Abstract
Introduction
Pregnant women will benefit from research on immunization during pregnancy because they will have more accurate information on the SARS-CoV-2 vaccine. The purpose of this study was to determine the risk factors and pregnant women's desire to get the SARS-CoV-2 vaccine in various countries.
Methods
A search of PubMed, ProQuest, and EBSCO for related publications published (January and December 2021) on risk factors and pregnant women's desire to get the SARS-CoV-2 vaccine in various countries. The Pooled Odds Ratio (POR) were calculated using fixed and random-effect analysis. The I-squared formula was used to calculate the heterogeneity. Egger's and Begg's tests were used to identify study bias. STATA 16.0 was used for data analysis.
Results
This study revealed good practice has the highest POR (8.99), followed by received influenza vaccine last year (2.72), high perception of SARS-CoV-2 vaccine (2.70), >35 years (2.01), sufficient information about the SARS-COV-2 vaccine (1.94), higher school education (1.84), and third trimester (1.35) with pregnant women's desire toward the SARS-CoV-2 vaccination. The heterogeneity analysis revealed homogenous among risk factors in >35 years, high perception of SARS-CoV-2 vaccine, good practice, and third trimester (I2 ≤ 50%). In the articles combined in this study, there was no indication of study bias.
Conclusion
The insights of this study might help the authorities in determining the most effective strategy to deploy SARS-CoV-2 mass immunization campaigns for pregnant women.
Keywords: Pregnant women, Risk factors, SARS-CoV-2, Vaccine, Willingness
1. Introduction
Many people have died as a condition of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak.1 Massive human death has resulted in public health issues, overwhelmed health systems, interrupted distribution chains and the economy.2 , 3 Pregnant women may be more vulnerable to SARS-CoV-2 infection than non-pregnant women.4, 5, 6 Severe illness is defined as a condition that necessitates hospitalization, intensive care, the use of a ventilator, special breathing equipment, and/or death. According to certain studies, infection with the SARS-CoV-2 in pregnant mothers is related to a higher chance of a severe illness course, requiring invasive ventilation or ecmo, and/or death.5 , 6
In another research, a link between SARS-CoV-2 and the likelihood of preterm and cesarean births was discovered.7 Vertical transmission of the virus, which can induce hydrops fetalis and fetal death.8 , 9 Children are more vulnerable to asymptomatic illnesses, but they also carry the SARS-COV-2 virus, which they may pass on to others, especially pregnant women.10 , 11 Pregnant women who are infected with SARS-CoV-2 have a higher risk of preterm delivery and other poor pregnancy outcomes than pregnant women who are not infected with SARS-CoV-2.1 It's also important to note that the SARS-CoV-2 pandemic leads pregnant women to be concerned about their fetus's and personal health, which has a substantial impact on their well-being.12
Immunization during pregnancy research will give information on vaccine safety and efficacy concerns. It has been produced information for healthcare practitioners and patients on how to utilize it in counseling.13 Unfortunately, insufficient study has been conducted to establish the global population's sentiments regarding vaccination among pregnant women. To our knowledge, no previously published study has been subjected to meta-analysis.
2. Materials and methods
2.1. Study design and research sample
A meta-analysis studies were undertaken to review current studies related to risk factors and pregnant women's desire to get the SARS-CoV-2 vaccine in various countries. This study follows the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines.14
2.2. Eligibility criteria
Original articles having a cross-sectional study design, English language, and human participants as study subjects were only considered for inclusion. Exclusion criteria for the study included the unavailability of a full text version, irrelevant topics, and data from articles that could not be used for further evaluation.
2.3. Search approach and study collection
A search of PubMed, ProQuest, and EBSCO for related publications published (January and December 2021) with keywords (“pregnant women” AND “COVID-19” OR “coronavirus” OR “SARS-CoV-2” AND “vaccine” AND “acceptance”). In this study, pregnant women's desire toward the SARS-CoV-2 vaccination was the dependent variable. The independent variables were the risk factors of SARS-CoV-2 vaccine willingness. The literature quest was carried out by two independent investigators. After the initial search, the duplicates were manually deleted, and the title/abstracts were screened for possible relevance. Following that, the full-texts of possible papers were evaluated using the criterion.
