Abstract
Background
As Parkinson's disease progresses the control of motor symptoms often requires the addition of other drugs to levodopa. The principle aim of COMT inhibitor therapy is to increase the duration of effect of each levodopa dose and thus reduce the time patients spend in the relatively immobile 'off' phase.
Objectives
To compare the efficacy and safety of adjuvant COMT inhibitor therapy versus placebo in patients with Parkinson's disease, already established on levodopa and suffering from motor complications.
Search methods
Electronic searches of the Cochrane Controlled Trials Register, (The Cochrane Library Issue 1, 2003), MEDLINE (1966‐2003), EMBASE (1974‐2003), were conducted. Grey literature was hand searched and the reference lists of identified studies and reviews examined. The manufacturers of COMT inhibitors were contacted.
Selection criteria
Randomised controlled trials of adjuvant COMT inhibitor therapy versus a placebo in patients with a clinical diagnosis of idiopathic Parkinson's disease and long‐term complications of levodopa therapy.
Data collection and analysis
Data were abstracted independently by the authors and differences settled by discussion. The outcome measures used included Parkinson's disease rating scales, levodopa dosage, 'off' time measurements and the frequency of withdrawals and adverse events.
Main results
Fourteen trials fulfilled the inclusion criteria. 2566 patients with Parkinson's disease and motor fluctuations were included in this review. Eight trials examined entacapone versus placebo in a total of 1560 patients. These trials were between two and twelve months in duration. Six trials examined tolcapone versus placebo in a total of 1006 patients. These trials were between six weeks and twelve months in duration.
Both tolcapone and entacapone reduced 'off' time, reduced levodopa dose and modestly improved motor impairments and disability. This was at the expense of increased risk of dyskinesias, nausea, vomiting, and diarrhoea. A few participants taking tolcapone were found to have raised liver enzyme levels.
Authors' conclusions
In the management of the motor complications seen in Parkinson's disease, tolcapone and entacapone can be used to reduce off time, reduce levodopa dose, and modestly improve motor impairment and disability. This is based on, at best, medium term evidence. However some participants on tolcapone had raised liver enzymes. This combined with three cases of fatal hepatic toxicity found during post‐marketing surveillance has raised concerns over the safety of tolcapone.
Plain language summary
The COMT inhibitors entacapone and tolcapone show similar benefits in relieving levodopa‐induced complications in Parkinson's disease but more data on the safety of tolcapone is required.
As Parkinson's disease progresses the control of the symptoms often requires the addition of other drugs to levodopa. The principle aim of COMT inhibitor therapy is to increase the duration of effect of each levodopa dose and thus reduce the time patients spend in the relatively immobile 'off' phase. Tolcapone and entacapone can be used to reduce off time, reduce levodopa dose, and modestly improve motor impairment and disability. This is based on, at best, medium term evidence. However some participants on tolcapone had raised liver enzymes. Post‐marketing surveillance identified three cases of fatal hepatic toxicity in patients treated with tolcapone. As a result, tolcapone has been withdrawn from some countries and severe restrictions on its use have been imposed in others.
Background
Over 20 years after its introduction, levodopa remains the most effective therapy in Parkinson's disease. However, with long‐term treatment, patients develop side effects comprised of motor and psychiatric complications. The former consist of involuntary writhing movements of the face, limbs and trunk (choreoathetosis), painful cramps often affecting the feet (dystonia) and a shortened response to each dose of levodopa (end‐of‐dose deterioration). These affect 50% of patients after 6 years of therapy (Rajput 1984) and 100% of young onset patients after a similar period of therapy (Quinn 1986).
In an attempt to reduce these adverse responses to fluctuations in dopaminergic stimulation various therapeutic strategies can be adopted. These include using more frequent smaller doses of levodopa, controlled release formulations, additional dopamine agonists or continuous subcutaneous infusions of apomorphine. Catechol‐O‐methyltransferase (COMT) is an enzyme that catalyses the metabolism of levodopa to 3‐O‐methyldopa. Drugs that inhibit COMT prolong the maintenance of serum levodopa levels and hence produce a longer and more stable clinical response (Bonifati 1999). There are two COMT inhibitors used in clinical practice at present: tolcapone and entacapone.
The efficacy and safety of COMT inhibitors have been examined in early Parkinson's disease, before motor complications have developed, and in the later stages of the condition, when patients have motor complications as a result of levodopa therapy. Trials in later disease have lead to COMT inhibitors being licensed for this indication in the expectation of a reduction in off time and improved motor function. There has been controversy over the licensing of tolcapone. Concerns over several deaths from liver toxicity thought to be induced by tolcapone led to the withdrawal of the European drug license, although the drug is still available in the USA provided stringent hepatic monitoring is performed (EAEM 1998).
The present systematic review examines all randomised controlled trials of adjuvant COMT inhibitor therapy compared with placebo in later Parkinson's disease with motor complications to establish its efficacy and tolerability. A separate review covers the effects of adjuvant COMT inhibitors versus active comparators.
Objectives
To compare the efficacy and safety of adjuvant COMT inhibitor therapy versus placebo in patients with Parkinson's disease, already established on levodopa and suffering from motor complications.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials comparing adjuvant COMT inhibitors with placebo were considered for inclusion in the study.
Types of participants
Patients with a clinical diagnosis of idiopathic Parkinson's disease who had developed long‐term motor complications of dyskinesia and/or end‐of‐dose deterioration. All ages were included. Any duration of levodopa therapy was included.
Types of interventions
Oral tolcapone or entacapone therapy versus placebo. Trial durations of greater than 4 weeks were included.
Types of outcome measures
1. Improvement in the time patients spend in the immobile 'off' state.
2. Changes in dyskinesia rating scales and the prevalence of dyskinesia.
3. Changes in Parkinsonian rating scales.
4. Reduction in levodopa dose.
5. Frequency of adverse events
6. Number of withdrawals due to lack of efficacy and/or side‐effects.
Search methods for identification of studies
1. The review was based on the search strategy of the Movement Disorders Group. This included computerised searches of MEDLINE (1966‐2003) and EMBASE (1974‐2003) and hand searching of appropriate neurology journals. Relevant trials were included on the Group's specialised register of randomised controlled trials. Further details are available in the Group's module on the Cochrane Database of Systematic Reviews.
2. The Cochrane Controlled Trials Register (The Cochrane Library Issue 1, 2003) was also searched for relevant trials.
3. The reference lists of located trials and of other COMT inhibitor reviews were searched.
4. Additional assistance was provided by the drug manufacturers, Orion and Roche.
Data collection and analysis
The two authors (CEC, KD) independently assessed the studies identified by the search strategy. Disagreements about inclusions were resolved by discussion. The full papers were assessed for methodological quality by recording the method of randomisation and blinding, whether an intention to treat analysis was used and the number of patients lost to follow up.
Eligible data was abstracted onto standardised forms by the authors independently, checked for accuracy and amalgamated. A weighted estimate (fixed effect model) of the typical treatment effect across trials was calculated for continuous (weighted mean difference) and dichotomous (Peto odds ratio) variables such as 'off' time and prevalence of adverse events.
Results
Description of studies
See also Characteristics of Included Studies Table and Results of Included Studies.
Fourteen trials fulfilled the inclusion criteria with a total of 2569 patients with Parkinson's disease and motor fluctuations included in this review.
ENTACAPONE Eight studies compared entacapone against placebo with 1563 participants (Brooks UK‐IRISH 2003, Fenelon 2002, Im 2002, Myllyla FILOMEN 2001, Poewe CELOMEN 2002, PSG SEESAW 1997, Rinne NOMECOMT 1998; Ruottinen 1996 (a)). One study had a randomised double blind cross‐over design (Ruottinen 1996 (a)), the remaining seven studies had a randomised, double‐blind parallel‐group design. One was a phase II study (Ruottinen 1996 (a)) the remaining seven were phase III studies.
Patients were well balanced across the arms of the studies in terms of age and Hoehn and Yahr score (see Characteristics of Included Studies).
Im 2002 did not state the dose of entacapone used in their study, however all of the remaining seven trials used 200 mg of entacapone with each of the patient's levodopa doses, up to 10 doses per day. This is comparable with the present recommended dose of 200 mg of entacapone with each of the patient's levodopa doses, to a maximum of 2g per day.
Levodopa dose reduction was not allowed in only one trial (Ruottinen 1996 (a)), the remaining trials all allowed levodopa dose reduction.
TOLCAPONE Six studies compared tolcapone against placebo with 1006 participants (Adler TFSGIII 1998, Baas 1997, Dupont TIPSII 1997, Kurth TFSGI 1997, Myllyla TIPS1 1997, Rajput 1997). All six studies had a randomised, double‐blind parallel‐group design. All were phase III studies.
Patients were well balanced across the arms of the studies in terms of age and Hoehn and Yahr score (see Characteristics of Included Studies). Patients received between 50 mg tid and 400 mg tid tolcapone. This is comparable with the present recommended dose of 100 mg tid tolcapone in the USA and up to 200 mg tid in Europe until it was withdrawn. Only one study (Adler TFSGIII 1998) did not add 0.5 mg riboflavin to the placebo tablet in order to mimic the yellow discolouration in the urine of participants that occurs with tolcapone as a harmless side‐effect.
Levodopa dose reduction was allowed in all six trials.
Risk of bias in included studies
For more details see Characteristics of Included Studies Table
ENTACAPONE The quality of the reporting of the entacapone trials was such that we had to contact the manufacturer for further details on both methodology and results.
The method of randomisation and concealment of allocation was adequately described in three studies (Brooks UK‐IRISH 2003; Poewe CELOMEN 2002; PSG SEESAW 1997), but five studies did not (Fenelon 2002; Im 2002; Myllyla FILOMEN 2001; Rinne NOMECOMT 1998; Ruottinen 1996 (a)). The majority of the reports were published after the first publication of the CONSORT guidelines in 1996 which recommend the full description of the method of randomisation and concealment of allocation. However it is recognised that two of the studies (Fenelon 2002; Im 2002) were only available as abstracts and so methodological details will necessarily be brief. This emphasises the importance of full publication of trials. As all of the studies were conducted by pharmaceutical companies in accordance with regulatory authorities' guidelines it is likely that the randomisation method and concealment of allocation were of high quality. However an accurate judgement of these methodological quality items cannot be made without the data.
Patients that participated in the trials were stated to have idiopathic Parkinson's disease but no diagnostic criteria were stated in any of the trials. Except for the two studies reported in abstract form (Fenelon 2002; Im 2002), the baseline characteristics were provided for all of the treatment groups and the number of patients who withdrew from the trials were stated along with the reasons for the withdrawal.
Fenelon 2002 and Im 2002 did not state the method used to analyse their data. Ruottinen 1996 (a) analysed the data on a per protocol basis which could have introduced performance and attrition bias. The remaining five trials analysed their data on an intention‐to‐treat basis. However Brooks UK‐IRISH 2003 and Poewe CELOMEN 2002 presented data from only 'observed cases' which means that withdrawals were not accounted for by using the so‐called 'last observation carried forward' method. Myllyla FILOMEN 2001, PSG SEESAW 1997, Rinne NOMECOMT 1998 used the last observation carried forward method which they considered to be a form of intention‐to‐treat analysis. The study by Ruottinen 1996 (a) was of cross‐over design. The results presented were a combination of the two phases of the trial and as such could have been affected by carry‐over effects.
TOLCAPONE All six trials were randomised using computer generated random number tables. A separate randomisation schedule was done for each centre; usually in block sizes twice the number of treatment arms. So if a trial used 10 centres for a study with four treatment arms, 10 sets of codes were created each in blocks of eight, i.e. with two of each treatment in each block of eight. The patients were allocated in a double‐blind method with the code not being broken until after the database was closed.
Patients were always diagnosed with idiopathic Parkinson's disease according to the United Kingdom's Brain Bank Criteria for Parkinson's Disease. The baseline characteristics were provided for all of the treatment groups. The number of patients who withdrew from the trials were stated along with the reasons for the withdrawal.
All of the trials analysed their data using the last observation carried forward method. Four of the trials (Adler TFSGIII 1998; Baas 1997; Dupont TIPSII 1997; Myllyla FILOMEN 2001) provided some or all of their data as least squares estimates of the mean treatment effects in each centre. This was described as "the unweighted averages of centre means with the covariate as its mean value". The nature of the covariate was not stated and was not clarified in our communications with the manufacturer. From the statistical advice we have gained, we believe it reasonable to assume that these least square means were closely equivalent to the true arithmetical means. The data for the results were presented in a manner that could be synthesised (i.e. mean change, standard deviation (SD) of change and patient numbers) in three trials (Adler TFSGIII 1998, Baas 1997, Myllyla TIPS1 1997). Additional data on Kurth TFSGI 1997 were provided by the manufacturer allowing analysis of levodopa dose, on and off times and adverse events. However, only 49 patients participated in the quality of life assessment in Kurth TFSGI 1997; the data for SIP and UPDRS motor scale were presented correctly for meta‐analysis, but the PAIS‐SR was given as baseline and endpoint mean and SD but no SD of change. Rajput 1997 provided the levodopa dose and UPDRS data correctly but 'off' time and Investigators Global Assessment (IGA) had no SD provided.
