Abstract
The role of wildlife species in the epidemiology of paratuberculosis has been the subject of increased research efforts following the discovery of natural paratuberculosis in free-living rabbits from farms in east Scotland. This paper describes the experimental inoculation of young calves with an isolate of Mycobacterium avium subsp. paratuberculosis recovered from a free-living rabbit. After a 6-month incubation period, all eight calves inoculated with the rabbit isolate had developed histopathological and/or microbiological evidence of M. avium subsp. paratuberculosis infection. Similar results were obtained from a group of calves infected with a bovine isolate of M. avium subsp. paratuberculosis. The virulence of the rabbit isolate for calves demonstrated in this study suggests that rabbits are capable of passing paratuberculosis to domestic ruminants and that wildlife reservoirs of M. avium subsp. paratuberculosis should therefore be considered when formulating control plans for the disease.
Paratuberculosis is a chronic enteritis of wild and domestic ruminants caused by infection with the bacterium Mycobacterium avium subsp. paratuberculosis. The disease is found worldwide, causing considerable economic loss to affected farms and industries. It has been estimated to cost the U.S. dairy industry alone between $200 and $250 million annually (17). Despite its importance, however, the epidemiology of this disease is still poorly understood.
Young ruminants are believed to be most susceptible to infection, and consequently most animals are thought to be infected soon after birth, usually by ingestion of M. avium subsp. paratuberculosis excreted in the feces of affected animals (8). A long subclinical period of infection precedes the development of clinical disease, which is usually only seen in animals over 2 years of age. Clinical paratuberculosis is characterized by intermittent diarrhea, a gradual loss in weight and condition, and eventually death (9). There is no effective treatment for paratuberculosis, and available vaccines do not prevent infection.
The role of wildlife in the epidemiology of paratuberculosis has not been widely investigated. A number of free-living ruminant species have been found to be infected with M. avium subsp. paratuberculosis, including big horn sheep (Ovis canadensis) (20), a number of species of deer (19), moufflon (Ovis musimon) (18), and bison (Bison bison) (4). Recently, the first reports of natural nonruminant paratuberculosis described M. avium subsp. paratuberculosis infection in up to 67% of free-living rabbits from paratuberculosis-affected farms in the east of Scotland (14). Subsequent investigations have revealed M. avium subsp. paratuberculosis infection in other free-living species, including the fox (Vulpes vulpes), stoat (Mustela erminea), weasel (Mustela nivalis), and badger (Meles meles) (2, 3). These findings indicate that M. avium subsp. paratuberculosis has a much wider natural host range than was previously realized and underline the need for further research into the role of these newly identified reservoirs.
Currently it is not known whether the infected wildlife species act as dead-end hosts of M. avium subsp. paratuberculosis, are part of a separate cycle of the organism unconnected with the disease in domestic ruminants, or are directly linked with ruminant paratuberculosis, with interspecies transmission of infection. To investigate further the role of wildlife and interspecies transmission in the epidemiology of paratuberculosis, an experiment was undertaken to determine if a leporine isolate of M. avium subsp. paratuberculosis could infect and cause lesions in young calves.
MATERIALS AND METHODS
Animals and experimental design.
Eighteen male Holstein-Friesian cross newborn calves (between 1 and 5 days old), having received colostrum from their dams, were acquired from a farm which had no known history of paratuberculosis and was regularly screened for evidence of M. avium subsp. paratuberculosis infection. The calves were allocated to one of three groups, and each animal received an oral dose of inoculum once a week for 3 weeks.
Eight calves were placed in group L (leporine group) and given the rabbit isolate (R7) of M. avium subsp. paratuberculosis; four calves were allocated to group B (bovine group) and administered a bovine isolate (F13) of M. avium subsp. paratuberculosis; and the remaining six calves were designated the control group, group C, and treated with phosphate-buffered saline (PBS). Isolates R7 and F13 were recovered from a naturally infected rabbit and bovid, respectively, from the same farm in east Scotland and are both of low passage and identical genotype (13). Each group of calves was housed separately, with animals penned individually until 6 weeks old. The calves were fed a proprietary brand of milk replacer until weaned onto a standard hay and concentrate ration when 4 to 6 weeks old.
