Abstract
Background:
Increasingly, women are undergoing contralateral prophylactic mastectomy for the treatment of unilateral breast cancer. The relationship between contralateral prophylactic mastectomy and breast reconstruction, postsurgical complications, additional breast-related procedures, and cost has not received the attention it deserves.
Methods:
Data from the New York comprehensive, all-age, all-payer, Statewide Planning and Research Cooperative System were queried to identify patients undergoing unilateral mastectomy or contralateral prophylactic mastectomy from 2008 to 2010. We identified the complications and breast-related procedures within a 2-y follow-up period. Costs of the index operation and subsequent follow-up were estimated. Univariate and multivariate analyses were conducted.
Results:
Of 12,959 women identified, 10.7% underwent contralateral prophylactic mastectomy. On univariate analysis, contralateral prophylactic mastectomy was positively associated with breast reconstruction, complications, and additional breast-related procedures. Rates of complications were greater for women who had contralateral prophylactic mastectomy (29.5% vs 20.8% for unilateral mastectomy group; P < .001), but not after stratifying by breast reconstruction. Additional breast-related procedures were more common in the contralateral prophylactic mastectomy group than in the unilateral mastectomy group, but only for those who underwent breast reconstruction (82.8% vs 72.1%; P < .001). Unadjusted costs were greater for women with contralateral prophylactic mastectomy than with unilateral mastectomy but did not differ between the groups after adjusting for breast reconstruction and additional breast-related procedures.
Conclusion:
Women who elected contralateral prophylactic mastectomy in this population-based study were more likely to have both breast reconstruction and additional breast-related procedures than women with unilateral mastectomy. The greater rates of complications and costs associated with contralateral prophylactic mastectomy were explained by breast reconstruction and additional breast-related procedures. Surgeons should counsel patients regarding the increased cost and likelihood of undergoing additional, non-complication–related procedures after contralateral prophylactic mastectomy with breast reconstruction.
Introduction
More women with unilateral breast cancer are undergoing not only removal of the affected breast with a unilateral mastectomy (UM) but also undergoing mastectomy of the contralateral, unaffected breast. The rate of contralateral prophylactic mastectomy (CPM) in 2012 was estimated to be 12.7%, increased from 3.9% in 2002.1 This increase has occurred despite a lack of evidence of survival benefit for most patients and the development well-defined, evidence-based clinical recommendations on the indications for CPM.2-7
The choice to undergo CPM is complex and associated with multiple, patient-driven factors.8-10 Most women report satisfaction with their operative decision, and regret is usually attributed to outcomes, body image and sexuality, and expectations.11-13 To better facilitate informed decision-making, awareness is growing of the need to inform patients of the negative outcomes associated with CPM,14 including greater rates of postoperative complications and reoperations,15-19 along with greater financial costs.20-23 Unfortunately, these reports have not studied the interaction between breast reconstruction (BR) and type of operation on costs, although the two are closely related.20,24,25 In addition, earlier work has not assessed postsurgical, non-complication, breast-related procedures as an outcome nor its impact on costs.
This study examined outcomes and costs associated with the operation and the BR for a large and racially diverse population residing in urban and rural settings. This data set provides the most inclusive picture of CPM costs to date because it includes information on patients from all payers, including Medicare and Medicaid, from all hospitals including those in academic and community settings, as well as data from both inpatient and outpatient visits.
Methods
This study utilized 2008 to 2012 Statewide Planning and Research Cooperative System (SPARCS) data. SPARCS is a comprehensive all-age, all-payer data set maintained by the New York State Department of Health. It contains information on discharges from inpatient and outpatient facilities (ie, ambulatory surgery, emergency department, and outpatient services), along with each ambulatory operation and outpatient services visit to a hospital extension clinic and diagnostic and treatment center licensed to provide ambulatory surgery services within the state of New York. SPARCS data includes patient-level information, such as patient demographics and insurance status, as well as diagnoses, procedures, services, and charges for each encounter. Individuals are assigned a de-identified unique patient identifier, allowing researchers to follow patients over time. This study was approved by the SPARCS Data Governance Committee through a data use agreement. The study was deemed not human subjects research by our institution and exempt from Institutional Review Board.
From these data, we selected female patients age 18 y or older undergoing mastectomy from 2008 to 2010. Patients were excluded if they received multiple mastectomies (ie, over multiple encounters/dates), resided outside of New York, or had no encounters within 2 y after the mastectomy. Health care utilization was identified through the following billing codes: International Classification of Diseases, Ninth Revision, Clinical Modification; Healthcare Common Procedures Classification System; Clinical Classifications Software; and Medicare Diagnosis Related Group.
Patients were categorized as having UM or CPM from billing codes (Appendix Table I). We required that those undergoing bilateral mastectomy have an accompanying diagnosis of prophylactic breast removal (International Classification of Diseases, Ninth Revision, Clinical Modification V50.41) in the same encounter to be classified as having CPM. The date of the index operation was set to this first surgical encounter. Patients were also categorized by whether they had BR and the type and timing of the procedure (immediate or delayed).
Primary outcomes included operative complications, additional breast-related procedures, and total costs within 2 y of the index mastectomy. Complications were identified using codes published elsewhere26 (Appendix Table I) and include outcomes, such as infection, fat necrosis, disruption of operation wound, etc. Additional breast-related procedures were identified by expert review of billing codes observed in our data and included other related procedures, such as revision of pedicle or flap graft, transposition of nipple, open periprosthetic capsulotomy, nipple/areola reconstruction, etc. Breast-related procedures were only defined as such for encounters with no indication of a complication.
Costs were calculated from index operation through 2 y of follow-up. Notably, SPARCS does not directly publish cost data, but rather hospital charges. We converted charges to costs using the SPARCS institutional-level Hospital Inpatient Cost Transparency data and Medicare Outpatient Prospective Payment System data.27 Encounters containing chemotherapy and radiation services were excluded because these treatments are not the focus of this report and can unduly inflate costs. All other follow-up encounters were included. We assumed non-surgery–related visits would be distributed similarly between the UM and CPM groups. We believe that including these costs better reflects the financial burden after mastectomy in the real world.