Effects of interventions
ENTACAPONE We found eight randomised controlled trials examining entacapone against placebo in a total of 1560 patients. Seven of the trials were parallel group design (n=1534), and one small trial was of a cross‐over design (n=26).
The Brooks UK‐IRISH 2003 study examined 300 participants but only 172 had fluctuating symptoms of Parkinson's disease. The study's results discussed below relate only to these 172 participants who met the inclusion criteria for the review. The study by Ruottinen 1996 (a) had a cross‐over design. The published results presented were a combination of the two phases of the trial and as such could have been affected by carry‐over effects. We therefore could not include these results in any meta‐analysis although we do discuss them in relation to the other trials' results.
The trials varied significantly in their duration. Two trials were two months long (Im 2002; Ruottinen 1996 (a)), Fenelon 2002 was three months long, four trials were six months long (Brooks UK‐IRISH 2003; Poewe CELOMEN 2002; PSG SEESAW 1997; Rinne NOMECOMT 1998), and Myllyla FILOMEN 2001 was one year long.
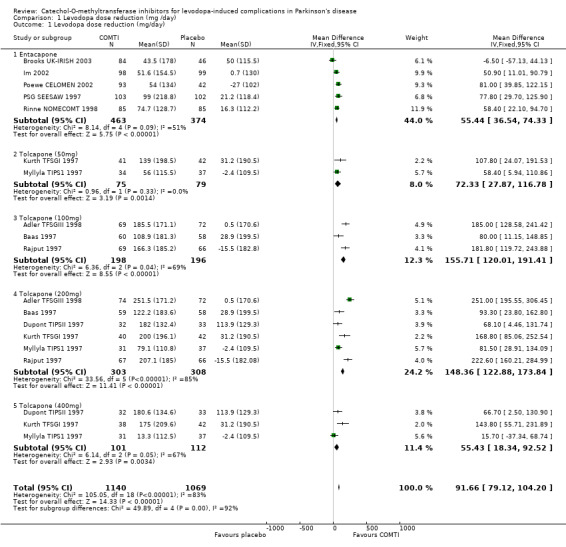
Only Ruottinen 1996 (a) did not allow levodopa dose reduction. Only two trials (Im 2002; Poewe CELOMEN 2002) provided published data in a manner that was amenable to meta‐analysis (i.e. the mean change, standard deviation and numbers of patients). The remaining four trials' data (Brooks UK‐IRISH 2003; PSG SEESAW 1997; Ruottinen 1996 (a); Rinne NOMECOMT 1998) was provided by the drug manufacturers at our request. From these trials the weighted mean difference in levodopa dose reduction between entacapone and placebo was 55 mg/day (95% CI: 37, 74 mg/day, P<0.00001, Figure 1). One trial provided insufficient data to calculate changes in levodopa dose but claimed statistically significant reductions (Myllyla FILOMEN 2001 P<0.01). Fenelon 2002 also claimed a significant difference in levodopa dose between the two groups (P=0.02) but provided no data to substantiate this.
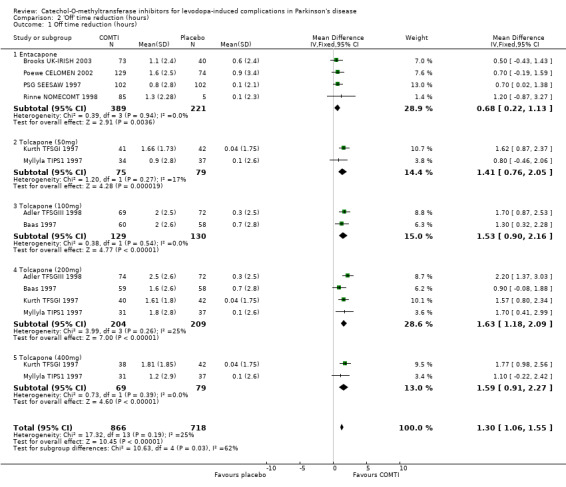
'Off' time reduction data were only published in Poewe CELOMEN 2002 in a manner amenable to meta‐analysis, but additional data was provided by the manufacturers for Brooks UK‐IRISH 2003, Rinne NOMECOMT 1998 and PSG SEESAW 1997. The mean difference was 41 minutes (95% CI: 13 min, 1 hour 8 min, P=0.004, Figure 2). Im 2002 provided the percentage change in 'off' time with its standard deviation, but the length of a 'day' was not stated and so the change in hours could not be calculated. Fenelon 2002 stated that more patients receiving entacapone improved in at least one category of UPDRS item 39 (proportion of day spent off) when compared to those participants on placebo, but the data was not amenable to further analysis. Myllyla FILOMEN 2001 and Ruottinen 1996 (a) did not measure this outcome.
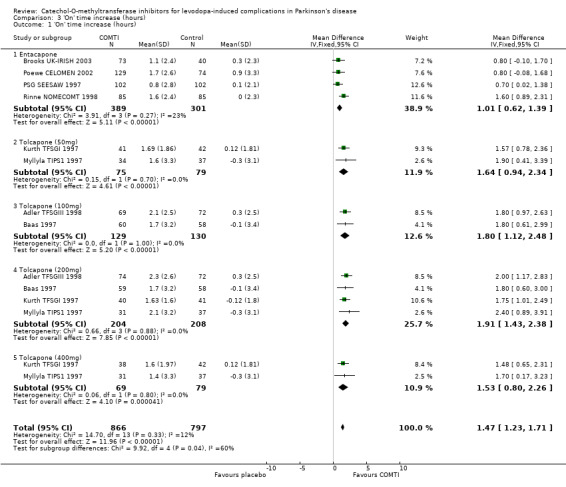
'On' time increase data were only published in Poewe CELOMEN 2002 in a manner amenable to meta‐analysis, but additional data was provided by the manufacturers for Brooks UK‐IRISH 2003, Rinne NOMECOMT 1998 and PSG SEESAW 1997. The mean difference was 1 hour 1 minute (95% CI: 37 min, 1 hour 23 min, P<0.00001, Figure 3). Im 2002 provided the percentage change in 'on' time with its standard deviation, but the length of a 'day' was not stated and so the change in hours could not be calculated. Ruottinen 1996 (a) provided the mean difference in 'on' time (34 minutes, 95% CI: 16 min, 54 min), but the data reported was from the end of the cross‐over trial. The data from the end of the first arm of the trial was not available. The results after the cross‐over could have been influenced by carry‐over effects. Fenelon 2002 provided no data for this outcome and Myllyla FILOMEN 2001 did not measure this outcome.
Sections of the UPDRS were assessed in five trials (Brooks UK‐IRISH 2003; Myllyla FILOMEN 2001; Poewe CELOMEN 2002; PSG SEESAW 1997; Rinne NOMECOMT 1998). Changes in activities of daily living were significant in four trials; Myllyla FILOMEN 2001 P<0.001; Poewe CELOMEN 2002 P<0.01; PSG SEESAW 1997 P=0.03; Rinne NOMECOMT 1998 P<0.01. Changes in the motor section were also significant in four trials; Myllyla FILOMEN 2001 P<0.01; Poewe CELOMEN 2002 P<0.01; PSG SEESAW 1997 P=0.02; Rinne NOMECOMT 1998 P<0.05. However we were unable to calculate accurately the mean difference in either the ADL or Motor sections of the UPDRS in any of these trials from the data provided.
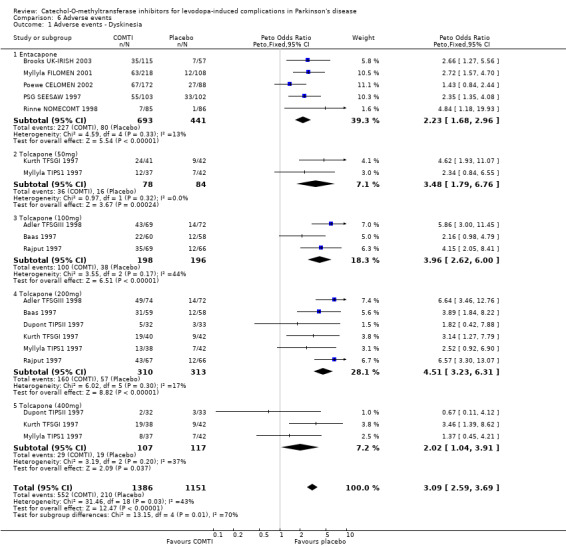
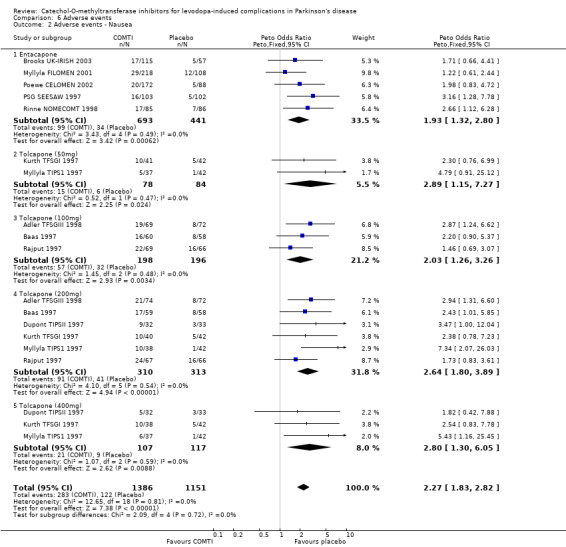
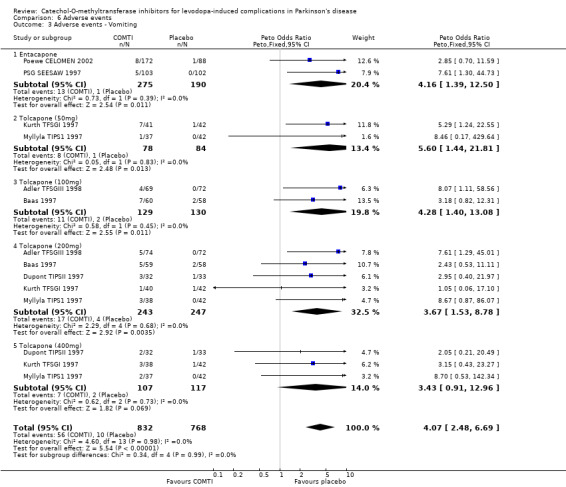
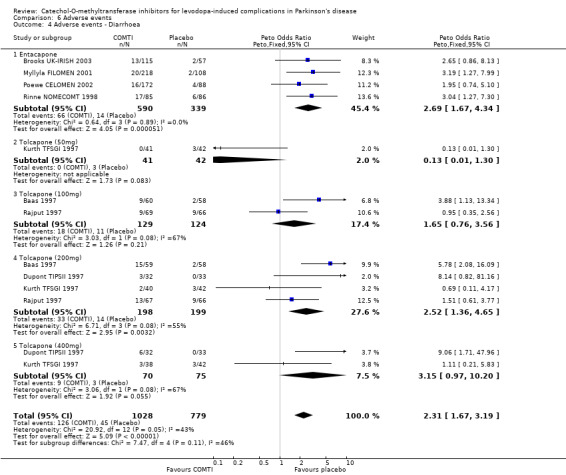
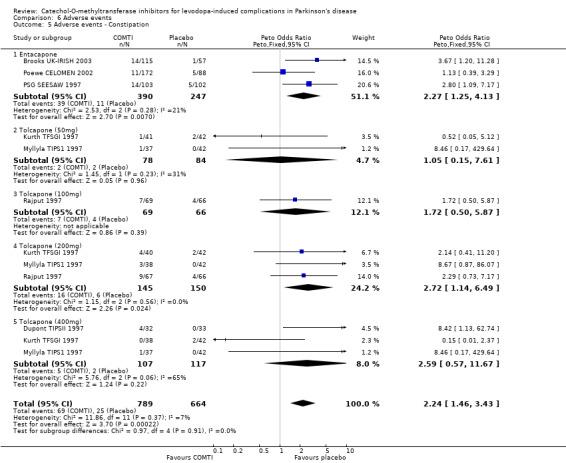
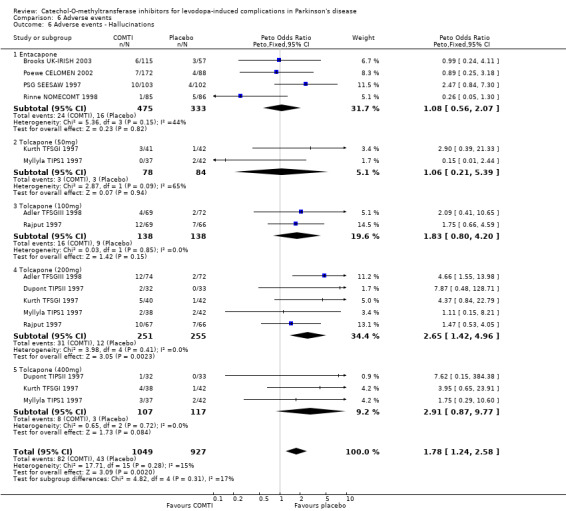
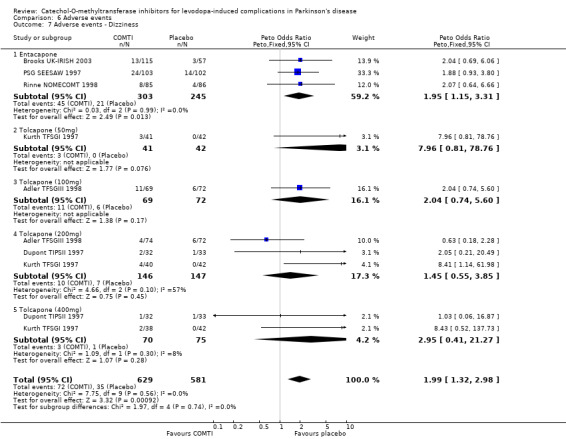
Tables summarising the most common adverse events were not available in the abstracts of Fenelon 2002 and Im 2002. In view of the large number of analyses, particularly of adverse events, we will only comment on those where the P value is smaller than 0.01. Entacapone increased significantly adverse events such as dyskinesia (Peto OR 2.23, P<0.00001, Figure 6.1). Also nausea (Peto OR 1.93, P=0.0006, Figure 6.2), vomiting (Peto OR 4.16, P=0.01, Figure 6.3), diarrhoea (Peto OR 2.69, P=0.0001, Figure 6.4), and constipation (Peto OR 2.27 P=0.007, Figure 6.5). Dizziness was of borderline significance (Peto OR 1.95 P=0.01, Figure 6.7). Hallucinations were not increased on entacapone (Peto OR 1.08, P=0.8, Figure 6.6).