Fecal samples from each calf were collected and cultured (see below) at the time of first inoculation and then weekly for the first 8 weeks and thereafter monthly until the calves were 6 months old. The calves were then euthanized with an overdose of intravenous barbiturate, a postmortem examination was carried out, and representative samples of tissues for both histopathological and microbiological analysis were taken from each animal. All experimental procedures and management protocols were examined and approved by the Moredun Research Institute Experiments and Ethics Committee and carried out under approved British Home Office licenses in accordance with the Animals (Scientific Procedures) Act of 1986.
Inoculum.
Both strains of M. avium subsp. paratuberculosis were propagated on modified Middlebrook 7H11 medium (2.1% 7H11 agar [Difco], 2.3 mM l-asparagine, 2.5% glycerol, and 2 μg of mycobactin J [Allied Monitor, St. Louis, Mo.] per ml supplemented with 10% Middlebrook OADC enrichment [Difco], 20% heat-inactivated newborn calf serum [Life Technologies], and two Selectatabs per liter [amphotericin B, polymixin B, carbenicillin, and trimethoprim; MAST Laboratories]). Cells were grown at 37°C for 8 to 10 weeks. M. avium subsp. paratuberculosis cells were resuspended in sterile PBS, and large clumps were allowed to settle at the bottom of the tube. The cell suspension was removed to a fresh tube, and the clumped cells were discarded. The optical density of the cell suspension was measured using a Densimat (Bio-Merieux) and adjusted to a McFarland standard of 3 to 3.5 with sterile PBS. An aliquot of the inoculum was titrated and plated in duplicate onto modified Middlebrook 7H11 medium to obtain a retrospective viable-cell count.
Microbiology.
Feces collected during the experiment and a sample of jejunal Peyer's patch (JPP) and mesenteric lymph node (MLN) taken from each calf during postmortem examination were cultured for the presence of M. avium subsp. paratuberculosis as follows. A 1-g sample of feces or 0.5 cm3 of tissue was homogenized for 30 s in 10 ml of sterile distilled water using a Colworth Stomacher 80 (Seward Medical). Homogenates were then decontaminated by adding 10 ml of 1.5% hexadecyl pyridinium chloride (Sigma-Aldrich) and left to stand overnight at room temperature. The resulting supernatants were centrifuged at 3,800 × g for 30 min at 4°C, and each pellet was resuspended in 10 ml of sterile distilled water. The centrifugation step was repeated, and each pellet was resuspended in 1 ml of sterile distilled water. Suspensions were then transferred to microcentrifuge tubes and centrifuged at 6,500 × g for 5 min at room temperature. The pellets were resuspended in 0.5 ml of sterile distilled water. A sample of 0.1 ml of each suspension was then inoculated onto two slopes of modified Middlebrook 7H11 agar. The cultures were incubated at 37°C for up to 16 weeks and examined regularly for bacterial growth.
To confirm the identity of colonies grown from the tissue and fecal samples, DNA from the colonies was extracted and screened for the presence of the M. avium subsp. paratuberculosis-specific insertion sequence IS900 (12). A 200-μl aliquot of sterile distilled water was inoculated with a single bacterial colony from a culture, then transferred to a screw-capped microcentrifuge tube containing 1 ml of zirconium-silica beads (0.1-mm diameter) (BioSpec Products), and cooled on ice. The tube was placed in a Hybaid ribolyser, ribolysed at 5.5 m/s for 20 s, and then cooled on ice again for 1 min. The DNA was extracted using guanidine hydrochloride as described previously (7) and then screened for the presence of IS900 using a PCR-enzyme-linked immunosorbent assay (ELISA) microplate assay (Stevenson et al., unpublished data). Briefly, the PCR was carried out using primers 90 and 91 as described previously (13) except that primer 91 was biotinylated. PCR product was captured on streptavidin-coated microplates after heat denaturation and hybridization to a 2,4-dinitrophenyl (DNP)-labeled oligonucleotide probe complementary to the amplified sequence. Amplified product was detected using peroxidase-conjugated rabbit anti-DNP and O-phenylenediamine dihydrochloride. The optical density at 450 nm readings were obtained using a standard laboratory microplate reader.