2.4. Data extraction
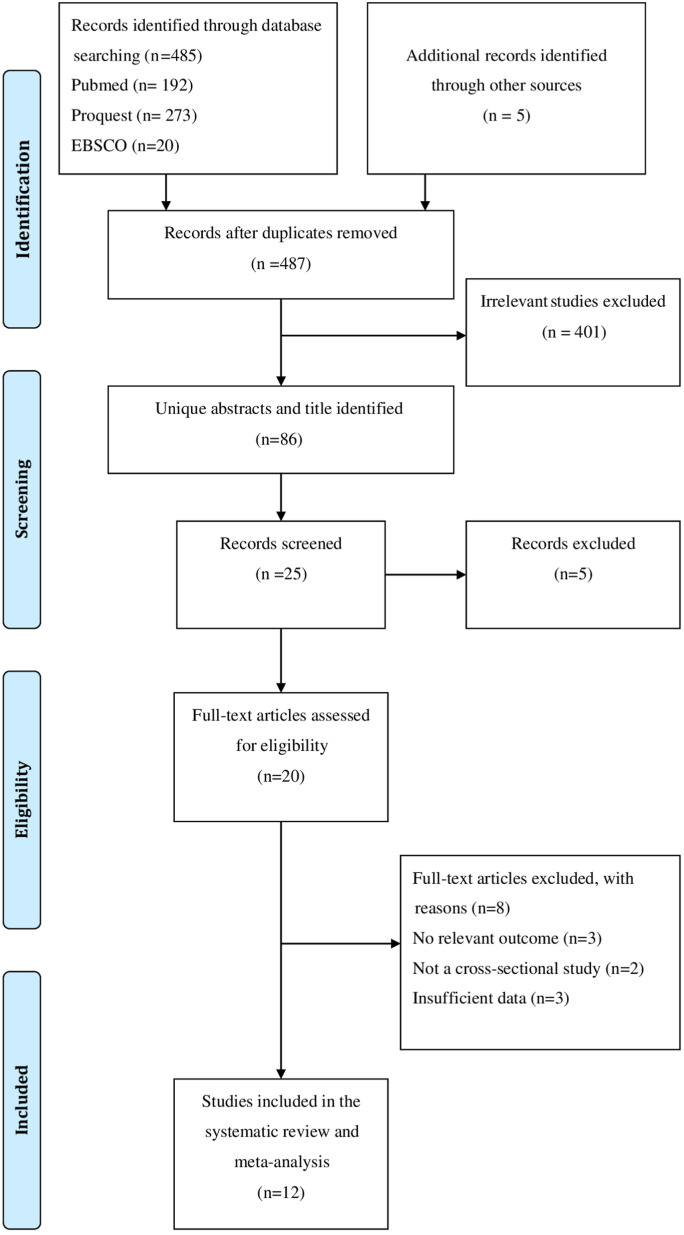
Data was retrieved by two separate authors using structured extraction forms. The quality of the publications was evaluated using the Newcastle-Ottawa Quality Assessment Scale (NOS). Articles were categorized into low, medium, and high quality groups using the numbers 0–3, 4–6, and 7–9.15 PRISMA flowcharts were used to show the stages required in looking for study publications (Fig. 1 ).
Fig. 1.
PRISMA flow diagram.
2.5. Data analysis
The Pooled Odds Ratio (POR) from the obtained data was calculated with a 95% confidence interval for further data analysis. I2 indicates that there was heterogeneity between publications if it was greater than 50%. The random effect analysis was used if the outcome was heterogeneous, and the fixed effect analysis was used if it was homogenous. Furthermore, the results were summarized as forest plots, and Egger's and Begg's tests were used to examine study bias. There was no publication bias among the studies, according to the p > 0.05 findings of the two tests. The role of covariate in lower middle income countries (LMICs) was investigated using restricted-maximum likelihood random effects meta-regression. Processing and analysis of all data, STATA 16.0 was used.
3. Results
A total of 12 recent studies were considered in this systematic review (Table 1 ). There were 15,444 participants in all that participated in the study.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27
Table 1.
