Abstract
Background
Cryptococcal meningitis causes high mortality in immunocompromised and immunocompetent patients. The objective of this study was to identify early predictors of clinical outcome, available at the first days of hospitalization, in patients with cryptococcal meningitis in a tertiary center in Brazil.
Methods
Ninety-six cases of cryptococcal meningitis with clinical, epidemiological and laboratory data, and identification and antifungal susceptibility of the strains were analyzed. Quantitative CSF yeast counts were performed by direct microscopic exam with a Fuchs-Rosenthal cell counting chamber using an institutional protocol. Univariable and multiple analyses using logistic regression were performed to identify predictors, available at the beginning of hospitalization, of in-hospital mortality. Moreover, we performed a secondary analysis for a composite outcome defined by hospital mortality and intensive care unit transfer.
Results
The species and the antifungal susceptibility were not associated with the outcomes evaluated. The variables significantly associated with the mortality were age (OR = 1.08, 95% CI 1.02–1.15), the cerebrospinal fluid (CSF) yeasts count (OR = 1.65, 95% CI 1.20–2.27), systemic arterial hypertension (OR = 22.63, 95% CI 1.64–312.91) and neurological impairment identified by computed tomography (OR = 41.73, 95% CI 3.10–561.65). At the secondary analysis, CSF yeast count was also associated with the composite outcome, in addition to the culture of Cryptococcus spp. from bloodstream and cerebral toxoplasmosis. The associations were consistent with survival models evaluated.
Conclusions
Age and CSF yeast count were independently associated with in-hospital mortality of patients with cryptococcal meningitis but Cryptococcus species identification and antifungal susceptibility were not associated with the outcomes. Quantitative CSF yeast counts used in this study can be evaluated and implemented in other low and middle-income settings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-022-07118-7.
Keywords: Cryptococcus, Cryptococcal meningitis, Predictors, Prognosis, Mortality
Background
Cryptococcosis is one of the major invasive diseases in humans. It is acquired by inhalation of fungal propagules, which can be deposited in the pulmonary alveoli to disseminate to cutaneous tissue, internal organs, and to the central nervous system, where is observed the most severe clinical form [1].
In people living with HIV/AIDS (PLWHA), cryptococcosis is one of the opportunistic AIDS-defining infections and one of the main causes of death [2–7].
The major agents of cryptococcosis are members of C. neoformans and C. gattii complex. This complex was recognized in 2015 into seven species, based on phenotypic and genotypic, geographic, epidemiological and virulence characteristics [8–15]. The new classification includes the species C. neoformans sensu strictu (s.s) (genotype VNI/VNII/VNB), hybrids between C. neoformans and C. deneoformans (genotype VNIII) and C. deneoformans (genotype VNIV), and C. gattii complex is formed by C. gattii s.s (VGI), C. deuterogattii (VGII), C. bacillisporus (VGIII), C. tetragattii (VGIV), and C. decagattii (VGIIIc/VGIV) [8, 16].
The major specie associated with immunocompromised remains C. neoformans s.s., mainly in PLWHA, followed by C. deneoformans, C. bacillisporus, C. tetragattii and C. decagattii. On the other hand, C. gattii and C. deuterogattii are known to affect apparently immunocompetent individuals due to increased virulence and less antifungal susceptibility [16–19].
The lethality rates vary and depend on several factors, among them: underlying disease, stage of diagnosis of the disease and therapeutic strategy. However, it remains the second most common cause of AIDS-related mortality, resulting in the death of an average of 15% of this population [20]. In developed countries, cryptococcal meningitis presents a lethality range of 9% to 20%, and this rate in developing countries is around 40% [7, 21–24].
The antifungal therapy depends on the immunity of the patient, occurrence of underlying disease, site of infection, toxicity and availability of the antifungal drugs [7, 25, 26]. The recommended treatment for patients with HIV is divided into phases of induction, consolidation and maintenance. The preferred induction regimen recommended by the World Health Organization consists in a short-course (one-week) with amphotericin B deoxycholate (AMBd) and 5-flucytosine (5FC), followed by one week of fluconazole (FCZ). In contrast, the preferred induction regimen recommended by the Infectious Disease Society of America consists in at least two weeks with lipidic formulations of amphotericin B and 5-flucytosine (5FC) [7, 25, 26]. However, lipidic formulations of amphotericin B and 5FC are usually unavailable in public health services of Latin America.
Despite the recommendation of some experts to the antifungal susceptibility determination by minimum inhibitory concentration (MIC) in the management of cryptococcal meningitis, the prognostic value of this test has not been defined and the correlation between the susceptibility results and the clinical outcomes remains uncertain [27–29]. Another limitation is about fungicidal drugs such as AMB. The reference test, the microdilution, determines de minimum inhibitory concentration of the antifungal, and it may not be appropriate since it determines the inhibitory activity and thus is unable to identify resistant strains [3, 30–32].
The level of heteroresistance to FCZ (LHF) refers to the ability of cells to grow at high concentrations of the drug. These cells can generate homogenous populations, or clones, able of adapting to higher concentrations [33–35]. The occurrence of resistant strains during therapy, therapeutic failure and relapses can be attributed to LHF, mainly due to FCZ exposure at the maintenance phase [33, 36–38]. Time-Kill (TK) method is capable of indicating the kill of the strains according to the time of exposure to the drug. In addition, the maximum concentration of AMB-d available in serum is 1 mg/L [39, 40]. This method can be promising because of reports that presented a correlation of TK with outcomes of the patients with cryptococcosis [41, 42].
Considering all these developments in diagnostic tests, it is necessary to estabilish which are useful tools to predict the prognosis and guide therapeutic alternatives. There are few data about the potential association between Cryptococcus species, antifungal susceptibility, fungal burden and clinical outcomes of cryptococcal meningitis in Brazil. Consequently, the purpose of this study was to identify predictors of clinical outcome in patients hospitalized with cryptococcal meningitis in a tertiary center of São Paulo, Brazil.
Methods
Study design and population
We developed a retrospective cohort study, including patients in their first episode of cryptococcal meningitis, admitted at the Emílio Ribas Institute of Infectious Diseases (ERIID) of São Paulo from August 2012 to December 2013 and January 2015 to August 2017. For administrative reasons, episodes from 2014 could not be evaluated for this study.
The ERIID is a public tertiary hospital and the main reference for the treatment of infectious diseases in the state of São Paulo, Brazil.
Data collection and clinical information
Demographic, clinical, and laboratory data were obtained from medical records. The variables evaluated included sex, age, duration of hospital stay, Glasgow coma scale, signs and symptoms of cryptococcosis at admission, antifungal therapy during the induction phase, information about HIV infection, viral load, CD4 values and occurrence of other infectious diseases or comorbidities. The antiretroviral therapy status was defined as antiretroviral naïve, regular, irregular and abandoned therapy.
The results of laboratory tests of cerebrospinal fluid (CSF) used were: opening pressure during the lumbar puncture, glucose and protein dosage, WBC count and differential count, Cryptococcus spp. antigen, culture, India ink test and quantitative CSF yeast cell counts using a validated protocol at ERIID [43]. This methodology is performed by direct microscopic exam with a Fuchs-Rosenthal cell counting chamber, quantifying how many yeast cells/mcL of CSF were present. Results of blood urea and creatinine level were also collected. All these variables were referred to the hospital admission. It was recorded results of brain computerized tomography (CT) and brain magnetic resonance imaging (MRI).
Identification and antifungal susceptibility profile of strains
The first strain obtained from each case was sent and identified in the Mycology Unit of Adolfo Lutz Institute of São Paulo, using classic methods [44]. All strains were genotyped by Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR–RFLP) with the URA5 gene amplification and treatment with enzymes Sau96I and HhaI [45]. The strains band profiles obtained by electrophoresis were plotted and compared with the profiles from C. neoformans reference strains: VNI-WM 148, VNII-WM 626, VNIII-WM 628 and VNIV-WM 629 and C. gattii: VGI- WM 179, VGII-WM 178, VGIII-WM 161 and VGIV-WM 779, provided by Oswaldo Cruz Foundation (FIOCRUZ) in Brazil.
The minimum inhibitory concentration (MIC) was determined by the broth microdilution described in E. Def. 7.3.1 of AFST-EUCAST [46] against FCZ and Etest® strips (bioMérieux, France) for AMB, following manufacturer's instructions. To interpret the results, breakpoints adopted in previous studies and clinically relevant were used: MIC < 8 mg/L and resistant MIC ≥ 16 mg/L for FCZ and MIC < 1 mg/L and resistant MIC > 2 mg/L for AMB [28, 29, 47]. The quality control strains C. krusei (Pichia kudriavzevii) ATCC 6258 and C. parapsilosis ATCC 22019 and the C. neoformans H99 and C. deuterogattii R265 were used in all tests.
The test to determine the LHF was performed in duplicate according to the method described in literature [34]. The strains were cultivated by spot test in plates of YPD agar with FCZ concentrations of 0, 8, 16, 32, 64, 128, and 256 mg/L and incubated at 30 °C for 72 h. The LHF was determined by the growth colonies on the plate with the highest FCZ concentration. These colonies, or clones, were cultivated in YPD agar plates with the FCZ concentration equivalent to the LHF.
The TK method was performed to determine the time to fungicide activity of 1 mg/L of AMBd [3, 41, 48]. The strains were cultivated in tubes with RPMI medium and the concentration of 1 mg/L of AMBd. These tubes were incubated at 30 °C and aliquots were diluted and cultivated in Sabouraud plates. This procedure was realized at time 0 (T0), as it refers to the moment without incubation of the cells with AMBd and repeated to 6 h later, i.e., T6, at T12, T24, T48 and T72. After 72 h of incubation, it was observed which the period of incubation with AMB was able to inhibit 99.9% of the UFC/mL, compared to the T0. The time required for this reduction was the endpoint of the test, the TK.