Histology.
A sample of retropharyngeal and submandibular lymph node, duodenum, jejunum, JPP, ileal Peyer's patch, terminal ileum, proximal and distal MLN, ileocaecal valve (ICV), ileocaecal lymph node (ICLN), and liver were taken from each calf during the postmortem examination and placed in 10% formol–saline. After a minimum of 24 h of fixation, tissues were trimmed, dehydrated through graded alcohols, and embedded in paraffin wax, and sections 5 μm thick were cut and stained with hematoxylin and eosin. Serial sections were stained by the Ziehl-Neelsen method.
RESULTS
No animal exhibited signs of diarrhea or weight loss during the 6-month incubation period, although three calves required antibiotic and anti-inflammatory treatment for unrelated conditions. In week 2 of the experiment, calf B2 (Table 1) showed signs consistent with a diagnosis of septic arthritis, and calf B4 developed suspected omphalitis. Both were treated with subcutaneous injections of a solution containing both clavulanic acid and amoxicillin (Synulox; Pfizer Ltd.) and intravenous flunixin (Finadyne; Schering-Plough Animal Health). Calf L5 showed signs of pneumonia in week 8 and was treated with subcutaneous enrofloxacin (Baytril; Bayer Plc.) and intravenous flunixin.
TABLE 1.
Microbiological and histopathological results for individual calves in groups L and B
| Calf no. | Age at first inoculation (days) | Total dose (CFU) |
M. avium subsp. paratuberculosis isolated (wk postinoculation) from:
|
Histopathology evident | |
|---|---|---|---|---|---|
| Feces | Tissues | ||||
| L6 | 2 | 9 × 108 | + (4) | + | + |
| L7 | 2 | 9 × 108 | + (22) | + | + |
| L3 | 1 | 1 × 109 | + (12) | + | − |
| L8 | 3 | 8 × 108 | + (1 and 2) | + | − |
| L1 | 4 | 1 × 109 | + (27) | − | − |
| L5a | 2 | 9 × 108 | − | + | + |
| L2 | 1 | 1 × 109 | − | + | − |
| L4 | 3 | 9 × 108 | − | + | − |
| B2a | 2 | 6 × 108 | − | + | + |
| B3 | 2 | 6 × 108 | − | + | + |
| B4a | 5 | 4 × 108 | − | + | − |
| B1 | 3 | 7 × 108 | − | − | − |
Animal received antibiotic therapy during the experiment.
Microbiology.
A total of 229 fecal samples were cultured from the calves during the experiment. M. avium subsp. paratuberculosis was isolated from six samples originating from five calves, all of which were in group L (Table 1). The positive fecal samples were produced at various time points throughout the experiment, from week 1 to week 27. No M. avium subsp. paratuberculosis was cultured from feces samples from calves in group B or C.
Samples of MLN and JPP were collected from all calves and cultured. M. avium subsp. paratuberculosis was isolated from the tissues of seven of eight animals in group L and three of four animals in group B. No bacterial isolates were recovered from any animal in group C (the control group). M. avium subsp. paratuberculosis was isolated from either JPP or MLN tissue from individual calves in group L, whereas the organism was isolated from both tissue types in individual animals in group B. The microbiological results for each group are summarized in Table 2, while the results for individual calves in groups L and B are compared in Table 1.
TABLE 2.