Multivariate analyses included variables such as patient demographics (age at undergoing mastectomy, race, and ethnicity), insurance, urban/rural residence, and year of index operation. For patients with multiple forms of insurance, we created a mutually exclusive insurance category based on a patient’s primary payment. Patients’ insurance status was defined by a hierarchical order of Medicaid, Medicare, other payments, private insurance, and self-pay.
Descriptive analyses and χ2 tests were conducted to compare baseline characteristics and outcomes between groups. Kolmogorov–Smirnov tests assessed the equality of cost distributions. Multivariate analyses were conducted controlling for the covariates described earlier in this report. In these models we included indicator variables for UM without BR (reference group), UM with BR, CPM without BR, and CPM with BR. Postestimation of average marginal effects were used to assess the independent contribution of CPM over UM, controlling for all covariates and BR. Logistic regressions were used to model complications and breast-related procedures. To address skewness of cost data, we applied a log transformation and fitted a log-linear regression model with robust standard errors to control for heteroscedasticity. Variables and the analytical data set were created using SAS software v 9.3 (SAS Institute Inc, Cary, NC, USA) and statistical analyses were performed using Stata Statistical Software: Release 15 (StataCorp LLC, College Station, TX, USA).
Results
Patient characteristics
In our final cohort of 12,959 women, 1,384 (10.7%) underwent CPM and 11,575 (89.3%) received UM (Table I). Median age for women electing to undergo CPM was 47 y compared with 60 y for the UM group (P < .001). On average, CPM patients were more likely to be white, not Hispanic, and have private insurance than women who underwent UM (all P < .001) and were also more likely to undergo BR (93.1% vs 46.2%; P < .001). For those who had BR, the vast majority in both groups (91.9% for UM and 98.4% for CPM group; P < .001) underwent BR at the time of mastectomy. The majority of women had implant-based reconstruction, with a slightly greater rate in the CPM group (76.2% vs 85.9%; P < .001). The rate of CPM increased each year, from 8.3% in 2008 to 12.8% in 2010.
Table I.
Characteristics of study sample by type of operation
| UM |
CPM |
P value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Sample size | 11,575 | 1,384 | |||
| Age (y) at time of operation | <.001 | ||||
| Age <40 | 632 | 5.5 | 291 | 21.0 | |
| Age 40–54 | 2,221 | 19.2 | 523 | 37.8 | |
| Age 55–64 | 2,785 | 24.1 | 390 | 28.2 | |
| Age 65–74 | 2,690 | 23.2 | 150 | 10.8 | |
| Age 75+ | 3,247 | 28.1 | 30 | 2.2 | |
| Race | <.001 | ||||
| White | 7,801 | 67.4 | 1,242 | 89.7 | |
| Black | 1,954 | 16.9 | 61 | 4.4 | |
| Other | 1,820 | 15.7 | 81 | 5.9 | |
| Ethnicity | <.001 | ||||
| Hispanic | 1,019 | 8.8 | 50 | 3.6 | |
| Not Hispanic | 10,083 | 87.1 | 1,311 | 94.7 | |
| Unknown | 473 | 4.1 | 23 | 1.7 | |
| Year of operation | <.001 | ||||
| 2008 | 3,832 | 33.1 | 347 | 25.1 | |
| 2009 | 4,027 | 34.8 | 492 | 35.5 | |
| 2010 | 3,716 | 32.1 | 545 | 39.4 | |
| Insurance | <.001 | ||||
| Private insurance | 5,363 | 46.3 | 1,219 | 88.1 | |
| Medicare | 3,588 | 31.0 | 99 | 7.2 | |
| Medicaid | 2,297 | 19.8 | 54 | 3.9 | |
| Other | 327 | 2.8 | 12 | 0.9 | |
| Breast reconstruction | <.001 | ||||
| No | 6,223 | 53.8 | 95 | 6.9 | |
| Yes | 5,352 | 46.2 | 1,289 | 93.1 | |
| Timing of reconstruction* | <.001 | ||||
| Immediate (at time of operation) | 4,920 | 91.9 | 1,269 | 98.4 | |
| Delayed | 432 | 8.1 | 20 | 1.6 | |
| Breast reconstruction type* | <.001 | ||||
| Not implant based | 1,275 | 23.8 | 182 | 14.1 | |
| Implant based | 4,077 | 76.2 | 1,107 | 85.9 | |
UM, unilateral mastectomy; CPM, contralateral prophylactic mastectomy.
For those who received reconstruction. Women who had an implant as any component of breast reconstruction (with or without additional autologous tissue) were categorized as having implant-based reconstruction.
Univariate analyses of complications, breast-related procedures, and costs
Overall, 21.7% of the study sample had a complication, with 11.0% requiring an inpatient stay as a result (Table II). The most common types of complication were infection (11.3%), hematoma/seroma (5.0%), removal of implant (4.4%), and wound complication (3.4%). Few women had 2 or more encounters for complications (5.9%, results not presented). Compared with those having undergone UM, women having undergone CPM were more likely to have a complication (29.5% vs 20.8%, results not presented; odds ratio [OR] = 1.6; P < .001). They were also more likely to have a complication that required an inpatient stay (OR = 1.5, P < .001). Some categories of complications were also more common in the CPM group, including wound complication, infection, graft/implant complication, and implant removal; however, this was not the case when stratified by BR (Fig 1). Rates of complications were 12.1% (UM) versus 12.6% (CPM) for those without BR (P = .863, results not presented), and 30.9% (UM) versus 30.7% (CPM) for those with BR (P = .898, results not presented). For women without BR, there were no differences between the CPM and UM groups across any measure of complication. For those with BR, only a single measure remained statistically significant, that of the odds of a graft/implant complication. Furthermore, this difference was driven by those with implant-based reconstruction.
Table II.