Systematic review of pregnant women's willingness toward the SARS-CoV-2 vaccination and associated factors.
| 1st author | Year | Study location | Study design | Sample size | Risk factors (OR, 95% CI) | NOS |
|---|---|---|---|---|---|---|
| Goncu Ayhan et al.16 | 2021 | Ankara, Turkey | Cross-sectional | 300 | Sufficient information about the SARS-CoV-2 vaccine (2.07, 1.22–3.51) | 7 |
| Battarbee et al.17 | 2021 | U.S. | Cross-sectional multicenter | 915 | Higher school education (2.40, 1.30–4.70) Received influenza vaccine last year (2.60, 1.90–3.60) |
8 |
| Levy et al.18 | 2021 | New York | Survey study | 653 | Sufficient information about the SARS-CoV-2 vaccine (30.95, 9.55–100.33) | 7 |
| Geoghegan et al.19 | 2021 | Dublin, Ireland | Online survey | 300 | >35 years old (1.36, 0.80–2.32) Higher school education (1.78, 1.09–2.92) |
6 |
| Hailemariam et al.20 | 2021 | Southwest Ethiopia | Cross-sectional | 412 | >35 years old (6.73, 3.84–11.79) Higher school education (5.87, 3.14–10.97) High perception of SARS-CoV-2 vaccine (4.35, 2.73–6.95) |
7 |
| Mose et al.21 | 2021 | Southwest Ethiopia | Cross-sectional | 396 | >35 years old (2.55, 1.06–6.08) Higher school education (3.28, 1.92–5.59) Sufficient information about the SARS-CoV-2 vaccine (3.24, 1.78–5.89) Good practice (9.15, 8.73–12.19) |
7 |
| Nguyen et al.22 | 2021 | Vietnam | Cross-sectional | 651 | Higher school education (1.98, 1.24–3.14) High perception of SARS-COV-2 vaccine (2.71, 1.93–3.82) |
7 |
| Skjefte et al.23 | 2021 | U.S., U.K., India, Brazil, Russia, Spain, Argentina, Colombia, Mexico, Peru, South Africa, Italy, Chile, Philippines, Australia and New Zealand | Cross-sectional | 5,294 | Higher school education (1.31, 1.12–1.54) Received influenza vaccine last year (3.29, 2.91–3.72) |
8 |
| Stuckelberger et al.24 | 2021 | Switzerland | Cross-sectional | 1,551 | >35 years old (2.00, 1.30–3.00) Higher school education (1.70, 1.30–2.20) Received influenza vaccine last year (3.60, 2.80–4.70) Third trimester (1.40,1.00–2.00) |
7 |
| Sutton et al.25 | 2021 | U.S. | Online survey | 1,012 | Received influenza vaccine last year (2.25, 1.66–3.05) | 7 |
| Tao et al.26 | 2021 | China | Cross-sectional | 1,392 | Higher school education (2.85, 1.45–5.59) Sufficient information about the SARS-CoV-2 vaccine (1.05, 1.01–1.10) Third trimester (1.49,1.03–2.16) |
7 |
| Wang et al.27 | 2021 | China | Cross-sectional | 2,568 | Sufficient information about the SARS-CoV-2 vaccine (2.63, 1.38–5.00) Received influenza vaccine last year (1.81, 1.18–2.80) High perception of SARS-CoV-2 vaccine (2.48, 1.83–3.35) Good practice (8.27, 5.35–12.77) Third trimester (1.27, 0.98–1.65) |
8 |
| Total samples | 15,444 | |||||
| NOS score | 7.17 ± 0.58 |
Abbreviation: CI = confidence interval; OR = odds ratio; NOS, Newcastle–Ottawa Quality Assessment Scale.
Table 1 is based on a review of 12 cross-sectional studies that looked at risk factors and pregnant women's desire to have the SARS-CoV-2 vaccine in various countries. This research revealed variables related to willingness of SARS-CoV-2 vaccination among pregnant women >35 years, higher school education, sufficient information about the SARS-COV-2 vaccine, high perception, good practice, received influenza vaccine last year and third trimester.