Summary of group microbiological and histopathological results for tissues collected postmortem
| Group | No. of animals positive/no. in groupa
|
|||
|---|---|---|---|---|
| Culture
|
Histopathology | |||
| JPP | MLN | JPP and/or MLN | ||
| L | 2/8 | 5/8 | 7/8∗ | 3/8‡ |
| B | 3/4 | 3/4 | 3/4† | 2/4§ |
| C | 0/6 | 0/6 | 0/6 | 0/6 |
Significant differences between each infected group and the control group were determined using Fisher's exact test. Probability values: ∗, P = 0.002; †, P = 0.02; ‡, P = 0.13; and §, P = 0.07.
Pathology.
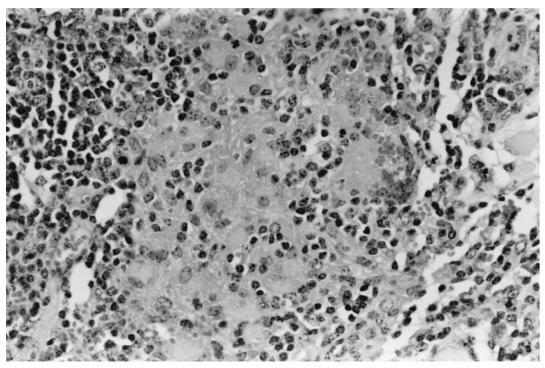
At the postmortem examination, no gross abnormalities were noted in any animal; however, three of the eight animals in group L and two of the four animals in group B had histopathological lesions consistent with a diagnosis of early M. avium subsp. paratuberculosis infection (Table 2). No lesions indicative of M. avium subsp. paratuberculosis infection were noted in any tissues from animals in group C. The pathological changes identified in groups L and B are summarized in Table 3 and consisted of clearly delineated, nonencapsulated granulomata containing macrophages, epithelioid cells, and occasional giant cells. A variable number of acid-fast bacteria (AFB) were present in certain cells of the granulomata, although no AFB were visible in some granulomata. The most common site for lesions was the lamina propria and underlying lymphoid tissue of the ICV (Fig. 1) and its draining lymph node, the ICLN. However, lesions were noted in the jejunum of one animal (B3), in which granulomata were identified in both the JPP and the overlying lamina propria, with a small number of AFB present in the granulomata of the JPP.
TABLE 3.
Summary of pathological changes noted in tissues examined from three calves in group L and two calves in group B 6 months after inoculation with M. avium subsp. paratuberculosis
| Animal no. | Scorea
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Jejunum
|
ICV
|
ICLN
|
||||||||
| Villi
|
Lymphoid tissue
|
Villi
|
Lymphoid tissue
|
|||||||
| Pathology | AFB | Pathology | AFB | Pathology | AFB | Pathology | AFB | Pathology | AFB | |
| L7 | − | − | − | − | ++ | + | + | − | + | + |
| L5 | − | − | − | − | ++ | ++ | − | − | − | − |
| L6 | − | − | − | − | − | − | − | − | − | + |
| B3 | ++ | − | ++ | + | ++ | + | + | − | +++ | ++ |
| B2 | − | − | − | − | − | − | + | − | +++ | + |
The scoring systems were as follows. Pathology: +, 1 or 2 small granulomata; ++, between 2 and 10 granulomata; +++, more than 10 granulomata. AFB: +, 1 to 5 clumps of bacteria identified; ++, moderate numbers of AFB present; +++, abundant numbers of AFB present.
FIG. 1.
Granuloma, consisting of macrophages and monocytes, in the lamina propria of the ICV in a calf infected with the rabbit isolate of M. avium subsp. paratuberculosis.
Histopathological lesions distinct from the above changes and consistent with chronic parasitism were evident in animals from all three groups. The most likely cause was considered to be intestinal coccidiosis, as the changes noted included pyogranulomata containing mainly polymorphonuclear cells in the mucosa-associated lymphoid tissue and coccidial oocysts in association with epithelial cells of the intestine. The extent and severity of these changes were consistent across the three groups.