Odds of complications and breast-related procedures by operation and breast reconstruction
| Overall Rate (%) | UM versus CPM | UM versus CPM, no BR |
UM versus CPM, BR |
UM versus CPM, BR, no implant |
UM versus CPM, BR with implant |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| OR | P value | OR | P value | OR | P value | OR | P value | OR | P value | ||
| Complication, any | 21.7 | 1.59 | <.001 | 1.06 | .863 | 0.99 | .899 | 1.02 | .909 | 0.98 | .817 |
| Complication, inpatient stay | 11.0 | 1.46 | <.001 | 1.09 | .836 | 1.03 | .725 | 1.05 | .835 | 1.01 | .924 |
| Type of complication | |||||||||||
| Wound complication | 3.4 | 1.79 | <.001 | 1.11 | .917 | 1.03 | .848 | 1.02 | .938 | 1.08 | .587 |
| Infection | 11.3 | 1.38 | <.001 | 1.48 | .265 | 0.92 | .340 | 0.96 | .863 | 0.89 | .202 |
| Hematoma/seroma | 5.0 | 1.10 | .441 | — | — | — | — | ||||
| Breast pain | 1.2 | 0.87 | .609 | — | — | — | — | ||||
| Fat necrosis | 2.4 | 1.23 | .229 | — | — | — | — | ||||
| Graft/implant complication | 1.8 | 2.75 | <.001 | * | 1.45 | .016 | 0.56 | .340 | 1.57 | .006 | |
| Implant removal | 4.4 | 2.48 | <.001 | * | 1.19 | .103 | * | 1.08 | .492 | ||
| Other | 1.8 | 1.36 | .111 | — | — | — | — | ||||
| Breast-related procedures | 44.1 | 5.26 | <.001 | 0.91 | .773 | 1.86 | <.001 | 1.35 | .062 | 1.77 | <.001 |
Note: The — indicates that these models were not fitted because the original model (UM versus CPM) was not statistically significant. OR, odds ratio.
These models were not fitted because the outcome does not apply.
Fig 1.
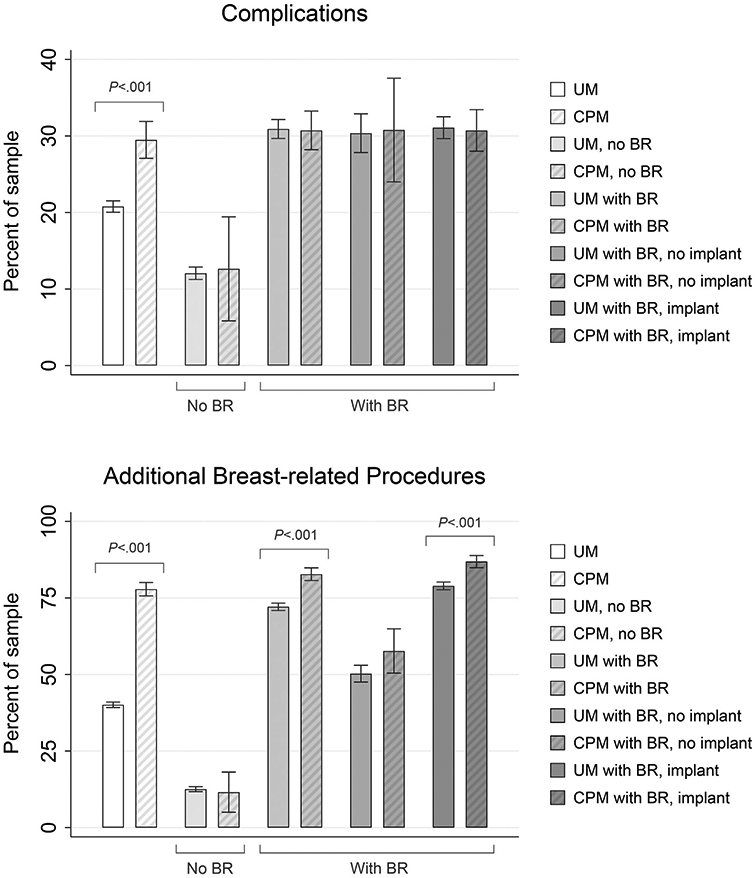
Percent of sample having complications and breast-related procedures by type of surgery and breast reconstruction. Whiskers represent values within 95% confidence interval. UM, unilateral mastectomy; CPM, contralateral prophylactic mastectomy; BR, breast reconstruction.
Almost half of the study sample overall (44.1%) had additional, breast-related procedures. Women in the CPM group were more likely to have breast-related procedures than women in the UM group (OR = 5.3, P < .001). Indeed, they also had more breast-related procedures. Of women with CPM, 29.7% had 2 or more encounters for breast-related procedures, compared with 12.4% of women with UM (P < .001, results not presented). The addition of BR greatly increased the rates of breast-related procedures, and these rates also differed by the type of operation (82.8% for CPM with BR versus 72.1% for UM with BR, results not presented; OR = 1.9; P < .001). Of those with BR, the CPM group was more likely to have had 2 or more encounters with breast-related procedures; 31.9% in the CPM with BR group versus 24.5% in the UM with BR group (P < .001, results not presented). This same pattern was observed in those with implant-based reconstruction (OR = 1.8; P < .001) and those whose reconstruction was not implant based (OR = 1.4; P = .062). Without BR, fewer women had additional, breast-related procedures; 11.6% in the CPM without BR group and 12.6% in the UM without BR group (OR = 0.9; P = .773).
We also identified the costs associated with complications and breast-related procedures (Table III). The majority of women, regardless of type of operation and BR, had no complication-related costs (ie, median costs equal to $0). The costs of complications differed between women with CPM and UM (P < .001), but not when stratified by receipt of BR or type of BR. This was not the case for costs associated with breast-related procedures. The median costs of breast-related procedures were $4,275 greater for women with CPM than with UM (P < .001) and $1,191 greater for those with BR (P < .001 both). Even when stratified by type of reconstruction, costs of breast-related procedures remained greater in the CPM group. Regardless of the type of operation, costs of breast-related procedures were greater in those with BR (difference in median costs of $3,580; P < .001), and within this group, costs of breast-related procedures were greater for those with implant-based reconstruction than those without implants (difference in median costs of $3,094; P < .001).
Table III.