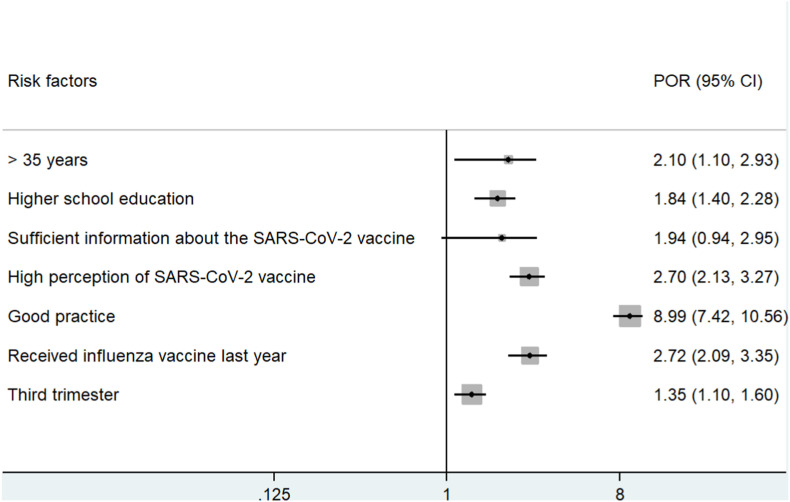
Meta-estimate of willingness of pregnant women toward the SARS-CoV-2 vaccination and associated factors (Table 2 and Fig. 2 ).
Table 2.
Meta-estimate of pregnant women's willingness toward the SARS-CoV-2 vaccination and associated factors.
| Risk factors | 1st author | OR (95% CI) | POR (95% CI) | Heterogeneity |
|
|---|---|---|---|---|---|
| I2 (%) | p-value | ||||
| >35 years | 2.01 (1.10–2.93) | 45.29 | 0.35 | ||
| Geoghegan et al.19 | 1.36 (0.80–2.32) | ||||
| Hailemariam et al.20 | 6.73 (3.84–11.79) | ||||
| Mose et al.21 | 2.55 (1.06–6.08) | ||||
| Stuckelberger et al.24 | 2.00 (1.30–3.00) | ||||
| Higher school education | 1.84 (1.40–2.28) | 51.98 | 0.02 | ||
| Battarbee et al.17 | 2.40 (1.30–4.70) | ||||
| Geoghegan et al.19 | 1.78 (1.09–2.92) | ||||
| Hailemariam et al.20 | 5.87 (3.14–10.97) | ||||
| Mose et al.21 | 3.28 (1.92–5.59) | ||||
| Nguyen et al.22 | 1.98 (1.24–3.14) | ||||
| Skjefte et al.23 | 1.31 (1.12–1.54) | ||||
| Stuckelberger et al.24 | 1.70 (1.30–2.20) | ||||
| Tao et al.26 | 2.85 (1.45–5.59) | ||||
| Sufficient information about the SARS-COV-2 vaccine | 1.94 (0.94–2.95) | 61.70 | 0.02 | ||
| Goncu Ayhan et al.16 | 2.07 (1.22–3.51) | ||||
| Levy et al.18 | 30.95 (9.55–100.33) | ||||
| Mose et al.21 | 3.24 (1.78–5.89) | ||||
| Tao et al.26 | 1.05 (1.01–1.10) | ||||
| Wang et al.27 | 2.63 (1.38–5.00) | ||||
| High perception of SARS-CoV-2 vaccine | 2.70 (2.13–3.27) | 0 | 0.26 | ||
| Hailemariam et al.20 | 4.35 (2.73–6.95) | ||||
| Nguyen et al.22 | 2.71 (1.93–3.82) | ||||
| Wang et al.27 | 2.48 (1.83–3.35) | ||||
| Good practice | 8.99 (7.42–10.56) | 0 | 0.67 | ||
| Mose et al.21 | 9.15 (8.73–12.19) | ||||
| Wang et al.27 | 8.27 (5.35–12.77) | ||||
| Received influenza vaccine last year | 2.72 (2.09–3.35) | 74.92 | <0.001 | ||
| Battarbee et al.17 | 2.60 (1.90–3.60) | ||||
| Skjefte et al.23 | 3.29 (2.91–3.72) | ||||
| Stuckelberger et al.24 | 3.60 (2.80–4.70) | ||||
| Sutton et al.25 | 2.25 (1.66–3.05) | ||||
| Wang et al.27 | 1.81 (1.18–2.80) | ||||
| Third trimester | 1.35 (1.10–1.60) | 0 | 0.78 | ||
| Stuckelberger et al.24 | 1.40 (1.00–2.00) | ||||
| Tao et al.26 | 1.49 (1.03–2.16) | ||||
| Wang et al.27 | 1.27 (0.98–1.65) | ||||
Abbreviation: CI = confidence interval; OR = odds ratio; POR= Pooled odds ratio; I2 > 50%, heterogeneity.
Fig. 2.
Forest plots of the risk factors and pregnant women's willingness toward the SARS-CoV-2 vaccination in various countries.
