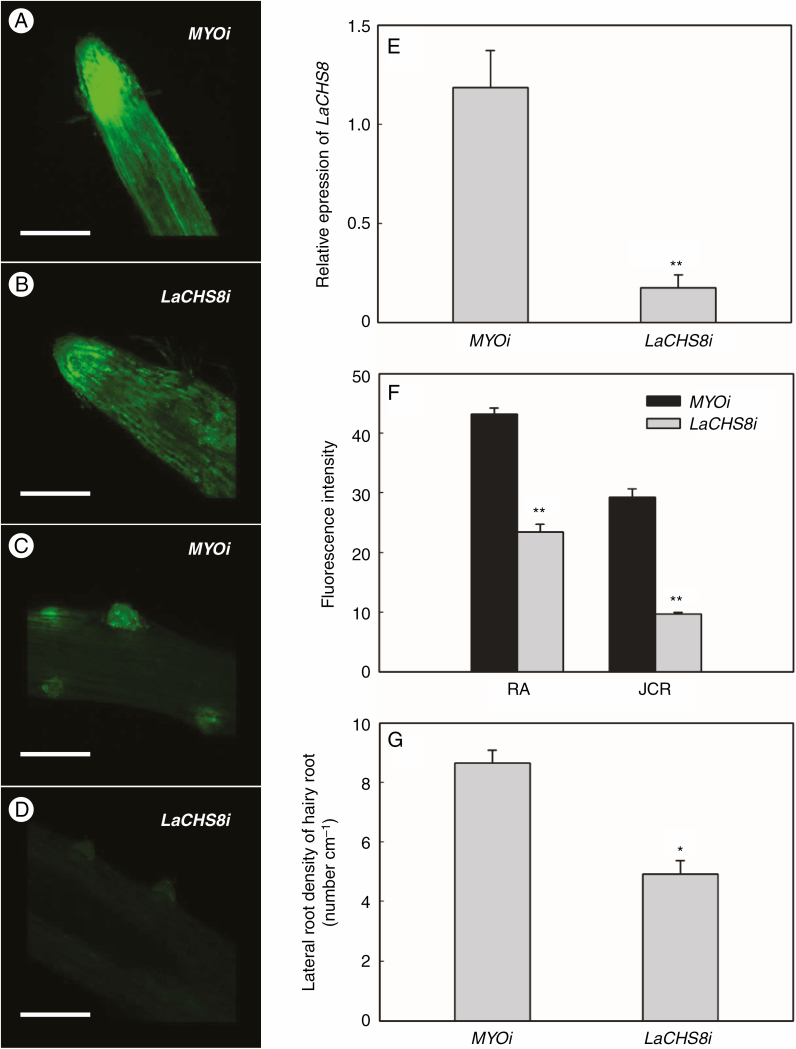
Both flavonoid accumulation and lateral root density were less in LaCHS8 RNAi hairy roots with attenuated expression of LaCHS8 than in control hairy roots (Fig. 3A–G). We used qRT–PCR to assess whether transgenic roots containing LaCHS8 RNAi constructs had altered transcript levels. As shown in Fig. 3E, the knockdown roots showed significantly lower LaCHS8 transcript levels in corresponding LaCHS8 RNAi roots than in control roots. The expression of LaCHS2 also tended to be reduced in LaCHS8 RNAi roots, albeit not significantly (Supplementary Data Fig. S3). In LaCHS8 RNAi hairy roots, the fluorescence of flavonoid–DPBA was weakened in both root apices and juvenile cluster roots (Fig. 3A–D), and the intensity of fluorescence in root apices and juvenile cluster roots was reduced by 47 and 67 %, respectively (Fig. 3F). The lateral root density was reduced by 43 % compared with the abundant rootlets in control transgenic cluster roots (Fig. 3G). However, the reduction of lateral root density in LaCHS8 RNAi roots was partially complemented after naringenin treatment (Supplementary Data Fig. S4).
Fig. 3.