Costs (in thousands) by type of operation and breast reconstruction.
| N | Total costs | Cost of complications | Costs of breast-related procedures |
||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Mean (median) [IQR] | P value* | Mean (median) [IQR] | P value* | Mean (median) [IQR] | P value* | ||
| Full sample | 12,959 | 24.7 (17.4) [17.7] | 2.0 (0) [0] | 2.8 (0) [3.9] | |||
| UM | 11,575 | 24.3 (16.3) [17.3] | <.001 | 2.0 (0) [0] | <.001 | 2.5 (0) [3.3] | <.001 |
| CPM | 1,384 | 27.7 (23.9) [14.0] | 2.6 (0) [1.8] | 5.2 (4.3) [5.0] | |||
| No BR | 6,318 | 22.9 (11.7) [16.7] | <.001 | 1.2 (0) [0] | <.001 | 0.7 (0) [0] | <.001 |
| BR | 6,641 | 26.3 (20.8) [15.2] | 2.8 (0) [1.8] | 4.7 (3.6) [6.1] | |||
| UM, no BR | 6,223 | 23.0 (11.7) [16.7] | .527 | 1.2 (0) [0] | 1.000 | 0.7 (0) [0] | 1.000 |
| CPM, no BR | 95 | 18.1 (11.0) [14.7] | 1.1 (0) [0] | 0.7 (0) [0] | |||
| UM with BR | 5,352 | 25.8 (19.6) [14.9] | <.001 | 2.8 (0) [1.7] | .410 | 4.5 (3.3) [5.7] | <.001 |
| CPM with BR | 1,289 | 28.4 (24.5) [13.8] | 2.7 (0) [2.3] | 5.5 (4.5) [4.6] | |||
| BR, not implant based | 1,457 | 30.8 (23.4) [18.7] | <.001 | 3.1 (0) [1.5] | .060 | 3.9 (0.9) [4.1] | <.001 |
| BR, implant based | 5,184 | 25.0 (20.1) [14.3] | 2.7 (0) [2.0] | 4.9 (4.0) [4.4] | |||
| UM with BR, not implant based | 1,275 | 30.9 (23.1) [18.9] | .035 | 3.2 (0) [1.5] | .894 | 4.0 (0.3) [4.2] | .024 |
| CPM with BR, not implant based | 182 | 30.4 (26.0) [18.6] | 2.3 (0) [2.2] | 2.9 (2.3) [4.0] | |||
| UM with BR, implant based | 4,077 | 24.2 (18.9) [13.7] | <.001 | 2.6 (0) [1.9] | .843 | 4.7 (3.7) [4.4] | <.001 |
| CPM with BR, implant based | 1,107 | 28.1 (24.3) [13.1] | 2.8 (0) [2.3] | 6.0 (4.8) [4.4] | |||
IQR, interquartile range.
P value from Kolmogorov-Smirnov test.
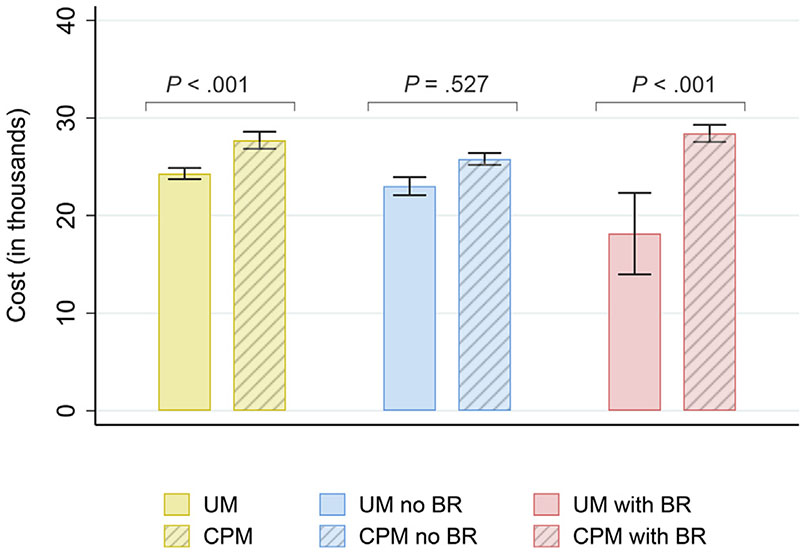
Unadjusted total costs varied by the type of operation and BR (Table III and Fig 2). Median total costs were $7,587 greater in the CPM than in the UM group overall (P < .001) and $4,898 greater in the CPM than in the UM group for those with BR (P < .001). The same pattern was observed regardless of type of reconstruction (implant based or not). In both cases, median total costs were greater in the CPM group than in the UM group. This was not the case for those without BR. Total costs did not differ between the UM without BR group and the CPM with our BR group.
Fig 2.
Unadjusted costs (in thousands) by type of surgery and breast reconstruction. Whiskers represent values within 95% confidence interval. UM, unilateral mastectomy; CPM, contralateral prophylactic mastectomy; BR, breast reconstruction.
Multivariate analyses of complications, breast-related procedures, and costs
Women who had CPM without BR were no more likely to have complications or additional breast-related procedures than women who had UM without BR after controlling for age, race, ethnicity, year of operation, and type of insurance (Table IV). For those who had UM, BR was associated with greater odds of complications (OR = 3.6; P < .001) and breast-related procedures (OR = 14.7; P < .001). Similarly, BR was associated with greater odds of complications (OR = 3.3; P < .001) and breast-related procedures (OR = 30.1; P < .001) for women who had CPM (model with CPM without BR as reference group not presented). CPM by itself was not independently associated with complications (P = .343) or breast-related procedures (P = .088) compared with UM.
Table IV.