Table 2 and Fig. 2 revealed good practice has the highest POR (95% CI) (8.99, 7.42–10.56), followed by received influenza vaccine last year (2.72, 2.09–3.35), high perception of SARS-CoV-2 vaccine (2.70, 2.13–3.27), >35 years (2.01, 1.10–2.93), sufficient information about the SARS-COV-2 vaccine (1.94, 0.94–2.95), higher school education (1.84, 1.40–2.28), and third trimester (1.35, 1.10–1.60) with desire of pregnant women toward the SARS-CoV-2 vaccination. The heterogeneity analysis revealed homogenous among risk factors in >35 years, high perception, good practice, and third trimester (I2 ≤ 50%).
The findings of Egger's and Begg's tests to identify study bias (Table 3 ).
Table 3.
The findings of Egger's and Begg's tests to identify study bias.
| Risk factors | Study bias |
|
|---|---|---|
| Egger's test | Begg's test | |
| >35 years | 0.766 | 0.857 |
| Higher school education | 0.934 | 0.054 |
| Sufficient information about the SARS-CoV-2 vaccine | 0.349 | 0.120 |
| High perception of SARS-CoV-2 | 0.445 | 0.065 |
| Good practice | 0.890 | 0.700 |
| Received influenza vaccine last year | 0.530 | 0.132 |
| Third trimester | 0.357 | 0.106 |
p > 0.05, no publication bias.
Table 3 revealed that based on the results of Egger's and Begg's tests (p > 0.05), an associated factors of >35 years, higher school education, sufficient information, high perception, good practice, received influenza vaccine last year, and third trimester had no study bias among publications included.
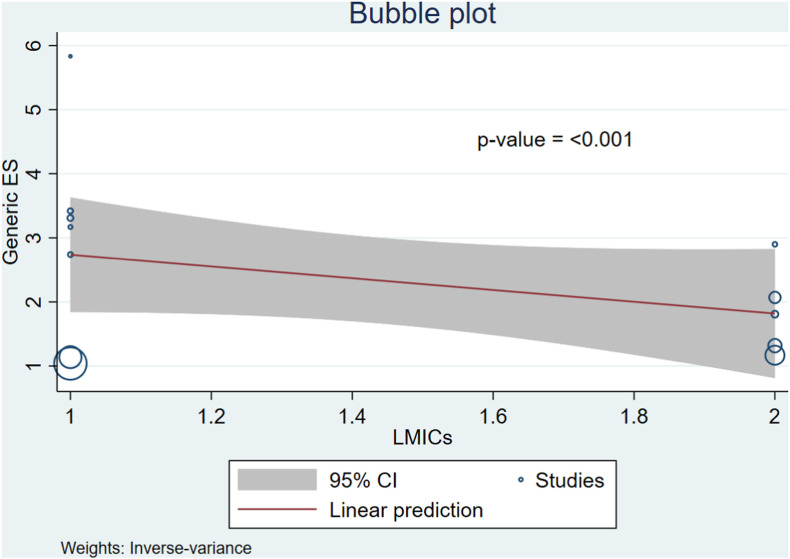
The relationship between LMICs and pregnant women's desire toward the SARS-CoV-2 vaccination based on meta-regression (Fig. 3 ).
Fig. 3.
The relationship between LMICs and pregnant women's desire to get vaccinated against SARS-CoV-2 based on meta-regression.
Fig. 3 revealed that the relationship between LMICs and decreased pregnant women's desire toward the SARS-CoV-2 vaccination (p=<0.001). This study found that pregnant women's desire toward the SARS-CoV-2 vaccination varies depending on the country type.
4. Discussion
Our findings revealed that pregnant women who received the SARS-CoV-2 immunization had a high level of practice about the vaccine. Vaccine practice rates can support in the planning of activities and initiatives that will assist increase knowledge and reassure people about the safety and advantages of vaccines, which will help prevent the spread of the virus and minimize the negative impacts of this historic pandemic.28 , 29 Identification of practice and vaccination rates for SARS-CoV-2 vaccines can assist in the selection of the most effective communication method for boosting vaccination confidence.30 Because mothers have the biggest effect on whether or not to vaccinate their children and other family members, it is equally critical to assess trust and the most important determinants of pregnant women's vaccination acceptance.23 , 31
According to the results of this investigation, there is a link between receiving influenza vaccine last year and pregnant women's acceptance of SARS-CoV-2 immunization. Women who had previously received an influenza vaccination reacted better to the SARS-CoV-2 vaccination. Women who were hesitant to obtain a SARS-CoV-2 vaccine expressed worries about vaccine safety and efficacy. Almost all pregnant women chose their obstetrician/gynecologist as their most trusted source of SARS-CoV-2 facts, with >40% choosing their gynecologist.23
The desire to get the SARS-CoV-2 vaccine was linked to high perception. This finding is consistent with prior studies in which high perception or sensitivity was linked to acceptance and willingness COVID-19 vaccination.32 , 33 Furthermore, women who saw COVID-19 as a danger among their acquaintances were more likely to get the immunization than others.