Statistics.
The results of the statistical analyses are summarized in Table 2. Using Fisher's exact test, a significant difference (P < 0.05) was found between the culture results of group C and group L, as well as between group C and group B. The corresponding statistical differences between the histopathological results were close to but not below the 5% mark. No significant differences were detected by either culture or histopathology between the two treated groups L and B.
DISCUSSION
This experiment has shown that an isolate of M. avium subsp. paratuberculosis from a naturally infected rabbit is capable of infecting young calves and causing lesions consistent with a diagnosis of early paratuberculosis. This supports the hypothesis that transmission of paratuberculosis from rabbits to cattle can occur under field conditions.
The pathological changes identified in animals inoculated with both the leporine and bovine isolates are consistent with early lesions of paratuberculosis described by other workers (1, 15). Tissues from all animals with histopathological changes indicative of early paratuberculosis yielded isolates of M. avium subsp. paratuberculosis when cultured, confirming the identity of the AFB noted in the tissue sections. The finding of lesions in the ICV and ICLN of the inoculated calves is noteworthy, since the terminal ileum and ICV are reported as the most common sites of paratuberculosis lesions in clinical cases (5, 10), suggesting in turn that the experimental infection in the calves was following the natural course of paratuberculosis.
Very few fecal samples from the calves yielded M. avium subsp. paratuberculosis on culture. From a total of 229 samples, only 6 yielded M. avium subsp. paratuberculosis organisms when cultured, and all six animals had been inoculated with the leporine isolate (Table 2). Additionally, the results from fecal cultures do not correlate well with the results from the postmortem analysis of samples. Animal L1 produced a fecal sample containing M. avium subsp. paratuberculosis in week 27, but no evidence of M. avium subsp. paratuberculosis infection was found by either the histopathological analysis or culture of tissues collected postmortem. Conversely, calves L5, B2, and B3 had lesions with AFB present in their intestines, and M. avium subsp. paratuberculosis was recovered from the MLN and JPP of all three calves. However, no organisms were recovered from fecal samples of these animals during the 6 months of the experiment. The interpretation of the results from fecal culture is complicated by the nature of the disease itself. Subclinically infected animals have been reported to shed M. avium subsp. paratuberculosis intermittently (6, 15), and it is notoriously difficult to culture organisms from low shedders. The fact that only the leporine isolate was recovered by fecal culture in this experiment is interesting and could reflect a strain characteristic. It is possible that the leporine strain is excreted more easily from the host or that the strain is more adapted to fecal transmission.
This experiment has established that a leporine isolate produces experimental paratuberculosis in calves that is indistinguishable from that produced by a bovine isolate. The results from the culture of tissues are comparable in both groups—samples from seven of eight calves in group L and three of four calves in group B yielded isolates of M. avium subsp. paratuberculosis. A similar proportion of animals in each group developed lesions—three of eight in group L, and two of four in group B. Consequently, no statistical differences were detected between the two treated groups, although some disparities were evident. The lesions noted in the two animals from group B were more extensive, with a higher number of AFB present than in group L. While it is tempting to speculate that the bovine isolate may produce more extensive lesions and the rabbit isolate has the potential to be more easily transmitted between animals, the data are insufficient to confirm this. Further experiments are required to investigate these observations and determine whether they represent the characteristics of different M. avium subsp. paratuberculosis strains.
There was a high prevalence of infection in the calves in groups L and B - all but 2 of 12 calves inoculated with M. avium subsp. paratuberculosis had evidence of infection at postmortem. This high prevalence of infection may reflect the suspected increased susceptibility of young animals to infection (8), as all calves in this study were exposed to M. avium subsp. paratuberculosis in the first week of life (Table 1). Further exposure undoubtedly occurred later when the calves were housed as groups, although any transmission between the calves would be much less than the original inoculum and therefore unlikely to influence the final outcome of the experiment.