Statistical analysis
The objective of this analysis was to identify predictors of prognosis. In that sense we considered as primary outcome in-hospital mortality. Moreover as a secondary analysis we explored additionally a composite outcome defined by the occurrence of in-hospital mortality and intensive care unit (ICU) transfer during hospitalization. The univariable analysis of categorical and quantitative variables was performed using Fisher's Exact Test and Wilcoxon-Mann–Whitney Test, respectively, and that presented a p-value ≤ 0.2 were selected to the logistic regression.
To avoid collinearity between the quantitative variables, the Pearson correlation coefficient was calculated and, among variables strongly correlated, the one with the highest association with the outcome was chosen. Consequently, the Glasgow scale was excluded from subsequent analysis since it was strongly correlated with the age (r = − 0.73) and presented lower association with mortality (age: OR = 1.06, 95% CI 1.02–1,11, p = 0.006; Glasgow scale OR = 0.59, 95% CI 0.35–0.99, p = 0.047).
Quantitative variables were analyzed regarding a possible linear relation with the outcome. Thus, the possibility of applying a transformation or defining cutoff points was explored. For this, the variables were categorized into deciles and then evaluated to about the odds of mortality.
The variables were evaluated by the logistic regression, and we obtained a multiple model only including those significantly associated with the outcome (p ≤ 0.05). The antifungal susceptibility profile and genotypes of the strains and the antifungal therapy were also evaluated in the multiple analysis, independently of the univariable results.
The goodness of fit was evaluated by the Pearson test and the Hosmer–Lemeshow test. The predicted values were used to elaborate the receiver operating characteristic (ROC) curves, and the accuracy of the model was calculated for the cutoff of 0.5. It was evaluated whether the associations were maintained by excluding the outliers (upper values three times the average of the leverage) and influential observations (with delta-deviance higher than the cutoff point of 4) [49].
Cox regression was performed to analyze whether these predictors were associated with time to the outcome and the Kaplan–Meier curves were obtained to represent graphically the survival time from the date of hospitalization to the occurrence of the outcomes. For this analysis, the patients who survived were censored on the day of hospital discharge, and for the secondary analysis, the patients who did not develop the corresponding outcome were censored on the day of hospital discharge.
All analyses were performed using the Stata® program (version 11.0, Stata Corp. LP, College Station, TX, USA).
Results
Clinical and laboratory information
We analyzed 96 cases of cryptococcal meningitis in patients aged 19 to 68 years old and the majority were male (74/96; 77.08%). The duration of hospital stay ranged from 1 to 202 days with a median of 38 days and an interquartile range (IQR) of 23–54, and ICU transfer occurred in 27 (30%) patients, with a stay period of 1 to 48 days and median of seven days in this unit. Hospital death occurred in 25 patients (25/93, 26.9%) who remained hospitalized between 1 and 202 days with a median of 23 days until the death. Relapse in up to 6 months was reported in 25.8% (16/62) of survival.
HIV-infection was described at 96.7% (87/90) of patients with viral load from undetectable (< 50 copies/mL), in eight of them, to 3.436.979 copies/mL (median 104.126, IQR 1824–332.156 copies/mL). CD4 values ranged from 2 to 722 cells/mm3 (median 42, IQR 16–78). Moreover, one or more comorbidities were recorded in 27 (30%) patients, being the most frequent: viral hepatitis infection (11/90, 12.2%), Kaposi's sarcoma (7/90; 7.8%) and systemic arterial hypertension (diastolic blood pressure equal to or above 130 mmHg by 80 mmHg [50]) (6/90, 6.7%). Other infections were diagnosed in 58.8% (53/90) of patients and the most frequently were oral/esophageal candidiasis (20; 22.1%), pulmonary tuberculosis (15; 16.7%), cytomegalovirus infection (12; 13.3%), bacterial pneumonia (11; 12.2%), and cerebral toxoplasmosis (9; 10%). About the cases with tuberculosis diagnosis, 2 cases presented pulmonary and meningeal co-infection. It was observed less than 10% of cases of syphilis, pneumocystosis and tuberculous meningitis. Regular antiretroviral therapy was recorded for 19.5% (17/87) of HIV-infected patients, 33.3% (29/87) cases were antiretroviral naive, 26.4% (23/87) related irregular treatment and 19.5% (17/87) abandoned the therapy. Combination antifungal therapy AMBd plus FCZ (AMBd 0.7–1 mg/kg/day + FCZ 800–1200 mg/day) was used in 47.2% (42/89) of patients and AMBd plus 5FC (AMBd 0.7–1 mg/kg/day + 5FC 100 mg/kg/day) was prescribed to 43.8% (39/89) of patients. Combination of AMBd plus FCZ plus 5FC (AMBd 0.7–1 mg/kg/day + FCZ 800–1200 mg/day + 5FC 100 mg/kg/day) was prescribed to 6.7% (6/89) patients. AMBd monotherapy was prescribed in only two (2.2%) patients.
The CSF opening pressure was recorded in 69 (76.7%) patients and the value ranged from 8 to 150 cmH2O (median 33, IQR 22.4–50 cm H2O). Intracranial hypertension (CSF opening pressure > 20 cm H2O) was observed in 54 (78.6%) patients. The CSF WBC count presented values from 0 to 800 cells/mL (median 55, IQR 3–55 cells/mL). The CSF yeast count presented values ranging from 0 to 11.520 yeasts/mm3 (median 192 yeasts/mm3). Cultures of Cryptococcus spp. from extra neural sites were observed in 20 (22.5%) patients, including 18 from the bloodstream, three from the skin, one from lymph nodes and one from lungs. All cases were isolated C. neoformans s.s.
Abnormalities in neuroimaging were observed in 50 patients. According to MRI results, the most observed alterations were the presence of pseudocysts and cryptococcomas with 5 (10%) occurrences from each. The most observed alterations by CT were cortical atrophy (15; 30%), cortical edema and ventricular dilatation (4; 8% each).
Identification and antifungal susceptibility of strains
It was identified 76 (81.7%) C. neoformans VNI, 17 (18.3%) C. neoformans VNII and three strains of C. deuterogattii (3.2%). The AMBd-MIC varied from 0.012 mg/mL to 0.94 mg/L, and resistance were not observed. The FCZ-MIC was 0.12 mg/L to 64 mg/L and 28.1% of strains (27/96) were FCZ-resistant (MIC ≥ 16 mg/L). LHF values ranged from 16 to 128 mg/L, in which 9.4% of strains with LHF 16 mg/L (9/96), 53.1% with 32 mg/L (51/96), 32.3% with 64 mg/L (31/96) and 5,2% (5/96) with LHF 128 mg/L. Fungicidal activity of AMBd at TK6 was observed to 20.8% (20/96), at TK12 to 19.8% (19/96), at TK24 to 23.9% (23/96), at TK48 to 17.7% (17/96) and at TK72 to 11.5% (11/96) of the strains. No fungicidal activity was observed in 6.2% of the strains (6/96).
Predictors of mortality
In the univariable analysis, the following variables were associated with in-hospital mortality: age, Glasgow coma scale, ICU transfer, torpor, systemic arterial hypertension, pneumonia, the culture of Cryptococcus spp. from extra neural sites, CSF yeasts count and urea level, and presence of cerebral edema and ventricular dilation by CT (Table 1).
Table 1.