Effect of LaCHS8 RNAi silencing on flavonoid accumulation and lateral rootlet density. Reduced expression of LaCHS8 was achieved by subcloning an mRNA fragment of LaCHS8 into an RNAi vector containing DsRED as a reporter and subsequent transformation of white lupin roots using A. rhizogenes. In parallel, roots transformed with a human myosin gene were used as a control to exclude effects due to the formation of hairy roots. Hairy roots were incubated in DPBA to show flavonoid accumulation. (A–D) Green fluorescence of flavonoid–DPBA was detected by confocal laser scanning microscopy (scale bar = 1.0 mm). Images of (A) flavonoid fluorescence in root apices of MYO RNAi (MYOi) hairy roots, (B) root apices of LaCHS8 RNAi (LaCHS8i) hairy roots, (C) lateral rootlet emergence zones of MYOi hairy roots and (D) lateral rootlet emergence zones of LaCHS8i hairy roots. (E) qRT–PCR confirmed the reduction of the LaCHS8 transcript in the corresponding RNAi roots relative to the control. (F) Fluorescence intensity (arbitrary fluorescence units) in different transgenic hairy root segments: root apexes (RA) and juvenile cluster roots (JCR). (G) Lateral rootlet density of LaCHS8i and MYOi hairy roots. Asterisks in (E–G) indicate significant differences compared with the control (MYOi) by Student’s t-test: *P < 0.05; **P < 0.01. Significant difference was based on four biological replicates. The bars on the graphs are standard errors.
Phosphorus deficiency alters flavonoid concentrations in cluster roots
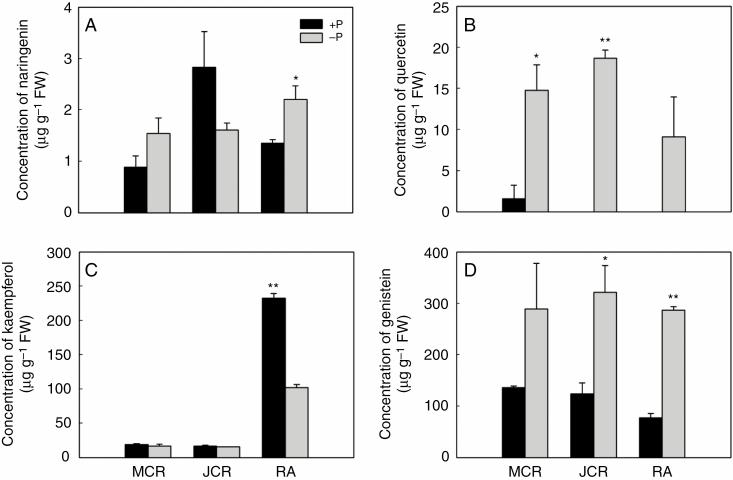
To further explore the relationship of the accumulation of flavonoids with cluster-root formation induced by P deficiency, some of the key flavonoids were quantified by HPLC, including naringenin (a flavanone), quercetin and kaempferol (flavonols), and genistein (an isoflavonoid). Supplementary Data Fig. S5 shows HPLC chromatograms of flavonoids in the extracts. Compared with P-sufficient plants, P deficiency increased the concentration of naringenin in root apices (Fig. 4A), that of quercetin in juvenile and mature roots (Fig. 4B) and genistein in juvenile roots and root apices (Fig. 4D). In contrast, P deficiency reduced the concentration of kaempferol by 56 % in root apices (Fig. 4C). Comparing the concentrations of four flavonoids in different root segments under the P-deficient condition, naringenin, quercetin and kaempferol showed a tissue-specific accumulation pattern in different root segments. In contrast, genistein was uniformly distributed and was the most abundant flavonoid (Fig. 4).
Fig. 4.
Quantification of flavonoids in roots of white lupin under P-sufficient and P-deficient conditions. Plants were grown in hydroponic solution with 75 μm P (+P) or 0 μm P (−P) for 21 d. Root samples including root apices (RA) and different developmental stages of cluster roots under +P and −P conditions were used for HPLC analysis of major flavonoids. Flavonoids analysed were (A) naringenin, (B) quercetin, (C) kaempferol and (D) genistein. Asterisks indicate significant differences between −P and +P by Student’s t-test: *P < 0.05; **P < 0.01. Significant difference was based on three biological replicates. The bars on the graphs are standard errors.