Transaminase levels were considered to be clinically significant if they were raised more than three times beyond the upper limit of the reference range. In Myllyla FILOMEN 2001 one patient on entacapone had a clinically significant rise in alanine transaminase (ALT) level, but as all values before and after were normal it was suspected that there was due to a laboratory error. Another patient had clinically significant elevations in ALT and aspartate transaminase (AST) due to acute cholecystitis unrelated to entacapone treatment. In Poewe CELOMEN 2002 one patient on entacapone and one on placebo had clinically significant rises in their transaminase levels for one visit each.
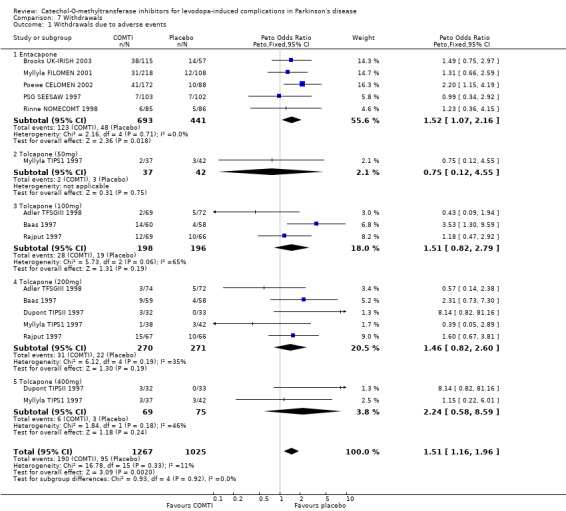
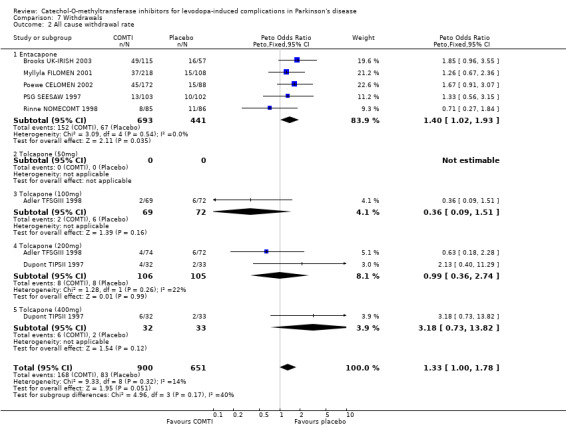
The two trials published as abstracts did not note the withdrawal rate due to adverse events or due to any causes (Fenelon 2002; Im 2002). Overall entacapone increased the likelihood that participants would withdraw due to adverse events (Peto OR 1.52, P=0.02, Figure 7.1) or due to all causes (Peto OR 1.40, P=0.04, Figure 7.2) although the levels of statistical significance were not high.
TOLCAPONE We found six parallel group randomised controlled trials examining tolcapone versus placebo in a total of 1006 patients.
The manufacturer provided us with unpublished methodological details but no additional data. All of the data were from relatively short term trials: four were 6 weeks long (Adler TFSGIII 1998; Dupont TIPSII 1997; Kurth TFSGI 1997; Myllyla TIPS1 1997), and one 3 months long (Baas 1997). Rajput 1997 the primary end point was 3 months when the efficacy data was recorded but patients could continue double‐blind treatment thereafter for up to a total of 12 months. The study was terminated on a common closing date when approximately 30% of patients had completed 1 year of treatment.
The tolcapone trials used a variety of doses of the drug. Three trials examined the efficacy and safety of 100 mg and 200 mg tolcapone (Adler TFSGIII 1998, Baas 1997 and Rajput 1997), two trials 50 mg, 200 mg and 400 mg tolcapone (Kurth TFSGI 1997 and Myllyla TIPS1 1997) and one trial 200 mg and 400 mg tolcapone (Dupont TIPSII 1997). The dose that was common to them all was 200 mg tolcapone. We have included the data on all of the doses of tolcapone from these trials in the Forest plots in Figures 1 to 7. This involves using the placebo data on more than one occasion. This may lead to potential problems if some imbalance had entered the placebo arm of one of the trials (e.g. higher proportion of patients on a large dose of levodopa); this potential bias will be perpetuated in multiple tolcapone dose comparisons. However, such imbalance is unlikely from the information available from the trials. We therefore feel justified in using this approach which has been used by other groups.
Levodopa dose reduction was allowed in all the six trials examined. In Baas 1997 there was an error in the footnote to the table which contained the levodopa dose reduction data. The footnote stated that the data was presented as mean and standard deviation. However this was in fact the standard error of the mean which was confirmed in correspondence with the authors of the study. The weighted mean difference in levodopa dose reduction showed a dose response trend (although this could not be tested further with RevMan software) and a fall off with the highest dose (Figure 1): 50 mg tolcapone produced a levodopa dose reduction of 72 mg/day (95% CI: 27 mg, 117 mg, P=0.001), 100 mg gave a 156 mg/day reduction (95% CI: 120 mg, 191 mg, P=0.00001), 200 mg gave a 148 mg/day reduction (95% CI: 123 mg, 174 mg, P=0.00001) and 400 mg gave a 55 mg/day reduction (95% CI: 18 mg, 93 mg, P=0.003). The analysis of the results for the 200 mg tolcapone dose showed significant heterogeneity (P<0.00001) which could not be resolved by the removal of any one study from the analysis.
'Off' time reduction was available for four trials (Adler TFSGIII 1998, Baas 1997, Kurth TFSGI 1997 and Myllyla TIPS1 1997). Rajput 1997 measured 'off' time but did not provide the standard error of means that would have enabled us to include the results. Dupont TIPSII 1997 did not measure this outcome in their study. Three of the trials used patient completed diaries for 16 hour days (Adler TFSGIII 1998, Baas 1997 and Myllyla TIPS1 1997). Although Kurth TFSGI 1997 also used patient completed diaries, these data were presented without any standard deviations. Therefore we used the investigator's evaluation of 'off' time over a 10 hour day. The weighted mean difference in 'off' time reduction (Figure 2) showed that the 50 mg dose was the least effective (1 hour 25 minutes, 95% CI: 46 min, 2 hours 3 minutes, P=0.00002), whilst the remaining doses were equivalent: 100 mg producing 1 hour 32 minutes off time reduction (95% CI: 54 min, 2 hours 10 minutes, P=0.00001), 200 mg giving 1 hour 38 minutes (95% CI: 1 hour 11 min, 2 hours 5 minutes, P=0.00001) and 400 mg giving 1 hour 35 minutes (95% CI: 55 min, 2 hours 16 minutes, P=0.00001) reduction in 'off' time. None of the analyses showed significant heterogeneity between studies.
'On' time increase was available for the same four trials as 'off' time. Dupont TIPSII 1997 and Rajput 1997 did not measure this outcome in their studies. As before Adler TFSGIII 1998, Baas 1997 and Myllyla TIPS1 1997 used patient completed diaries for 16 hour days whilst Kurth TFSGI 1997 provided the investigator's evaluation of 'off' time over a 10 hour day. Overall the 'on' time increases were similar to the 'off' time reduction (Figure 3): 50 mg tolcapone produced a 1 hour 38 minutes increase in 'on' time ( 95% CI: 56 min, 2 hours 20 minutes, P=0.00001), 100 mg gave 1 hour 48 minutes (95% CI: 1 hour 7 min, 2 hours 29 minutes, P=0.00001), 200 mg gave 1 hour 55 minutes ( 95% CI: 1 hour 26 min, 2 hours 23 minutes, P=0.00001) and 400 mg gave 1 hour 32 minutes (95% CI: 48 min, 2 hours 16 minutes, P=0.00004). None of the analyses showed significant heterogeneity between the studies.
Activities of daily living were assessed using the UPDRS in four studies (Adler TFSGIII 1998, Dupont TIPSII 1997, Myllyla TIPS1 1997 and Rajput 1997). Only Dupont TIPSII 1997 found a statistically significant difference in UPDRS ADL scale (P<0.05) at 200 mg: placebo change increase of 0.4 versus tolcapone 200 mg reduction of 1.1 points. The Motor section of the UPDRS was recorded in five studies (Adler TFSGIII 1998, Baas 1997, Dupont TIPSII 1997, Myllyla TIPS1 1997 and Rajput 1997). Only one study (Baas 1997) found a significant difference (P<0.01) at 200 mg: placebo reduction 2.1 versus tolcapone 200 mg reduction of 6.5 points.
In view of the large number of analyses, particularly of adverse events, we will only comment on those where the P value is small than 0.01. Tolcapone significantly increased the risk of dyskinesia (Figure 6.1) at the 50 mg, 100 mg and 200 mg doses (Peto Odds ratios (P values) of 3.48 (0.0002), 3.96 (0.00001) and 4.51 (0.00001) respectively). Tolcapone significantly increased the risk of nausea (Figure 6.2) at the 100 mg, 200 mg and 400 mg doses (Peto Odds ratios (P values) of 2.03 (0.003), 2.64 (0.00001)and 2.80 (0.009) respectively. Tolcapone produced a borderline increase in the risk of vomiting (Figure 6.3) at the 50 mg, 100 mg and 200 mg doses (Peto Odds ratios (P values) of 5.60 (0.01), 4.28 (0.01) and 3.67 (0.003) respectively). Tolcapone significantly increased the risk of diarrhoea (Figure 6.4) at the 200 mg dose (Peto Odds ratio (P value) 2.52 (0.003)). Tolcapone significantly increased the risk of hallucinations (Figure 6.6) at the 200 mg dose (Peto Odds ratio (P value) 2.65 (0.002)).
The levels of transaminases were considered to be clinically significant if they were raised more than three times beyond the reference range. No changes in liver function tests were reported in four trials (Kurth TFSGI 1997, Dupont TIPSII 1997, Adler TFSGIII 1998, Myllyla TIPS1 1997). In Rajput 1997 tolcapone was associate with raised AST and ALT concentrations in three patients in the tolcapone 100 mg group and two in the 200 mg group, one of whom (from the 200 mg group) withdrew from the study as a result. In this patient, the values were three to five times the upper limit of normal but the patient was asymptomatic. No follow up data on these patients were given. Baas 1997 reported an elevation of mean AST and ALT levels at Week 6 in the tolcapone 200 mg group compared with placebo; this resolved throughout the rest of the trial. However they found three patients (one on 100 mg and two on 200 mg) with consistently abnormal AST and ALT levels. One of these on 200 mg tolcapone withdrew on Day 113 but transaminases were normal by Day 169.
Withdrawals due to adverse events were reported in all six trials. There was no significant increase in withdrawals due to adverse events in those patients on tolcapone (Figure 7.1). Only Adler TFSGIII 1998 and Dupont TIPSII 1997 provided any data on all cause withdrawal rates. There was no significant increase in withdrawals in those patients on tolcapone (Figure 7.2).
Discussion
Eight randomised controlled trials have attempted to establish the efficacy and safety of entacapone versus placebo in Parkinson's disease with motor complications. The total number of randomised patients was 1560. One of these (Ruottinen 1996 (a)) was a small short term phase II study of two months duration. The remaining phase III trials varied significantly in their duration; between two months and one year. Six randomised controlled trials have attempted to establish the efficacy and safety of tolcapone versus placebo in Parkinson's disease with motor complications. The total number of randomised patients was 1006. All of these phase III studies were of relatively short duration, between 6 weeks and 3 months long.
The principle aim of COMT‐inhibitor therapy is to increase the duration of effect of the levodopa dose and thus reduce the time patients spend in the relatively immobile 'off' phase. Entacapone provided a statistically and clinically significant reduction in 'off' time of 41 minutes when compared to placebo at the only dose examined (200 mg with each dose of levodopa). Tolcapone provided a statistically significant reduction in 'off' time when compared to placebo at all of the doses assessed. There appeared to be a dose response effect with the most effective dose (200 mg) producing a clinically significant 1 hour 38 minute reduction in 'off' time. Corresponding increases in 'on' time that were statistically and clinically significant were seen with both agents; entacapone 1 hour 1 minute, tolcapone 1 hour 55 minutes.