Univariable analysis of prognostic variables related to hospital mortality in 96 patients with cryptococcal meningitis at Emilio Ribas Institute of Infectious Diseases, São Paulo, Brazil
| Variables | All | Deaths | Survivors | p* |
|---|---|---|---|---|
| Age (years), average (IQR) | 38.7 (29–46) | 44.2 (36–52.5) | 36.4 (28–44.5) | 0.007 |
| Glasgow coma scale, average (IQR) | 15 (15–15) | 14 (14–15) | 15 (15–15) | < 0.001 |
| ICU transfer, n (%) | 27/90 (30%) | 15/22 (68.2%) | 12/68 (17.7%) | < 0.001 |
| Signs and symptoms at admission | ||||
| Nausea | 28/90 (31.1%) | 6/22 (27.5%) | 22/68 (32.4%) | 0.07 |
| Seizures | 12/90 (13.3%) | 6/22 (27.5%) | 6/68 (8.8%) | 0.06 |
| Mental confusion | 19/90 (21.1%) | 8/22 (36.4%) | 11/68 (16.2%) | 0.07 |
| Torpor | 4/90 (4.4%) | 3/22 (13.6%) | 1/68 (1.5%) | 0.004 |
| Clinical-laboratory data | ||||
| Systemic arterial hypertension | 6/90 (6.7%) | 5/22 (22.7%) | 1/68 (1.5%) | 0.003 |
| Pneumonia | 11/90 (12.2%) | 6/22 (27.3%) | 5/68 (7.4%) | 0.02 |
| Culture of Cryptococcus spp. from extra neural sites | 20/89 (22.5%) | 9/22 (40.9%) | 11/67 (16.4%) | 0.04 |
| Culture of Cryptococcus spp. from bloodstream | 18/89 (20.2%) | 8/22 (36.4%) | 10/67 (14.9%) | 0.06 |
| Culture of Cryptococcus spp. from skin | 3/89 (3.4%) | 2/22 (9.1%) | 1/67 (1.5%) | 0.15 |
| CSF opening pressure (cmH2O)-average (IQR) | 39.4 (22.4–50) | 47.9 (26.2–60.5) | 36.8 (20.5–50) | 0.20 |
| CSF yeasts cout (yeasts/mm3)-average (IQR) | 749 (6–665) | 1696 (340–2240) | 443.2 (3–384) | < 0.001 |
| Urea level (mg/dL)-average (IQR) | 33.41 (21–40) | 42.9 (22–48) | 30.0 (21–35) | 0.06 |
| Time-kill of 1 mg/L AMB (hours)-average (IQR) | 27.9 (12–48) | 24 (6–24) | 29.8 (12–48) | 0.17 |
| Neuroimage-computed tomography | ||||
| Cerebral edema | 4/90 (4.4%) | 3/22 (13.6%) | 1/68 (1.5%) | 0.04 |
| Ventricular dilation | 4/90 (4.4%) | 3/22 (13.6%) | 1/68 (1.5%) | 0.04 |
*p ≤ 0.2; IQR, interquartile range 25–75%; CSF, cerebrospinal fluid; AMB, amphotericin B; ICU, intensive care unit
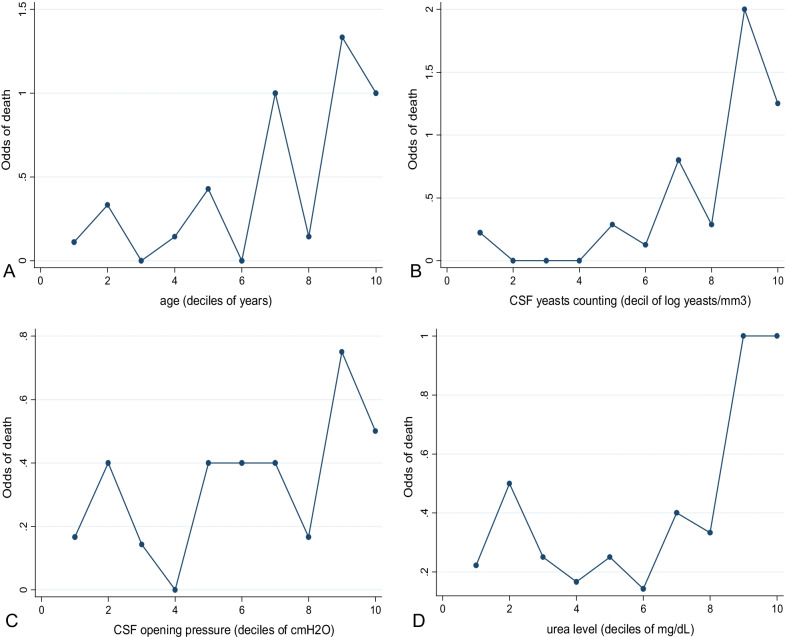
We observed trends compatible with a linear shape when odds of mortality were plotted regarding the deciles of age, CSF yeasts count and CSF opening pressure (Fig. 1A–C respectively). The variable urea level showed stabilization of odds at 40 mg/dL. For this reason, it was possible to categorize it (deciles nine and ten at Fig. 1D).
Fig. 1.
Graphs of the quantitative variables associated with mortality, transformed into deciles A Age, B CSF yeast count, C CSF opening pressure, and D Blood urea level
The CSF yeast count was transformed to a logarithm version. However, a unit was added to all observations before transforming into logarithm, to avoid losses related to the natural logarithm of 0. This variable, hereafter named as “CSF yeast count-log”, had a higher association with the outcome than the original version.
The variables cerebral edema and ventricular dilation by CT were unified, due to the low number of observations. This variable was defined by “neurological impairment identified by computed tomography”.
The variables associated with hospital mortality in the multiple analysis were: age, CSF yeast count-log, systemic arterial hypertension and neurological impairment by computed tomography (Table 2). The antifungal susceptibility profile, the genotypes strains, antifungal therapy regimen and antiretroviral therapy were not associated with mortality.
Table 2.
Multiple analysis by logistic regression of variables associated with hospital mortality in 96 patients with cryptococcal meningitis at Emilio Ribas Institute of Infectious Diseases, São Paulo, Brazil
| Variable | Odds ratio | Confidence interval 95% | p |
|---|---|---|---|
| Age (years) | 1.08 | 1.02–1.15 | 0.007 |
| CSF yeast count-log | 1.65 | 1.20–2.27 | 0.002 |
| Systemic arterial hypertension | 22.63 | 1.64–312.91 | 0.02 |
| Neurological impairment by computed tomography | 41.73 | 3.10–561.65 | 0.005 |
CSF, cerebrospinal fluid
The model showed an area under the ROC curve of 91.9% (95% Confidence Interval [95% CI] 0.86–0.97%, Fig. 2) and presented an accuracy of 83.3%. The sensitivity and specificity were 54.5% and 92.6%, respectively (positive predictive value = 70.6%, negative predictive value = 86.6%). The model presented goodness of fit at the Pearson test and the Hosmer–Lemeshow test with p = 0.95 and 0.99, respectively.
Fig. 2.
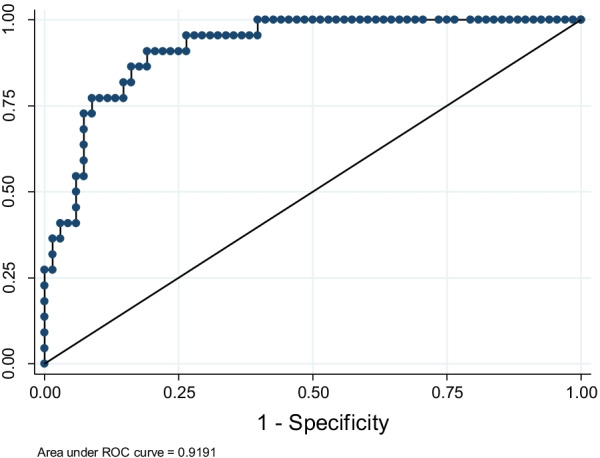
ROC curve using the variables age in years, CSF yeast count-log, systemic arterial hypertension and neurological impairment by computed tomography associated with hospital mortality
By excluding the extreme and influential observations, the associations with the outcome were maintained (see Additional file 1: Fig. S1, Tables S1 and S2).
The categorical variable of urea level was associated with the outcome (Odds Ratio [OR] = 6.16, 95% CI 1.31–29.00, p = 0.02), however, its inclusion resulted in the loss of precision due to the increase at the standard error of other estimates, such as systemic arterial hypertension (95% CI increased to 2.655–26.91) and neurological impairment by computed tomography (95% CI increased to 4.03–1063.87). Also, the number of observations for this variable was lower (n = 83), and the goodness of fit decreased (Hosmer–Lemeshow p = 0.08). For these reasons, it was not maintained in the model.
The associations were consistent at Cox regression, in which the following variables were associated with the time to event: neurological impairment by computed tomography (HR = 3.69, 95% CI 1.32–10.35; p = 0.01), CSF yeast count-log (HR = 1.37, 95% CI 1 1.10–1.70, p = 0.004) and age in years (HR = 1.06, 95% CI 1.01–1.10, p = 0.01) and systemic arterial hypertension but with lower precision (HR = 2.56, 95% CI 0.88–7.47, p = 0.08).
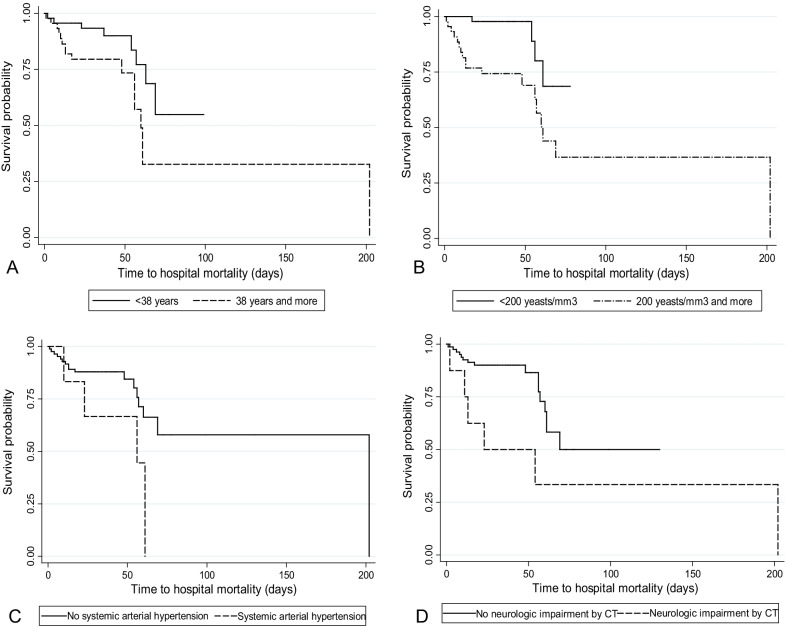
For the representation with Kaplan–Meier curves, the quantitative variables were categorized according to the respective medians (i. e. 38 years for age and 200 yeasts/mm3 for CSF yeast count). Since the curves did not intersect, it was assumed that the proportionality of the risks was maintained throughout the time of hospitalization (Fig. 3). A similar result was observed in survival curves adjusted (see Additional file 1: Fig. S2).
Fig. 3.
Survival curves until the hospital mortality, according to the variables selected for the multiple analysis model. A Age, B CSF yeast count and C Systemic arterial hypertension and D Neurologic impairment by computed tomography (CT)
Secondary analysis—predictors of the composite outcome
The variables that presented statistically significant difference regarding the composite outcome were: age, Glasgow scale, headache, cerebral toxoplasmosis, systemic arterial hypertension, pneumonia, culture of Cryptococcus spp. from bloodstream, culture of Cryptococcus spp. from extra neural sites, CSF yeasts count, urea level and cerebral edema and ventricular dilation by computed tomography (Table 3).