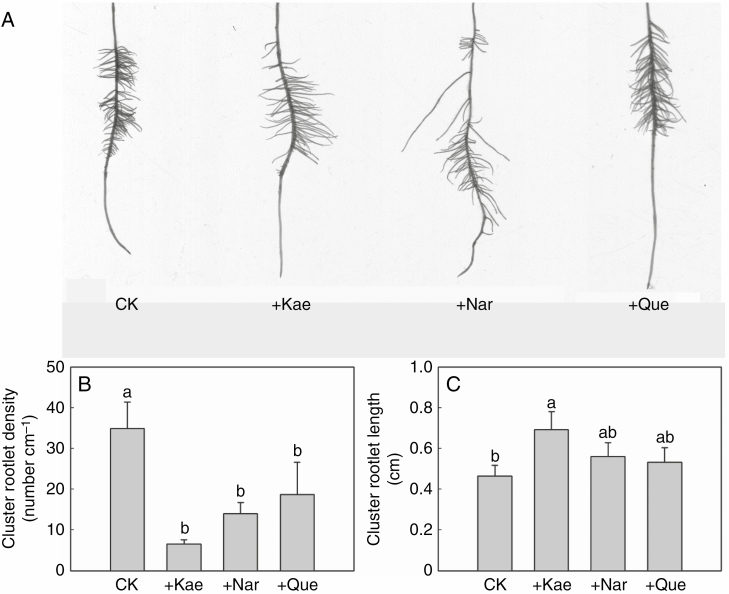
Effect of exogenous flavonoids on cluster-root growth
The cluster-root phenotype was significantly changed after treatments with naringenin, quercetin and kaempferol (Fig. 5A, Supplementary Data Fig. S6). The three flavonoids significantly reduced lateral root density, by 47–81 % (Fig. 5B). In addition, the kaempferol treatment significantly increased lateral rootlet length by 49 % (Fig. 5C). Distinct from the effect of kaempferol, exogenous naringenin and quercetin had no effect on the length of lateral rootlets (Fig. 5C). Overall, applications of naringenin, quercetin and kaempferol decreased the formation of cluster roots.
Fig. 5.
Changes in cluster-root morphology of white lupin upon addition of different flavonoids. Plants grown in 0 μm P (−P) nutrient solution were treated with a mock solution or with 10 μm flavonoid (kaempferol, naringenin or quercetin) for 14 d. (A) Scanning image of one cluster of rootlets in each treatment. (B) Cluster rootlet density and (C) cluster rootlet length of roots after mock or flavonoid treatment. CK, control (mock treatment); Kae, kaempferol; Nar, naringenin; Que, quercetin. Lowercase letters denote significant differences among treatments (P < 0.05). Significant difference was based on four biological replicates. The bars on the graphs are standard errors.
Accumulation of flavonoids in epidermis, primordia and lateral root caps of cluster roots
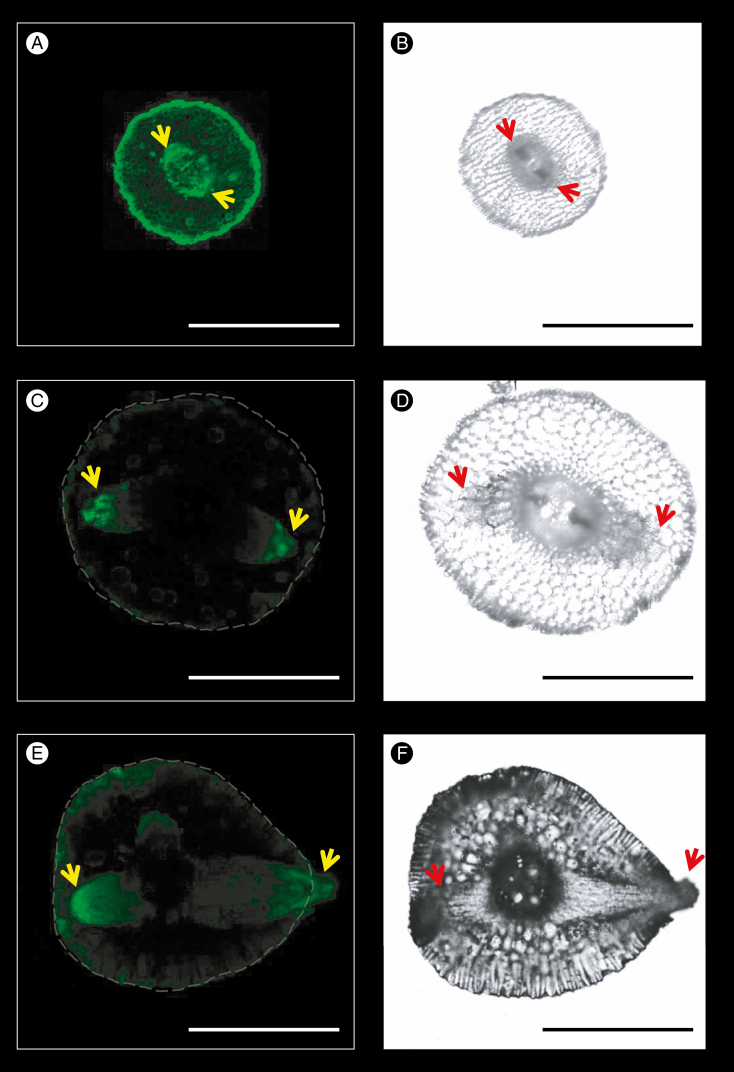
More details of flavonoid distribution within cluster roots were examined to explore the potential mechanism of cluster-root formation. Flavonoids in fresh cross-sections of cluster roots were visualized by staining with DPBA. Different growth stages, including primordium initiation (Fig. 6A, B), primordium development (Fig. 6C, D) and lateral rootlet emergence (Fig. 6E, F), showed strong fluorescence intensity in primordia, particularly in lateral root caps, indicating a tissue-specific distribution pattern of flavonoids related to cluster-root development.