Our research also found that as people become older, they are more likely to develop chronic conditions including hypertension, renal disease, and heart disease, which can lower a pregnant woman's immunity and raise the risk of SARS-CoV-2 related morbidity and death. As a result, it may instill fear in the elderly population, leading to a greater willingness to receive the SARS-CoV-2 vaccination.21
When compared to pregnant women who had limited understanding about SARS-CoV-2, those who had high information were more likely to receive SARS-CoV-2 vaccine. This might be explained by the fact that pregnant women with good understanding of SARS-CoV-2 would be aware of the virus's severity to themselves and their fetus, allowing them to readily receive SARS-CoV-2 vaccination to mitigate the pandemic's effects.34
Higher-educated pregnant women were more likely to wish to acquire the SARS-CoV-2 vaccine. Previous research have found a significant level of concern against the SARS-CoV-2 immunization among the less educated. This might be because more educated people have easier access to vaccination facts and are better able to interpret facts about the SARS-CoV-2 vaccine's advantages and risks. On the contrary, vaccination misinformation is more likely to affect persons who are less informed.35
We revealed that being pregnant in the second trimester was a negative predictor of SARS-CoV-2 vaccination uptake when compared to the third trimester, indicating a potential concern of caused fetal abnormalities. Fear of any potential detrimental negative consequences of the vaccination on their pregnancy or newborn, as well as worries about safety and advantages, have been identified as important factors for vaccine aversion in various studies.17 , 36
The essential battle against the pandemic is to figure out what factors influence pregnant women's willingness to get COVID-19 vaccines.20 As more information on the safety and effectiveness of the COVID-19 immunization becomes known, immunization acceptance, perceived motivators, and barriers to acceptance may change among pregnant women.37 Acceptance of the COVID-19 vaccination and its determinants among pregnant women differs throughout the world. As a result, vaccination efforts targeting this community should be tailored to the needs of each country in order to have the most effect.38 , 39
Our study's strength was performed in various countries. As a result, this is a good setting for investigating potential relationships between pregnant women's willingness toward the SARS-CoV-2 vaccination among LMICs and non-LMICs. There are a few limitations in this meta-analysis study. Three publications appeared to be acceptable in this study, but they lacked sufficient evidence and yielded negligible data estimation findings. The possibility of selection bias will be exacerbated as a result of this issue. Furthermore, we excluded articles written in other languages. This may limit epidemiological data from nations that do not speak English.
The findings suggest that health authorities should establish immediate promotion of health programs and distribute more accurate information. Authorities should take steps to ensure that individuals have access to sufficient facts, adopt positive vibes, and hold high perception about SARS-CoV-2 vaccinations. Another recommendation is for obstetric care specialists to make a clear advice to pregnant women to get the vaccination. This will likely enhance COVID-19 vaccine uptake. Apart from that, community-based engagement activities may be necessary to adapt pregnant women education materials and increase communication and shared decision-making in order to accomplish universal health coverage in pregnant immunization.
5. Conclusion
This finding results revealed the risk factors for pregnant women's willingness toward the SARS-CoV-2 vaccination, good practice has the highest risk, followed by received influenza vaccine last year, high perception, >35 years, sufficient information, higher school education and third trimester. The heterogeneity analysis revealed homogenous among risk factors in >35 years, high perception, good practice, and third trimester. The insights of this research might help the authorities in determining the most effective strategy to deploy SARS-CoV-2 mass immunization campaigns for pregnant women.
Funding/financial support
This research received no funding from public, private, or non-profit sources.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank Riyani Betri Novialita, MA, for her help with data collecting.