Table 3.
Univariable analysis of prognostic variables related to hospital mortality and/or ICU transfer during hospitalization in 96 patients with cryptococcal meningitis at Emilio Ribas Institute of Infectious Diseases, São Paulo, Brazil
| Variables | All | Death or ICU transfer (n = 37) | Survivor or no ICU transfer (n = 56) | p* |
|---|---|---|---|---|
| Age (years)-average (IQR) | 38.7 (29–46) | 41.8 (30.5–51) | 36.3 (28–44.5) | 0.06 |
| Glasgow coma scale (IQR) | 14 (15) | 14 (14–15) | 15 (15–15) | < 0.001 |
| Signs and symptoms at admission | ||||
| Headache | 77/90 (85.56%) | 25/34 (73.53%) | 52/56 (92.86%) | 0.03 |
| Mental confusion | 19/90 (21.11%) | 11/34 (32.35%) | 8/56 (14.29%) | 0.06 |
| Seizure | 12/90 (13.33%) | 7/34 (20.59%) | 5/56 (8.93%) | 0.2 |
| Nausea | 28/90 (31.11%) | 7/34 (20.59%) | 21/56 (37.5%) | 0.11 |
| Cough | 2/90 (2.22%) | 2/34 (5.88%) | 0/56 (0%) | 0.14 |
| Neck stiffness | 3/90 (3.33%) | 3/34 (8.82%) | 0/56 (0%) | 0.05 |
| Storpor | 4/90 (4.44%) | 3/34 (8.82%) | 1/56 (1.79%) | 0.15 |
| Clinical-laboratory data | ||||
| Cerebral toxoplasmosis | 9/90 (10%) | 7/34 (20.59%) | 2/56 (3.57%) | 0.02 |
| Systemic arterial hypertension | 6/90 (6.67%) | 5/34 (14.71%) | 1/56 (1.79%) | 0.03 |
| Lymphoma | 3/90 (3.33%) | 3/34 (8.82%) | 0/56 (0%) | 0.05 |
| Pneumonia | 11/90 (12.22%) | 9/34 (26.47%) | 2/56 (3.57%) | 0.002 |
| Culture of Cryptococcus spp. from bloodstream | 18/89 (20.22%) | 12/34 (35.29%) | 6/55 (10.91%) | 0.007 |
| Culture of Cryptococcus spp. from extra neural sites | 20/89 (22.47%) | 12/34 (35.29%) | 8/55 (14.55%) | 0.03 |
| CSF yeasts count (yeasts/mm3) average (IQR) | 749 (6–665) | 1531 (73–2240) | 275 (2.5–330) | 0.002 |
| Urea level (mg/dL) average (IQR) | 33.41 (21–40) | 40.91 (22–48) | 28.46 (21–34) | 0.04 |
| HIV viral load average (IQR) | 242,556 (1824–332,156) | 280,197 (7912–3,826,251) | 221,890 (495 -311,000) | 0.18 |
| Regular antiretroviral therapy | 17/90 (18.89%) | 3/34 (8.82%) | 14/56 (25%) | 0.09 |
| C. neoformans VNI | 77/96 (80.21%) | 27/37 (72.97%) | 48/56 (85.71%) | 0.18 |
| AMB-FCZ therapy | 42/89 (47.19%) | 12/34 (35.29%) | 30/55 (54.55%) | 0.09 |
| FCZ MIC (mg/L) average (IQR) | 9 (1–16) | 8 (1–8) | 10 (1–16) | 0.20 |
| FCZ MIC ≥ 16 mg/L | 27/96 (28.13%) | 7/37 (18.92%) | 20/56 (35.71%) | 0.10 |
| Neuroimaging-computed tomography | ||||
| Cerebral edema | 4/90 (4.44%) | 4/34 (11.76%) | 0/56 (0%) | 0.02 |
| Ventricular dilation | 4/90 (4.44%) | 4/34 (11.76%) | 0/56 (0%) | 0.02 |
| No alterations | 42/90 (46.67%) | 11/34 (32.35%) | 31/56 (55.36%) | 0.05 |
| Neuroimaging-magnetic resonance imaging | ||||
| Pseudocysts | 4/90 (4.44%) | 3/34 (8.82%) | 1/56 (1.79%) | 0.15 |
*p ≤ 0.2; IQR, interquartile range 25–75%; CSF, cerebrospinal fluid; AMB, amphotericin B; FCZ, fluconazole; MIC, minimum inhibitory concentration
At the multiple model, the CSF yeast count-log (OR = 1.37, 95% CI 1.11–1.71, p = 0.004), culture of Cryptococcus spp. from bloodstream (OR = 3.66, 95% CI 1.08–12.30, p = 0.04) and cerebral toxoplasmosis (OR = 12.53, 95% CI 2.04–76.93, p = 0.006) were associated with this outcome. This model showed the area under the ROC curve of 76.2% (95% CI 0.70–0.90). The accuracy was 78.65%, with a sensitivity of 61.7% and specificity of 89.1% (positive predictive value 77.8%, negative predictive value 79.0%). This model showed the goodness of fit with p = 0.71 and p = 0.74 to the Pearson and the Hosmer–Lemeshow test, respectively.
When the extreme and the influential observations were excluded, the CSF yeast count-log remained associated with the composite outcome (Additional file 1: Fig. S3, Table S3 and S4).
The increase of one unit of CSF yeasts count-log was associated with the acceleration of 14% to the occurrence of one of the events, observed in the Cox regression model (Hazard ratio [HR] = 1.14, 95% CI 0.99–1.33 p = 0.07). The culture of Cryptococcus spp. exhibited an HR of 2.1 in the same model (95% CI 0.98–4.53, p = 0.05). Diagnosis of cerebral toxoplasmosis showed an HR of 1.44 (95% CI 0.546–3.82, p = 0.46).The patients who had CSF yeast count ≥ 200 yeasts/mm3, culture of Cryptococcus spp. from bloodstream or cerebral toxoplasmosis presented lower survival time related to the occurrence of one of the events of the composite outcome (Fig. 4).
Fig. 4.
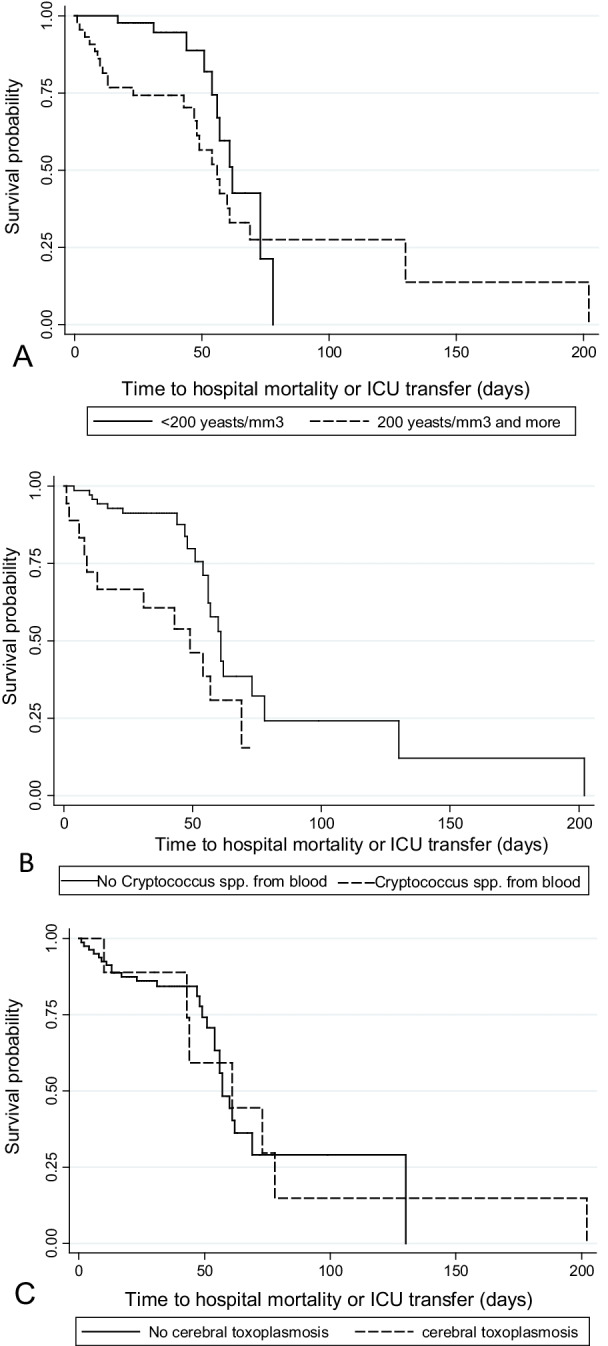
Survival curves until the occurrence of the composed outcome (hospital mortality and/orICU transfer), according to the variables selected for the multiple analysis model. A CSF yeasts count, B culture of Cryptococcus spp. from bloodstream and C concomitant cerebral toxoplasmosis
Discussion
We studied a cohort of patients with cryptococcal meningitis, which were predominantly from PLWHA and, as in other studies, the majority were males, also reflecting the population most affected by the AIDS epidemic [51–57]. The in-hospital mortality observed was 26.9% and, according to other Brazilian studies, this rate may vary from 25 to 74%, especially in the first weeks of therapy [4, 55, 58–62].