It was not possible to determine the effect of intestinal coccidiosis on the experimental results, as no suitable control group was available for comparison and no studies have yet been published on the effect of concurrent intestinal disease on the susceptibility of animals to M. avium subsp. paratuberculosis infection.
The results of this study therefore support the findings of earlier investigations, which reported a link between paratuberculosis in cattle and rabbits. Greig et al. (13) examined rabbits from farms with and without a history of paratuberculosis in domestic ruminants and found a statistically significant association between farms with a history of paratuberculosis in their domestic cattle and the presence of M. avium subsp. paratuberculosis infection in rabbits collected from the farm.
Cattle normally show a strong aversion to pasture contaminated with feces. However, Daniels et al. (11) showed that cattle do not avoid pasture contaminated with rabbit pellets and that between 3 and 7% of rabbit feces disappeared daily from monitored swards, suggesting some ingestion by cattle. Further studies have shown that the mean number of M. avium subsp. paratuberculosis in rabbit feces is approximately 104 CFU g−1 (Daniels et al., unpublished data), suggesting that ingestion of a small number of contaminated rabbit fecal pellets could constitute an infective dose. Therefore, evidence is accumulating to support the hypothesis that rabbits play a role in the epidemiology of paratuberculosis in domestic ruminants and that this should be taken into consideration when planning strategies for controlling M. avium subsp. paratuberculosis.
ACKNOWLEDGMENTS
Grateful thanks are extended to Alison Baird and Valerie Forbes for preparing the histological sections and to Jock McCracken and the clinical division of Moredun Research Institute for the excellent husbandry of the calves. Iain McKendrick provided advice and assistance with the statistical analyses.
This research was funded by the Scottish Executive Rural Affairs Department.
REFERENCES
- 1.Angus K W, Gilmour N J L. Effect of the rimino phenazine B663 ( G30320) on Mycobacterium johnei infection and reinfection in sheep. II. Pathology. J Comp Pathol. 1971;81:227–234. doi: 10.1016/0021-9975(71)90096-x. [DOI] [PubMed] [Google Scholar]
- 2.Beard P M, Daniels M J, Henderson D, Pirie A, Rudge K, Buxton D, Rhind S, Greig A, Hutchings M R, McKendrick I, Stevenson K, Sharp J M. Paratuberculosis infection of non-ruminant wildlife in Scotland. J Clin Microbiol. 2001;39:1519–1521. doi: 10.1128/JCM.39.4.1517-1521.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beard P M, Henderson D, Daniels M J, Pirie A, Buxton D, Greig A, Hutchings M R, McKendrick I, Rhind S, Stevenson K, Sharp J M. Evidence of paratuberculosis in fox (Vulpes vulpes) and stoat (Mustela erminea) Vet Rec. 1999;145:612–613. [Google Scholar]
- 4.Buergelt C D, Ginn P. The histopathologic diagnosis of subclinical Johne's disease in North American bison (Bison bison) Vet Microbiol. 2000;77:325–331. doi: 10.1016/s0378-1135(00)00317-5. [DOI] [PubMed] [Google Scholar]
- 5.Buergelt C D, Hall C, McEntree K, Duncan J R. Pathological evaluation of paratuberculosis in naturally infected cattle. Vet Pathol. 1978;15:196–207. doi: 10.1177/030098587801500206. [DOI] [PubMed] [Google Scholar]
- 6.Chaitaweesub P, Abbott K A, Whittington R, Marshall D J. Shedding of organisms and subclinical effects on production on preclinical merino sheep affected with paratuberculosis. In: Manning E J B, Collins M T, editors. Proceedings of the Sixth International Colloquium on Paratuberculosis. Madison, Wis: International Association for Paratuberculosis; 1999. pp. 126–131. [Google Scholar]