Fig. 6.
Localization of flavonoids in primordia of cluster roots. Green fluorescence of flavonoid–DPBA was detected by confocal laser scanning microscopy (scale bar = 1.0 mm). The images show the high flavonoid–DPBA fluorescence in primordia apices of lateral roots. (A, B) Flavonoid fluorescence and light-field images of primordia initiation zones near the root apex. (C, D) Flavonoid fluorescence and light-field images of rootlet primordia development stage. (E, F) Flavonoid fluorescence and light-field images of rootlet emergence stage. Yellow arrows in the fluorescence images and red arrows in the light fields indicate the apex of the rootlet primordia.
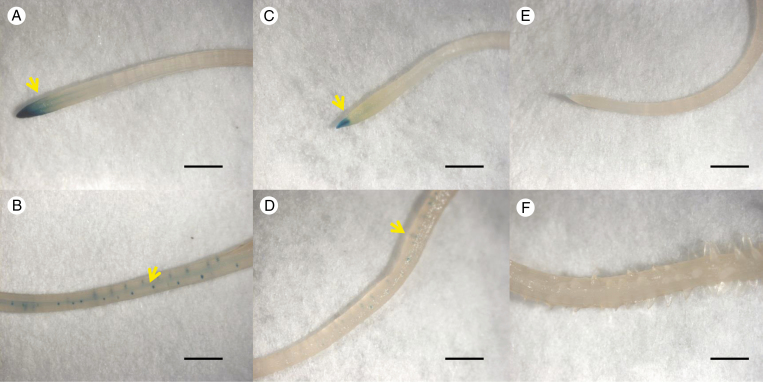
Negative effects of exogenous flavonoid treatments on DR5:GUS expression
To test the effect of flavonoids on the auxin response, DR5:GUS transgenic hairy roots were treated with naringenin, quercetin, kaempferol or genistein. Histochemical staining of GUS signals showed that DR5:GUS expression was predominantly located in the root apex, stele and lateral root meristem regions when plants were grown in a P-deficient solution without flavonoids (Fig. 7A, B). A decreased auxin response was observed in naringenin-treated roots, especially in the stele and lateral root meristem regions (Fig. 7C, D), and there was no auxin response signal at all in negative control transgenic hairy roots without DR5:GUS (Fig. 7E, F). Compared with the effects of the other three flavonoids, application of naringenin showed the strongest inhibition effect on DR5:GUS expression (Fig. 7, Supplementary Data Fig. S7).
Fig. 7.
Effects of naringenin on the auxin response in white lupin hairy roots. Auxin reporter DR5:GUS was transformed in white lupin roots using A. rhizogenes. Roots transformed with the auxin reporter DR5:GUS in 0 μm P (−P) nutrient solution were treated with mock or naringenin (10 μm) for 24 h. (A, B) DR5:GUS expression was predominantly located in the stele and lateral root meristem regions of roots with mock treatment. (C, D) A decreased auxin response was observed in naringenin-treated roots. (E, F) No auxin response in negative control (transgenic hairy roots without DR5:GUS). Yellow arrows indicate auxin response sites in the root apex and the root stele. Scale bar = 5.0 mm.
DISCUSSION
Cluster roots are similar to non-cluster lateral roots at the early initiation stage until the cluster of rootlets reaches maturity. The mature cluster rootlets are highly differentiated ephemeral root structures, densely covered by root hairs and lacking active meristems; thus they stop elongating but mobilize and take up P until senescence (Watt and Evans, 1999; Neumann and Martinoia, 2002; Gallardo et al., 2019). Therefore, it is important to reveal the regulatory pattern of cluster-root formation, and white lupin offers a great model. Auxin is an important regulator in the induction and development of cluster roots in white lupin; cluster-root development has a specific expression profile of auxin-related genes, and the use of the DR5 marker by Gallardo et al. (2019) indicated the establishment of a maximum of auxin is necessary to maintain rootlet development. However, how the distribution of auxin in cluster roots is regulated remained unclear. Flavonoids are abundant metabolites that accumulate in cluster roots, and affect natural auxin transport, but the mechanism underlying cluster-root formation fine-tuned by interactions between flavonoids and auxin remains unknown. In this study, we characterized the distribution profile of flavonoids in cluster roots and found that it was affected by P status. This is evidenced by quantitative results showing precise spatial localization of flavonoid accumulation with different patterns. Disturbance of flavonoid distribution negatively regulated cluster-root formation, as evidenced by the effects of exogenous flavonoids and RNAi-attenuated flavonoid accumulation. A tissue-specific accumulation pattern of flavonoids in cluster-root primordia was further characterized, which resembled the localization of the auxin responses. Together with the inhibitory effect of naringenin on the auxin response, which is related to cluster-root formation, the present results provide evidence that flavonoids and auxin interact during cluster-root formation in white lupin under P deficiency.