In Brazil, the etiologic agents of cryptococcal meningitis are, in their vast majority, C. neoformans s.s., also confirmed in this series of patients [4, 11, 55, 62–68]. Of the 3 cases of C. deuterogattii infection, 2 were negative HIV, and among them, one of them was diagnosed with primary immunodeficiency. About the gravity of these patients, no association was observed between the species and the outcomes. No influence was observed at the analysis without the C. deuterogatti isolates (data no shown). The presence of cryptococcomas is most usual in C. deuterogattii infections and it can cause additional neurological damage, impair the antifungal drugs access to infection sites and need prolonged therapy [69–74]. The cryptococcomas were described at 5 patients, 2 C. neoformans VNI, 2 C. neoformans VNII and 1 case who resulted in the only death with C. deuterogattii isolation. Among the 25 cases of in-hospital death, the majority presented C. neoformans VNI (17), followed by 7 C. neoformans VNII and 1 C. deuterogattii isolates. It is known that C. deuterogattii is the second specie most frequent, and it shows greater virulence when compared to C. neoformans, which could justify the individuals without apparent immunosuppression can be infected by this specie. However, other factors may be associated with and interfere with the immune response of patients, such as age and the presence of other comorbidities [63, 75–77]. As described in studies conducted in Southern Brazil, C. neoformans VNI overtake the others in this study, and among C. gattii complex, C. deuterogattii was exclusive [78–82].
The multiple analysis revealed important variables potentially available in the first week of hospitalization, including age, CSF yeast count, systemic arterial hypertension and neurological impairment identified by CT. The survival analysis indicated that these variables were consistent predictors of time to in-hospital mortality, even when adjusted. The Cox regression indicated that these variables maintained the associations, even after the exclusion of influential observations and outliers. In the secondary analysis, CSF yeast count, culture of Cryptococcus spp. from the bloodstream and cerebral toxoplasmosis were associated with the composed outcome. All these associations were consistent in the survival models.
The age was significantly associated with in-hospital death, and this result was also observed at the multiple analysis confirming that age can be a risk factor for death in these patients [52, 74, 83, 84]. A study performed in Taiwan observed that age > 60 years was a predictor of poor prognosis [52]. Another research with cryptococcal meningitis patients showed age > 50 years independently associated with death within two weeks [22]. Therefore, our study is consistent with these associations and we also suggest that age exhibited a biological gradient since the increase in years was progressively associated with the odds of death.
Fungal burden has been associated with therapeutic failure and death [4, 22, 43, 85–88]. However, fungal burden is formally evaluated with quantitative CSF cultures [22] but this methodology is usually unavailable in routine clinical practice of low and middle-income countries. In the present study, quantitative CSF yeast counts performed by direct microscopic exam was associated with mortality in multiple analysis by logistic regression. This association was suggested in the univariable analysis of a previous study performed at our institution, which include 46 HIV-infected patients with cryptococcal meningitis [88]. Thus, our results extend the value of quantitative CSF yeast counts performed by direct microscopic exam.
The presence of a high number of Cryptococcus spp. yeasts at CSF contribute to increase intracranial pressure, it being a mechanism proposed to the mechanical obstruction of CSF flow [89, 90]. Probably thick capsules and giant cells also contribute to increased intracranial pressure [91, 92]. Although there was no difference between increased intracranial pressure and death in this study, there was a trend of a greater number of cases of this variable related to death, as reported in the literature [7, 93–95]. Probably due to the sample size, it was not possible to estimate this association with precision. Furthermore, aggressive control of measures to control the increased intracranial pressure may have masked the effect of this variable on mortality.
The presence of Cryptococcus spp. antigen in CSF was not associated with outcomes in a prior study, suggesting that serum or CSF antigen dosage cannot be considered a predictive factor for failure to treat or relapse of cryptococcosis in this specific hospitalized population [7]. However, the dissemination of cryptococcosis, identified by the culture of Cryptococcus spp. from extra neural sites, was associated with an increased hazard of the composite outcome. This is consistent with previous reports in which the isolation of Cryptococcus spp. from the bloodstream was and predictor of poor clinical outcome [83, 94, 96–99].
The CD4 < 200 cells/mm3 was not associated with the outcomes of our study. However, it is known that when a variable presents low variability or values predominantly reduced, as in this case, the capacity of discrimination is reduced, which could explain the lack of association of this variable with the outcomes.
World Health Organization recommends screening all PLWHA who have a CD4 < 200 cells/mm3 for cryptococcal antigen to identify those patients who could benefit from preemptive fluconazole treatment prior to the onset of meningitis [7, 100]. Nevertheless, where cryptococcal antigen testing is not available, FCZ primary prophylaxis should be used in some epidemiological settings [7, 96, 100].
All patients were treated with AMBd during the therapy induction and the combined therapy was administered in almost all patients. According to recommendations, AMB-combination is the most appropriate therapy for cryptococcal meningitis [7, 26]. Due to the partial availability of 5FC at the hospital, this drug was given to almost half of the patients. In this study, in contrast to current recommendations [7], the use of 5FC or FCZ as a second drug was not associated with the outcomes (in-hospital mortality: 5FC p = 1 and FCZ p = 0.6; composite outcome: 5FC p = 0.83 and FCZ p = 0.09). This result can indicate multiples intervening factors in this retrospective observational study. Similarly, antifungal susceptibility was not associated with the outcomes. Currently, the determination of AMB-MIC and FCZ-MIC are not recommended at the hospital routine, since the correlation between these results and the clinical response remains uncertain. Also, the determination of clinical breakpoints for Cryptococcus spp. remains non-existent due to the absence of studies correlating MIC or other susceptibility measures with the clinical prognosis [28, 29, 42, 47, 83, 101, 102]. All strains presented FCZ-heteroresistant clones however, even with high values (53.1% of strains with LHF ≥ 32 mg/L) the LHF was not associated with the severity of cryptococcal meningitis. In a previous study, LHF clones were obtained from Cryptococcus neoformans strains of the first episode of no-AIDS patient and from another patient with relapses of cryptococcosis. The LHF was similar to both [33]. On the other hand, a study showed distinct LHF at FCZ-resistant and susceptible strains and higher FCZ-MIC at respective heteroresistant clones [36]. Likewise, the fungicidal activity of AMB by Time-kill methodology showed no association with outcomes. Similar result was previously described [3]. On the other hand, a study reported an association of AMB-tolerant strains and mortality [41, 42]. Possibly the sample size can be responsible for the absence of this association in our study.
The sample size was a limiting factor that may have affected the precision of estimates, for instance, at the 95% CI of systemic arterial hypertension and that of the neurological impairment by CT, that it was wide. Despite this, associations were consistent among the models evaluated including those with binary and time-to-event outcomes. All associations with the outcomes were maintained in all models, like OR and OR adjusted and the survival models. These observations were maintained even when the extreme and influential observations were excluded and, therefore, these findings indicate these predictors are associated with the frequency and the velocity of the outcomes. Moreover, the associations were coherent with the expected according to the literature. For this reason, it is plausible that the associations observed could be extrapolated to other populations.
Conclusion
We identified clinical and laboratorial factors observed in the first days of hospitalization able to predict the prognosis of cryptococcosis in a predominantly PLWHA. The associations remained consistent when evaluated in survival analyses. CSF yeast count was a consistent predictor of in- hospital mortality and severity and the methodology used in this study can be implemented in low and middle-income settings.
Supplementary Information
Additional file 1. SUPPLEMENTARY DATA Figure S1- Delta-deviance and leverage of observations generating the multiple model using the variables age, CSF yeast count-log, systemic arterial hypertension and neurological impairment by computed tomography associated with hospital mortality. Figure S2- Survival curves until the in-hospital mortality, according to the variables selected for the multiple analysis model adjusted. Figure S3 - Delta-deviance and leverage of observations generating the multiple model using the CSF yeast count-log, positive blood culture for Cryptococcus spp. and cerebral toxoplasmosis, associated with composed outcome. Table S1- Multiple analysis by logistic regression of variables associated to in-hospital mortality, without the influential observations. Table S2 - Multiple analysis by logistic regression of variables associated to in-hospital mortality, without the outliers. Table S3 – Multiple analysis by logistic regression of variables associated to composed outcome, without the influential observations. Table S4 - Multiple analysis by logistic regression of variables associated to composed outcome, without the outliers.
Acknowledgements
We thank Marilena dos Anjos Martins and Jefferson Sabino Rodrigues for the collaboration in the molecular genotyping tests and Maria Walderez Szeszs, Lucas Xavier Bonfietti and Mirian Rando Araujo for helping with the antifungal susceptibility tests.
Authors' contributions
LO conceived the study, participated of the design, performed the laboratory experiments, collected of the clinical and laboratory data from medical records, created, carried out the database and its interpretation and prepared the first draft of the manuscript. RB e OJC performed the collect of clinical and laboratory data from medical records and contributed to the database elaboration. MSCM participated in design study, supported in development and interpretation of the antifungal susceptibility tests. JEV collaborate to collect the clinical data from medical records, FADQ participated in its design and coordination of the study, carried out the data analysis and collaborate with preparation of the manuscript. All authors participated at the study implementation, contributed to editing and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
LO was supported by National Council for Scientific and Technological Development -CNPq, process identification: 165381/2015-9. FADQ is beneficiary of a fellowship for research productivity from the National Council for Scientific and Technological Development—CNPq, process/contract identification: 312656/2019-0.