Increasing the duration of effect of the levodopa dose can lead to smaller doses of levodopa being required by patients. The entacapone trials showed that treatment with entacapone resulted in a weighted mean difference of 55 mg/day reduction in levodopa which was statistically significant. The trials of tolcapone showed that treatment resulted in statistically significant reductions in levodopa at all the doses assessed. Again there appeared to be a dose response curve with the greatest reduction (156 mg/day) being produced by 100 mg/day tolcapone. The chi‐square test for heterogeneity was highly significant for the 200 mg tolcapone dose. There is nothing obvious in the trial designs which could have produced this and it was not seen with the other doses of tolcapone.
The trials also examined whether adjuvant COMT‐inhibitor therapy could improve motor impairments and disability. Statistical significant improvements in UPDRS ADL and motor scores were demonstrated in four out of the five entacapone trials in which this was measured. It was not possible to perform meta‐analysis of these improvements from the data provided, but it appeared likely that they were of sufficient size to be clinically as well as statistically significant. However improvements in the UPDRS ADL and motor score were seen far less often in the tolcapone trials. Only one out of five studies demonstrated statistically significant improvements at the 200 mg dose.
Thus regarding the comparative efficacy of the two COMT inhibitors, entacapone produced clinically and statistically significant reductions in 'off' time and levodopa dose and improvements in 'on' time. Measures of motor function and activities of daily living were significantly improved in 4 of the 5 studies. Tolcapone produced slightly larger significant reductions in 'off' time and levodopa dose and improvements in 'on' time. However motor function and activities of daily living improved in fewer studies. It would be unwise to draw any conclusions about the relative efficacy of these two COMT inhibitors from this review. Such indirect comparisons from placebo‐controlled trials would be prone to error since the agents were not compared head‐to‐head.
The benefits of COMT inhibitor therapy are produced at the expense of increased adverse events. Some of the adverse events are related to the increased availability of levodopa and it is likely that as soon as the levodopa dose was reduced these symptoms may have improved. As adverse events are reported for the trial as a whole, irrespective of their duration, it is difficult to interpret these results in a clinically useful manner. However the profile of adverse events appears to be similar for both drugs. Both entacapone and tolcapone increased the risk of dyskinesias, nausea and vomiting by similar degrees. Entacapone increased the risk of diarrhoea (Peto odds ratio 2.69) but tolcapone only significantly increased the risk of diarrhoea at the 200 mg dose (Peto odds ratio 2.52). Entacapone increased the risk of constipation and dizziness, whilst tolcapone increased the risk of hallucinations at the 200 mg dose.
Relatively few abnormalities in transaminases were recorded in the tolcapone trials and these appeared to be reversible on drug withdrawal. It was only post‐marketing surveillance that identified three cases of fatal hepatic toxicity which led to the withdrawal of tolcapone's product license in some markets and dose limitations in others (EAEM 1998).
Overall entacapone increased the likelihood that participants would withdraw due to adverse events (Peto OR 1.52, P=0.02) or due to all causes (Peto OR 1.40, P=0.04), whilst patients on tolcapone showed no significant increase in withdrawals due to adverse events or due to all causes. However it should be noted that all cause withdrawal rates were only available for two of the six tolcapone trials.
In summary, entacapone produced reductions in 'off' time and levodopa dose accompanied by a meaningful improvement in motor impairments and disability, but at the cost of increased adverse events such as dyskinesias, nausea, vomiting, diarrhoea, constipation and dizziness. Tolcapone produced a slightly larger reduction in 'off' time and levodopa dose which was occasionally accompanied by measurable functional improvements but at the cost of an increased risk of dyskinesias, nausea, vomiting, diarrhoea and hallucinations.
Authors' conclusions
Implications for practice.
In the management of the motor complications seen in Parkinson's disease, tolcapone and entacapone can be used to reduce off time, reduce levodopa dose, and modestly improve motor impairment and disability. This is based on, at best, medium term evidence. However Phase IV surveillance has raised concerns over the safety of tolcapone with regard to hepatic toxicity.
Implications for research.
We gather that a further study is underway to assess the benefits of tolcapone in patients who have been withdrawn from entacapone. If this presumably non‐randomised study shows significantly greater benefits with tolcapone over entacapone then there may be a case for using tolcapone in spite of the risks of hepatic damage. This type of study cannot replace the more rigorous approach of a randomised controlled trial comparing these drugs.
In the future, adjuvant therapy trials in Parkinson's disease should:‐
Be published in full to avoid publication bias.
Should be reported using the CONSORT guidelines (CONSORT 2001).
Include valid sample size calculations which also take into account safety issues.
Provide full data on outcome measures including mean change and its standard deviation/error.
Express results in the original unit of measurement (hours rather than percentage off time).
Firm diagnostic criteria should be used (e.g. UK Parkinson's Disease Brain Bank Criteria, Gibb 1988).
What's new
| Date | Event | Description |
|---|---|---|
| 13 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 7 June 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Our thanks are due to Orion Pharmaceuticals and Roche Pharmaceuticals for access to unpublished data.
Data and analyses
Comparison 1. Levodopa dose reduction (mg /day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Levodopa dose reduction (mg/day) | 11 | 2209 | Mean Difference (IV, Fixed, 95% CI) | 91.66 [79.12, 104.20] |
| 1.1 Entacapone | 5 | 837 | Mean Difference (IV, Fixed, 95% CI) | 55.44 [36.54, 74.33] |
| 1.2 Tolcapone (50mg) | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | 72.33 [27.87, 116.78] |
| 1.3 Tolcapone (100mg) | 3 | 394 | Mean Difference (IV, Fixed, 95% CI) | 155.71 [120.01, 191.41] |
| 1.4 Tolcapone (200mg) | 6 | 611 | Mean Difference (IV, Fixed, 95% CI) | 148.36 [122.88, 173.84] |
| 1.5 Tolcapone (400mg) | 3 | 213 | Mean Difference (IV, Fixed, 95% CI) | 55.43 [18.34, 92.52] |
1.1. Analysis.
Comparison 1 Levodopa dose reduction (mg /day), Outcome 1 Levodopa dose reduction (mg/day).
Comparison 2. 'Off' time reduction (hours).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Off time reduction (hours) | 8 | 1584 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [1.06, 1.55] |
| 1.1 Entacapone | 4 | 610 | Mean Difference (IV, Fixed, 95% CI) | 0.68 [0.22, 1.13] |
| 1.2 Tolcapone (50mg) | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | 1.41 [0.76, 2.05] |
| 1.3 Tolcapone (100mg) | 2 | 259 | Mean Difference (IV, Fixed, 95% CI) | 1.53 [0.90, 2.16] |
| 1.4 Tolcapone (200mg) | 4 | 413 | Mean Difference (IV, Fixed, 95% CI) | 1.63 [1.18, 2.09] |
| 1.5 Tolcapone (400mg) | 2 | 148 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [0.91, 2.27] |
2.1. Analysis.
Comparison 2 'Off' time reduction (hours), Outcome 1 Off time reduction (hours).
Comparison 3. 'On' time increase (hours).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 'On' time increase (hours) | 8 | 1663 | Mean Difference (IV, Fixed, 95% CI) | 1.47 [1.23, 1.71] |
| 1.1 Entacapone | 4 | 690 | Mean Difference (IV, Fixed, 95% CI) | 1.01 [0.62, 1.39] |
| 1.2 Tolcapone (50mg) | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | 1.64 [0.94, 2.34] |
| 1.3 Tolcapone (100mg) | 2 | 259 | Mean Difference (IV, Fixed, 95% CI) | 1.8 [1.12, 2.48] |
| 1.4 Tolcapone (200mg) | 4 | 412 | Mean Difference (IV, Fixed, 95% CI) | 1.91 [1.43, 2.38] |
| 1.5 Tolcapone (400mg) | 2 | 148 | Mean Difference (IV, Fixed, 95% CI) | 1.53 [0.80, 2.26] |
3.1. Analysis.
Comparison 3 'On' time increase (hours), Outcome 1 'On' time increase (hours).
Comparison 4. UPDRS ADL section (Part II).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 UPDRS ADL section (Part II) | Other data | No numeric data | ||
| 1.1 Entacapone | Other data | No numeric data | ||
| 1.2 Tolcapone (all doses) | Other data | No numeric data |
4.1. Analysis.
Comparison 4 UPDRS ADL section (Part II), Outcome 1 UPDRS ADL section (Part II).
| UPDRS ADL section (Part II) | ||||||
|---|---|---|---|---|---|---|
| Study | Placebo | Entacapone | Tolcapone (50mg) | Tolcapone (100mg) | Tolcapone (200mg) | Tolcapone (400mg) |
| Entacapone | ||||||
| Brooks UK‐IRISH 2003 | Placebo n = 57 | Entacapone n = 115; not significant | ||||
| Myllyla FILOMEN 2001 | 'On' state. Placebo n = 93 | Entacapone n = 182, P<0.001 | ||||
| Poewe CELOMEN 2002 | 'On' state. Placebo n = 88 | Entacapone n= 172, P<0.05 (95% CI ‐2.46; ‐0.02) | ||||
| PSG SEESAW 1997 | 'On' state. Placebo n= 102 | Entacapone n=103; P=0.03 | ||||
| Rinne NOMECOMT 1998 | Placebo n = 86 | Entacapone n = 85; P<0.01 | ||||
| Tolcapone (all doses) | ||||||
| Adler TFSGIII 1998 | Placebo n=72, least‐squares mean = ‐0.7 (SD 3.4) | Tolcapone 100mg n=69, least squares mean = ‐0.4 (SD 3.3); not significant | Tolcapone 200mg n=74, least‐squares mean =‐0.5 (SD 3.4); not significant | |||
| Baas 1997 | Placebo n=58 (no data available) | Tolcapone 100mg n=60 (no data available); not significant | Tolcapone 200mg n=59 (no data available); not significant | |||
| Dupont TIPSII 1997 | 'On' state. Placebo least‐squares mean = 0.4 (SD 2.3) | Tolcapone 200mg n=32 least‐squares mean = 1.1 (SD 2.3); p<0.05 | Tolcapone 400mg n=32, least square mean = 0.1 (SD 2.3); not significant | |||
| Myllyla TIPS1 1997 | 'On' state. Placebo least‐squares mean = ‐0.8 (SD 3.0) | Tolcapone 50mg n=34, least squares mean = ‐0.6 (SD 2.9); not significant | Tolcapone 200mg n=31 least‐squares mean = ‐1.4 (SD 2.8); not significant | Tolcapone 400mg n=31, least squares mean = ‐1.5 (SD 2.8); not significant | ||
| Rajput 1997 | 'On' state. Placebo n=66, mean = ‐0.3 (SD 4.1) | Tolcapone 100mg n=69, mean =‐0.8 (SD 3.3); not significant | Tolcapone 200mg n=67 mean = 0.2 (SD 3.3); not significant | |||
Comparison 5. UPDRS Motor section (Part III).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 UPDRS Motor section (Part III) | Other data | No numeric data | ||
| 1.1 Entacapone | Other data | No numeric data | ||
| 1.2 Tolcapone (all doses) | Other data | No numeric data |
5.1. Analysis.
Comparison 5 UPDRS Motor section (Part III), Outcome 1 UPDRS Motor section (Part III).