Fluorescence analysis of cluster roots stained with DPBA provides a tool to examine the accumulation of flavonoids in root tissues (Buer et al., 2007; Yang et al., 2009; Ng et al., 2015a). Fluorescence intensity of flavonoid–DPBA compounds qualitatively reflects accumulation of flavonoids, or semi-quantitatively reflects the concentration of several specific flavonoids by detecting particular wavelengths of emission light from 510 to 580 nm (Buer et al., 2007). We adopted the method of collecting the DPBA-enhanced green fluorescence of unspecific flavonoids at 515 nm, and combined this with HPLC analyses to reveal the distribution characteristics of flavonoids in the roots. We showed an accumulation of flavonoids in the root apex, where the high intensity of the fluorescence signal was the result of overlay of different flavonoid compounds. The similar fluorescence signals in root apices between P-deficient and P-sufficient plants was possibly due to saturation of the fluorescence signal (Fig. 1C, D); hence, HPLC analysis was employed in the study. The difference in flavonoid fluorescence intensity was observed in other root parts between P treatments. In general, the difference in fluorescence intensity reflects the accumulation of flavonoids during root development. The greater fluorescence signal in juvenile and mature cluster roots in P-deficient plants than in P-sufficient ones (Fig. 1E–H) indicated a spatial pattern that strongly depended on P status. Through a HPLC assays, we found that the accumulation of naringenin, quercetin and genistein in cluster roots was upregulated by P deficiency (Figs 1 and 4), as observed in other studies (Stewart et al., 2001; Weisskopf et al., 2006; Jiang et al., 2007; Yang et al., 2009). Localized distribution of flavonoids was also developmentally regulated. The flavonoids were mainly located in young root tissues, including root apices, primordia, juvenile cluster roots and apices of mature cluster rootlets (Figs 1 and 6). Different flavonoids showed a tissue-specific pattern, with naringenin and kaempferol accumulating in root apices, and quercetin concentrations being higher in cluster roots; however, the genistein concentration was similar throughout the whole of the cluster roots, and this flavonoid was the most abundant (Fig. 4). The strong expression of LaCHS8, which encodes CHS, in cluster roots receiving no P (Fig. 2) further supports the contention that flavonoid accumulation in cluster roots was developmentally regulated by P deficiency. The accumulation of flavonoids in the root tissues suggests that white lupin regulated flavonoid spatial distribution in response to P deficiency. However, this effect does not mean a similar trend for downstream-specific flavonoids. The most abundant flavonoid in cluster roots, genistein, was evenly distributed in cluster roots. This distribution was not surprising, since the most important role of genistein in legumes is to communicate with rhizobia rather than regulating lateral root formation (Hassan and Mathesius, 2012).
Flavonoids play a distinct role in cluster-root formation, as evidenced by the modified root phenotype through silencing of LaCHS8 and exogenous application of flavonoids. We observed a reduced accumulation of flavonoids in LaCHS8 RNAi roots by DPBA staining, leading to a significant inhibition of lateral root density (Fig. 3). However, supplementation with exogenous naringenin, the upstream product of flavonoid metabolism, to LaCHS8 RNAi roots showed a trend of a complementary effect (Supplementary Data Fig. S4), suggesting a role of flavonoids in regulating lateral root development. This suggests that a certain concentration of a flavonoid is required for lateral root formation, which might be linked to local accumulation or a response to auxin during cluster-root development under P deficiency. Effects of flavonoids on lateral root development have been studied in different species with genetic defects in the synthesis of flavonoids. In tomato, flavonols play a positive role in lateral root formation, evidenced by a reduced initiation of lateral roots in a mutant with lower levels of flavonols (Maloney et al., 2014). In contrast, the flavonoid-deficient transparent testa (tt4) mutants in Arabidopsis thaliana develop more lateral roots than wild-type Arabidopsis (Brown et al., 2001; Jain et al., 2007). The observation that flavonoid deficiency results in contrasting lateral root phenotypes in white lupin, tomato and Arabidopsis reflects the range of effects of flavonoids on lateral root development among different species (Brown et al., 2001; Jain et al., 2007). Application of the flavonoids naringenin, quercetin and kaempferol strongly inhibited cluster-root formation with reduced lateral rootlet density (Fig. 5). The uptake and movement of exogenous flavonoids affect local accumulation of endogenous flavonoids (Buer et al., 2007). Therefore, we assume that the uptake and movement of exogenous flavonoids by white lupin roots may affect the local accumulation pattern of endogenous flavonoids mediated by P deficiency, and thus decreased the effect of flavonoids as the auxin regulator in response to P deficiency. In conclusion, disturbing flavonoid accumulation in lateral roots through both exogenous application of flavonoids and LaCHS8 RNAi treatment caused a negative effect on auxin accumulation in rootlet primordia, leading to a decrease in lateral root density.