Multivariate logistic regressions of complications and breast-related procedures, and log-linear regression of costs
| Complications |
Breast-related procedures |
Costs |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | Parameter estimate | 95% CI | P value | |
| Operation and BR | |||||||||
| UM without BR | Ref | Ref | Ref | ||||||
| UM with BR | 3.58 | 3.20–4.01 | <.001 | 14.67 | 13.14–16.25 | <.001 | 0.31 | 0.27–0.35 | <.001 |
| CPM without BR | 1.18 | 0.65–2.17 | .586 | 0.76 | 0.40–1.43 | .391 | 0.04 | −0.11 to 0.18 | .602 |
| CPM with BR | 3.91 | 3.30–4.63 | <.001 | 22.81 | 19.07–27.28 | <.001 | 0.55 | 0.50–0.59 | <.001 |
| Age (y) at time of operation | |||||||||
| <40 | Ref | Ref | Ref | ||||||
| 40–54 | 1.04 | 0.88–1.23 | .624 | 0.83 | 0.69–0.99 | .035 | 0.03 | −0.01 to 0.07 | .196 |
| 55–64 | 1.12 | 0.94–1.33 | .215 | 0.67 | 0.55–0.81 | <.001 | 0.0004 | −0.05 to 0.05 | .987 |
| 65–74 | 0.89 | 0.71–1.12 | .335 | 0.74 | 0.58–0.94 | .013 | 0.06 | −0.01 to 0.12 | .092 |
| ≥75 | 0.89 | 0.69–1.14 | .348 | 0.58 | 0.44–0.75 | <.001 | 0.18 | 0.10–0.25 | <.001 |
| Race | |||||||||
| White | Ref | Ref | Ref | ||||||
| Black | 1.12 | 0.98–1.26 | .086 | 0.80 | 0.70–0.92 | .001 | 0.22 | 0.18–0.26 | <.001 |
| Other | 0.92 | 0.80–1.05 | .214 | 0.82 | 0.71–0.95 | .008 | 0.07 | 0.03–0.11 | .001 |
| Ethnicity | |||||||||
| Hispanic | Ref | Ref | Ref | ||||||
| Not Hispanic | 1.08 | 0.92–1.28 | .352 | 1.11 | 0.94–1.31 | .236 | −0.16 | −0.21 to −0.11 | <.001 |
| Unknown | 1.27 | 0.97–1.67 | .083 | 1.01 | 0.76–1.34 | .941 | −0.21 | −0.29 to −0.12 | <.001 |
| Year of operation | |||||||||
| 2008 | Ref | Ref | Ref | ||||||
| 2009 | 0.92 | 0.83–1.02 | .116 | 0.99 | 0.89–1.10 | .845 | 0.04 | 0.01–0.07 | .009 |
| 2010 | 0.91 | 0.82–1.01 | .084 | 1.03 | 0.92–1.15 | .614 | 0.09 | 0.06–0.12 | <.001 |
| Insurance | |||||||||
| Private insurance | Ref | Ref | Ref | ||||||
| Medicare | 1.31 | 1.10–1.56 | .003 | 0.76 | 0.64–0.91 | .003 | 0.07 | 0.01–0.12 | .014 |
| Medicaid | 1.74 | 1.53–1.98 | <.001 | 0.81 | 0.71–0.93 | .003 | 0.20 | 0.16–0.24 | <.001 |
| Other | 1.46 | 1.12–1.92 | .006 | 0.66 | 0.50–0.86 | .002 | −0.04 | −0.12 to 0.04 | .339 |
| Complications | |||||||||
| No | * | * | Ref | ||||||
| Yes | * | * | 0.46 | 0.43–0.49 | <.001 | ||||
| Breast-related procedures | |||||||||
| No | * | * | Ref | ||||||
| Yes | * | * | 0.22 | 0.19–0.25 | <.001 | ||||
Variable not included in model.
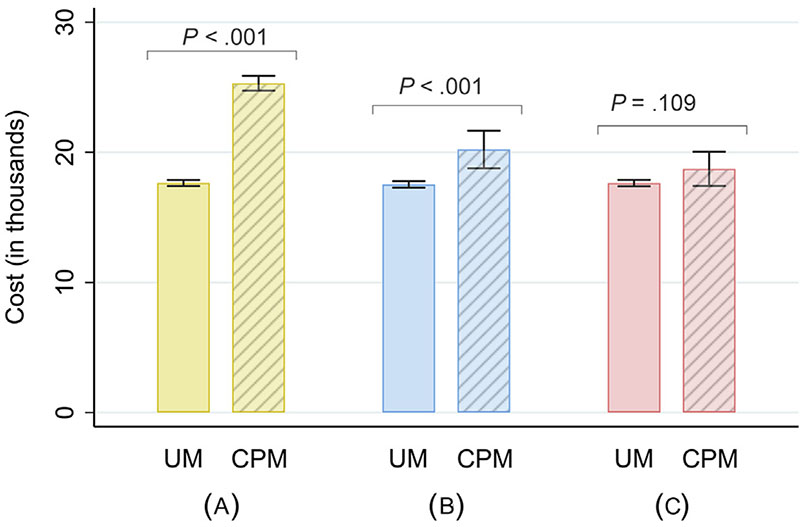
On multivariate analysis, there was no difference in total costs for women who had CPM without BR (P = .600). Costs were 31.1% greater for women undergoing UM with BR (P < .001) and 54.6% greater for women with CPM and BR, compared with the UM without BR group (P < .001). CPM itself was independently associated with a 13.9% increase in total costs (95% confidence interval [CI] 6.8%–21.1%, P < .001), corresponding to a difference of $3,201 (95% CI $2,102–$4,301). Predicted costs were greater for the CPM group even after adjusting for BR ($20,214 vs $17,530; P < .001; Fig 3); however, this difference was no longer observed after adjusting for both BR and breast-related procedures: $17,631 (UM) versus $18,726 ([CPM] P = .109), further suggesting that the increased costs associated with CPM are from the additional breast-related procedures after BR.
Fig 3.
Predicted costs (in thousands) from multivariate analyses by type of surgery. Whiskers represent values within 95% confidence interval. (A) No adjustments included. (B) Adjusts for breast reconstruction.(C) adjusts for breast reconstruction and additional breast-related procedures. UM, unilateral mastectomy; CPM, contralateral prophylactic mastectomy.