Availability of data and materials
The datasets used and analyzed during the current study are part of the patient's medical records. They are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent participate
This research was developed by the search for the clinical data from retrospective cases of cryptococcosis consequently, the consent to participate was not required of this study. The need of informed consent was waived by the ethical committee from Emílio Ribas Institute of Infectious Diseases. Besides, a declaration of confidentiality and use of information was required and signed by the authors, before submitting the research to the committees. All methods were performed in accordance with the relevant guidelines and regulations. This study was approved by Research Ethics Committees of the School of Public Health of the University of São Paulo (Certificate of Presentation for Ethical Appreciation: 54949716.8.0000.5421), Adolfo Lutz Institute (Certificate of Presentation for Ethical Appreciation: 54949716.8.3001.0059) and of Emílio Ribas Institute of Infectious Diseases (Certificate of Presentation for Ethical Appreciation: 54949716.8.2001.0061).
Consent for publication
Not applicable.
Competing interests
The authors report no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lin X, Heitman J. The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol. 2006;60(1):69–105. doi: 10.1146/annurev.micro.60.080805.142102. [DOI] [PubMed] [Google Scholar]
- 2.Valle LD. HIV disorders of the brain; pathology and pathogenesis. Front Biosci. 2006;11(1):718. doi: 10.2741/1830. [DOI] [PubMed] [Google Scholar]
- 3.Pappalardo MCSM, Szeszs MW, Martins MA, et al. Susceptibility of clinical isolates of Cryptococcus neoformans to amphotericin B using time–kill methodology. Diagn Microbiol Infect Dis. 2009;64(2):146–151. doi: 10.1016/j.diagmicrobio.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Mora DJ, da Cunha Colombo ER, Ferreira-Paim K, Andrade-Silva LE, Nascentes GAN, Silva-Vergara ML. Clinical, epidemiological and outcome features of patients with Cryptococcosis in Uberaba, Minas Gerais, Brazil. Mycopathologia. 2012;173(5–6):321–327. doi: 10.1007/s11046-011-9504-9. [DOI] [PubMed] [Google Scholar]
- 5.Domingues CSB, Waldman EA. Causes of death among people living with aids in the pre- and post-HAART eras in the city of São Paulo, Brazil. In: Vermund SH, editor. PLoS ONE. 2014;9(12):e114661. 10.1371/journal.pone.0114661 [DOI] [PMC free article] [PubMed]
- 6.Opintan JA, Awadzi BK, Biney IJK, et al. High rates of cerebral toxoplasmosis in HIV patients presenting with meningitis in Accra, Ghana. Trans R Soc Trop Med Hyg. 2017;111(10):464–471. doi: 10.1093/trstmh/trx083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Guidelines on the Diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection; 2018. [PubMed]
- 8.Hagen F, Khayhan K, Theelen B, et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol. 2015;78:16–48. doi: 10.1016/j.fgb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Byrnes EJ, Bartlett KH, Perfect JR, Heitman J. Cryptococcus gattii: an emerging fungal pathogen infecting humans and animals. Microbes Infect. 2011;13(11):895–907. doi: 10.1016/j.micinf.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trilles L, Meyer W, Wanke B, Guarro J, Lazéra M. Correlation of antifungal susceptibility and molecular type within the Cryptococcusneoformans/C. gattii species complex. Med Mycol. 2012;50:328. doi: 10.3109/13693786.2011.602126. [DOI] [PubMed] [Google Scholar]
- 11.Cogliati M. Global molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii: an atlas of the molecular types. Scientifica. 2013;2013:1–23. doi: 10.1155/2013/675213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beale MA, Sabiiti W, Robertson EJ, et al. Genotypic diversity is associated with clinical outcome and phenotype in cryptococcal meningitis across southern Africa. In: Vinetz JM, ed. PLOS Negl Trop Dis. 2015;9(6):e0003847. 10.1371/journal.pntd.0003847 [DOI] [PMC free article] [PubMed]
- 13.Nishikawa MM, Almeida-Paes R, Brito-Santos F, et al. Comparative antifungal susceptibility analyses of Cryptococcus neoformans VNI and Cryptococcus gattii VGII from the Brazilian Amazon Region by the Etest, Vitek 2, and the Clinical and Laboratory Standards Institute broth microdilution methods. Medical Mycology. 2019 doi: 10.1093/mmy/myy150. [DOI] [PubMed] [Google Scholar]
- 14.Pakshir K, Fakhim H, Vaezi A, et al. Molecular epidemiology of environmental Cryptococcus species isolates based on amplified fragment length polymorphism. J de Mycologie Médicale. 2018;28(4):599–605. doi: 10.1016/j.mycmed.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Firacative C, Lizarazo J, Illnait-Zaragozí MT, Castañeda E. The status of cryptococcosis in Latin America. Memórias do Instituto Oswaldo Cruz. 2018 doi: 10.1590/0074-02760170554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagen F, Lumbsch HT, Arsic Arsenijevic V, et al. Importance of resolving fungal nomenclature: the case of multiple pathogenic species in the Cryptococcus genus. In: Lorenz M, editor. mSphere. 2017;2(4). 10.1128/mSphere.00238-17 [DOI] [PMC free article] [PubMed]
- 17.Ellis DH, Pfeiffer TJ. Natural habitat of Cryptococcus neoformans var. gattii. J Clin Microbiol. 1990;28(7):1642–1644. doi: 10.1128/jcm.28.7.1642-1644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montenegro H, Paula CR. Environmental isolation of Cryptococcusneoformans var. gattii and C. neoformans var. neoformans in the city of São Paulo, Brazil. Med Mycol. 2000;38:385. doi: 10.1080/mmy.38.5.385.390. [DOI] [PubMed] [Google Scholar]
- 19.Ngamskulrungroj P, Meyer W. Melanin production at 37°C is linked to the high virulent Cryptococcus gattii Vancouver Island outbreak genotype VGIIa. Australasian Mycologist. 2009;28:9–14. [Google Scholar]
- 20.Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17(8):873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corbett EL, Churchyard GJ, Charalambos S, et al. Morbidity and mortality in South African gold miners: impact of untreated disease due to human immunodeficiency virus. Clin Infect Dis. 2002;34(9):1251–1258. doi: 10.1086/339540. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis JN, Bicanic T, Loyse A, et al. Determinants of Mortality in a combined cohort of 501 patients with hiv-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis. 2014;58(5):736–745. doi: 10.1093/cid/cit794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 24.Pyrgos V, Seitz Ae, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal Meningitis in the US: 1997–2009. In: Arez AP, ed. PLoS ONE. 2013;8(2):e56269. 10.1371/journal.pone.0056269. [DOI] [PMC free article] [PubMed]
- 25.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lortholary O, Fernández-Ruiz M, Perfect JR. The current treatment landscape: other fungal diseases (cryptococcosis, fusariosis and mucormycosis) J Antimicrob Chemother. 2016;71(suppl 2):31–36. doi: 10.1093/jac/dkw394. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller M, Zhang J, Messer S, et al. Molecular epidemiology and antifungal susceptibility of Cryptococcus neoformans isolates from Ugandan AIDS patients. Diagn Microbiol Infect Dis. 1998;32(3):191–199. doi: 10.1016/S0732-8893(98)00095-9. [DOI] [PubMed] [Google Scholar]
- 28.Aller AI, Martin-Mazuelos E, Lozano F, et al. Correlation of fluconazole MICs with Clinical outcome in cryptococcal infection. Antimicrob Agents Chemother. 2000;44(6):1544–1548. doi: 10.1128/AAC.44.6.1544-1548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dannaoui E, Abdul M, Arpin M, et al. Results Obtained with various antifungal susceptibility testing methods do not predict early clinical outcome in patients with cryptococcosis. Antimicrob Agents Chemother. 2006;50(7):2464–2470. doi: 10.1128/AAC.01520-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodero L, Córdoba S, Cahn P, et al. In vitro susceptibility studies of Cryptococcus neoformans isolated from patients with no clinical response to amphotericin B therapy. J Antimicrob Chemother. 2000;45(2):239–242. doi: 10.1093/jac/45.2.239. [DOI] [PubMed] [Google Scholar]
- 31.Silva DC, Martins MA, Szeszs MW, Bonfietti LX, Matos D, Melhem MSC. Susceptibility to antifungal agents and genotypes of Brazilian clinical and environmental Cryptococcus gattii strains. Diagn Microbiol Infect Dis. 2012;72(4):332–339. doi: 10.1016/j.diagmicrobio.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira L, Santos DCS, dos Martins MA, Szeszs MW, Melhem MSC. Time-Kill Curves studies with amphotericin b against Cryptococcus neoformans/C. gattii species Complex clinical isolates. Curr Fungal Infect Rep. 2017;11(4):158–162. doi: 10.1007/s12281-017-0296-3. [DOI] [Google Scholar]
- 33.Mondon P, Petter R, Amalfitano G, et al. Heteroresistance to fluconazole and voriconazole in Cryptococcus neoformans. Antimicrob Agents Chemother. 1999;43(8):1856–1861. doi: 10.1128/AAC.43.8.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sionov E, Chang YC, Garraffo HM, Kwon-Chung KJ. Heteroresistance to fluconazole in Cryptococcus neoformans is intrinsic and associated with virulence. Antimicrob Agents Chemother. 2009;53(7):2804–2815. doi: 10.1128/AAC.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira GF, Santos JRA, da Costa MC, et al. Heteroresistance to itraconazole alters the morphology and increases the virulence of Cryptococcus gattii. Antimicrob Agents Chemother. 2015;59(8):4600–4609. doi: 10.1128/AAC.00466-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamazumi T, Pfaller MA, Messer SA, et al. Characterization of heteroresistance to fluconazole among clinical isolates of Cryptococcus neoformans. J Clin Microbiol. 2003;41(1):267–272. doi: 10.1128/JCM.41.1.267-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheong JWS, McCormack J. Fluconazole resistance in cryptococcal disease: emerging or intrinsic? Med Mycol. 2013;51(3):261–269. doi: 10.3109/13693786.2012.715763. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira GF, Santos DA. Heteroresistance and fungi. Mycoses. 2017;60(9):562–568. doi: 10.1111/myc.12639. [DOI] [PubMed] [Google Scholar]
- 39.Schiave LA, Nascimento E, Gaspar GG, et al. Minimum concentration of Amphotericin B in serum according to the formulation, dose, and daily or prolonged intermittent therapeutic regimen. Rev Soc Bras Med Trop. 2020;53:e20180463. doi: 10.1590/0037-8682-0463-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fields BT, Bates JH, Abernathy RS. Amphotericin B Serum concentrations during therapy. Appl Microbiol. 1970;19(6):955–959. doi: 10.1128/am.19.6.955-959.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodero L, Cordoba S, Cahn P, et al. Timed-kill curves for Cryptococcus neoformans isolated from patients with AIDS. Med Mycol. 2000;38(3):201–207. doi: 10.1080/mmy.38.3.201.207. [DOI] [PubMed] [Google Scholar]
- 42.Córdoba S, Vivot W, Szusz W, Isla G, Davel G. Comparison of different in vitro tests to detect Cryptococcus neoformans not susceptible to amphotericin B. Mycopathologia. 2015;179(5–6):359–371. doi: 10.1007/s11046-015-9871-8. [DOI] [PubMed] [Google Scholar]
- 43.Vidal JE, Gerhardt J, de Miranda PÉJ, et al. Role of quantitative CSF microscopy to predict culture status and outcome in HIV-associated cryptococcal meningitis in a Brazilian cohort. Diagn Microbiol Infect Dis. 2012;73(1):68–73. doi: 10.1016/j.diagmicrobio.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurtzman C, Fell JW, Boekhout T. The yeasts. 5 edn. Elsevier Science; 2011.