Naringenin might be an important factor regulating cluster-root formation which is related to the action of auxin. The tissue-specific accumulation of flavonoids in cluster-root primordia (Fig. 6) coincided with regions of auxin response (Fig. 7); a similar auxin response was identified in white lupin recently and shown to be involved in cluster-root formation (Gallardo et al., 2019). Exogenous supply of flavonoids, particularly naringenin, inhibited the expression of the auxin response reporter DR5:GUS (Fig. 7) and strongly disturbed the auxin concentration gradient in primordia zones and cluster rootlet emergence zones induced by P deficiency (Supplementary Data Fig. S8). These results indicate the interaction between flavonoids and auxin during rootlet development in transgenic hairy roots that express the DR5:GUS auxin reporter, suggesting a role of flavonoids in auxin accumulation and response. Previous studies have reported that auxin is involved in cluster-root formation as demonstrated by the positive effect of auxin application and inhibitory effect of auxin antagonists (Gilbert et al., 2000; Skene, 2001; Gallardo et al., 2019). Genes related to auxin synthesis, transport and signalling are regulated by P deficiency in cluster roots of white lupin (Meng et al., 2013; Wang et al., 2015; Hufnagel et al., 2020; Xu et al., 2020). Flavonoids are well suited to modulate auxin transport by affecting the expression, localization and cycling of auxin efflux facilitators such as PIN proteins (Geldner et al., 2001; Peer et al., 2004; Santelia et al., 2008). Flavonoids also play a pivotal role in inhibiting auxin transport during nodulation (Zhang et al., 2009; Ng et al., 2015a), thus increasing local auxin levels, which in turn promote nodule organogenesis (Hassan and Mathesius, 2012). Clearly, flavonoids show an interaction with auxin in plant or nodule development, but, at least till now, there was no report on how the interaction affects cluster-root formation. Here, we are the first to examine the consistent localization of flavonoids and auxin in cluster roots, and inhibitory effects of flavonoids on the auxin response in cluster-root formation. Taken together, the results suggest that flavonoid–auxin interactions as affected by P deficiency regulate cluster-root initiation and subsequent development in white lupin (Supplementary Data Fig. S9).
CONCLUSIONS
We propose that flavonoids mediate P-deficiency-induced cluster-root formation in white lupin. The negative effects of LaCHS8 RNAi on flavonoid production and rootlet formation indicate a key role of flavonoids in cluster-root formation. Phosphorus deficiency induces a spatial pattern of flavonoid concentrations in different root segments of cluster roots, and promotes accumulation of flavonoids in young root tissues. This specific accumulation profile of flavonoids in root segments would fine-tune the local auxin response in roots and subsequent cluster-root formation. These findings provide valuable insight into the mechanisms of P-deficiency-induced cluster-root formation.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following. Figure S1: fluorescence intensity in different root segments including root apices, juvenile cluster roots and mature cluster roots. Figure S2: amino acid sequence similarity of LaCHS2 and LaCHS8. Figure S3: expression of the LaCHS2 transcript in the LaCHS8 RNAi roots relative to that in the control. Figure S4: effects of naringenin on lateral root formation of white lupin hairy roots grown without P. Figure S5: HPLC chromatograms of the root extracts and flavonoid standards. Figure S6: effects of naringenin, quercetin and kaempferol on the growth of white lupin under P-deficient conditions. Figure S7: effects of quercetin, kaempferol and genistein on the auxin response in white lupin hairy roots. Figure S8: effects of naringenin on auxin concentrations in cluster roots of white lupin plants grown without P. Figure S9: model for the role of flavonoids in P-deficiency-induced cluster-root formation in white lupin. Table S1: primers used in this study.