Discussion
With the increase in CPM rates in the United States and no clear survival benefit,1 there is continued interest in the operative and financial outcomes of this procedure. Our study used a population-based, state-wide, all-payor insurance database. The longitudinal nature of these data also allowed us to observe health care utilization for patients over time. The demographics of our cohort and annual increase in the rate of CPM observed in our study are similar to others. We found that CPM patients were younger, more likely to be white, and more likely to have private insurance than UM patients. As in other studies, we also observed that patients who had CPM underwent BR at a much greater rate than UM patients (93.1% vs 46.3%; P < .001).20,28
Utilization of BR after mastectomy increased markedly after passage of the Women’s Health and Cancer Rights Act in 1998,29 and BR, particularly successful reconstruction, has been shown to be associated with satisfaction with operative outcomes30,31 and improved quality of life in breast cancer survivors.32 As others have found, however, the addition of BR in our cohort was associated with more operative complications.16 However, there was no difference in the complication rate in our study among the types of operation (UM versus CPM) when stratified by BR. Billig et al23 reported a similar finding for women who received immediate BR, along with Osman et al15 who included only those without BR. These observations would suggest that the larger number of complications associated with CPM is attributable to the greater rate of BR in this group, which we confirmed with univariate and multivariate analyses. Our rates of complications (30.9% for those with BR) also mirrored those reported in a recent, multicenter, prospective, cohort study (32.9%).19
Consistent with other studies,20,22,23 CPM was associated with greater costs, both overall and for those with BR, even though this latter group was more likely to have implant-based reconstruction. Our study design, however, differed from earlier work in important ways. In addition to controlling for BR, we included an interaction term for the type of operation and reconstruction. This interaction term was statistically significant, indicating that costs differed between the UM and CPM groups overall and for those who had BR. In addition, we also identified subsequent breast-related procedures in addition to complications as outcomes. On multivariate analysis, CPM was associated with additional breast-related procedures, but only for women with BR. After adjusting for BR and breast-related procedures, costs no longer differed between the CPM and UM groups. These results suggest that the greater unadjusted costs observed with CPM are driven by additional procedures performed for women with BR, particularly in those with implant-based reconstruction, and not owing to complications, CPM itself, or CPM with reconstruction.
Although we were unable to identify the indications for the breast-related procedures in our data, by construction they were distinct from codes associated with complications. It is possible that these procedures were planned, staged procedures, performed to improve cosmetic outcome. Multiple studies report cosmesis and breast symmetry to be major factors in the choice of CPM, and it may be that these patients are more motivated to pursue additional operative interventions to achieve these goals.12,14,33 Although satisfaction is consistently high in women who elect CPM,12 poor cosmetic result was the most commonly reported reason for decisional regret and dissatisfaction in two studies.34,35 Additional research is needed to validate our findings in other settings and to assess the impact beyond cost for these additional procedures performed for women with CPM.
This study has limitations. Costs were calculated from hospital charges and a provider/insurer perspective. Others have used reimbursed amount,22 allowable amount,23 charges,21 amount reimbursable by Medicare,20 and patient-reported out-of-pocket expenditures.36 Although our data include all payers in a population-based cohort, the sample was limited to residents of New York State. Other claims-based studies may represent a larger geographic area,22,23 but are still not nationally representative and do not include all payers.37 We also relied on billing codes to define our cohort and outcomes so that misclassification and miscoding of diagnosis and procedure codes are possible, along with excluded codes. SPARCS data do not include all encounters. We were unable to include costs from routine office visits or medications. In addition, being from an administrative database, these data do not provide information on important patient characteristics such as the Charlson/Deyo comorbidity score. Our multivariate analyses were also limited by the variables available in these data.
Cost-effectiveness analyses comparing CPM and UM with surveillance report mixed findings.38-40 These studies model women diagnosed around the age of 45 y and vary widely in important parameters, such as BR and complication rates. None include costs associated with additional breast-related procedures or the value of psychosocial outcomes such as worry about recurrence, a strong factor in women’s surgical decisions.8,14,33,41,42 Multiple organizations including the Society of Surgical Oncology5 and American Society of Breast Surgeons13,43 have released consensus guidelines on counseling or actively discouraging CPM in breast cancer patients with average risk,6 but there remains disagreement on the best approach for individual patients.44-47 Understanding the risks, likelihood of additional breast-related procedures, and financial cost associated with the decision to undergo CPM is crucial for both surgeons and patients when discussing surgical options. Total versus out-of-pocket cost vary widely and are often not transparent to either surgeon or patient. Shared decision-making requires communication, education, and mutual respect for patient autonomy and values from surgeons and for surgeon expertise from patients. In decisions as complicated and deeply personal as those related to breast cancer surgery, this is no small task.
Acknowledgments
The authors acknowledge the efforts of the Bureau of Health Informatics, Office of Quality and Patient Safety, New York State Department of Health in providing the Statewide Planning and Research Cooperative System data. The data used to produce this publication were purchased from or provided by the New York State Department of Health (NYSDOH). However, the conclusions derived and the views expressed herein are those of the authors and do not reflect the conclusions or views of NYSDOH. NYSDOH, its employees, officers, and agents make no representation, warranty or guarantee as to the accuracy, completeness, currency, or suitability of the information provided here.
Funding/Support
This work was supported in part by the University of Iowa Holden Comprehensive Cancer Center Alberhasky Breast Cancer Fund.
Appendix
Appendix Table 1.