- 45.Meyer W, Castañeda A, Jackson S, et al. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9(2):189. doi: 10.3201/eid0902.020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arendrup MC, Guinea J, Cuenca-Estrella M, et al. Eucast definitive document e.def 7.3. 21.
- 47.Pfaller MA, Messer SA, Boyken L, et al. Global trends in the antifungal susceptibility of Cryptococcus neoformans (1990 to 2004) J Clin Microbiol. 2005;43(5):2163–2167. doi: 10.1128/JCM.43.5.2163-2167.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klepser ME, Ernst EJ, Lewis RE, Ernst ME, Pfaller MA. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob Agents Chemother. 1998;42(5):1207–1212. doi: 10.1128/AAC.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann JP. Regression models for categorical, count, and related variables : an applied approach. University of California Press; 2016.
- 50.Précoma DB, de Oliveira GMM, Simão AF, et al. Updated cardiovascular prevention guideline of the brazilian society of cardiology—2019. Arquivos Brasileiros de Cardiologia. 2019 doi: 10.5935/abc.20190204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mdodo R, Moser SA, Jaoko W, et al. Antifungal susceptibilities of Cryptococcus neoformans cerebrospinal fluid isolates from AIDS patients in Kenya. Mycoses. 2011;54(5):e438–442. doi: 10.1111/j.1439-0507.2010.01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee CH, Chang TY, Liu JW, et al. Correlation of anti-fungal susceptibility with clinical outcomes in patients with cryptococcal meningitis. BMC Infect Dis. 2012;12(1):361. doi: 10.1186/1471-2334-12-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agudelo CA, Muñoz C, Ramírez A, et al. Response to therapy in patients with cryptococcosis and AIDS: association with in vitro susceptibility to fluconazole. Rev Iberoam Micol. 2015;32(4):214–220. doi: 10.1016/j.riam.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Chen YC, Chang TY, Liu JW, et al. Increasing trend of fluconazole-non-susceptible Cryptococcus neoformans in patients with invasive cryptococcosis: a 12-year longitudinal study. BMC Infect Dis. 2015 doi: 10.1186/s12879-015-1023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aguiar de PADF, Pedroso dos RS, Borges AS, Moreira de TA, Araújo de LB, Röder de DVDB. The epidemiology of cryptococcosis and the characterization of Cryptococcus neoformans isolated in a Brazilian University Hospital. Revista do Instituto de Medicina Tropical de São Paulo. 2017. 10.1590/s1678-9946201759013. [DOI] [PMC free article] [PubMed]
- 56.Arechavala A, Negroni R, Messina F, et al. Cryptococcosis in an infectious diseases hospital of Buenos Aires, Argentina. Revision of 2041 cases: diagnosis, clinical features and therapeutics. Revista Iberoamericana de Micología. 2018;35(1):1–10. doi: 10.1016/j.riam.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Shaheen AA, Somayaji R, Myers R, Mody CH. Epidemiology and trends of cryptococcosis in the United States from 2000 to 2007: a population-based study. Int J STD AIDS. 2018;29(5):453–460. doi: 10.1177/0956462417732649. [DOI] [PubMed] [Google Scholar]
- 58.de Moreira TA, Ferreira MS, Ribas RM, Borges AS. Criptococose: estudo clínico-epidemiológico, laboratorial e das variedades do fungo em 96 pacientes. Revista da Sociedade Brasileira de Medicina Trop. 2006;39(3):255–258. doi: 10.1590/S0037-86822006000300005. [DOI] [PubMed] [Google Scholar]
- 59.da Silva PR, de Rabelo RAS, Terra APS, Teixeira DNS. Susceptibility to antifungal agents among Cryptococcus neoformans varieties isolated from patients at a university hospital. Revista da Sociedade Brasileira de Medicina Tropical. 2008;41(2):158–162. doi: 10.1590/S0037-86822008000200005. [DOI] [PubMed] [Google Scholar]
- 60.Mascarenhas-Batista AV, Mascarenhas Souza N, Sacramento E. Fatores prognósticos na meningite criptocócica em hospital de referência para doenças infecciosas. Revista Baiana de Saúde Pública. 2013;37:68. doi: 10.22278/2318-2660.2013.v37.n0.a591. [DOI] [Google Scholar]
- 61.de Azambuja AZ, Wissmann Neto G, Watte G, Antoniolli L, Goldani LZ. cryptococcal meningitis: a retrospective cohort of a Brazilian reference hospital in the post-HAART era of Universal Access. Canadian J Infect Dis Med Microbiol. 2018;2018:1–5. doi: 10.1155/2018/6512468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Nunes JO, de Tsujisaki RAS, de Nunes MO, et al. Cryptococcal meningitis epidemiology: 17 years of experience in a state of the Brazilian Pantanal. Revista da Sociedade Brasileira de Medicina Trop. 2018;51(4):485–492. doi: 10.1590/0037-8682-0050-2018. [DOI] [PubMed] [Google Scholar]
- 63.Oliveira RV, Trilles L, dos Lázera MS, et al. Regional pattern of the molecular types of Cryptococcus neoformans and Cryptococcusgattii in Brazil. Mem Inst Oswaldo Cruz. 2008;103(5):455–462. doi: 10.1590/S0074-02762008000500002. [DOI] [PubMed] [Google Scholar]
- 64.Freire AKL, dos Santos BA, de Lima SI, et al. Molecular characterisation of the causative agents of Cryptococcosis in patients of a tertiary healthcare facility in the state of Amazonas-Brazil: cryptococcosis in the state of Amazonas-Brazil. Mycoses. 2012;55(3):e145–e150. doi: 10.1111/j.1439-0507.2012.02173.x. [DOI] [PubMed] [Google Scholar]
- 65.Matos CS, Andrade SA, Oliveira NS, Barros TF. Microbiological characteristics of clinical isolates of Cryptococcus spp. in Bahia, Brazil: molecular types and antifungal susceptibilities. Eur J Clin Microbiol Infect Dis. 2012;31(7):1647–1652. doi: 10.1007/s10096-011-1488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andrade-Silva L, Ferreira-Paim K, Mora DJ, et al. Susceptibility profile of clinical and environmental isolates of Cryptococcus neoformans and Cryptococcus gattii in Uberaba, Minas Gerais, Brazil. Medical Mycology. 2013;51(6):635–640. doi: 10.3109/13693786.2012.761737. [DOI] [PubMed] [Google Scholar]
- 67.Wirth F, Azevedo MI, Goldani LZ. Molecular types of Cryptococcus species isolated from patients with cryptococcal meningitis in a Brazilian tertiary care hospital. Brazilian J Infect Dis. 2018 doi: 10.1016/j.bjid.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herkert PF, Hagen F, Pinheiro RL, Muro MD, Meis JF, Queiroz-Telles F. Ecoepidemiology of Cryptococcus gattii in developing countries. J Fungi. 2017;3(4):62. doi: 10.3390/jof3040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen YC, Chang SC, Shih CC, et al. Clinical features and in vitro susceptibilities of two varieties of Cryptococcus neoformans in Taiwan. Diagn Microbiol Infect Dis. 2000;36(3):175–183. doi: 10.1016/S0732-8893(99)00137-6. [DOI] [PubMed] [Google Scholar]
- 70.Jain N, Wickes BL, Keller SM, et al. Molecular epidemiology of clinical cryptococcus neoformans strains from India. J Clin Microbiol. 2005;43(11):5733–5742. doi: 10.1128/JCM.43.11.5733-5742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galanis E. Epidemiology of Cryptococcusgattii, British Columbia, Canada, 1999–2007. Emerg Infect Dis. 2010 doi: 10.3201/eid1601.090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srivanitchapoom P, Pitakpatapee Y, Sitthinamsuwan P, Sathornsumetee S. Brainstem dysfunction heralding disseminated cryptococcosis. Clin Neurol Neurosurg. 2018;167:62–64. doi: 10.1016/j.clineuro.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 73.Wei J, Li XY, Zhang Y. Central nervous system Cryptococcoma mimicking demyelinating disease: a case report. BMC Neurol. 2020;20(1):297. doi: 10.1186/s12883-020-01880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chau TT, Mai NH, Phu NH, et al. A prospective descriptive study of cryptococcal meningitis in HIV uninfected patients in Vietnam—high prevalence of Cryptococcus neoformans var grubii in the absence of underlying disease. BMC Infect Dis. 2010 doi: 10.1186/1471-2334-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paula CR, Domingues-Ferreira M, Soares MCP, et al. Cryptococcusgattii: immunological and microbiological study in a patient with neurocryptococcosis. JMM Case Rep. 2015 doi: 10.1099/jmmcr.0.000099. [DOI] [Google Scholar]
- 76.de Vasconcelos DM, Domingues-Ferreira M, Soares MCP, et al. Cryptococcusgattii: immunological and microbiological study in a patient with neurocryptococcosis. JMM Case Rep. 2015 doi: 10.1099/jmmcr.0.000099. [DOI] [Google Scholar]