| UPDRS Motor section (Part III) | ||||||
|---|---|---|---|---|---|---|
| Study | Placebo | Entacapone | Tolcapone (50mg) | Tolcapone (100mg) | Tolcapone (200mg) | Tolcapone (400mg) |
| Entacapone | ||||||
| Brooks UK‐IRISH 2003 | 'On' state. Placebo n = 57 | Entacapone n = 115; not significant | ||||
| Myllyla FILOMEN 2001 | 'On' state. Placebo n = 108 | Entacapone n = 218; P<0.01 | ||||
| Poewe CELOMEN 2002 | 'On' state. Placebo n = 88 | Entacapone n = 172; P<0.01 (95% CI ‐5.13; ‐0.46) | ||||
| PSG SEESAW 1997 | 'On' state. Placebo n = 102 | Entacapone n = 103; P=0.02 | ||||
| Rinne NOMECOMT 1998 | Placebo n = 86 | Entacapone n = 85; P<0.05 | ||||
| Tolcapone (all doses) | ||||||
| Adler TFSGIII 1998 | 'On' state. Placebo n=72, least‐squares mean = ‐1.2 (SD 5.9) | Tolcapone 100mg n=69, least‐squares mean = ‐2.3 (SD 5.8); not significant | Tolcapone 200mg n=74 least‐squares mean = ‐2.4 (SD 6.0); not significant | |||
| Baas 1997 | 'On' state. Placebo n=58, least‐squares mean = ‐2.1 (SD 1.1) | Tolcapone 100mg n=60, least‐squares mean = ‐4.2 (SD1.0); not significant | Tolcapone 200mg n=59, least‐squares mean = ‐6.5 (SD 1.0); P<0.01 | |||
| Dupont TIPSII 1997 | Placebo n=33, least‐squares mean = ‐1.5 (SD 7.5) | Tolcapone 200mg n=32, least‐squares mean = ‐1.0 (SD 7.4); not significant | Tolcapone 400mg n=32, least squares mean = ‐1.0 (SD 7.9); not significant | |||
| Myllyla TIPS1 1997 | 'On' state. Placebo n=37, least‐squares mean = ‐0.3 (SD 10.3) | Tolcapone 50mg n=34, least squares mean ‐2.6 (SD 11.1); not significant | Tolcapone 200mg n=31, least‐squares mean ‐5.8 (SD 10.6); not significant | Tolcapone 400mg n=31, least squares mean = ‐3.7 (SD 11.1); not significant | ||
| Rajput 1997 | 'On' state. Placebo n=66, mean ‐0.4 (SD 7.3) | Tolcapone 100mg n=69, mean ‐1.9 (SD 7.5); not significant | Tolcapone 200mg n=67, mean ‐2.0 (SD 7.4); not significant | |||
Comparison 6. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events ‐ Dyskinesia | 11 | 2537 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.09 [2.59, 3.69] |
| 1.1 Entacapone | 5 | 1134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.23 [1.68, 2.96] |
| 1.2 Tolcapone (50mg) | 2 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.48 [1.79, 6.76] |
| 1.3 Tolcapone (100mg) | 3 | 394 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.96 [2.62, 6.00] |
| 1.4 Tolcapone (200mg) | 6 | 623 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.51 [3.23, 6.31] |
| 1.5 Tolcapone (400mg) | 3 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.02 [1.04, 3.91] |
| 2 Adverse events ‐ Nausea | 11 | 2537 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.27 [1.83, 2.82] |
| 2.1 Entacapone | 5 | 1134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.93 [1.32, 2.80] |
| 2.2 Tolcapone (50mg) | 2 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.89 [1.15, 7.27] |
| 2.3 Tolcapone (100mg) | 3 | 394 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.03 [1.26, 3.26] |
| 2.4 Tolcapone (200mg) | 6 | 623 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.64 [1.80, 3.89] |
| 2.5 Tolcapone (400mg) | 3 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.80 [1.30, 6.05] |
| 3 Adverse events ‐ Vomiting | 7 | 1600 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.07 [2.48, 6.69] |
| 3.1 Entacapone | 2 | 465 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.16 [1.39, 12.50] |
| 3.2 Tolcapone (50mg) | 2 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.60 [1.44, 21.81] |
| 3.3 Tolcapone (100mg) | 2 | 259 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.28 [1.40, 13.08] |
| 3.4 Tolcapone (200mg) | 5 | 490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.67 [1.53, 8.78] |
| 3.5 Tolcapone (400mg) | 3 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.43 [0.91, 12.96] |
| 4 Adverse events ‐ Diarrhoea | 8 | 1807 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.31 [1.67, 3.19] |
| 4.1 Entacapone | 4 | 929 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.69 [1.67, 4.34] |
| 4.2 Tolcapone (50mg) | 1 | 83 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 1.30] |
| 4.3 Tolcapone (100mg) | 2 | 253 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [0.76, 3.56] |
| 4.4 Tolcapone (200mg) | 4 | 397 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.52 [1.36, 4.65] |
| 4.5 Tolcapone (400mg) | 2 | 145 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.15 [0.97, 10.20] |
| 5 Adverse events ‐ Constipation | 7 | 1453 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.24 [1.46, 3.43] |
| 5.1 Entacapone | 3 | 637 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.27 [1.25, 4.13] |
| 5.2 Tolcapone (50mg) | 2 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.15, 7.61] |
| 5.3 Tolcapone (100mg) | 1 | 135 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [0.50, 5.87] |
| 5.4 Tolcapone (200mg) | 3 | 295 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.72 [1.14, 6.49] |
| 5.5 Tolcapone (400mg) | 3 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.59 [0.57, 11.67] |
| 6 Adverse events ‐ Hallucinations | 9 | 1976 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [1.24, 2.58] |
| 6.1 Entacapone | 4 | 808 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.56, 2.07] |
| 6.2 Tolcapone (50mg) | 2 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.21, 5.39] |
| 6.3 Tolcapone (100mg) | 2 | 276 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.83 [0.80, 4.20] |
| 6.4 Tolcapone (200mg) | 5 | 506 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.65 [1.42, 4.96] |
| 6.5 Tolcapone (400mg) | 3 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.91 [0.87, 9.77] |
| 7 Adverse events ‐ Dizziness | 6 | 1210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.99 [1.32, 2.98] |
| 7.1 Entacapone | 3 | 548 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [1.15, 3.31] |
| 7.2 Tolcapone (50mg) | 1 | 83 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.96 [0.81, 78.76] |
| 7.3 Tolcapone (100mg) | 1 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.04 [0.74, 5.60] |
| 7.4 Tolcapone (200mg) | 3 | 293 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [0.55, 3.85] |
| 7.5 Tolcapone (400mg) | 2 | 145 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.95 [0.41, 21.27] |
6.1. Analysis.
Comparison 6 Adverse events, Outcome 1 Adverse events ‐ Dyskinesia.
6.2. Analysis.
Comparison 6 Adverse events, Outcome 2 Adverse events ‐ Nausea.
6.3. Analysis.
Comparison 6 Adverse events, Outcome 3 Adverse events ‐ Vomiting.
6.4. Analysis.
Comparison 6 Adverse events, Outcome 4 Adverse events ‐ Diarrhoea.
6.5. Analysis.
Comparison 6 Adverse events, Outcome 5 Adverse events ‐ Constipation.
6.6. Analysis.
Comparison 6 Adverse events, Outcome 6 Adverse events ‐ Hallucinations.
6.7. Analysis.
Comparison 6 Adverse events, Outcome 7 Adverse events ‐ Dizziness.
Comparison 7. Withdrawals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawals due to adverse events | 10 | 2292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.51 [1.16, 1.96] |
| 1.1 Entacapone | 5 | 1134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [1.07, 2.16] |
| 1.2 Tolcapone (50mg) | 1 | 79 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.12, 4.55] |
| 1.3 Tolcapone (100mg) | 3 | 394 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.51 [0.82, 2.79] |
| 1.4 Tolcapone (200mg) | 5 | 541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.82, 2.60] |
| 1.5 Tolcapone (400mg) | 2 | 144 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.24 [0.58, 8.59] |
| 2 All cause withdrawal rate | 7 | 1551 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [1.00, 1.78] |
| 2.1 Entacapone | 5 | 1134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [1.02, 1.93] |
| 2.2 Tolcapone (50mg) | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Tolcapone (100mg) | 1 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.09, 1.51] |
| 2.4 Tolcapone (200mg) | 2 | 211 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.36, 2.74] |
| 2.5 Tolcapone (400mg) | 1 | 65 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.18 [0.73, 13.82] |
7.1. Analysis.
Comparison 7 Withdrawals, Outcome 1 Withdrawals due to adverse events.
7.2. Analysis.
Comparison 7 Withdrawals, Outcome 2 All cause withdrawal rate.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adler TFSGIII 1998.
| Methods | Randomised double‐blind parallel group study. Patient randomisation numbers were generated in blocks of 6 for each centre by the sponsor's drug‐packaging department and incorporated into double‐blind labelling. These randomisation numbers were then assigned sequentially in each centre in the order in which the patients were enrolled. 15 centres, USA. Intention‐to‐treat analysis. Duration: 6 weeks | |
| Participants | 215 patients, 12 withdrew. 6 withdrew from placebo group, 5 due to adverse effects. 2 withdrew from the 100 mg entacapone group both due to adverse effects. 4 withdrew from 200 mg entacapone, 3 due to adverse events. 149 males. Mean 62 years, mean duration of disease 10.5 years. Mean H&Y can be calculated. Inclusion criteria: Patients were at least 30 years old, had at least 2 of the cardinal signs of idiopathic PD, had been treated with levodopa‐carbidopa for at least one year with clear clinical improvement. They were required to be taking at least 4 daily doses of levodopa‐carbidopa, or 3 doses if at least 2 were controlled release, and to show predictable end‐of‐dose wearing off that could not be eliminated by adjusting their existing antiparkinsonian medications. They had to be able to keep reliable diaries of 'off' and 'on' times. The minimal acceptable dose of carbidopa was 20 mg with each dose of levodopa or a total daily dose of 70 mg. Medications must have been stable 4 weeks prior to randomisation. Women had to be sterile or using effective contraception Exclusion criteria: Nonidiopathic Parkinson's disease or parkinsonian variants, sudden and unpredictable off/on fluctuations, or a diphasic pattern of dyskinesias. Treatment with centrally acting dopamine antagonists or MAO inhibitors (other than selegiline) within previous 2 months, drug or alcohol abuse within previous 2 years, psychotic illness or major depression within last 6 months, and any other clinically significant medical or neurological abnormalities. | |
| Interventions | Patients received 200 mg tid tolcapone (n=74), 100 mg tid tolcapone (n=69) or placebo tid (n=72). The first dose was given with the first dose of levodopa‐carbidopa (either immediate‐release or controlled‐release formulations). The second and third doses were taken at 6‐hour intervals thereafter. Patients could also receive a stable regime of dopamine agonists, selegiline or other antiparkinsonian drugs. | |
| Outcomes | Primary outcome: 'On' and 'Off' times Secondary outcomes: levodopa dose number of levodopa doses UPDRS ADL UPDRS motor (on) UPDRS total SIP physical SIP psychosocial IGA ‐ wearing off IGA ‐ symptom severity IGA ‐ overall efficacy Adverse events | |
| Notes | TOLCAPONE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Baas 1997.
| Methods | Randomised double‐blind parallel group study. Randomised by computer generated random number tables with the participants being double‐blinded and the code not being broken until after the database was closed. Data analysis unclear 24 centres in Europe. Duration: 3 months | |
| Participants | 177 patients, 27 withdrew. 4 patients withdrew from placebo group, 14 from the 100 mg tolcapone group and 9 from the 200 mg tolcapone group all because of adverse effects. 99 men (56%), man age 63 years, mean duration of PD 9.8 years, mean levodopa dose 668 mg/day. Inclusion criteria: At least 30 years old at onset of symptoms, treated with levodopa for at least one year, responded to levodopa treatment, on a stable regime of levodopa/benserazide and any other antiparkinsonian drugs for 4 weeks. Women were either post menopausal or using a reliable method of contraception. Exclusion criteria: Non‐idiopathic parkinsonism, patients with sudden, unpredictable 'on/off' fluctuations or diphasic dyskinesia. MMSE score of 25 or less. Patients who had had a major depressive episode in the preceding 6 months, had had neurosurgery in previous year, or had any psychiatric or medical condition that might interfere with assessments. Treatment with a centrally acting dopamine antagonist during previous 6 months, a monoamine oxidase inhibitor (except selegiline) in the preceding 2 months, apomorphine in the preceding 7 months, or any investigational agent within the preceding 4 weeks. | |
| Interventions | Patients received 200 mg tid tolcapone (n=59), 100 mg tid tolcapone (n=60) or placebo tid (n=58). Riboflavin (0.5 mg) was included in the placebo tablets to mimic the yellow discolouration in urine that occurs with tolcapone as a harmless side‐effect. The first daily doe of tolcapone or placebo was taken with the first dose of levodopa, the remaining two doses were taken at six hourly intervals thereafter. | |
| Outcomes | 'Off' time 'On' time Levodopa dose UPDRS motor UPDRS total SIP Adverse events | |
| Notes | TOLCAPONE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Brooks UK‐IRISH 2003.
| Methods | Randomised double‐blind parallel group study. The patients were stratified into two groups fluctuators and non‐fluctuators and randomised in a 2:1 ratio to entacapone or placebo using a computerised method. Multi‐centred, UK and Ireland. Intention‐to‐treat analysis. Duration: 6 months | |
| Participants | 300 patients, 172 fluctuators, 128 non‐fluctuators. 49 receiving entacapone withdrew (24%), 38 due to adverse events, 16 receiving placebo withdrew (17%), 14 due to adverse events. In the fluctuating patients, 109 were male (63%), average age 65.3 years, average duration of PD 9.4 years, average levodopa dose 697 mg/day. Inclusion criteria: Patients aged 30‐80 years, with levodopa responsive idiopathic fluctuating or non‐fluctuating PD, who would benefit from levodopa enhancement. Number of levodopa doses could vary from 2‐10 daily. | |
| Interventions | Patients received 200 mg entacapone (n=203) or placebo (n=97) with each levodopa dose. Both standard and controlled‐release levodopa preparations with either of the DDC inhibitors and any other concomitant antiparkinsonian medication were allowed. | |
| Outcomes | 'On' time UPDRS (all parts) Levodopa dose Adverse events | |
| Notes | ENTACAPONE Fluctuating and non‐fluctuating patients but the data is segregated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dupont TIPSII 1997.
