Introduction
Vitiligo is a depigmentation disorder that affects approximately 1% of the population of the United States and Europe, with a peak age of onset earlier in females compared to males.1 It presents in females typically in the first decade of life, while males tend to present in the fifth decade of life.1 It is characterized by a patchy distribution of depigmented macules and is subclassified by location and distribution of affected skin.1 Vitiligo is hypothesized to be autoimmune in nature, provoked by an environmental or biologic trigger that results in destruction of melanocytes.1 Vitiligo is associated with other autoimmune disorders and has been reported to develop following the onset of viral illnesses, such as HIV, hepatitis C virus, and cytomegalovirus.2 SARS-CoV-2 infection has been linked to a rise in autoimmunity, with many patients reporting novel postviral autoimmune conditions.3 In this report, we present a patient with no history of autoimmune disease, who developed vitiligo after contracting SARS-CoV-2 infection.
Case report
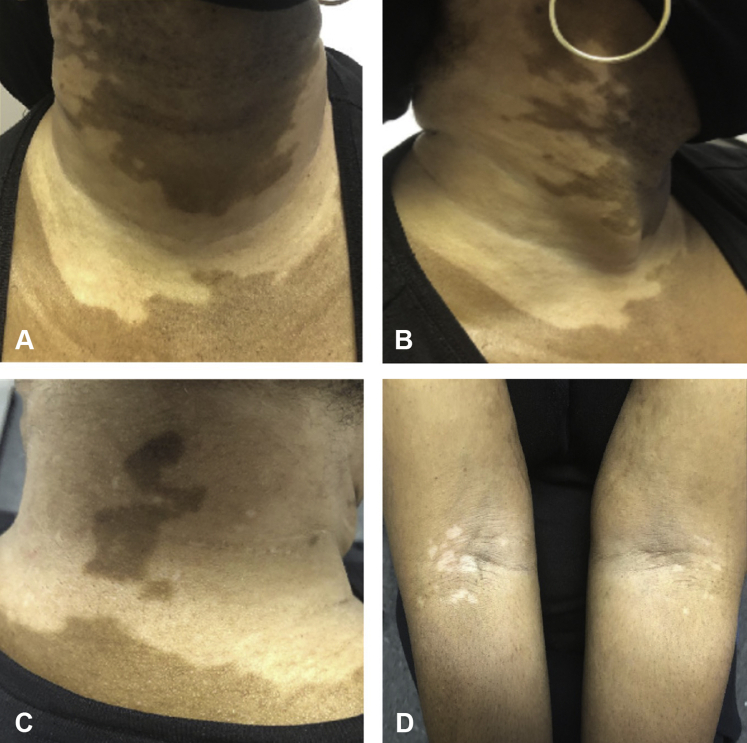
A 52-year-old woman presented to the dermatology clinic for evaluation of a 10-month history of asymptomatic white patches that began on her neck and spread to the face and trunk. Notably, one month prior to developing these cutaneous manifestations, the patient was diagnosed with COVID-19 after SARS-CoV-2 infection was confirmed by nasopharyngeal swab real-time polymerase chain reaction testing. During this time, she experienced COVID-19-related symptoms, including fatigue and dyspnea that did not require hospitalization but kept her from working for approximately 3 months. She reported first noticing the hypopigmented patches during her convalescence. The patient’s past medical history was significant for iatrogenic hypothyroidism that developed following thyroidectomy for a benign nodule. Her medications included levothyroxine and metoprolol. She had no personal or family history of rheumatologic or autoimmune conditions. On physical examination, macules and patches of central depigmentation and peripheral hypopigmentation ranging in size from 0.2 to 18 cm were observed on the neck, axilla, face, antecubital fossae, and inframammary and inguinal folds (Fig 1); there were confirmed with Wood’s lamp. A biopsy was not performed, as these findings were clinically consistent with a diagnosis of trichrome intertriginous vitiligo. Topical tacrolimus 0.1%, topical hydrocortisone 2.5%, and a 3-month course of oral dexamethasone treatment (4 mg twice weekly) were initiated. Upon return examination 7 months later, partial regimentation was evident on the face, anterolateral aspect of the neck, and inframammary and inguinal folds. There were no areas of new involvement at this time.
Fig 1.
Vitiligo. Macules and patches of central depigmentation and peripheral hypopigmentation were observed on the patient’s neck (A-C), antecubital fossae (D), axilla, face, and inframammary and inguinal folds (not pictured).