- 77.Amaral do DM, Rocha de RCC, Carneiro LEP, Vasconcelos DM, Abreu de MAMM. Disseminated cryptococcosis manifested as a single tumor in an immunocompetent patient, similar to the cutaneous primary forms. Anais Brasileiros de Dermatologia. 2016;91(5 suppl 1):29–31. 10.1590/abd1806-4841.20164582 [DOI] [PMC free article] [PubMed]
- 78.Grizante Barião PH, Tonani L, Cocio TA, Martinez R, Nascimento É, von Zeska Kress MR. Molecular typing, in vitro susceptibility and virulence of Cryptococcus neoformans/Cryptococcus gattii species complex clinical isolates from south-eastern Brazil. Mycoses. 2020;63(12):1341–1351. doi: 10.1111/myc.13174. [DOI] [PubMed] [Google Scholar]
- 79.Nascimento E, Barião PHG, von Kress MRZ, et al. Cryptococcosis by Cryptococcusneoformans/Cryptococcusgattii species complexes in non-HIV-infected patients in southeastern Brazil. Rev Soc Bras Med Trop. 2021;54:e0169–2021. doi: 10.1590/0037-8682-0169-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herkert PF, Meis JF, de Salvador LOG, et al. Molecular characterization and antifungal susceptibility testing of Cryptococcusneoformans sensu stricto from southern Brazil. J Med Microbiol. 2018;67(4):560–569. doi: 10.1099/jmm.0.000698. [DOI] [PubMed] [Google Scholar]
- 81.Andrade-Silva LE, Ferreira-Paim K, Ferreira TB, et al. Genotypic analysis of clinical and environmental Cryptococcus neoformans isolates from Brazil reveals the presence of VNB isolates and a correlation with biological factors. In: Nielsen K, editor. PLoS ONE. 2018;13(3):e0193237. 10.1371/journal.pone.0193237. [DOI] [PMC free article] [PubMed]
- 82.Damasceno-Escoura AH, de Souza ML, de Oliveira NF, et al. Epidemiological, clinical and outcome aspects of patients with cryptococcosis caused by Cryptococcus gattii from a non-endemic area of Brazil. Mycopathologia. 2019;184(1):65–71. doi: 10.1007/s11046-018-0304-3. [DOI] [PubMed] [Google Scholar]
- 83.Tseng HK, Liu CP, Ho MW, et al. Microbiological, Epidemiological, and clinical characteristics and outcomes of patients with cryptococcosis in Taiwan, 1997–2010. In: Chaturvedi V, editor. PLoS ONE. 2013;8(4):e61921. 10.1371/journal.pone.0061921. [DOI] [PMC free article] [PubMed]
- 84.Tsai WC, Lien CY, Lee JJ, et al. The clinical characteristics and therapeutic outcomes of cryptococcal meningitis in elderly patients: a hospital-based study. BMC Geriatr. 2019;19(1):91. doi: 10.1186/s12877-019-1108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pitisuttithum P, Tansuphasawadikul S, Simpson AJH, Howe PA, White NJ. A prospective study of aids-associated cryptococcal meningitis in thailand treated with high-dose amphotericin b. J Infect. 2001;43(4):226–233. doi: 10.1053/jinf.2001.0916. [DOI] [PubMed] [Google Scholar]
- 86.Brouwer AE, Rajanuwong A, Chierakul W, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363(9423):1764–1767. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 87.Dromer F, Mathoulin-Pélissier S, Launay O, Lortholary O, the French Cryptococcosis study group. Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D Study. In: Perfect JR, editor. PLoS Med. 2007;4(2):e21. 10.1371/journal.pmed.0040021. [DOI] [PMC free article] [PubMed]
- 88.Chagas OJ, Buccheri R, de Souza Melhem CM, et al. Usefulness of yeast cell counting and lack of clinical correlation of the antifungal susceptibility testing results in management of aids-associated cryptococcal meningitis. Curr Fungal Infect Rep. 2020;14(1):1–8. 10.1007/s12281-020-00368-5.
- 89.Bicanic T, Brouwer AE, Meintjes G, et al. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS. 2009 doi: 10.1097/QAD.0b013e32832605fe. [DOI] [PubMed] [Google Scholar]
- 90.Loyse A, Wainwright H, Jarvis JN, et al. Histopathology of the arachnoid granulations and brain in HIV-associated cryptococcal meningitis: correlation with cerebrospinal fluid pressure. AIDS. 2010;24(3):405–410. doi: 10.1097/QAD.0b013e328333c005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robertson EJ, Najjuka G, Rolfes MA, et al. Cryptococcus neoformans ex vivo capsule size is associated with intracranial pressure and host immune response in hiv-associated cryptococcal meningitis. J Infect Dis. 2014;209(1):74–82. doi: 10.1093/infdis/jit435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bouklas T, Fries BC. Aging as an emergent factor that contributes to phenotypic variation in Cryptococcus neoformans. Fungal Genet Biol. 2015;78:59–64. doi: 10.1016/j.fgb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee YC, Wang JT, Sun HY, Chen YC. Comparisons of clinical features and mortality of cryptococcal meningitis between patients with and without human immunodeficiency virus infection. J Microbiol Immunol Infect. 2011;44(5):338–345. doi: 10.1016/j.jmii.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 94.Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. In: Feldmesser M, editor. PLoS ONE. 2013;8(3):e60431. 10.1371/journal.pone.0060431. [DOI] [PMC free article] [PubMed]
- 95.Rolfes MA, Hullsiek KH, Rhein J, et al. The Effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis. 2014;59(11):1607–1614. doi: 10.1093/cid/ciu596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12(8):929–935. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 97.Govender NP, Patel J, van Wyk M, Chiller TM, Lockhart SR, for the Group for enteric, respiratory and meningeal disease surveillance in South Africa (GERMS-SA). Trends in Antifungal Drug Susceptibility of Cryptococcus neoformans Isolates obtained through population-based surveillance in South Africa in 2002–2003 and 2007–2008. Antimicrob Agents Chemother. 2011;55(6):2606–2611. 10.1128/AAC.00048-11. [DOI] [PMC free article] [PubMed]
- 98.Hashemi R, Majidi A, Tabatabaey A, Motamed H. Fatal disseminated Cryptococcus infection in an immunocompetent patient. Arch Clin Infect Dis. 2014 doi: 10.5812/archcid.20246. [DOI] [Google Scholar]
- 99.Badali H, Alian S, Fakhim H, et al. Cryptococcal meningitis due to Cryptococcus neoformans genotype AFLP1/VNI in Iran: a review of the literature. Mycoses. 2015;58(12):689–693. doi: 10.1111/myc.12415. [DOI] [PubMed] [Google Scholar]
- 100.Ford N, Shubber Z, Jarvis JN, et al. CD4 cell count threshold for cryptococcal antigen screening of HIV-infected individuals: a systematic review and meta-analysis. Clin Infect Dis. 2018;66(suppl_2):S152–S159. doi: 10.1093/cid/cix1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vena A, Muñoz P, Guinea J, et al. Fluconazole resistance is not a predictor of poor outcome in patients with cryptococcosis. Mycoses. 2018 doi: 10.1111/myc.12847. [DOI] [PubMed] [Google Scholar]
- 102.O’Connor L, Van Anh D, Chau TTH, et al. Antifungal susceptibility does not correlate with fungal clearance or survival in aids-associated cryptococcal meningitis. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. SUPPLEMENTARY DATA Figure S1- Delta-deviance and leverage of observations generating the multiple model using the variables age, CSF yeast count-log, systemic arterial hypertension and neurological impairment by computed tomography associated with hospital mortality. Figure S2- Survival curves until the in-hospital mortality, according to the variables selected for the multiple analysis model adjusted. Figure S3 - Delta-deviance and leverage of observations generating the multiple model using the CSF yeast count-log, positive blood culture for Cryptococcus spp. and cerebral toxoplasmosis, associated with composed outcome. Table S1- Multiple analysis by logistic regression of variables associated to in-hospital mortality, without the influential observations. Table S2 - Multiple analysis by logistic regression of variables associated to in-hospital mortality, without the outliers. Table S3 – Multiple analysis by logistic regression of variables associated to composed outcome, without the influential observations. Table S4 - Multiple analysis by logistic regression of variables associated to composed outcome, without the outliers.
Data Availability Statement
The datasets used and analyzed during the current study are part of the patient's medical records. They are available from the corresponding author on reasonable request.