ACKNOWLEDGEMENTS
C.X., C.P.V., J.S. and L.C. designed the study. C.X., X.L., X.W. and J.W. performed the experiments. C.X, J.S. and L.C. analysed the data. C.X., J.S. and L.C. wrote the manuscript. C.X., J.S., L.C. and H.L. revised the manuscript. The authors declared there is no any conflict of interest to be included in the manuscript.
FUNDING
This work was supported by the National Science Foundation of China (No. 31972496, 32130094, 31772402, 31572190), Hainan Provincial Natural Science Foundation of China (321CXTD443), Project of New Fertilizer Research and Development of Yun-Tian-Hua Group of Yunnan of China and The 2115 Talent Development Program of China Agricultural University. L.C and J.S. are also supported by the project of Beijing’s Advanced Disciplines.
LITERATURE CITED
- Brown DE, Rashotte AM, Murphy AS, et al. 2001. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiology 126: 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Muday GK. 2004. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16: 1191–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Muday GK, Djordjevic MA. 2007. Flavonoids are differentially taken up and transported long distances in Arabidopsis. Plant Physiology 145: 478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Imin N, Djordjevic MA. 2010. Flavonoids: new roles for old molecules. Journal of Integrative Plant Biology 52: 98–111. [DOI] [PubMed] [Google Scholar]
- Buer CS, Kordbacheh F, Truong TT, Hocart CH, Djordjevic MA. 2013. Alteration of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long-term effects on root and shoot architecture. Planta 238: 171–189. [DOI] [PubMed] [Google Scholar]
- Cheng L, Bucciarelli B, Liu J, et al. 2011. White lupin cluster root acclimation to phosphorus deficiency and root hair development involve unique glycerophosphodiester phosphodiesterases. Plant Physiology 156: 1131–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardanelli MS, Fernández de Córdoba FJ, Espuny MR, et al. 2008. Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biology & Biochemistry 40: 2713–2721. [Google Scholar]
- Dinkelaker B, Römheld V, Marschner H. 1989. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant, Cell & Environment 12: 285–292. [Google Scholar]
- Gallardo C, Hufnagel B, Casset C, et al. 2019. Anatomical and hormonal description of rootlet primordium development along white lupin cluster root. Physiologia Plantarum 165: 4–16. [DOI] [PubMed] [Google Scholar]
- Gardner WK, Parbery DG, Barber DA. 1981. Proteoid root morphology and function in Lupinus albus. Plant and Soil 60: 143–147. [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K. 2001. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428. [DOI] [PubMed] [Google Scholar]
- Gilbert GA, Diane KJ, Vance CP, Allan DL. 2000. Proteoid root development of phosphorus deficient lupin is mimicked by auxin and phosphonate. Annals of Botany 85: 921–928. [Google Scholar]
- Grunewald W, De Smet I, Lewis DR, et al. 2012. Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proceedings of the National Academy of Sciences of the USA 109: 1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S, Mathesius U. 2012. The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant-microbe interactions. Journal of Experimental Botany 63: 3429–3444. [DOI] [PubMed] [Google Scholar]
- Heuer S, Gaxiola R, Schilling R, Herrera-Estrella L, López-Arredondo D, Wissuwa M. 2017. Improving phosphorus use efficiency: a complex trait with emerging opportunities. Plant Journal 90: 868–885. [DOI] [PubMed] [Google Scholar]
- Hinsinger P. 2001. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant and Soil 237: 173–195. [Google Scholar]
- Hufnagel B, Marques A, Soriano A, et al. 2020. High-quality genome sequence of white lupin provides insight into soil exploration and seed quality. Nature Communications 11: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imin N, Nizamidin M, Wu T, Rolfe BG. 2007. Factors involved in root formation in Medicago truncatula. Journal of Experimental Botany 58: 439–451. [DOI] [PubMed] [Google Scholar]
- Jain A, Poling MD, Karthikeyan AS, et al. 2007. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiology 144: 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Gao X, Liao L, Harberd NP, Fu X. 2007. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiology 145: 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. 2006. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98: 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont BB. 2003. Structure, ecology and physiology of root clusters – a review. Plant and Soil 248: 1–19. [Google Scholar]
- Li H, Shen J, Zhang F, Tang C, Lambers H. 2008. Is there a critical level of shoot phosphorus concentration for cluster-root formation in Lupinus albus? Functional Plant Biology 35: 328–336. [DOI] [PubMed] [Google Scholar]