References
- 1.Nindrea R.D., Usman E., Katar Y., Sari N.P. Acceptance of COVID-19 vaccination and correlated variables among global populations: a systematic review and meta-analysis. Clin Epidemiol Glob Health. 2021;12:100899. doi: 10.1016/j.cegh.2021.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nindrea R.D., Sari N.P. How does family planning services respond to the SARS-CoV-2 pandemic in Indonesia? Asia Pac J Publ Health. 2021;2021:1–2. doi: 10.1177/10105395211051254. [DOI] [PubMed] [Google Scholar]
- 3.Nindrea R.D., Usman E., Firdawati Sari NP. The challenges: management of infectious medical waste during the pandemic COVID-19 in health care facilities in Indonesia. Asia Pac J Publ Health. 2021;33(5):681–682. doi: 10.1177/10105395211014697. [DOI] [PubMed] [Google Scholar]
- 4.Kotlar B., Gerson E., Petrillo S., Langer A., Tiemeier H. The impact of the COVID-19 pandemic on maternal and perinatal health: a scoping review. Reprod Health. 2021;18(1):10. doi: 10.1186/s12978-021-01070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellington S., Strid P., Tong V.T., et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, january 22-june 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):769–775. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin J., Byström E., Carnahan A., Ahrne M. Public Health Agency of Sweden's Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99(7):819–822. doi: 10.1111/aogs.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury R., Bernstein P.S., Debolt C., et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York city medical centers. Obstet Gynecol. 2020;136(2):273–282. doi: 10.1097/AOG.0000000000004025. [DOI] [PubMed] [Google Scholar]
- 8.Ferraiolo A., Barra F., Kratochwila C., et al. Report of positive placental swabs for SARS-CoV-2 in an asymptomatic pregnant woman with COVID-19. Medicina (Kaunas) 2020;56(6):306. doi: 10.3390/medicina56060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popescu D.E., Cioca A., Muresan C., et al. A case of COVID-19 pregnancy complicated with hydrops fetalis and intrauterine death. Medicina (Kaunas). 2021;57(7):667. doi: 10.3390/medicina57070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta N.S., Mytton O.T., Mullins E.W.S., et al. SARS-CoV-2 (COVID-19): what do we know about children? A systematic review. Clin Infect Dis. 2020;71(9):2469–2479. doi: 10.1093/cid/ciaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludvigsson J.F. Children are unlikely to be the main drivers of the COVID-19 pandemic - a systematic review. Acta Paediatr. 2020;109(8):1525–1530. doi: 10.1111/apa.15371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortazavi F., Mehrabadi M., KiaeeTabar R. Pregnant women's well-being and worry during the COVID-19 pandemic: a cross-sectional study. BMC Pregnancy Childbirth. 2021;21(1):59. doi: 10.1186/s12884-021-03548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chervenak F.A., McCullough L.B., Bornstein E., et al. Professionally responsible coronavirus disease 2019 vaccination counseling of obstetrical and gynecologic patients. Am J Obstet Gynecol. 2021;224(5):470–478. doi: 10.1016/j.ajog.2021.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions:explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells G.A., Shea B., O'Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Last cited Feb 28, 2021]. Available from:
- 16.Goncu Ayhan S., Oluklu D., Atalay A., et al. COVID-19 vaccine acceptance in pregnant women. Int J Gynaecol Obstet. 2021;154(2):291–296. doi: 10.1002/ijgo.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battarbee A.N., Stockwell M.S., Varner M., et al. Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during august-december 2020. Am J Perinatol. 2021;2021:1–22. doi: 10.1055/s-0041-1735878. [DOI] [PubMed] [Google Scholar]
- 18.Levy A.T., Singh S., Riley L.E., Prabhu M. Acceptance of COVID-19 vaccination in pregnancy: a survey study. Am J Obstet Gynecol MFM. 2021;3(5):100399. doi: 10.1016/j.ajogmf.2021.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geoghegan S., Stephens L.C., Feemster K.A., Drew R.J., Eogan M., Butler K.M. "This choice does not just affect me." Attitudes of pregnant women toward COVID-19 vaccines: a mixed-methods study. Hum Vaccin Immunother. 2021;17(10):3371–3376. doi: 10.1080/21645515.2021.1924018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hailemariam S., Mekonnen B., Shifera N., et al. Predictors of pregnant women's intention to vaccinate against coronavirus disease 2019: a facility-based cross-sectional study in southwest Ethiopia. SAGE Open Med. 2021;9 doi: 10.1177/20503121211038454. 