| Methods | Randomised double‐blind parallel group study. Randomised by computer generated random number tables with the participants being double‐blinded and the code not being broken until after the database was closed. Multi‐centred ‐ 15 centres in 7 countries. Intention‐to‐treat data analysis. Duration: 6 week parallel group design followed by 3 weeks where the tolcapone groups were crossed over for exploratory purposes. | |
| Participants | 97 patients, 12 patients withdrew, 6 for adverse events (three in each of the tolcapone groups), the remainder withdrew for other reasons (2 from placebo, 1 in the 200 mg group, 3 in the 400 mg group). 62 men. Mean age 66 years, mean H&Y 'on' 2.2, 'off' 2.5, Mean levodopa dose 662 mg/day. Inclusion criteria: Patients with moderately advanced PD whose wearing‐off of levodopa effects had been successfully controlled by relatively frequent dosage. | |
| Interventions | Patients received 200 mg tid tolcapone (n=32), 400 mg tid tolcapone (n=32) or placebo (n=33). Riboflavin (0.5 mg) was included in the placebo tablets to mimic the yellow discolouration in urine that occurs with tolcapone as a harmless side‐effect. Selegiline and apomorphine were not permitted. Concomitant use of other types of antiparkinsonian medications was allowed if the dosage regime had been stable for 2 months before study entry and remained unchanged throughout the study. Nonselective MAOI's were prohibited during the study. High protein‐binding drugs were avoided. | |
| Outcomes | Levodopa dose Number of doses IGA motor signs severity UPDRS motor UPDRS ADL (on) UPDRS mood Adverse events | |
| Notes | TOLCAPONE Cross‐over 200‐400 mg ‐ weeks 6‐9. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Fenelon 2002.
| Methods | Randomised double‐blind parallel group study. Method of randomisation not stated. Patients were randomised according to a 3:2 ratio, entacapone: placebo. Multi‐centred, France, Spain & Switzerland. Description of data analysis not stated. Duration: 3 months. | |
| Participants | 162 patients, no withdrawals noted. No baseline characteristics available. Inclusion criteria: PD patients showing end‐of‐dose deterioration whilst on L‐Dopa and dopamine agonist therapy. | |
| Interventions | Patients received 200 mg entacapone (n=99) or placebo (n=63) with each dose of levodopa. | |
| Outcomes | 'On' time 'Off' time (measured with patient diaries and item 39 of UPDRS) Investigators global assessment SF‐36 Change in L‐Dopa dosage Adverse events | |
| Notes | ENTACAPONE Abstract only ‐ summary results. Levodopa + dopamine agonist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Im 2002.
| Methods | Randomised double‐blind parallel group study. Method of randomisation not stated. Multi‐centred, South Korea. Method of data analysis not stated. Duration: 2 months | |
| Participants | 197 patients, no withdrawals noted. No baseline characteristics available. Inclusion criteria: PD patients experiencing end‐of‐dose deterioration. | |
| Interventions | Patients recieved entacapone (dose not given; n=98) or placebo (n=99) as an adjunct to levodopa (with each dose?). | |
| Outcomes | 'On' time 'Off' time Levodopa dose Total UPDRS UPDRS motor CGI Adverse events | |
| Notes | ENTACAPONE Abstract only Dose not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kurth TFSGI 1997.
| Methods | Randomised double‐blind parallel group study. Randomised by computer generated random number tables with the participants being double‐blinded and the code not being broken until after the database was closed. Intention‐to‐treat data analysis of those that had taken at least one dose and were assessed at baseline and 6 weeks. Multi‐centre, 12 sites Duration: 6 weeks | |
| Participants | 161 patients (151 analysed), 5 patients withdrew due to adverse effects. 105 participants were male (65%), mean age 64.5 years, mean disease duration 9.2 years, mean Hoehn and Yahr 'on' 2.15, 'off' 2.81. No baseline mean levodopa dose was stated. Inclusion criteria: Patients with idiopathic PD occurring after age 30, experiencing predictable 'on' response to the first morning dose of levodopa/carbidopa, with at least two episodes of predictable end‐of‐dose 'off' periods. Total 'off' time while awake had to exceed 2 hours per day. Patients were on a stable regime of at least 3 doses per day of standard levodopa/carbidopa. Exclusion criteria: Unpredictable motor fluctuations. Treatment with dopamine agonists, amantadine, anticholinergics, selegiline, carbidopa or levodopa alone, Sinemet CR, Sinemet 1:10 ratio and agents used to treat tremor (primidone, beta‐blockers) was not allowed. | |
| Interventions | Patients received placebo (n=42), 50 mg tid tolcapone (n=41), 200 mg tid tolcapone (n=40), 400 mg tid tolcapone (n=38). Riboflavin (0.5 mg) was included in the placebo tablets to mimic the yellow discolouration in urine that occurs with tolcapone as a harmless side‐effect. No other antiparkinsonian medications were allowed. | |
| Outcomes | UPDRS motor 'Off' time 'On' time 'On' time with dyskinesia Levodopa dose Number of levodopa doses Adverse events UPDRS mentation UPDRS ADL IGA (Welsh QOL paper n=49 SIP PAIS‐SR UPDRS H&Y) | |
| Notes | TOLCAPONE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Myllyla FILOMEN 2001.
| Methods | Randomised double‐blind parallel group study. Method of randomisation not described. Patients were stratified into those recieving 2‐4 or 5‐10 daily doses of levodopa/ DDC inhibitor. They were then randomised in a 2:1 ratio, entacapone: placebo. Intention‐to‐treat analysis. Duration: 12 months | |
| Participants | 326 patients, 37 withdrew from the entacapone group and 15 from the placebo group. 66% male, 34% female. Mean age 56.5 years old. Mean duration of PD 6.1 years. Mean levodopa dose 634 mg. Inclusion criteria: Outpatients aged 30‐80 years, with levodopa‐responsive idiopathic PD, needing enhancement and/or smoothing of levodopa effects. Exclusion criteria: Using apomorphine. Secondary parkinsonism, dementia or other significant neurological disease that could interfere with the evaluation. Patients with major psychiatric disorders, such as depression, or other clinically unstable major concurrent illnesses. Patients treated with neuroleptic agents in previous 6 months or with alpha‐methyldopa or reserpine within the previous month. | |
| Interventions | Patients received 200 mg entacapone (n=218) or placebo (n=108) with each levodopa dose. The use of any levodopa preparation, and all other anti‐Parkinsonian treatments except apomorphine were allowed. Concurrent treatment with catechol‐structured drugs was prohibited. Treatment with MAO‐A inhibitors and/or non‐selective MAO inhibitors within one month prior to the initiation of the study medication was prohibited. | |
| Outcomes | Primary outcome: Safety i.e. Adverse events Frequency of elevated liver transaminases. Secondary outcomes: Levodopa dose Interval between 1st 2 morning l‐dopa doses UPDRS motor UPDRS ADL UPDRS mental UPDRS total | |
| Notes | ENTACAPONE Fluctuating and non‐fluctuating patients, fluctuation not defined, results not separated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Myllyla TIPS1 1997.
| Methods | Randomised double‐blind parallel group study. Randomised by computer generated random number tables with the participants being double‐blinded and the code not being broken until after the database was closed. 22 centres in Europe and Australia Intention‐to‐treat data analysis Duration: 6 weeks | |
| Participants | 154 patients, 10 withdrawals due to adverse effects. 3 from placebo group, 2 from 50 mg tid tolcapone group, 1 from 200 mg tid tolcapone group and 4 from 400 mg tid tolcapone group. 95 men. Mean age 63 years, mean disease duration 11 years, mean levodopa dose 734 mg/day. Inclusion criteria: Patients aged 40 or more with clinically idiopathic PD, and presented the 'wearing‐off' phenomenon despite 'optimal' antiparkinsonian therapy, with 'off' time comprising more than 25% of the waking day, despite at least 5 doses of levodopa. Have used levodopa for at least 2 years and have reached or almost reached the threshold of tolerability as shown by mild‐to‐moderate dyskinesia or newly occurring dyskinesia after slight increase in levodopa dose. Patients must have been receiving a stable dose of a standard levodopa/decarboxylase inhibitor formulation, in a 4:1 ratio, for at least 2 months prior to enrolment, although a bedtime dose of slow‐release formulation was permitted. Disease severity of no more than 3 on H&Y scale. Women had to have been amenorrhoeic for at least 1 year or surgically sterile for at least 6 months. Patients were required to keep reliable 'on/off' charts. Exclusion criteria: Non‐idiopathic parkinsonism, predominately trembling symptomatology, unpredictable motor fluctuations, or diphasic dyskinesia in response to levodopa. Patients treated with levodopa alone, levodopa/carbidopa 10:1 ratio, levodopa in a controlled release formulation during the day, a total daily dose of greater than 1200 mg levodopa, fewer than 5 or more than 8 daily intakes. Treatment with neuroleptics, antidepressants (except low‐dose tricyclic antidepressants at bedtime), selegiline, or any investigational drug within preceding 2 months, apomorphine in the preceding week, antiemetics, high protein binding drugs(>90%), or moderately high protein‐binding drugs with a narrow therapeutic range. Patients with unstable medical problems, significant organic disease or related treatments, a history of alcoholism or drug abuse, or evidence of previous myocardial infarction, arrhythmia, or conductance defects on electrocardiography. | |
| Interventions | Patients received placebo (n=42), 50 mg tid tolcapone (n=37), 200 mg tid tolcapone (n=38), 400 mg tid tolcapone. Riboflavin (0.5 mg) was included in the placebo tablets to mimic the yellow discolouration in urine that occurs with tolcapone as a harmless side‐effect. | |
| Outcomes | 'On' time 'Off' time levodopa dose IGA ‐ wearing off phenomenon IGA ‐ symptom severity UPDRS mood UPDRS ADL UPDRS motor Adverse effects | |
| Notes | TOLCAPONE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Poewe CELOMEN 2002.
| Methods | Randomised double‐blind parallel group study. The computer‐generated randomisation procedure was performed separately for each centre. Only the sponsor‐employed person who generated the plan was aware of a given individual's assignment during the study. The randomisation envelopes were printed in quadruplicate: one for the investigator, one for the monitor, one for the clinical research manager of the sponsor and one for the Safety Monitoring Committee. Intention‐to‐treat analysis. Multi‐centred, 30 centres in Germany & Austria. Duration: 6 months | |
| Participants | 301 patients, 63 patients withdrew. 15 withdrew from the placebo arm, 10 due to adverse events , 5 due to other reasons. 48 patients withdrew from the entacapone arm, 41 due to adverse events, 7 due to other reasons. 44% male. Mean age 61 years, mean duration of PD 8.9 years, mean levodopa dose 571 mg/day. Inclusion criteria: levodopa responsive patients with idiopathic PD who needed enhancement and/or smoothening of levodopa effects; aged 30‐80 years; use 2‐10 daily doses of standard and/or CR levodopa preparations; stable levodopa treatment for 1 month before entering study. Exclusion criteria: treatment with neuroleptics, neuroleptic antiemetics, catechol‐structured drugs, MAO‐A inhibitors or non‐selective MAO inhibitors. Patients with other major neurological, psychiatric or medical disorders. | |
| Interventions | Patients received 200 mg entacapone (n=197; 172 fluctuating) or placebo (n=104; 88 fluctuating) with each levodopa dose. Patients treated with amantadine, memantine, anticholinergics, selegiline or dopamine agonists in addition to levodopa were allowed. | |
| Outcomes | UPDRS total (on) levodopa dose number of daily doses 'on' time 'off' time Adverse effects blood pressure heart rate | |
| Notes | ENTACAPONE Fluctuating & non‐fluctuating patients but results segregated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
PSG SEESAW 1997.
| Methods | Randomised double‐blind parallel group study. The computer‐generated randomisation scheme included stratification (by centre) and blocking (block size = 4). Those randomised to recieve entacapone were further randomised to recieve either 24 or 26 weeks of active therapy followed respectively by either 4 or 2 weeks of placebo. Multi‐centred, Finland. Intention‐to‐treat analysis. Duration: 24 weeks. | |
| Participants | 205 patients, 23 patients withdrew. 10 patients in placebo group withdrew, 7 due to adverse events, 3 due to other reasons. 13 patients withdrew from the entacapone group, 7 due to adverse events and 6 due to other reasons. 64.9% male. Mean age 63.3 years. Disease duration 11.1 years. Mean years since motor fluctuations 4.4 years. Mean Modified Hoehn & Yahr stage 2.4 (SD 0.6). Mean levodopa dose 772 mg/day. Inclusion criteria: Patients with idiopathic PD in (modified) Hoehn & Yahr stage 1.5 to 4 (when in an 'off' state), who were responsive to levodopa, who had motor fluctuations paralleling their levodopa dosing, and who were taking a stable regime of 4‐10 daily doses of carbidopa/levodopa. Exclusion criteria: Patients with atypical or secondary parkinsonism, pronounced dementia, or other significant neurologic disease, major psychiatric disorders such as severe depression, or clinically severe or unstable systemic illness. Treated within 6 months with neuroleptic agents, or within one month with alpha‐methyldopa or reserpine. | |
| Interventions | Patients received 200 mg entacapone (n= 103) or placebo (n=102) with each levodopa dose. Patients were allowed to continue with amantadine, anticholinergics, selegiline or dopamine agonists at a constant dosage in addition to their levodopa doses. Patients were not allowed to receive controlled‐release formulations of carbidopa/levodopa. Concurrent treatment with catechol‐structured drugs was prohibited. | |
| Outcomes | Primary outcome: % 'on' time Secondary outcomes: % 'on' time while awake in morning, afternoon, and evening % asleep time levodopa dose number of levodopa dose failures UPDRS total UPDRS mental UPDRS motor UPDRS ADL Global evaluation | |
| Notes | ENTACAPONE abstracts 'QOL' data combined with Rinne & Nomecomt 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rajput 1997.