- Maloney GS, DiNapoli KT, Muday GK. 2014. The anthocyanin reduced tomato mutant demonstrates the role of flavonols in tomato lateral root and root hair development. Plant Physiology 166: 614–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng ZB, You XD, Suo D, et al. 2013. Root-derived auxin contributes to the phosphorus-deficiency-induced cluster-root formation in white lupin (Lupinus albus). Physiologia Plantarum 148: 481–489. [DOI] [PubMed] [Google Scholar]
- Neumann G, Martinoia E. 2002. Cluster roots – an underground adaptation for survival in extreme environments. Trends in Plant Science 7: 162–167. [DOI] [PubMed] [Google Scholar]
- Ng JLP, Hassan S, Truong TT, et al. 2015. a. Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre1. Plant Cell 27: 2210–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JLP, Perrine-Walker F, Wasson AP, Mathesius U. 2015b. The control of auxin transport in parasitic and symbiotic root-microbe interactions. Plants 4: 606–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Murphy AS. 2007. Flavonoids and auxin transport: modulators or regulators? Trends in Plant Science 12: 556–563. [DOI] [PubMed] [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Murphy AS. 2004. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16: 1898–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routaboul JM. 2006. Flavonoid diversity and biosynthesis in seed of Arabidopsis thaliana. Planta 224: 96–107. [DOI] [PubMed] [Google Scholar]
- Santelia D, Henrichs S, Vincenzetti V, et al. 2008. Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. Journal of Biological Chemistry 283: 31218–31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, Lambers H. 2005. Cluster roots: a curiosity in context. Plant and Soil 274: 101–125. [Google Scholar]
- Shen J, Yuan L, Zhang J, et al. 2011. Phosphorus dynamics: from soil to plant. Plant Physiology 156: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene KR. 1998. Cluster roots: some ecological considerations. Journal of Ecology 86: 1060–1064. [Google Scholar]
- Skene KR. 2001. Cluster roots: model experimental tools for key biological problems. Journal of Experimental Botany 52: 479–485. [DOI] [PubMed] [Google Scholar]
- Stewart AJ, Chapman W, Jenkins GI, Graham I, Martin T, Crozier A. 2001. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant, Cell & Environment 24: 1189–1197. [Google Scholar]
- Tomasi N, Weisskopf L, Renella G, et al. 2008. Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biology & Biochemistry 40: 1971–1974. [Google Scholar]
- Uhde-Stone C, Liu J, Zinn KE, Allan DL, Vance CP. 2005. Transgenic proteoid roots of white lupin: a vehicle for characterizing and silencing root genes involved in adaptation to P stress. Plant Journal 44: 840–853. [DOI] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157: 423–447. [DOI] [PubMed] [Google Scholar]
- Wang BL, Tang XY, Cheng LY, et al. 2010. Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytologist 187: 1112–1123. [DOI] [PubMed] [Google Scholar]
- Wang Z, Rahman ABMM, Wang G, Ludewig U, Shen J, Neumann G. 2015. Hormonal interactions during cluster-root development in phosphate-deficient white lupin (Lupinus albus L.). Journal of Plant Physiology 177: 74–82. [DOI] [PubMed] [Google Scholar]
- Watt M, Evans JR. 1999. Proteoid roots. Physiology and development. Plant Physiology 121: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf L, Abou-Mansour E, Fromin N, et al. 2006. White lupin has developed a complex strategy to limit microbial degradation of secreted citrate required for phosphate acquisition. Plant, Cell & Environment 29: 919–927. [DOI] [PubMed] [Google Scholar]
- Williams CA, Grayer RJ. 2004. Anthocyanins and other flavonoids. Natural Product Reports 21: 539–573. [DOI] [PubMed] [Google Scholar]
- Xu W, Zhang Q, Yuan W, et al. 2020. The genome evolution and low-phosphorus adaptation in white lupin. Nature Communications 11: 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Liu H, Li G, et al. 2009. Reduction of root flavonoid level and its potential involvement in lateral root emergence in Arabidopsis thaliana grown under low phosphate supply. Functional Plant Biology 36: 564–573. [DOI] [PubMed] [Google Scholar]
- Zhang J, Subramanian S, Stacey G, Yu O. 2009. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant Journal 57: 171–183. [DOI] [PubMed] [Google Scholar]
- Zhou K, Yamagishi M, Osaki M, Masuda K. 2008. Sugar signalling mediates cluster root formation and phosphorus starvation-induced gene expression in white lupin. Journal of Experimental Botany 59: 2749–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Sarker U, Neumann G, Ludewig U. 2018. The LaCEP1 peptide modulates cluster root morphology in Lupinus albus. Physiologia Plantarum 166: 525–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.