Discussion
The pathophysiology of vitiligo is thought to be autoimmune. This hypothesis is supported by the fact that patients with vitiligo often have a personal or family history of other autoimmune conditions, as well as studies demonstrating both increased levels of oxidative stress markers and other autoimmune indicators such as CD8+ cytotoxic T cells specific to melanocytes.4 In autoimmune disease, oxidative stress may be induced by some outside “trigger,” such as exposure to environmental toxins, chemical agents, and UV radiation.4,5 Viral infections have also been implicated as potential triggers. The development of vitiligo has been shown to be associated with viral infections such as cytomegalovirus, Epstein-Barr virus, HIV, and many others.2 Proposed mechanisms for HIV-associated development of vitiligo include direct viral infection of melanocytes, overproduction of inflammatory cytokines leading to oxidative stress, and viral disruption of CD4+ and CD8+ T cell balance.2 This latter mechanism is plausible given the increased levels of CD8+ cytotoxic T cells observed in vitiligo, as mentioned previously.2
While much is still unknown about long-term sequelae of infection with SARS-CoV-2, emerging evidence suggests potential autoimmune complications. Recent literature describes the development of multiple autoimmune conditions following COVID-19, including systemic lupus erythematosus, autoimmune hemolytic anemia and thrombocytopenia, Guillain-Barré syndrome, and Kawasaki disease.3,6 Multiple possible etiologies have been proposed to explain these post-COVID-19 autoimmune phenomena. For example, severe infection with SARS-CoV-2 can cause transient immunosuppression and dysregulation of CD8+ lymphocytes, which then allows for inappropriate immune reconstitution and disrupts previously established tolerance to self-antigens.6 Similar dysregulation of CD8+ T cells has been described in the pathogenesis of vitiligo, particularly in association with HIV infection.2 Hence, this may represent a common mechanism between postviral autoimmunity and the development of vitiligo.
Oxidative stress is another potential catalyst in the development of post-COVID-19 autoimmune disease. Notably, oxidative stress has been implicated in the pathogenesis of disease resulting from respiratory viruses, such as the influenza virus, human respiratory syncytial virus, and the rhinovirus family.7 Infection with SARS-CoV-2 can stimulate overactivity of the immune response and subsequent overproduction of reactive oxygen species.7 Severely dysregulated immune responses due to SARS-CoV-2 infection can lead to organ damage via “cytokine storm,” or the overproduction of inflammatory cytokines and overactivation of immune cells. It is hypothesized that the subsequent, excessive oxidative stress produced by this process contributes to the development of COVID-19 sequelae, such as acute respiratory distress syndrome.7 Given that oxidative stress has also been described in the pathogenesis of vitiligo, this suggests another potential linkage between vitiligo and COVID-19.
Oftentimes the “stressor” that triggers vitiligo remains unknown; it is therefore possible that this case presents as a timely coincidence. However, our patient’s age (52 years) is above the average age of 20 to 30 years for the initial presentation of vitiligo.5 Further, patients of advanced age (>50 years) with new-onset vitiligo are more likely to have a family history of vitiligo and/or personal history of other autoimmune disorders, which was not the case.8 Thus, this case demonstrates new-onset vitiligo following confirmed infection with SARS-CoV-2. Of interest, there have been multiple cases reporting new-onset of vitiligo within several days to 1 week after patients received first doses of COVID-19 messenger RNA vaccinations.9,10 We postulate that this patient’s atypical history and sequential relationship of SARS-CoV-2 infection to onset of vitiligo, in conjunction with the existence of robust pathophysiologic theories to explain the phenomenon, support the diagnosis of COVID-induced vitiligo.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Alikhan A., Felsten L.M., Daly M., Petronic-Rosic V. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65(3):473–491. doi: 10.1016/j.jaad.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Dwivedi M., Laddha N.C., Begum R. Viral causes of vitiligo: a new perspective for vitiligo pathogenesis. Virol Immunol J. 2018;2(8) doi: 10.23880/VIJ-16000181. [DOI] [Google Scholar]
- 3.Galeotti C., Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16(8):413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rashighi M., Harris J.E. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35(2):257–265. doi: 10.1016/j.det.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergqvist C., Ezzedine K. Vitiligo: a review. Dermatology. 2020;236(6):571–592. doi: 10.1159/000506103. [DOI] [PubMed] [Google Scholar]
- 6.Cañas C.A. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med Hypotheses. 2020;145 doi: 10.1016/j.mehy.2020.110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernyak B.V., Popova E.N., Prikhodko A.S., Grebenchikov O.A., Zinovkina L.A., Zinovkin R.A. COVID-19 and oxidative stress. Biochemistry (Mosc) 2020;85(12):1543–1553. doi: 10.1134/S0006297920120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dogra S., Parsad D., Handa S., Kanwar A.J. Late onset vitiligo: a study of 182 patients. Int J Dermatol. 2005;44(3):193–196. doi: 10.1111/j.1365-4632.2004.01948.x. [DOI] [PubMed] [Google Scholar]
- 9.Ciccarese G., Drago F., Boldrin S., Pattaro M., Parodi A. Sudden onset of vitiligo after COVID-19 vaccine. Dermatol Ther. 2022;35(1) doi: 10.1111/dth.15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaminetsky J., Rudikoff D. New-onset vitiligo following mRNA-1273 (Moderna) COVID-19 vaccination. Clin Case Rep. 2021;9(9) doi: 10.1002/ccr3.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]