| Methods | Randomised double‐blind parallel group study. Randomised by computer generated random number tables with the participants being double‐blinded and the code not being broken until after the database was closed. 11 centres in USA and Canada Intention‐to‐treat data analysis Duration: 3 months | |
| Participants | 202 patients, 37 withdrew due to adverse effects. 10 in placebo group, 12 in tolcapone 100 mg tid group, and 15 in tolcapone 200 mg tid group. 139 males. Mean age 64 years, mean disease duration 10.9 years, mean levodopa dose 867 mg/day. Inclusion criteria: At least 30 years old, had two of the three cardinal features of PD, and were clinically diagnosed with idiopathic PD. Patients had to have been treated with levodopa for at least 1 year and shown clear improvement with levodopa therapy. Patients had to be receiving at least 4 doses of the standard levodopa/carbidopa ($/1) preparation or , if CR formulation was used, at least 3 daily intakes, of which at least two were CR. Patients had to have predictable motor fluctuations at the end of the dosing interval that could not be eliminated by adjusting existing antiparkinsonian medications. Exclusion criteria: Non‐idiopathic parkinsonism, sudden unpredictable 'off/on' fluctuations or disabling diphasic dyskinesias, a MMSE score of 25 or less, and treatment with centrally acting dopamine antagonists during the previous 6 months or MAOI (other than selegiline) during previous 2 months. | |
| Interventions | Patients received placebo (n=66), 100 mg tid tolcapone (n=69), 200 mg tid tolcapone (n=67). Riboflavin (0.5 mg) was included in the placebo tablets to mimic the yellow discolouration in urine that occurs with tolcapone as a harmless side‐effect. | |
| Outcomes | Levodopa dose Number of daily intakes Daily 'off' time IGA ‐ wearing off effect IGA ‐ PD symptom severity IGA ‐ overall efficacy UPDRS total UPDRS mental UPDRS ADL UPDRS motor Adverse events | |
| Notes | TOLCAPONE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rinne NOMECOMT 1998.
| Methods | Randomised double‐blind parallel group study. Method of randomisation not described. 16 centres in Nordic countries. Intention‐to‐treat analysis. Duration: 6 months. | |
| Participants | 171 patients, 19 patients withdrew. 8 from entacapone group, 6 due to adverse events 2 due to other reasons. 11 withdrew from the placebo group, five due to adverse events, 6 due to other reasons. 47% male. Mean age 62.7, mean duration of PD 10.8 years, mean duration of fluctuations 4.5 years, mean levodopa dose 703 mg/day. Inclusion criteria: levodopa responsive patients with idiopathic PD with motor fluctuations of the end‐of‐dose type (wearing‐off phenomenon), Hoehn & Yahr stage 1.5‐4.0 during 'on' phase, and average 'on' time after each single dose of levodopa less than 4 hours. Patients taking 4‐10 daily doses of standard levodopa. Patients treated with amantadine, selegiline, or dopamine agonists in addition to levodopa were permitted. Exclusion criteria: Using controlled release levodopa preparations. | |
| Interventions | Patients received 200 mg entacapone (n=85) or placebo (n=86) with each levodopa dose. | |
| Outcomes | Primary outcomes: Daily 'on' time Duration of 'on' time after first morning levodopa dose. Secondary outcomes: Daily 'off' time Patients estimation of benefit from single levodopa dose UPDRS (all parts) ('on') Daily fluctuations in disability evaluation. Patient global score Clinician global score. Levodopa dose Levodopa dose frequency Adverse events | |
| Notes | ENTACAPONE abstracts 'QOL' data combined with PSG 1997 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ruottinen 1996 (a).
| Methods | Randomised double‐blind cross‐over study with two four‐week treatment periods without a wash‐out period. The method of randomisation was not described. Per‐protocol analysis of data. Single‐centre, Finland. Duration: 8 weeks | |
| Participants | 26 patients, three withdrew due to adverse events or intercurrent disease, 1 from the placebo group and 2 from the entacapone group. Number of males and females not stated. Mean age 61.3 years. Mean disease duration 14 years. Duration of fluctuations ranged from 3‐19 years. Inclusion criteria: Idiopathic Parkinson's disease and levodopa‐related fluctuations in disability. Exclusion criteria: Psychiatric or severe physical illnesses. | |
| Interventions | Patients received 200 mg entacapone or placebo with each levodopa dose. All patients were receiving levodopa. Amantadine, anticholinergics, dopamine agonists and selegiline were allowed and remained constant. Test days were preceded with an overnight fast and at least 6 hours without Parkinsonian medications. The standard constant levodopa dose was then given at 8am. | |
| Outcomes | Primary clinical outcome: 'on' time Secondary outcomes: motor scores change Time to reach lowest score during levodopa test Duration, magnitude and starting time of dyskinesia . UPDRS Adverse events ECG Blood pressure Heart rate | |
| Notes | ENTACAPONE Cross‐over trial data presented as combined results of both treatment arms and both placebo arms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ceravolo 2002 | Single dose study |
| Davis 1995 | Single dose study |
| Dingemanse 1995 | Participants were all healthy males. |
| Durif 2001 | Uncontrolled study. |
| Gasparini 1997 | Uncontrolled study. |
| Gasser 1999 | Single dose study. |
| Hauser 1998 | Participants had early Parkinson's disease with no fluctuations and they were not on levodopa. |
| Jorga 1998 | Participants were healthy males. |
| Kaakkola 1994 | Uncontrolled study. |
| Larsen NOMESAFE 2001 | Open label continuation of NOMECOMT study. |
| Lee 2002 | Uncontrolled study. |
| Limousin 1993 | Less than 4 weeks duration. |
| Limousin 1995 | Less than 4 weeks duration. |
| Lyytinen 1997 | No placebo control arm, study compared levodopa and entacapone with and without selegiline. |
| Lyytinen 2001 | Less than 4 weeks duration. |
| Merello 1994 | Single dose study. |
| Nutt 1994 | Uncontrolled study. |
| Piccini 2000 | Single dose study. |
| Roberts 1993 | Single dose study. |
| Ruottinen 1996 (b) | Uncontrolled study. |
| Sawle 1994 | Not placebo‐controlled, non‐randomised, healthy control group. |
| Suchowersky 2001 | Participants had non‐fluctuating symptoms of Parkinson's disease. |
| Troconiz 1998 | Single dose study. |
| Waters TSSG 1997 | Participants had non‐fluctuating symptoms of Parkinson's disease. |
| Yamamoto 1997 | Uncontrolled study. |
Sources of support
Internal sources
University of Birmingham, UK.
External sources
Orion, UK.
The Cadbury Foundation, UK.
Roche, UK.
Hull Branch, Parkinson's Disease Society, UK.
Declarations of interest
The department of Dr C E Clarke has received unrestricted grants (£3000 from each) from Orion Pharmaceuticals and Roche Pharmaceuticals.
Edited (no change to conclusions)
References
References to studies included in this review
Adler TFSGIII 1998 {published and unpublished data}
- Adler CH, Singer C, O'Brien C, Hauser RA, Lew MF, Marek KL, Dorflinger E, Pedder S, Deptula D, Yoo K, for the Tolcapone Fluctuator Study Group III. [Randomized, placebo‐controlled study of tolcapone in patients with fluctuating Parkinson's disease treated with levodopa‐carbidopa]. Archives of Neurology 1998;55:1089‐1095. [DOI] [PubMed] [Google Scholar]
Baas 1997 {published and unpublished data}
- Baas H, Beiske AG, Ghika J, Jackson M, Oertel WH, Poewe W, Ransmayr G. Catechol‐O‐methytransferase inhibition with tolcapone reduces the 'wearing‐off' phenomenon and levodopa requirements in fluctuating Parkinsonian patients. Journal of Neurology, Neurosurgery and Psychiatry 1997;63(4):421‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brooks UK‐IRISH 2003 {published and unpublished data}
- Brooks D J, Sagar H. Entacapone is beneficial in both fluctuating and non‐fluctuating patients with Parkinson's disease. A randomized, placebo‐controlled, double‐blind six‐month study. Journal of Neurology, Neurosurgery and Psychiatry 2003;74:1071‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar H, Brooks D, UK‐Irish Entacapone Study Group. The UK‐Irish double‐blind study of entacapone in Parkinson's disease. Movement Disorders 2000;15(Supplement 3):135. [ENT 0317] [Google Scholar]
- Sagar H, Brooks DJ, and the UK‐Irish Entacapone Group. Efficacy and safety of entacapone in non‐fluctuating and fluctuating Parkinsonian patients. The UK‐Irish long‐term multicentre Study. European Journal of Neurology 2000;7(3):17. [ENT 0347] [Google Scholar]
Dupont TIPSII 1997 {published and unpublished data}
- Dupont E, Burgunder J‐M, Findley LJ, Olsson J‐E, Dorflinger E, and the Tolcapone in Parkinson's Disease Study Group II (TIPS II). Tolcapone added to levodopa in stable Parkinsoian patients: A double‐blind placebo‐controlled study. Movement Disorders 1997;12(6):928‐934. [DOI] [PubMed] [Google Scholar]
Fenelon 2002 {published data only}
- Fenelon G, Bourdiex I, Pere JJ, Galiano L, Shadarack J. Entacapone provides additional symptomatic benefits in Parkinson's disease (PD) patients treated with L_DOPA and a dopamine agonist who are experiencing wearing‐off motor fluctuations: Results of a randomized, double‐blind study. AAN conf??? 2002. [Google Scholar]
Im 2002 {published data only}
- Im JH, Jeon BS, Lee MS, Lee WY, Kim JW, Lee MC. A multicentre, double‐blind, placebo‐controlled study to assess efficacy and safety of entacapone in Korean patients with Parkinson's disease experiencing end‐of‐dose deterioration. Movement Disorders 2002;17(5):S40. [Google Scholar]
Kurth TFSGI 1997 {published and unpublished data}
- Kurth MC, Adler CH, Saint‐Hilaire M, Singer C, Waters C, LeWitt P, Chernik DA, Dorflinger MD, Yoo K, and the Tolcapone Fluctator Study Group I. Tolcapone improves motor fluctuation and reduces levodopa requirement in patients with Parkinson's disease experiencing motor fluctuations: A multicenter, double‐blind, randomized, placebo‐controlled trial. Neurology 1997;48(1):81‐87. [DOI] [PubMed] [Google Scholar]
- Welsh MD, Dorflinger E, Chernik D, Waters C. Illness impact and adjustment to Parkinson's disease: before and after treatment with Tolcapone. Movement Disorders 2000;15(3):497‐502. [DOI] [PubMed] [Google Scholar]
Myllyla FILOMEN 2001 {published data only}
- Myllyla VV and the Filomen Study Group. Long‐term safety of entacapone as an adjunct to levodopa in non‐fluctuating and fluctuating patients with Parkinson's disease. Movement Disorders 1998;13(Supplement 2):294. [Google Scholar]
- Myllyla VV, Kultalahti E‐R, Haapaniemi H, Leinonen M, and the FILOMEN Study Group. Twelve‐month saftey of entacapone patients with Parkinson's disease. European Journal of Neurology 2001;8:53‐60. [ENT 0415] [DOI] [PubMed] [Google Scholar]
Myllyla TIPS1 1997 {published and unpublished data}
- Myllyla V V, Jackson M, Larsen J P, Baas H, for the Tolcapone International Parkinson's Disease Study (TIPS) Group 1. Efficacy and safety of tolcapone in levodopa‐treated Parkinson's disease patients with 'wearing‐off' phenomenon: a multicentre, double‐blind, randomized, placebo‐controlled trial. European Journal of Neurology 1997;4:333‐341. [Google Scholar]
Poewe CELOMEN 2002 {published data only}
- Deuschl G, Poewe W, Poepping M, and the Celomen Study Group. Efficacy and safety of entacapone as an adjunct to levodopa treatment in Parkinson's disease (PD): Experience from the Austrain‐German six‐moths study. Parkinsonism and Related Disorders 1999;5:S75. [Google Scholar]
- Poewe WH, Deuschl G, Gordin A, Kultalahti E‐R, Leinonen M, the Celomen Study Group. Efficacy and saftey of entacapone in Parkinson's disease patients with suboptimal levodopa response: a 6‐moth randomized placebo‐controlled double‐blind study in Germany and Austria (Celomen study). Acta Neurologica Scandinavica 2002;105:245‐255. [ENT 0281] [DOI] [PubMed] [Google Scholar]
PSG SEESAW 1997 {published data only}
- Kieburtz K, Hubble J. Benefits of COMT inhibitors in levodop‐treated parkinsonian patients. Results of clinical trials. Neurology 2000;55(Supplement 4):S42‐S45. [PubMed] [Google Scholar]