Billing codes used to identify operative procedures and outcomes
| Category | Code | Type of code |
|---|---|---|
| Unilateral mastectomy (UM) | 85.33, 85.34, 85.4, 85.41, 85.43, 85.45, 85.47 | ICD-9 procedure code |
| 19180, 19182, 19184-19187, 19200, 19211, 19216, 19220, 19224, 19229, 19240, 19250, 19255, 19271, 19272, 19300, 19303-19307 | CPT/HCPCS | |
| 257, 258, 582, 583 | DRG | |
| 167 | CCS procedure | |
| Contralateral prophylactic mastectomy (CPM) | V5041 | ICD-9 diagnosis code |
| 8535, 8536, 8542, 8544, 8546, 8548 | ICD-9 procedure code | |
| Breast reconstruction* | 85.51, 85.52, 85.79, 85.8, 85.82, 85.53, 85.86, 85.87, 85.89, 85.89, 86.02 (NOS) 85.33, 85.35, 85.50, 85.53, 85.54, 85.93-85.96 (implant) 85.55, 85.7, 85.70 (autolgous, NOS) 85.71, 85.72, 85.84, 85.85, 86.70-86.72, 86.74 (autologous, pedicled) 85.73-85.76 (aotologous, free) | ICD-9 procedure code |
| 19324, 19350, 19366, 19380 (NOS) 19325, 19340, 19342, 19567, L8600 (implant) 19361, 19367-19369 (autologous, pedicled) 19364, S2066-S2068 (autologous, free) | CPT/HCPCS | |
| Complications** | 611.0, 680.2, 682.2, 682.3, 998.50, 998.51, 998.59, 998.69 (infection) 611.3 (fat necrosis) 611.71 (breast pain) 996.52, 996.79 (graft/implant complication) 998.11, 998.12, 998.13 (hematoma/seroma) 998.3, 998.32, 998.83, 998.6 (wound complication) 998.89, 998.9 (other) | ICD-9 diagnosis code |
| 85.0 (infection) 85.82, 85.84, 85.85 (wound complication) 85.91, 86.01, 86.04 (hematoma/seroma) 85.94 (implant removal) | ICD-9 procedure code | |
| 10030, 10140 (hematoma/seroma) 10060, 10180, 19020 (infection) 12020, 12021, 13160 (wound complication) 19328, 19330 (implant removal) | CPT/HCPCS | |
| Additional breast-related procedures | 34.23, 34.79, 40.22, 40.23, 40.29, 40.3, 40.51, 53.41, 53.42, 53.51, 53.59, 54.0, 54.3, 54.72, 70.95, 83.82, 85.20, 85.21-85.24, 85.31, 85.32, 85.41, 85.50, 85.52-85.55, 85.6, 85.70, 85.71-85.76, 85.79, 85.81, 85.83, 85.86, 85.87, 85.89, 85.93, 85.96, 85.99, 86.09, 86.59, 86.67, 86.69, 86.70, 86.71, 86.72, 86.74, 86.75, 86.83, 86.84, 86.89, 86.93, 88.66, 92.29, 93.56, 99.00 | ICD-9 procedure code |
| 11954, 11970, 11971, 14000, 14001, 14301, 14302, 15200, 15330, 15734, 19120, 19318, 19325, 19340, 19342, 19350, 19357, 19366, 19370, 19371, 20926, C1762, C1763, C1789, C9354, L8000, L8020, L8030, L8600, L8699, Q4100, Q4116 | CPT/HCPCS |
Abbreviations: ICD-9, International Classification of Diseases Ninth Revision; CPT/HCPCS, Current Procedural Terminology/Healthcare Common Procedures Classification System; CCS,Clinical Classifications Software; DRG, Medicare Diagnosis Related Group.
Women could have both implant and autologous reconstruction procedure codes in the same encounter. Those with any implant as a component of breast reconstruction were categorized as having implant-based reconstruction.
Complications identified and categorized from codes published in Smith et al. JNCI 2017:109(1).
Footnotes
Conflict of interest/Disclosure
The authors have no financial or other conflict of interest to disclose.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.surg.2020.06.030.
References
- 1.Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann Surg. 2017;265:581–589. [DOI] [PubMed] [Google Scholar]
- 2.Pesce C, Liederbach E, Wang C, Lapin B, Winchester DJ, Yao K. Contralateral prophylactic mastectomy provides no survival benefit in young women with estrogen receptor-negative breast cancer. Ann Surg Oncol. 2014;21:3231–3239. [DOI] [PubMed] [Google Scholar]
- 3.Carbine NE, Lostumbo L, Wallace J, Ko H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev. 2018;4:CD002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boughey JC, Attai DJ, Chen SL, et al. Contralateral prophylactic mastectomy (CPM) consensus statement from the American Society of Breast Surgeons: data on CPM outcomes and risks. Ann Surg Oncol. 2016;23:3100–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt KK, Euhus DM, Boughey JC, et al. Society of Surgical Oncology Breast Disease Working Group statement on prophylactic (risk-reducing) mastectomy. Ann Surg Oncol. 2017;24:375–397. [DOI] [PubMed] [Google Scholar]
- 6.Wright FC, Look Hong NJ, Quan ML, et al. Indications for contralateral prophylactic mastectomy: a consensus statement using modified Delphi methodology. Ann Surg. 2018;267:271–279. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN) Web site. Breast cancer. Version 3 2018. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed January 11, 2019.
- 8.Parker PA, Peterson SK, Shen Y, et al. Prospective study of psychosocial outcomes of having contralateral prophylactic mastectomy among women with nonhereditary breast cancer. J Clin Oncol. 2018;36:2630–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tesson S, Richards I, Porter D, et al. Women’s preferences for contralateral prophylactic mastectomy following unilateral breast cancer: what risk-reduction makes it worthwhile? Breast. 2017;31:233–240. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg SM, Sepucha K, Ruddy KJ, et al. Local therapy decision-making and contralateral prophylactic mastectomy in young women with early-stage breast cancer. Ann Surg Oncol. 2015;22:3809–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braude L, Kirsten L, Gilchrist J, Juraskova I. A systematic review of women’s satisfaction and regret following risk-reducing mastectomy. Patient Educ Couns. 2017;100:2182–2189. [DOI] [PubMed] [Google Scholar]
- 12.Ager B, Butow P, Jansen J, Phillips KA, Porter D. Contralateral prophylactic mastectomy (CPM): a systematic review of patient reported factors and psychological predictors influencing choice and satisfaction. Breast. 2016;28:107–120. [DOI] [PubMed] [Google Scholar]
- 13.Boughey JC, Attai DJ, Chen SL, et al. Contralateral prophylactic mastectomy consensus statement from the American Society of Breast Surgeons: additional considerations and a framework for shared decision making. Ann Surg Oncol. 2016;23:3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg SM, Greaney ML, Patenaude AF, Sepucha KR, Meyer ME, Partridge AH. “I don’t want to take chances.”: a qualitative exploration of surgical decision making in young breast cancer survivors. Psychooncology. 2018;27:1524–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osman F, Saleh F, Jackson TD, Corrigan MA, Cil T. Increased postoperative complications in bilateral mastectomy patients compared to unilateral mastectomy: an analysis of the NSQIP database. Ann Surg Oncol. 2013;20:3212–3217. [DOI] [PubMed] [Google Scholar]
- 16.Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. 2013;20:4113–4120. [DOI] [PubMed] [Google Scholar]