- 7.Challans J A, Stevenson K, Reid H W, Sharp J M. A rapid method for the extraction and detection of Mycobacterium avium subspecies paratuberculosis from clinical specimens. Vet Rec. 1994;134:95–96. doi: 10.1136/vr.134.4.95. [DOI] [PubMed] [Google Scholar]
- 8.Chiodini R J, Van Kruiningen H J, Merkal R S. Ruminant paratuberculosis (Johne's disease)—the current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- 9.Clarke C J. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Pathol. 1997;116:217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 10.Clarke C J, Little D. The pathology of ovine paratuberculosis: Gross and histological changes in the intestine and other tissues. J Comp Pathol. 1996;114:419–437. doi: 10.1016/s0021-9975(96)80017-x. [DOI] [PubMed] [Google Scholar]
- 11.Daniels M J, Ball N, Hutchings M R, Greig A. The grazing response of cattle to pasture contaminated with rabbit faeces and the implications for the transmission of paratuberculosis. Vet J. 2001;161:306–313. doi: 10.1053/tvjl.2000.0550. [DOI] [PubMed] [Google Scholar]
- 12.Green E P, Tizard M L, Moss M T, Thompson J, Winterbourne D J, McFadden J J, Hermon-Taylor J. Sequence and characteristics of IS900, an insertion element identified in a human Crohn's disease isolate of Mycobacterium paratuberculosis. Nucleic Acids Res. 1989;17:9063–9073. doi: 10.1093/nar/17.22.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greig A, Stevenson K, Henderson D, Perez V, Hughes V, Pavlik I, Hines M E, McKendrick I, Sharp J M. Epidemiological study of paratuberculosis in wild rabbits in Scotland. J Clin Microbiol. 1999;37:1746–1751. doi: 10.1128/jcm.37.6.1746-1751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greig A, Stevenson K, Perez V, Pirie A A, Grant J M, Sharp J M. Paratuberculosis in wild rabbits (Oryctolagus cuniculus) Vet Rec. 1997;140:141–143. doi: 10.1136/vr.140.6.141. [DOI] [PubMed] [Google Scholar]
- 15.Juste R A, Marin J F G, Peris B, Deocariz C S, Badiola J J. Experimental infection of vaccinated and non-vaccinated lambs with Mycobacterium paratuberculosis. J Comp Pathol. 1994;110:185–194. doi: 10.1016/s0021-9975(08)80189-2. [DOI] [PubMed] [Google Scholar]
- 16.McDonald W L, Ridge S E, Hope A F, Condron R J. Evaluation of diagnostic tests for Johne's disease in young cattle. Aust Vet J. 1999;77:113–119. doi: 10.1111/j.1751-0813.1999.tb11679.x. [DOI] [PubMed] [Google Scholar]
- 17.Ott S L, Wells S J, Wagner B A. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev Vet Med. 1999;40:179–192. doi: 10.1016/s0167-5877(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 18.Pavlik I, Bartl J, Dvorska L, Svastova P, DuMaine R, Machackova M, Yayo Ayele W, Horvathova L. Epidemiology of paratuberculosis in wild ruminants studied by restriction fragment length polymorphism in the Czech republic during the period 1995–1998. Vet Microbiol. 2000;77:231–251. doi: 10.1016/s0378-1135(00)00309-6. [DOI] [PubMed] [Google Scholar]
- 19.Sharp J M, Stevenson K, Challans J A, Ramage C, Hitchcock D, Reid H W. Mycobacterial infections of free living deer in Scotland. In: Chiodini R J, Hines M E, Collins M T, editors. Proceedings of the 5th International Colloquium on Paratuberculosis. Madison, Wis: International Association for Paratuberculosis; 1997. pp. 180–182. [Google Scholar]
- 20.Williams E S, Spraker T R, Schoonveld G G. Paratuberculosis (Johne's disease) in bighorn sheep and a rocky mountain goat in Colorado. J Wildl Dis. 1979;15:221–227. doi: 10.7589/0090-3558-15.2.221. [DOI] [PubMed] [Google Scholar]