- McDermott M, Kieburtz K, on behalf of the PSG Steering Committee. Reply (to Entacapone and Motor Fluctuations in Parkinson's disease, Riggs 1998). Annals of Neurology 1998:292. [DOI] [PubMed] [Google Scholar]
- Parkinson's Study Group. Entacapone improves motor fluctuations in levodopa‐treated Parkinson's disease patients. Annals of Neurology 1997;42(5):747‐755. [ENT 0139, SEESAW Study] [DOI] [PubMed] [Google Scholar]
- Riggs J E. Entacapone and Motor Fluctuations in Parkinson's Disease (Letter). Annals of Neurology 1998:292. [DOI] [PubMed] [Google Scholar]
- The Nomecomt Study Group, The Parkinson Study Group. Effect of entacapone on factors related to quality of life in Parkinson's disease. Movement Disorders 2000;15(Supplement 3):125. [Google Scholar]
Rajput 1997 {published and unpublished data}
- Rajput AH, Martin W, Saint‐Hillaire M‐H, Dorflinger E, Pedder S. Tolcapone improves motor function in Parkinsonian patients with 'wearing‐off' phenomenon: a double‐blind, placebo‐controlled, multicentre trial. Neurology 1997;49(4):1066‐1071. [DOI] [PubMed] [Google Scholar]
Rinne NOMECOMT 1998 {published data only}
- Kieburtz K, Hubble J. Benefits of COMT inhibitors in levodopa‐treated parkinsonian patients. Results of clinical trials. Neurology 2000;55(Supplement 4):S42‐S45. [PubMed] [Google Scholar]
- Rinne UK, Larsen JP, Siden, A, Worm‐Petersen J, and the Nomecomt Study Group. Entacapone enhances the response to levodopa in Parkinsonian patients with motor fluctuations. Neurology 1998;51(5):1309‐1314. [ENT 0185] [DOI] [PubMed] [Google Scholar]
- The Nomecomt Study Group, The Parkinson Study Group. Effect of entacapone on factors related to quality of life in Parkinson's disease. Movement Disorders 2000;15(Supplement 3):125. [Google Scholar]
Ruottinen 1996 (a) {published data only}
- Ruottinen HM, Rinne UK. Entacapone prolongs levodopa response in a one month double blind study in Parkinsonian patients with levodopa related fluctuations. Journal of Neurology, Neurosurgery and Psychiatry 1996;60:36‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ceravolo 2002 {published data only}
- Ceravolo R, Piccini P, Bailey DL, Jorga KM, Bryson H, Brooks DJ. 18F‐dopa PET evidence that tolcapone acts as a central COMT inhibitor in Parkinson's disease. Synapse 2002;43(4):201‐207. [DOI] [PubMed] [Google Scholar]
Davis 1995 {published data only}
- Davis TL, Roznoski M, Burns RS. Acute effects of COMT inhibition on L‐DOPA pharmacokinetics in patients treated with carbidopa and selegeline. Clinical Neuropharmacology 1995;18(4):333‐337. [DOI] [PubMed] [Google Scholar]
- Davis TL, Roznoski M, Burns RS. Effects of tolcapone in Parkinson's patients taking L‐dihydroxyphenylalanine/carbidopa and selegeline. Movement Disorders 1995;10(3):349‐351. [DOI] [PubMed] [Google Scholar]
Dingemanse 1995 {published data only}
- Dingemanse J, Jorga K, Zurcher G, Schmitt M, Sedek G, Prada M, Brummelen P. Pharmacokinetic‐pharmacodynamic interaction between the COMT inhibitor tolcapone and single‐dose levodopa. British Journal of Clinical Pharmacology 1995;40:253‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Durif 2001 {published data only}
- Durif F, Devaux I, Pere JJ, Delumeau JC, Bourdeix I, on behalf of the F‐01 Study Group. Efficacy and tolerability of entacapone as adjuvant therapy to levodopa in patients with Parkinson's disease and end‐of‐dose deterioration in daily medical practice: An open multicentre study. European Neurology 2001;45(2):111‐118. [ENT 0389] [DOI] [PubMed] [Google Scholar]
Gasparini 1997 {published data only}
- Gasparini M, Fabrizio E, Bonifati V, Meco G. Cognitive inprovement during Tolcapone treatment in PArkinson's disease. Journal of Neural Transmission ‐ General Section 1997;104(8‐9):887‐894. [DOI] [PubMed] [Google Scholar]
Gasser 1999 {published data only}
- Gasser UE, Jorga K, Crevoisier C, Hovens SE, Giersbergen PL. COMT inhibition by tolcapone further improves levodopa pharmacokinetics when combined with a dual‐release formulation of levodopa/benserazide. A novel principle in the treatment of Parkinson's disease. European Neurology 1999;41(4):206‐211. [DOI] [PubMed] [Google Scholar]
Hauser 1998 {published data only}
- Hauser RA, Molho E, Shale H, Pedder S, Dorflinger EE, and the Tolcapone De Novo Study Group. A pilot evaluation of the tolerability, safety, and efficacy of Tolcapone alone and in combination with oral Selegeline in untreated Parkinson's disease patients. Movement Disorders 1998;13(4):643‐647. [DOI] [PubMed] [Google Scholar]
Jorga 1998 {published data only}
- Jorga JM, Fotteler B, Heizmann P, Zurcher G. Pharmacokinetics and pharmacodynamics after oral and intravenous administration of tolcapone, a novel adjunct to Parkinson's disease therapy. European Journal of Clinical Pharmacology 1998;54(5):443‐447. [DOI] [PubMed] [Google Scholar]
Kaakkola 1994 {published data only}
- Kaakkola S, Teravainen H, Ahtila S, Rita H, Gordin A. Effect of entacapone, a COMT inhibitor, on clinical disability and levodopa metabolism in Parkinsonian patients. Neurology 1994;44(1):77‐80. [DOI] [PubMed] [Google Scholar]
Larsen NOMESAFE 2001 {published data only}
- Larsen JP, Siden A, Worm‐Petersen J, Gordin A, Reinikainen K, Kultalahti E‐R. Long‐term efficacy and safety of entacapone in Parkinsonian patients with motor fluctuations: a Nordic open study of 3 years duration. Proceedings of the XIV International Congress on Parkinson's Disease. Helsinki, Finland, 2001.
Lee 2002 {published data only}
- Lee MS, Kim HS, Cho EK, Lim JH, Rinne JO. COMT genotype and effectiveness of entacapone in patients with fluctuating Parkinson's disease. Neurology 2002;58:564‐567. [DOI] [PubMed] [Google Scholar]
Limousin 1993 {published data only}
- Limousin P, Pollak P, Gervason‐Tournier C‐L, Hommel M, Perret JE. Ro 40‐7592, a COMT inhibitor, plus levodopa in Parkinson's disease. The Lancet 1993;341:1605. [DOI] [PubMed] [Google Scholar]
Limousin 1995 {published data only}
- Limousin P, Pollack P, Pfefen JP, Tournier‐Gervason CL, Dubuis R, Perret JE. Acute administration of levodopa‐benserazide and tolcapone, a COMT inhibitor, in Parkinson's disease. Clinical Neuropharmacology 1995;18(3):285‐265. [DOI] [PubMed] [Google Scholar]
Lyytinen 1997 {published data only}
- Lyytinen J, Kaakkola S, Ahtila S, Tuomainen P, Teravainen H. Simultaneous MAO‐B and COMT‐Inhibition in L‐Dopa‐treated patients with Parkinson's disease. Movement Disorders 1997;12(4):497‐505. [DOI] [PubMed] [Google Scholar]
Lyytinen 2001 {published data only}
- Lyytinen J, Sovijarvi A, Kaakkola S, Gordin A, Teravainen H. The effect of catechol‐O‐methyltransferase inhibition with entacapone on cardiovascular autonomic responses in L‐Dopa‐treated patients with Parkinson's disease. Clinical Neuropharmacology 2001;24(1):50‐57. [DOI] [PubMed] [Google Scholar]
Merello 1994 {published data only}
- Merello M, Lees AJ, Webster R, Bovingdon M, Gordin A. Effect of entacapone, a peripherally acting catechol‐O‐methyltransferase inhibitor, on the motor response to acute treatment with levodopa in patients with Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry 1994;57(2):186‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nutt 1994 {published data only}
- Nutt JG, Woodward WR, Becknar RM, Stone CK, Berggren BA, Carter JH, Gancher ST, Hammerstad JP, Gordin A. Effect of peripheral catechol‐O‐methytransferase inhibition on the pharmacokinetics and pharmacodynamics of levodopa in Parkinsonian patients. Neurology 1994;44:913‐919. [DOI] [PubMed] [Google Scholar]
Piccini 2000 {published data only}
- Piccini P, Brooks DJ, Korpela K, Pavese N, Karlsson M, Gordin A. The catechol‐o‐methyltransferase (COMT) inhibitor entacapone enhances that pharmacokinetic and clinical response to Sinemet CR in Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 2000;68:589‐594. [ENT 0300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 1993 {published data only}
- Roberts JW, Cora‐Locatelli G, Bravi D, Amantea MA, Mouradian MM, Chase TN. Catechol‐O‐methytransferase inhibitor tolcapone prolongs levodopa/carbidopa action in parkinsonian patients. Neurology 1993;43(12):2685‐2688. [DOI] [PubMed] [Google Scholar]
Ruottinen 1996 (b) {published data only}
- Ruottinen HM, Rinne UK. Effect of one month's treatment with peripherally acting catechol‐O‐methyltransferase inhibitor, entacapone, on pharmacokinetics and motor response to levodopa in advanced Parkinsonian patients. Clinical Neuropharmacology 1996;19(3):222‐233. [DOI] [PubMed] [Google Scholar]
Sawle 1994 {published data only}
- Sawle GV, Burn DJ, Morrish PK, Lammersma AA, Snow BJ, Luthra S, Osman S, Brooks DJ. The effect of entacapone (OR‐611) on brain [18F]‐6‐L‐fluorodopa metabolism: Implications for levodopa therapy of Parkinson's disease. Neurology 1994;44:1292‐1297. [DOI] [PubMed] [Google Scholar]
Suchowersky 2001 {published data only}
- Suchowersky O, Bailey P, Pourcher E, Bulger L, Facciponte G. Comparison of two dosages of tolcapone added to levodopa in nonfluctuating patients with PD. Clinical Neuropharmacology 2001;24(4):214‐220. [DOI] [PubMed] [Google Scholar]
Troconiz 1998 {published data only}
- Troconiz IF, Naukkarien TH, Ruottinen HM, Rinne UK, Gordin A, Karlsson MO. Population pharmacodynamic modeling of levodopa in patients with Parkinson's disease recieving entacapone. Clinical Pharmacology and Therapeutics 1998;64(1):106‐116. [DOI] [PubMed] [Google Scholar]
Waters TSSG 1997 {published data only}
- Waters CH, Kurth M, Bailey P, Shulman LM, LeWitt P, Dorflinger E, Deptula D, Pedder S, and the Tolcapone Study Group. Tolcapone in stable Parkinson's disease: efficacy and safety of long‐term treatment. Neurology 1997;49:665‐671. [DOI] [PubMed] [Google Scholar]
Yamamoto 1997 {published data only}
- Yamamoto M, Yokochi M, Kuno S, Hattori Y, Tsukamoto Y, Narabayashi H, Tohgi H, Mizuno Y, Kowa H, Yanagisawa N, Kanazawa I. Effects of tolcapone, a catechol‐O‐methyltransferase inhibitor, on motor symtoms and pharmacokinetics of levodopa patients with Parkinson's disease. Journal of Neural Transmissions ‐ General Section 1997;104(2‐3):229‐236. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Zhang 2003 {published data only}
- Zhang ZX, Li H, Luo Y, Jiang YP, Chen SD, Chen HB, Sun B, Wen HB, Wang J, Weng ZF, Wang XD. A randomised placebo‐controlled double‐blind parallel group trial of entacapone in patients with fluctuating Parkinson's disease. Chinese Journal of Neurology 2003;36:406‐410. [Google Scholar]
Additional references
Assal 1998
- Assal F, Spahr L, Hadengue A, Rubbici‐Brandt L, Burkhard PR. Tolcapone and fulminant hepatitis. Lancet 1998;352:958. [DOI] [PubMed] [Google Scholar]
Bonifati 1999
- Bonifati V, Meco G. New, selective catechol‐O‐methyltransferase inhibitors as therapeutic agents in Parkinson's disease. Pharmacological therapies 1999;81(1):1‐36. [DOI] [PubMed] [Google Scholar]
Colosimo 1999
- Colosimo C. The rise and fall of tolcapone. Journal of Neurology 1999;246(10):880‐882. [DOI] [PubMed] [Google Scholar]
CONSORT 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357:1191‐1194. [PubMed] [Google Scholar]
EAEM 1998
- The European Agency for the Evaluation of Medicines. CPMP/2457/98: Recommendation for the suspension of the marketing authorisation for Tasmar (Tolcapone). Press release (London) 17th November 1998:EU/1/97/044/001‐006.
Fisher 2002
- Fisher A, Croft‐Baker J, Davis M, Purcell P, McLean AJ. Entacapone‐induced hepatotoxicity and hepatic dysfunction. Movement Disorders 2002;17(6):1362‐1365. [DOI] [PubMed] [Google Scholar]
Gibb 1988
- Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 1988;51:745‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinn 1986
- Quinn N, Critchley P, Parkeds D, Marsden CD. When should levodopa be started?. Lancet 1984;ii:985‐986. [DOI] [PubMed] [Google Scholar]
Rajput 1984
- Rajput AH, Stern W, Laverty WH. Chronic low‐dose levodopa therapy in Parkinson's disease. Neurology 1984;34:991‐996. [DOI] [PubMed] [Google Scholar]