20503121211038454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mose A., Yeshaneh A. COVID-19 vaccine acceptance and its associated factors among pregnant women attending antenatal care clinic in Southwest Ethiopia: institutional-based cross-sectional study. Int J Gen Med. 2021;14:2385–2395. doi: 10.2147/IJGM.S314346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen L.H., Hoang M.T., Nguyen L.D., et al. Acceptance and willingness to pay for COVID-19 vaccines among pregnant women in Vietnam. Trop Med Int Health. 2021;26(10):1303–1313. doi: 10.1111/tmi.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skjefte M., Ngirbabul M., Akeju O., et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36(2):197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuckelberger S., Favre G., Ceulemans M., et al. SARS-CoV-2 vaccine willingness among pregnant and breastfeeding women during the first pandemic wave: a cross-sectional study in Switzerland. Viruses. 2021;13(7):1199. doi: 10.3390/v13071199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton D., D'Alton M., Zhang Y., et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am J Obstet Gynecol MFM. 2021;3(5):100403. doi: 10.1016/j.ajogmf.2021.100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao L., Wang R., Han N., et al. Acceptance of a COVID-19 vaccine and associated factors among pregnant women in China: a multi-center cross-sectional study based on health belief model. Hum Vaccin Immunother. 2021;17(8):2378–2388. doi: 10.1080/21645515.2021.1892432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang R., Tao L., Han N., et al. Acceptance of seasonal influenza vaccination and associated factors among pregnant women in the context of COVID-19 pandemic in China: a multi-center cross-sectional study based on health belief model. BMC Pregnancy Childbirth. 2021;21(1):745. doi: 10.1186/s12884-021-04224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brewer N.T., Chapman G.B., Gibbons F.X., Gerrard M., McCaul K.D., Weinstein N.D. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26(2):136–145. doi: 10.1037/0278-6133.26.2.136. [DOI] [PubMed] [Google Scholar]
- 29.Prematunge C., Corace K., McCarthy A., Nair R.C., Pugsley R., Garber G. Factors influencing pandemic influenza vaccination of healthcare workers--a systematic review. Vaccine. 2012;30(32):4733–4743. doi: 10.1016/j.vaccine.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Lin C., Tu P., Beitsch L.M. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines (Basel) 2020;9(1):16. doi: 10.3390/vaccines9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nindrea R.D., Usman E., Katar Y., Darma I.Y., Warsiti Hendriyani H., Sari N.P. Dataset of Indonesian women's reproductive, high-fat diet and body mass index risk factors for breast cancer. Data Brief. 2021;36:107107. doi: 10.1016/j.dib.2021.107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harapan H., Wagner A.L., Yufika A., et al. Willingness-to-pay for a COVID-19 vaccine and its associated determinants in Indonesia. Hum Vaccines Immunother. 2020;16(12):3074–3080. doi: 10.1080/21645515.2020.1819741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harapan H., Wagner A.L., Yufika A., et al. Acceptance of a COVID-19 vaccine in Southeast Asia: a crosssectional study in Indonesia. Front Public Health. 2020;8:381. doi: 10.3389/fpubh.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Crosta A., Ceccato I., Marchetti D., et al. Psychological factors and consumer behavior during the COVID-19 pandemic. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0256095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik A.A., McFadden S.M., Elharake J., Omer S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26:100495. doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutz C.S., Carr W., Cohn A., Rodriguez L. Understanding barriers and predictors of maternal immunization: identifying gaps through an exploratory literature review. Vaccine. 2018;36(49):7445–7455. doi: 10.1016/j.vaccine.2018.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geoghegan S., O'Callaghan K.P., Offit P.A. Vaccine safety: myths and misinformation. Front Microbiol. 2020;11:372. doi: 10.3389/fmicb.2020.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Figueiredo A., Simas C., Karafllakis E., Paterson P., Larson H.J. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898–908. doi: 10.1016/S0140-6736(20)31558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nindrea R.D., Sari N.P., Lazuardi L., Aryandono T. Validation: the use of google trends as an alternative data source for COVID-19 surveillance in Indonesia. Asia Pac J Publ Health. 2020;32(6-7):368–369. doi: 10.1177/1010539520940896. [DOI] [PMC free article] [PubMed] [Google Scholar]