- 17.Zion SM, Slezak JM, Sellers TA, et al. Reoperations after prophylactic mastectomy with or without implant reconstruction. Cancer. 2003;98:2152–2160. [DOI] [PubMed] [Google Scholar]
- 18.Momoh AO, Cohen WA, Kidwell KM, et al. Tradeoffs associated with contralateral prophylactic mastectomy in women choosing breast reconstruction: results of a prospective multicenter cohort. Ann Surg. 2017;266:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett KG, Qi J, Kim HM, Hamill JB, Pusic AL, Wilkins EG. Comparison of 2-year complication rates among common techniques for postmastectomy breast reconstruction. JAMA Surg. 2018;153:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshmukh AA, Cantor SB, Crosby MA, et al. Cost of contralateral prophylactic mastectomy. Ann Surg Oncol. 2014;21:2823–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucknor A, Chattha A, Ultee K, et al. The financial impact and drivers of hospital charges in contralateral prophylactic mastectomy and reconstruction: a nationwide inpatient sample hospital analysis. Breast Cancer Res Treat. 2017;165:301–310. [DOI] [PubMed] [Google Scholar]
- 22.Boughey JC, Schilz SR, Van Houten HK, Zhu L, Habermann EB, Lemaine V. Contralateral prophylactic mastectomy with immediate breast reconstruction increases healthcare utilization and cost. Ann Surg Oncol. 2017;24:2957–2964. [DOI] [PubMed] [Google Scholar]
- 23.Billig JI, Duncan A, Zhong L, et al. The cost of contralateral prophylactic mastectomy in women with unilateral breast cancer. Plast Reconstr Surg. 2018;141: 1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albornoz CR, Matros E, Lee CN, et al. Bilateral mastectomy versus breast-conserving surgery for early-stage breast cancer: the role of breast reconstruction. Plast Reconstr Surg. 2015;135:1518–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal S, Kidwell KM, Kraft CT, et al. Defining the relationship between patient decisions to undergo breast reconstruction and contralateral prophylactic mastectomy. Plast Reconstr Surg. 2015;135:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith BD, Jiang J, Shih YC, et al. Cost and complications of local therapies for early-stage breast cancer. J Natl Cancer Inst. 2016;109:djw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hospital Inpatient Cost Transparency. New York State Department of Health Web site. https://health.data.ny.gov/Health/Hospital-Inpatient-Cost-Transparency-Beginning-200/7dtz-qxmr. Accessed November 15, 2018. [Google Scholar]
- 28.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29:2158–2164. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Yuexin T, L. WG. Federal health coverage mandates and health care utilization: the case of the women’s health and cancer rights act and use of breast reconstruction surgery. J Womens Health (Larchmt). 2015;24:655–662. [DOI] [PubMed] [Google Scholar]
- 30.Koslow S, Pharmer LA, Scott AM, et al. Long-term patient-reported satisfaction after contralateral prophylactic mastectomy and implant reconstruction. Ann Surg Oncol. 2013;20:3422–3429. [DOI] [PubMed] [Google Scholar]
- 31.Eltahir Y, Werners LLCH, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg. 2013;132:201e–209e. [DOI] [PubMed] [Google Scholar]
- 32.Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2000;106:1014–1025;discussion 1026-1017. [DOI] [PubMed] [Google Scholar]
- 33.Beesley H, Holcombe C, Brown SL, Salmon P. Risk, worry and cosmesis in decision-making for contralateral risk-reducing mastectomy: analysis of 60 consecutive cases in a specialist breast unit. Breast. 2013;22:179–184. [DOI] [PubMed] [Google Scholar]
- 34.Frost MH, Slezak JM, Tran NV, et al. Satisfaction after contralateral prophylactic mastectomy: the significance of mastectomy type, reconstructive complications, and body appearance. J Clin Oncol. 2005;23:7849–7856. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery LL, Tran KN, Heelan MC, et al. Issues of regret in women with contralateral prophylactic mastectomies. Ann Surg Oncol. 1999;6:546–552. [DOI] [PubMed] [Google Scholar]
- 36.Huang J, Chagpar A. Impact of anticipated financial burden on patient decision to undergo contralateral prophylactic mastectomy. Surgery. 2018;164:856–865. [DOI] [PubMed] [Google Scholar]
- 37.Blewett LA, Call KT, Turner J, Hest R. Data resources for conducting health services and policy research. Annu Rev Public Health. 2018;39:437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zendejas B, Moriarty JP, O’Byrne J, Degnim AC, Farley DR, Boughey JC. Cost-effectiveness of contralateral prophylactic mastectomy versus routine surveillance in patients with unilateral breast cancer. J Clin Oncol. 2011;29:2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts A, Habibi M, Frick KD. Cost-effectiveness of contralateral prophylactic mastectomy for prevention of contralateral breast cancer. Ann Surg Oncol. 2014;21:2209–2217. [DOI] [PubMed] [Google Scholar]
- 40.Keskey RC, LaJoie AS, Sutton BS, et al. Cost-effectiveness analysis of contralateral prophylactic mastectomy compared to unilateral mastectomy with routine surveillance for unilateral, sporadic breast cancer. Ann Surg Oncol. 2017;24:3903–3910. [DOI] [PubMed] [Google Scholar]
- 41.Fisher CS, Martin-Dunlap T, Ruppel MB, Gao F, Atkins J, Margenthaler JA. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol. 2012;19:3246–3250. [DOI] [PubMed] [Google Scholar]
- 42.Covelli AM, Baxter NN, Fitch MI, McCready DR, Wright FC. ‘Taking control of cancer’: understanding women’s choice for mastectomy. Ann Surg Oncol. 2015;22:383–391. [DOI] [PubMed] [Google Scholar]
- 43.American Society of Breast Surgeons recommendation. Choosing wisely: promoting conversations between patients and clinicians Web site. Updated June 27, 2016. Accessed May 13, 2019 https://www.choosingwisely.org/clinician-lists/breast-surgeons-mastectomies-for-single-breast-cancer-patients/. [Google Scholar]
- 44.Tuttle TM, Barrio AV, Klimberg VS, et al. Guidelines for guidelines: an Assessment of the American Society of Breast Surgeons Contralateral Prophylactic Mastectomy Consensus Statement. Ann Surg Oncol. 2017;24:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg SM, Partridge AH. Contralateral prophylactic mastectomy: an opportunity for shared decision making. JAMA Surg. 2014;149:589–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramaswami R, Morrow M, Jagsi R. Contralateral prophylactic mastectomy. N Engl J Med. 2017;377:1288–1291. [DOI] [PubMed] [Google Scholar]
- 47.Nass SJ, Nekhlyudov L. Commentary on the consensus statement of the American Society of Breast Surgeons on contralateral prophylactic mastectomy. Ann Surg Oncol. 2017;24:611–613. [DOI] [PubMed] [Google Scholar]